Abstract
The goal of this article is to provide the reader a snapshot of recent studies on axonal actin—largely emerging from superresolution and live-imaging experiments—and place this new information in context with earlier studies.
Actin in axon shafts has been the black sheep of the neuronal actin family for decades. Although the creeping egress of actin at axonal growth cones or the fluctuating morphology of actin at dendritic spines have captured the imagination of generations of scientists, the patchy, focal distribution of actin along axon shafts, as seen by routine light microscopy, has been somewhat uninteresting. However recent superresolution studies and live-imaging experiments using F-actin probes have uncovered a dramatic world of axonal actin, replete with intricate architectural assemblies and surprisingly dynamic behaviors. These findings have led to entirely new conceptual models of actin anatomy and physiology in axons, complementing information on actin at growth cones and synapses. This short article will clarify three major axonal actin assemblies—actin “waves,” “rings,” and “trails”—two of which have been recognized only recently, and highlight some unanswered questions that have emerged as a result of new information.
One of the most abundant proteins in neurons, actin has established roles in axon elongation, signaling, and synaptic homeostasis. Although axonal growth cones are capable of limited local actin synthesis, the vast majority of neuronal actin is synthesized in the perikarya and conveyed into the axon via slow axonal transport, as shown by in vivo pulse-chase radiolabeling studies (Black and Lasek, 1979; Willard et al., 1979). Three axonal actin assemblies are briefly discussed here: actin waves, rings, and trails. Another actin assembly in developing axons, called actin patches, was reviewed recently (Arnold and Gallo, 2014) and is not discussed here. More details on neuronal actin in general can be found in recent reviews (Coles and Bradke, 2015; Kevenaar and Hoogenraad, 2015).
What are actin waves, rings, and trails?
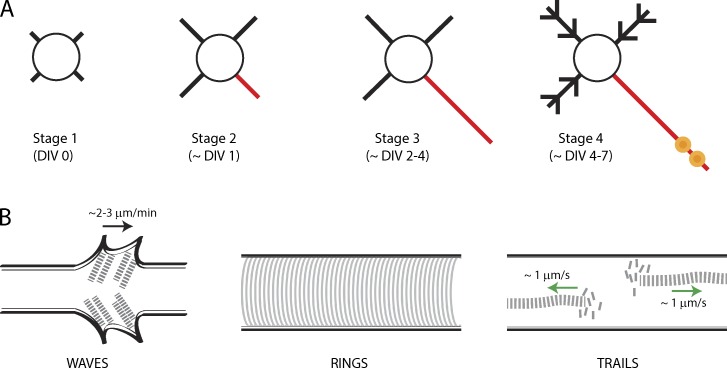
Actin assemblies have been best described in cultured hippocampal neurons where they can be visualized at high resolution, although most are also documented in situ. Immediately after plating, the cell bodies of these cultured neurons extend multiple processes (neurites); one of which differentiates into the axon while the others morph into dendrites (Fig. 1 A; Dotti et al., 1988). This model system has been a workhorse for neurobiologists and various neuronal actin assemblies have been characterized in the setting of this predictable pattern of differentiation.
Figure 1.
Various actin assemblies in axons. (A) Schematic depicting maturation of hippocampal neurons in culture. The circle represents the soma while the black lines represent neurites/dendrites. Red line denotes putative/actual axon and yellow circles represent presynaptic boutons. (B) Schematic of axonal actin assemblies described in the text. The black arrow (left) points anterogradely and the green arrows (right) indicate direction of actin polymer growth.
Actin waves are growth cone–like structures that emerge at the base of neurites, migrating slowly up to the tip, flaring the plasma membrane during transit (Fig. 1 B, left; Ruthel and Banker, 1998, 1999; Flynn et al., 2009; Katsuno et al., 2015). These waves move slowly, at ∼2–3 µm/min, but are strikingly periodic, with approximately one to two waves appearing every hour. Actin filaments within the waves fan out, with individual filaments generally oriented at acute angles to the long axis (Katsuno et al., 2015). Single filaments within a wave undergo directional treadmilling, with monomers added at filament tips and disassembled at the filament bases (Katsuno et al., 2015), much like F-actin dynamics at axonal growth cones and leading edges of migrating nonneuronal cells (Pollard and Borisy, 2003). Waves are critically dependent on actin dynamics, but are also disrupted by microtubule-depolymerizing agents (Ruthel and Banker, 1998). Indeed single microtubules extend into actin waves (Ruthel and Banker, 1998) and are enriched in doublecortin, a cytoskeletal-stabilizing protein that binds to both microtubules and actin (Tint et al., 2009).
Collectively, the data suggest an intricate interplay of actin and microtubule cytoskeleton in the biogenesis and progression of axonal actin waves, though many mechanistic details remain unclear. Interestingly, waves of actin have also been described in many nonneuronal cells including neutrophils, fibroblasts, keratinocytes, and Dictyostelium discoideum, where they are called traveling waves (t-waves; Allard and Mogilner, 2013). T-waves travel along the perimeter of these cells and bear striking resemblance to the actin waves described in neurons. Many interesting ideas have emerged from experiments in these nonneuronal cells, for instance, clues into processes that trigger the t-waves and the biophysical rules dictating wave generation and propagation (Allard and Mogilner, 2013). Some of these ideas (e.g., the role of membrane tension in wave initiation) may be particularly relevant to neurons, as actin waves mysteriously but consistently emerge from the somato-neuritic junction where membrane tension might be a factor. Unfortunately, none of these ideas have been seriously explored in neurons. Despite the fact that axonal actin waves were first described over 15 years ago, have been documented in situ, and have a precedence in other cell types, they have been often regarded as a “cell culture artifact,” which is a lost opportunity to learn more about this intriguing phenomenon.
In 2013, using stochastic optical reconstruction microscopy, Xu et al. (2013) reported periodic, circumferential lattices of F-actin and spectrin wrapping underneath the axonal plasma membrane, a previously unknown structure they called actin rings (Fig. 1 B, middle). Actin rings are spaced at ∼190 nm (below the resolution of light microscopy) and can only be clearly seen by superresolution imaging. The mechanism by which adjacent rings are held together at such precise intervals is intriguing. Data suggest that actin rings are connected by spectrin multimers that may act as “spacers” and that the capping protein adducin may stabilize the filaments (Xu et al., 2013; Leterrier et al., 2015). This intricate “wire mesh–type” assembly likely makes this structure stable, although ring components are still susceptible to actin-depolymerizing agents (Zhong et al., 2014). The lattice architecture of actin rings immediately suggests a structural role (discussed later) and their discovery is a major insight into the structure and function of actin in axons. However, most eukaryotic cells have both stable subplasmalemmal and flexible deep-actin assemblies. Although deep axonal actin filaments were observed in initial superresolution studies of fixed neurons, the reports were understandably focused on actin rings (Xu et al., 2013; D’Este et al., 2015). Thus it was unclear if the deep filaments were also stable static structures or if they represented a distinct, more dynamic pool.
We have also been looking at axonal actin, but by live imaging, using F-actin selective fluorescent probes. Using these methods we saw focal hotspots of F-actin in axons, spaced ∼3–4 µm apart, where actin undergoes continuous polymerization and depolymerization (Ganguly et al., 2015). These hotspots are a nidus for rapid F-actin assembly, with long actin filaments spurting bidirectionally along the axon shaft, a phenomenon we call actin trails (Ganguly et al., 2015). Actin trails are surprisingly dynamic and are exquisitely sensitive to actin-depolymerizing drugs but unaffected by microtubule-disrupting agents. Moreover, actin trails are dependent on a family of rapid actin nucleators and elongators called formins (Chesarone et al., 2010). However, only the elongation, and not the nucleation, of actin trails is formin dependent. How are the F-actin hotspots generated? We found that the hotspots colocalize with stationary axonal endosomes when visualized by two-color live imaging of F-actin and endosomal markers. F-actin nucleates on the surface of endosomes in nonneuronal cells (Hong et al., 2015), and we propose a model where axonal actin is nucleated on the surface of stationary axonal endosomes and subsequently elongated by formins, generating the actin trails. 3D stochastic optical reconstruction microscopy of axonal actin also revealed deep actin filaments that were distinct from the subplasmalemmal actin rings (Ganguly et al., 2015). Thus the data suggest a two-tier assembly of axonal actin, with subplasmalemmal rings and deep dynamic actin filaments.
How do the various neuronal actin assemblies arise and evolve during development?
It is intriguing that the same cytoskeletal protein gives rise to such morphologically distinct structures. Although mechanisms by which these assemblies initiate remain obscure, a closer examination of the underlying dynamic process offers some clues. Because the majority of axonal actin is synthesized in the perikarya and delivered into the axon shaft, it is likely that this dynamic pool participates in development and turnover of the myriad axonal actin assemblies. Therefore we think that it is important to consider this dynamism in models of axonal actin assemblies; however, this is rarely taken into account.
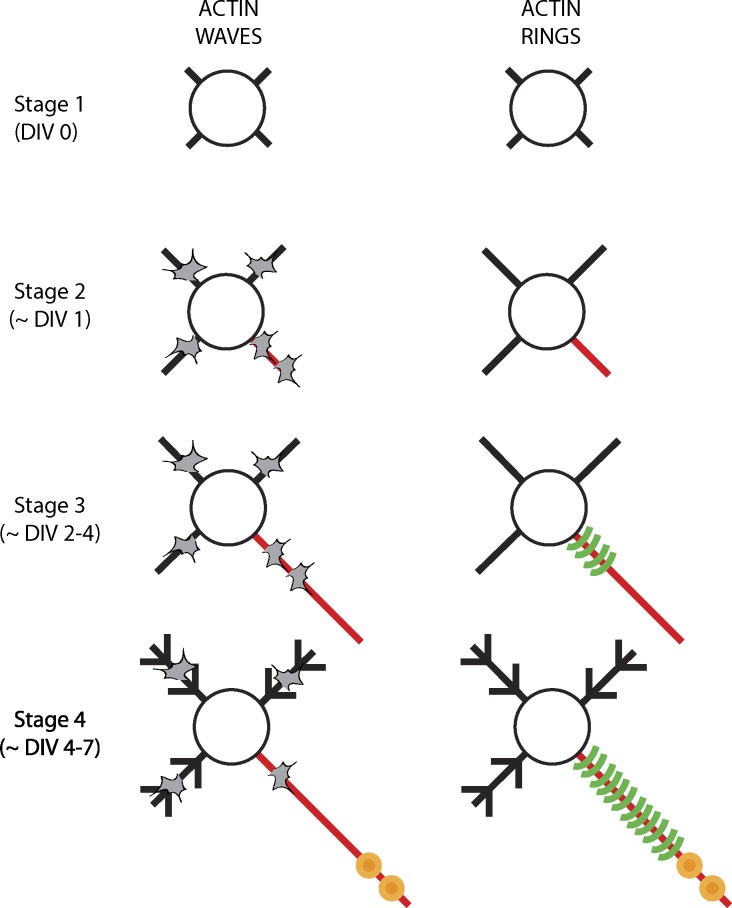
The evolution of actin waves and rings has been examined in cultured hippocampal neurons during development. Actin waves first appear at stage 2 (at ∼1 day in vitro [DIV 1]), where they can be seen in any of the developing neurites. However, prospective live imaging has shown that the waves are more frequent in the process that would later differentiate into the axon (Flynn et al., 2009; Fig. 2, left). As the neurites mature, actin waves continue to be relatively more frequent in axons, but by stage 4 (approximately DIV 4–7), their frequency and rate is diminished (Ruthel and Banker, 1999). We did not see actin waves in older (DIV 7–10) neurons, though our imaging conditions were not ideal to capture the slow kinetics of actin waves (Ganguly et al., 2015). The molecular mechanisms by which axonal actin waves originate at the soma–neurite junction are unclear. Though knockdown experiments have identified some individual components that may be involved in actin wave progression—cofilin, doublecortin, and shootin (Flynn et al., 2009; Tint et al., 2009; Katsuno et al., 2015)—the overall biophysical and biological principles triggering and driving this phenomenon have not garnered much attention.
Figure 2.
Evolution of actin assemblies in axons. (left) Schematic showing evolution of actin waves (gray shapes) in cultured hippocampal neurons; number of waves shown reflects relative frequency. Note that waves are seen in all processes but are more frequent in putative/actual axon until stage 3, after which their frequency diminishes. (right) Actin rings first appear in stage 3 neurons, but are restricted to the proximal axons. At stage 4 and later, the rings are seen along distal axonal shafts as well.
Components of the circumferential lattice structure in actin rings first appear in stage 3 (approximately DIV 2) neurons, but are restricted to the proximal axon. Over time (approximately DIV 6), the periodicity is seen all along the axon shaft, suggesting time-dependent propagation of the rings as the axons mature (Fig. 2, right; Zhong et al., 2014). One possibility is that new, fully assembled rings are synthesized in the soma and added at the base of the axon, essentially “pushing” the entire ring assembly toward the tip of the axon like a ratchet. However, this seems unlikely as ring components do not translocate when visualized directly by live imaging or indirectly by photobleaching experiments (Zhong et al., 2014). The other, more likely, possibility is that the rings are initiated and sustained by local actin pools in the axon, likely delivered and maintained by axonal transport. The rate of actin ring propagation in growing axons over several days (Fig. 2, right) is also consistent with the known slow axonal transport of actin. Interestingly, disassembly of dynamic actin trails (Ganguly et al., 2015) could provide a readily available pool of assembly-competent actin monomers all along the axon, and the trails themselves may represent the slow axonal transport of actin. Rigorous testing of these ideas will probably have to await a deeper understanding of actin transport mechanisms.
Actin trails have been described in DIV 7–10 neurons, but their evolution over the course of development has not been reported yet. However, there is some understanding of the initiating mechanisms. Specifically, the data advocate a model where F-actin nucleates on the surface of stationary axonal endosomes, generating the hotspots, and this F-actin is subsequently elongated by formins to generate linear polymers (actin trails). However, many questions remain. For instance, the evidence that F-actin nucleates on endosomes is based only on correlational light microscopy; and it is generally unclear how the hotspots are initiated or how the F-actin is stabilized there. The exact polarity (location of barbed ends) of the elongating actin trails is also unclear. Because F-actin hotspots and trails are the only dynamic events visible in axons, it seems likely that this dynamism would also generate the slow transport of this cytoskeletal protein. In that regard, though the rate of actin elongation is similar for both anterograde and retrograde trails, relative frequencies of anterograde actin trails in axons is greater (Ganguly et al., 2015). However, it is not clear how this would lead to a slow, biased actin transit, and a clearer understanding of actin transport would probably need a deconstruction of the mechanisms underlying the hotspots and the trails. Characteristics of the three actin assemblies are summarized in Table 1.
Table 1. Characteristics of the three actin assemblies.
| Waves | Rings | Trails | |
|---|---|---|---|
| Location | Undifferentiated neurites, axons, and dendritesa | Mainly axons, ∼10–20% dendritesb,c | Axonsd, ?dendrites unknown |
| Dynamics | Move at ∼2–3 µm/mina | None observede | Elongate at ∼1 µm/sd |
| Mode of actin polymerization | Treadmilling, nucleators (Arp2/3, formins) involvedf | Actin/spectrin scaffold, capping proteinsa | Nucleators (formins)d |
| Effect of actin-disrupting agents | Yesa | Yesb | Yesd |
| Effect of microtubule-disrupting agents | Yesa | Yese | Nod |
| Initiating triggers | ? | ? | ?Actin nucleation on endosomesd |
| Putative function | Deliver actin to developing growth conesa,f,g,h | Support axonal plasma membrane, sodium channels in axon initiation segmentb | Deliver actin to axons and synapses (axonal transport)d |
What are the functions of these actin assemblies?
Axonal actin waves have always been likened to growth cones. Many proteins in the actin waves are also identical to those in growth cones, further underlining the anatomical and molecular resemblance of these two structures (Katsuno et al., 2015). Indeed, the arrival of actin waves at growth cones leads to small but significant bursts of axon elongation (although they are not necessary for axon growth), suggesting that waves may help deliver actin to growing tips (Ruthel and Banker, 1999; Flynn et al., 2009; Katsuno et al., 2015). Because the velocity of actin waves is generally similar to the rates at which actin is conveyed in slow axonal transport, it has been proposed that the waves may represent slow transport (Ruthel and Banker, 1998; Flynn et al., 2009). However, several issues are worth considering. First, actin waves with similar overall kinetics are seen in both axons and dendrites, and even in undifferentiated neurites. Second, periodic waves of actin (t-waves) are also seen in a host of nonneuronal cells, where they result from activation and inhibition feedbacks in actin dynamics, resembling biochemical reaction–diffusion systems; and it’s not clear that this would lead to the transport of material over long distances. Third, the periodic nature of the waves, emerging from the axon base and moving up to the tip every ∼30 min, seems inconsistent with the single radiolabeled peak of actin transport seen in the in vivo pulse-chase experiments. Fourth, axonal transport is a constitutive process occurring throughout the life of the neuron, but the kinetics of actin waves diminish over time and it’s unclear if they are seen in more mature neurons with established axons and dendrites. Nevertheless, it is clear that further efforts are needed to understand this phenomenon and clarify its role.
Although the function of actin rings is not established, the architecture and location strongly suggest that it is a scaffold supporting the overlying plasma membrane (Xu et al., 2013). As axons can grow to enormous lengths and are probably under incredible mechanical stress, it seems reasonable that they have evolved specialized architectural elements to support themselves. Additionally, rings in the axon initiation segment may help tether sodium channels that are critical for initiating action potentials (Xu et al., 2013). Speculatively, actin rings along more distal axons may also help tether vesicles along the axon shaft, particularly stationary endosomes, thus, perhaps linking actin rings and trails. The function of actin trails is also not established. Our data suggest that these dynamic assemblies supply actin into presynaptic boutons and we posit that the overall kinetics are also involved in generating the slow biased transport of actin in axons. Interestingly, the remarkable dynamism of actin trails can create a scenario where free actin monomers are readily available throughout the axon shaft. Assuming that the monomers are assembly competent, they can be used to construct or replenish other axonal actin assemblies (for instance the actin rings) or to initiate anatomical fluctuations along the axon (such as filopodia or new branches). Finally, this mobile pool of actin is also well suited to supply actin to growth cones and support the myriad signaling cascades dependent on actin. Many of these ideas are now testable using live-imaging and superresolution microscopy.
After languishing in relative obscurity for years, axonal actin has come of age. Recent experiments have unraveled an intricate organization of actin along axon shafts, offering new and unexpected insights into neuroanatomy and neurobiology. Interestingly, the advances have almost entirely emerged from simply being able to visualize the static and dynamic architecture at a high-enough resolution, returning to the basic principles that laid the foundation of modern cell biology.
Acknowledgments
I thank many colleagues who have generously shared their knowledge and reagents, either directly or via Addgene.
Work in the Roy laboratory is currently supported by grants from the National Institute of Neurological Disorders and Stroke/National Institutes of Health (R01NS075233) and National Institute on Aging/National Institutes of Health (R01AG048218).
The authors declare no competing financial interests.
References
- Allard J., and Mogilner A.. 2013. Traveling waves in actin dynamics and cell motility. Curr. Opin. Cell Biol. 25:107–115. 10.1016/j.ceb.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D.B., and Gallo G.. 2014. Structure meets function: actin filaments and myosin motors in the axon. J. Neurochem. 129:213–220. 10.1111/jnc.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M.M., and Lasek R.J.. 1979. Axonal transport of actin: slow component b is the principal source of actin for the axon. Brain Res. 171:401–413. 10.1016/0006-8993(79)91045-X [DOI] [PubMed] [Google Scholar]
- Chesarone M.A., DuPage A.G., and Goode B.L.. 2010. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat. Rev. Mol. Cell Biol. 11:62–74. 10.1038/nrm2816 [DOI] [PubMed] [Google Scholar]
- Coles C.H., and Bradke F.. 2015. Coordinating neuronal actin–microtubule dynamics. Curr. Biol. 25:R677–R691. 10.1016/j.cub.2015.06.020 [DOI] [PubMed] [Google Scholar]
- D’Este E., Kamin D., Göttfert F., El-Hady A., and Hell S.W.. 2015. STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Reports. 10:1246–1251. 10.1016/j.celrep.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Dotti C.G., Sullivan C.A., and Banker G.A.. 1988. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8:1454–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn K.C., Pak C.W., Shaw A.E., Bradke F., and Bamburg J.R.. 2009. Growth cone-like waves transport actin and promote axonogenesis and neurite branching. Dev. Neurobiol. 69:761–779. 10.1002/dneu.20734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A., Tang Y., Wang L., Ladt K., Loi J., Dargent B., Leterrier C., and Roy S.. 2015. A dynamic formin-dependent deep F-actin network in axons. J. Cell Biol. 210:401–417. 10.1083/jcb.201506110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong N.H., Qi A., and Weaver A.M.. 2015. PI(3,5)P2 controls endosomal branched actin dynamics by regulating cortactin–actin interactions. J. Cell Biol. 210:753–769. 10.1083/jcb.201412127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuno H., Toriyama M., Hosokawa Y., Mizuno K., Ikeda K., Sakumura Y., and Inagaki N.. 2015. Actin migration driven by directional assembly and disassembly of membrane-anchored actin filaments. Cell Reports. 12:648–660. 10.1016/j.celrep.2015.06.048 [DOI] [PubMed] [Google Scholar]
- Kevenaar J.T., and Hoogenraad C.C.. 2015. The axonal cytoskeleton: from organization to function. Front. Mol. Neurosci. 8:44 10.3389/fnmol.2015.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C., Potier J., Caillol G., Rueda Boroni F., and Dargent B.. 2015. Nanoscale architecture of the axon initial segment reveals an organized and robust scaffold. Cell Reports. In Press. 10.1016/j.celrep.2015.11.051 [DOI] [PubMed] [Google Scholar]
- Pollard T.D., and Borisy G.G.. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 112:453–465. 10.1016/S0092-8674(03)00120-X [DOI] [PubMed] [Google Scholar]
- Ruthel G., and Banker G.. 1998. Actin-dependent anterograde movement of growth-cone-like structures along growing hippocampal axons: a novel form of axonal transport? Cell Motil. Cytoskeleton. 40:160–173. [DOI] [PubMed] [Google Scholar]
- Ruthel G., and Banker G.. 1999. Role of moving growth cone-like “wave” structures in the outgrowth of cultured hippocampal axons and dendrites. J. Neurobiol. 39:97–106. [DOI] [PubMed] [Google Scholar]
- Tint I., Jean D., Baas P.W., and Black M.M.. 2009. Doublecortin associates with microtubules preferentially in regions of the axon displaying actin-rich protrusive structures. J. Neurosci. 29:10995–11010. 10.1523/JNEUROSCI.3399-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard M., Wiseman M., Levine J., and Skene P.. 1979. Axonal transport of actin in rabbit retinal ganglion cells. J. Cell Biol. 81:581–591. 10.1083/jcb.81.3.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Zhong G., and Zhuang X.. 2013. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 339:452–456. 10.1126/science.1232251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G., He J., Zhou R., Lorenzo D., Babcock H.P., Bennett V., and Zhuang X.. 2014. Developmental mechanism of the periodic membrane skeleton in axons. eLife. 3:e04581 10.7554/eLife.04581 [DOI] [PMC free article] [PubMed] [Google Scholar]