Abstract
Considerable attention has been focused on long‐term use of proton pump inhibitor (PPI) medications in relation to increased risk of cancer via stimulation of DNA‐damaged cells. The aim of this study is to examine the dose‐dependent effect of PPI on periampullary cancers in a national population‐based cohort. A nested case–control analysis was constructed based on Taiwan's National Health Insurance Research Database and the Taiwan Cancer Registry between the years 2000 and 2010. Cases involving patients diagnosed with periampullary cancers were selected and controls were matched to cases according to age, sex and observational period. A “PPI user” was defined as any patient receiving more than 28 cumulative defined daily doses as measured by prescription drug claims. Conditional logistic regression analysis was conducted to calculate odds ratios (ORs) and 95% confidence intervals (CIs) according to the level of PPI exposure. A total of 7,681 cases and 76,762 matched controls were included with a mean follow‐up period of 6.6 years (SD: 2.0). The odds of PPI exposure in patients with periampullary cancers were higher than that of control patients with an adjusted OR of 1.35 (95% CIs: 1.16–1.57). Our results also showed that PPI exposure was slightly linked to periampullary cancers in dose‐dependent manner. A similar association was observed in patients who solely took PPI but no eradication therapy for Helicobacter pylori infection. Long‐term PPI use was associated with an increased risk of periampullary cancers in the current population‐based study. Physicians must weigh potential risks of long‐term maintenance against therapeutic benefit.
Keywords: proton pump inhibitors, periampullary cancers, nested case–control study, H. pylori eradication therapy
Short abstract
What's new?
Proton pump inhibitor (PPI) medications are a common treatment for gastroesophageal reflux disease and peptic ulcer disease. However, some evidence indicates that long‐term use of PPIs might increase cancer risk. This large Taiwanese study found that PPI exposure was indeed slightly linked to an increased risk of periampullary cancers, in a dose‐dependent manner. (This was not seen in patients undergoing H. pylori eradication therapy, however.) These results indicate that physicians must weigh the potential risks of long‐term maintenance use of PPIs against their therapeutic benefit.
The use of proton pump inhibitor (PPI) medications has rapidly increased in recent years because of its efficacy in treating gastroesophageal reflux disease (GERD) and peptic ulcer disease (PUD).1 Because PPIs have minimal side effects and few significant drug interactions, they are generally considered safe for long‐term treatment.2 Since their introduction in the late 1980s, millions of individuals worldwide have been using these medications on a continuous or long‐term basis. Recently, studies have explored the appropriateness and judiciousness in the use of PPI in hospital and outpatient practices.3, 4
Significant current concerns have focused on the long‐term effect of PPIs and whether these medications can change gastric physiology,5 potentially leading to cell transformation, gastric adenocarcinoma, bacterial overgrowth, enteric infections or malabsorption (e.g., fat, minerals and vitamins). Many retrospective observational studies found several adverse outcomes linked to PPI therapy, including hip fracture,6 pneumonia,7 acute interstitial nephritis8 and hypomagnesemia.9 Thus, the safety of the long‐term use of PPI has recently been questioned.
Physiologically, long‐term PPI use can induce acid–base imbalance in the gastrointestinal tract. Normal gastric acid has pH below 4 and has a powerful bactericidal effect. The strong acidity has the ability to kill exogenous, acid‐sensitive bacteria in the stomach within a 15‐min time span.10 PPIs reduce the amount of gastric acid secretion in the stomach, thereby increasing the survival of various microbes and allowing at least 50% of ingested bacterial to survive gastric trap.10 Optimal physiological processes in the gastrointestinal tract require coordination and fine balance between intracellular and extracellular pH. All cells must maintain cytoplasmic pH within a narrow range to survive.11 This is particularly true in the upper gastrointestinal tract, where the pH range varies greatly. Theoretically, prolonged pH imbalance from long‐term PPI use can decrease enzymatic activities in upper gastrointestinal tract and ultimately increase the possibility of DNA damage and harmful cell mutation.12, 13
Although postulated mechanisms of cancer related to PPI remain unclear, one theory suggests that PPIs allow the proliferation of cells with deadly mutations, in the upper gastrointestinal tract, especially in the ampulla of Vater and increase risk to developing periampullary adenocarcinomas.14, 15 Given the widespread use of PPIs, the aim of this study is to examine the effect of PPIs on periampullary adenocarcinomas in a dose‐dependent manner, based on a national population‐based cohort.
Material and Methods
Data source
The National Health Insurance Research Database (NHIRD) of Taiwan is a nationwide claims database, maintained by the National Health Insurance Administration (NHIA). It covers almost every medical reimbursement claim received by beneficiaries under the regulation of National Health Insurance (NHI) program. Since 1995, all citizens of Taiwan are required by law to enroll in the NHI. In 2012, the NHI coverage rate was 99%. The NHIRD contains claims data on beneficiary demographics, disease diagnoses, treatment procedures, prescription medications, date‐of‐service, reimbursement amounts and beneficiary‐ and provider‐encrypted identifiers. To verify the accuracy of diagnoses and the rationale for treatments, the NHIA routinely audits a proportion of the NHI claims.
The Taiwan Cancer Registry (TCR) is a population‐based cancer registry that standardizes medical definitions and terminology, coding and procedures of the registry's reporting system and tracking of patients with a cancer diagnosis. Following the enactment of the Cancer Control Act in 2003, all hospitals are mandated to submit cancer data to TCR. Additionally, TCR data are subject to periodic quality‐control audits and is processed according to the standard guidelines of the International Agency for Research on Cancer, resulting in 2‐year time lag between collection and publication of data.
Cohort selection
Of the 23 million beneficiaries enrolled in NHI from 2000 to 2010, the eligible cohort in this study included patients who were 40 years or older and met the following criteria: (i) continuously enrolled in NHI since 2000; (ii) absence of any periampullary cancer diagnosis before 2002 and (iii) had no PPI prescription claims between 2000 and 2001 to create a new‐user cohort without PPI exposure before entering the cohort.
A new‐user design can eliminate two major biases by restricting the analysis to persons under observation at the start of the current course of treatment. The first bias was the healthy user effect that patients who decided to use PPI have a more favorable risk factors profile than do nonusers that is common in many observational studies. The second bias was due to the covariates for drug users at study entry often are plausibly affected by the drug itself. Investigators often do not adjust for these factors on the causal pathway, which may introduce confounding.16, 17
Patients diagnosed with periampullary cancers within a year of follow‐up were excluded. This cohort was screened for any occurrence of periampullary cancers and if so, death up to December 2012. All deaths were confirmed by using the National Death Registry (NDR). The completeness and accuracy of death records of Taiwan is high as it is mandatory to register all death with the NDR.
Case identification and control selection
According to the International Classification of Disease of Oncology (ICD‐O), periampullary cancer is classified as extrahepatic cholangiocarcinoma (ICD‐O‐3: C24.0), ampullary (ICD‐O‐3: C24.1), duodenum (ICD‐O‐3: C17.0), jejunum (ICD‐O‐3: C17.1) or pancreatic (ICD‐O‐3: C25.0). By definition, periampullary cancers arise within 2 cm of the major papilla in the duodenum and encompass four different types of cancers according to location: ampullary (ampulla of Vater), biliary (intrapancreatic distal bile duct), pancreatic (head–uncinate process) and duodenal (mainly from the second portion). Although these tumors have different origins, the complex regional anatomy and their proximity to other organs generally dictate the operative approach.18 Therefore, this study considered a broader definition of periampullary cancers.
Up to ten control subjects with no previous or existing diagnosis of cancer were selected from the study cohort. We used the incidence density sampling approach to match controls with each case according to age (±1 year), sex and the follow‐up period of PPI exposure. This method not only reduced potential bias in the observational studies but decreased the time‐window bias by differentiating exposure opportunity time windows between subjects.19 The date of cancer diagnosis was treated as the index date. All control patients were assigned a pseudo‐index date (referred as the index date here after) which corresponded to the index date of their matched cases.
PPI exposure
The duration of use for PPI was determined based on prescription claims in the NHIRD. For each prescription claim, we recorded the start and withdrawal dates, drug name and dosage. The type of PPI was selected based on the Anatomical Therapeutic Chemical (ATC) system of medications of A02BC from NHIRD. The PPIs include: Rabeprazole, Pantoprazole, Lansoprazole, Esomeprazole and Omeprazole—all covered by NHI in Taiwan.
A PPI user was defined as a patient taking PPI for ≥28 days during follow‐up periods. We considered used the patients had no PPI exposure and those exposed PPI <28 cumulative defined daily dose (cDDD) as the nonuser group. The major idea of this study was long‐term, cumulative and high‐dose exposure of PPI might change the acid–base imbalance in gastrointestinal tract resulting in cell damage. Of the patients who use PPI due to Helicobacter pylori eradication therapy or other indications usually being prescribed in a short‐term, temporal and low‐dose exposure were treated as non‐PPI users in this study.
The dose–response effect was calculated using the cDDD, which is the assumed maintenance dose per day for adults. Three cDDD categories were used: 28–90 cDDD, 91–180 cDDD and >180 cDDD.
Covariates
Covariates considered in our analysis include known risk factors associated with the study cancers, such as choledochal cysts, cholangitis, cholelithiasis, cirrhosis, alcoholic liver disease, nonalcoholic liver disease (NAFLD), hepatitis B virus (HBV), hepatitis C virus (HCV), diabetes, chronic pancreatitis, inflammatory bowel disease, PUD, GERD and cardiovascular diseases.20, 21 Each disease condition was defined by having two or more diagnostic claims within 2 years before index date. Other medications like H. pylori eradication therapy, histamine‐2 receptor antagonists (H2RAs), aspirin, nonsteroidal anti‐inflammatory drug (NSAID), statin, metformin, insulin and other antidiabetic drugs were included in our analysis in patients with ≥28 cDDD each year. Detailed information related to these variables and ICD‐9 diagnostic codes is provided in Supporting Information Table 1.
Subgroup and sensitivity analysis
Special attention and a subgroup analysis focused on H. pylori eradication therapy. Standard therapy for H. pylori eradication involves PPI in conjunction with antibiotic combination.22 Patients with H. pylori eradication therapy are more likely to have a peptic ulcer disease. Furthermore, H. pylori is an important risk factor to the development of gastric malignancy and dyspeptic symptoms. Thus, the use of PPI to eradicate H. pylori infection is necessary to significantly reduce the acid in the stomach.
To increase the robustness of our analysis, we performed two sensitivity analyses to strengthen the validity of our findings. First, we employed a 3‐year washout period to exclude any patients who had been exposed to PPI before entering the cohort. A washout period avoids potential carryover effect of drugs during initial observational period. Second, we used lung cancer as a negative cancer case to ensure that any association observed in the initial analysis was not random. This is also a tool to detect confounding variables in observational studies.23 Lung cancer was selected as its demographic characteristic is very similar to periampullary cancers.
Statistical analysis
For all variables of interest, risk estimates were computed via (i) univariate analyses based on matching factors and (ii) multivariate analyses with additional adjustments for potential confounders. Conditional logistic regression analysis was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) in the risk of developing periampullary cancers with long‐term PPI use. In a nested case–control study, controls are selected using incidence density sampling. Because of the low incidence of periampullary cancers, the calculated estimated incidence ratio was roughly equal to the OR.24 All analyses were performed using SAS/STAT 9.2 software (SAS Institute, Cary, NC). p Values < 0.05 were considered significant.
Ethics statement
This study was approved by the Taipei Medical University Joint Institutional Review Board (approval no. 201503054). Confidentiality was ensured by abiding to data regulations of the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare, Executive Yuan, Taiwan. The HWDC encrypts individual identifiers to protect privacy before releasing information to investigators for research purposes. The informed consents of the participants were exempted under the full review process of the Joint Institutional Review Board of Taipei Medical University.
Results
Sample size
More than 8,000,000 NHI beneficiaries were eligible for the study and the final cohort for our analysis comprised of 7,681 cases with periampullary cancers and 76,762 matched controls (Fig. 1).
Figure 1.
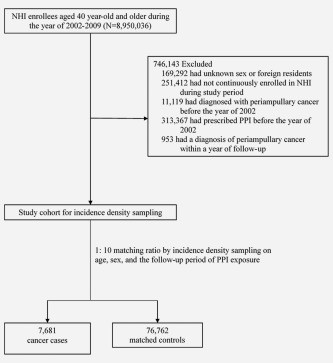
Study flow diagram.
Basic characteristics
Table 1 presents the basic characteristics of cases and their matched controls. Among cases with periampullary cancers, 17.8% were diagnosed with extrahepatic cholangiocarcinoma, 21.7% with ampullary cancer, 10.1% with duodenal cancer and 50.4% with pancreatic head cancer. The mean age of the cohort was 69.5 years (SD: 11.6); 58.1% of the patients were male. Periampullary cancer patients in our study were more likely to have a disease related to bile and liver, such as cholangitis, cholelithiasis, cholecystitis, alcoholic liver disease and HBV but not NAFLD and HCV. In addition, case patients were more likely than controls to have diabetes, chronic pancreatitis, inflammatory bowel disease and PUD. The results also showed that case patients were more likely to receive H. pylori eradication therapy, H2RAs and antidiabetic drugs than controls.
Table 1.
Basic characteristics of periampullary cancer cases and matched controls
| Cancer cases | Matched controls | ||
|---|---|---|---|
| N (%) | N (%) | Unadjusted OR (95% CIs) | |
| No. | 7,681 | 76,762 | |
| Male | 4,463(58.1) | 44,607(58.1) | – |
| Age, mean (SD) | 69.47(11.6) | 69.53(11.6) | – |
| Cancer site | |||
| Extrahepatic cholangiocarcinoma | 1,370(17.8) | ||
| Ampullary cancer | 1,665(21.7) | ||
| Duodenal cancer | 775(10.1) | ||
| Pancreatic head cancer | 3,871(50.4) | ||
| Previous or coexisting medical condition | |||
| Choledochal cysts | 3(<1) | 31(<1) | 0.97(0.30–3.17) |
| Cholangitis | 200(2.6) | 126(<1) | 16.8(13.4–21.1)a |
| Cholelithiasis | 553(7.2) | 1,284(1.7) | 4.71(4.25–5.23)a |
| Cholecystitis | 58(<1) | 57(<1) | 10.3(7.13–14.9)a |
| Hemochromatosis | 4(<1) | 39(<1) | 1.03(0.37–2.87) |
| Cirrhosis | 108(1.4) | 1,033(1.3) | 1.05(0.86–1.28) |
| Alcoholic liver disease | 49(<1) | 272(<1) | 1.82(1.34–2.47)a |
| NAFLD | 7(<1) | 269(<1) | 1.38(0.98–1.94) |
| HBV | 127(1.7) | 812(1.1) | 1.58(1.31–1.91)a |
| HCV | 98(1.3) | 860(1.1) | 1.14(0.93–1.41) |
| Diabetes | 1,923(25.0) | 13,900(18.1) | 1.53(1.45–1.62)a |
| Chronic pancreatitis | 84(1.1) | 61(<1) | 14.6(10.4–20.5)a |
| Inflammatory bowel disease | 52(<1) | 286(<1) | 1.83(1.36–2.46)a |
| PUD | 2,968(38.6) | 26,360(34.3) | 1.43(1.35–1.53)a |
| GERD | 213(2.8) | 1,855(2.4) | 1.16(1.00–1.35) |
| Cardiovascular disease | 1,663(21.7) | 16,110(21.0) | 1.04(0.98–1.11) |
| Medication | |||
| H. pylori eradication therapy | 1,826(23.8) | 16,656(21.7) | 1.27(1.13–1.44)a |
| H2RAs | 322(4.2) | 2,568(3.3) | 1.00(0.94–1.06) |
| Aspirin | 1,496(19.5) | 14,987(19.5) | 1.09(1.03–1.15) |
| NSAIDs | 1,733(22.6) | 16,282(21.2) | 1.03(0.94–1.13) |
| Statins | 532(6.9) | 5,194(6.8) | 1.44(1.33–1.55)a |
| Metformin | 906(11.8) | 6,579(8.6) | 1.61(1.37–1.89)a |
| Insulins | 178(2.3) | 1,118(1.5) | 1.38(1.29–1.47) |
| Other antidiabetic drug | 1,197(15.6) | 9,116(11.9) | 1.46(1.36–1.57)a |
p < 0.001.
Abbreviations: H2RAs: histamine‐2 receptor antagonists; HBV: hepatitis B virus; HCV: hepatitis C virus; GERD: gastroesophageal reflux disease; NAFLD: nonalcoholic fatty liver disease; NSAID: nonsteroidal anti‐inflammatory drug; OR: odds ratio; PUD: peptic ulcer disease.
PPI exposure and the risk of periampullary cancer
Table 2 indicates the odds of PPI exposure in periampullary cancers. Among this study cohort, 537 of 7,681 (7.0%) cancer patients and 4,449 of 76,762 (5.8%) controls have exposed to PPI ≥28 cDDD. The odds of PPI use with periampullary cancer cases were higher than matched control patients, with an adjusted OR of 1.35 (95% CIs: 1.16–1.57, p < 0.001). In considering the use of PPI according to cDDD subgroups, the highest dose–response effect was found in patients with PPI exposure of 91–180 cDDD but not in patients exposed to PPI >180 cDDD.
Table 2.
The odds of PPI exposure of periampullary cancer cases and matched controls
| Cancer cases | Matched controls | |||||
|---|---|---|---|---|---|---|
| N (%) | N (%) | Unadjusted OR (95% CIs) | p Values | Adjusted OR (95% CIs)a | p Values | |
| Sample size | 7,681(100) | 76,762(100) | ||||
| PPI exposure | ||||||
| Non‐PPI user | 7,144(93.0) | 72,313(94.2) | 1.00(Ref.) | 1.00(Ref.) | ||
| PPI user | 537(7.0) | 4,449(5.8) | 1.56(1.35–1.81) | <0.001 | 1.35(1.16–1.57) | <0.001 |
| cDDD | ||||||
| 0–27 | 7,144(93.0) | 72,313(94.2) | 1.00(Ref.) | <0.001b | 1.00(Ref.) | 0.001b |
| 28–90 | 96(1.2) | 936(1.2) | 1.34(1.04–1.74) | 1.33(1.01–1.73) | ||
| 91–180 | 221(2.9) | 1,784(2.3) | 1.61(1.34–1.94) | 1.48(1.22‐1.79) | ||
| >180 | 220(2.9) | 1,729(2.3) | 1.60(1.34–1.91) | 1.26(1.04–1.52) |
Adjusted for choledochal cysts, cholangitis, cholelithiasis, cholecystitis, cirrhosis, alcoholic liver disease, NAFLD, HBV, HCV, diabetes, chronic pancreatitis, inflammatory bowel disease, PUD, GERD, cardiovascular disease, H2RAs, aspirin, NSAIDs, statins, metformin, insulins, other antidiabetic drugs and H. pylori eradication therapy.
p for trend.
Abbreviations: cDDD: cumulative defined daily dose; H2RAs: histamine‐2 receptor antagonists; HBV: hepatitis B virus; HCV: hepatitis C virus; GERD: gastroesophageal reflux disease; NAFLD: nonalcoholic fatty liver disease; NSAID: nonsteroidal anti‐inflammatory drug; OR: odds ratio; PUD: peptic ulcer disease.
Figure 2 displays the dose–response curves for risk of periampullary cancers as a function of PPI use measured by cDDD during follow‐up period. The highest risk of periampullary cancer occurs in patients with PPI exposure over cDDD 180 days; the risk slightly decreased as cDDD increases past 180 days. The wider 95% CIs were due to the smaller sample size as cDDD increased.
Figure 2.
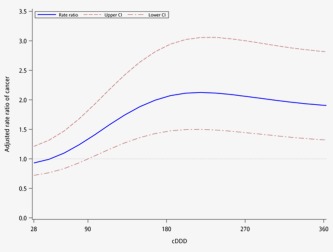
Dose–response curve for the rate ratio (solid line) and 95% CIs (dashed lines) of periampullary cancers as a function of PPI dose (measured in cDDD) estimated by cubic splines model fit by conditional logistic regression. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Subgroup analysis
Another analysis was performed by stratifying patients into two groups: (i) patients with H. pylori eradication therapy and (ii) patients without H. pylori eradication therapy. We found that patients with PPI use but not H. pylori eradication therapy had significantly higher odds of having periampullary cancers than their matched control group, with an adjusted OR of 1.33 (95% CIs: 1.11–1.60, p = 0.003) (see Table 3).
Table 3.
Subgroup analysis of the odds of PPI exposure of periampullary cancer cases and matched controls by H. Pylori eradication therapy
| Cancer cases | Matched controls | ||||
|---|---|---|---|---|---|
| Group | PPI exposure | N (%) | N (%) | Adjusted OR (95% CIs)a | p Values |
| With H. Pylori eradication therapy | |||||
| Non‐PPI user | 1,086(84.3) | 8,601(86.3) | 1.00(Ref.) | ||
| PPI user | 203(15.7) | 1,363(13.7) | 1.14(0.80–1.64) | 0.469 | |
| cDDD | |||||
| 0–27 | 1,086(84.3) | 8,601(86.3) | 1.00(Ref.) | 0.592b | |
| 28–90 | 38(2.9) | 363(3.6) | 1.10(0.67–1.81) | ||
| 91–180 | 78(6.1) | 480(4.8) | 1.31(0.85–2.02) | ||
| >180 | 87(6.7) | 520(5.2) | 1.02(0.65–1.60) | ||
| Without H. Pylori eradication therapy | |||||
| Non–PPI user | 6,058(94.8) | 63,712(95.4) | 1.00(Ref.) | ||
| PPI user | 334(5.2) | 3,086(4.6) | 1.33(1.11–1.60) | 0.003 | |
| cDDD | |||||
| 0–27 | 6,058(94.8) | 63,712(95.4) | 1.00(Ref.) | 0.015b | |
| 28–90 | 58(0.9) | 573(0.9) | 1.36(0.96–1.93) | ||
| 91–180 | 143(2.2) | 1,304(2.0) | 1.44(1.14–1.82) | ||
| >180 | 133(2.1) | 1,209(1.8) | 1.23(0.97–1.55) |
Adjusted for choledochal cysts, cholangitis, cholelithiasis, cholecystitis, cirrhosis, alcoholic liver disease, NAFLD, HBV, HCV, diabetes, chronic pancreatitis, inflammatory bowel disease, PUD, GERD, cardiovascular disease, H2RAs, aspirin, NSAIDs, statins, metformin, insulins, other antidiabetic drugs and H. pylori eradication therapy.
p for trend.
Abbreviations: cDDD: cumulative defined daily dose; H2RAs: histamine‐2 receptor antagonists; HBV: hepatitis B virus; HCV: hepatitis C virus; GERD: gastroesophageal reflux disease; NAFLD: nonalcoholic fatty liver disease; NSAID: nonsteroidal anti‐inflammatory drug; OR: odds ratio; PUD: peptic ulcer disease.
Sensitivity analysis
Sensitivity analysis was performed by selecting another matched case–control cohort with no PPI exposure for 3 years (i.e., a longer washout period) before the date of entry. Results showed a similar result to our primary analysis. In addition, we also used lung cancer as a negative cancer case and conducted another nested case–control study to confirm that use of PPI was not associated with increased risk of lung cancer when compared to the matched control patients (see Table 4).
Table 4.
Sensitivity analysis
| Cancer cases | Matched controls | |||
|---|---|---|---|---|
| PPI exposure | N (%) | N (%) | Adjusted OR (95% CIs)a | p Value |
| Use a 3‐year washout period to select cancer cases | ||||
| Non‐PPI user | 6,206(92.2) | 62,935(93.5) | 1.00(Ref.) | |
| PPI user | 527(7.8) | 4,346(6.5) | 1.27(1.09–1.48) | 0.003 |
| Use the lung cancer as a negative cancer cases | ||||
| Non‐PPI user | 50,126(94.8) | 502,499(95.1) | 1.00(Ref.) | |
| PPI user | 2,750(5.2) | 26,117(4.9) | 1.03(0.97–1.09) | 0.360 |
Adjusted for choledochal cysts, cholangitis, cholelithiasis, cholecystitis, cirrhosis, alcoholic liver disease, NAFLD, HBV, HCV, diabetes, chronic pancreatitis, inflammatory bowel disease, PUD, GERD, cardiovascular disease, H2RAs, aspirin, NSAIDs, statins, metformin, insulins, other antidiabetic drugs and H. pylori eradication therapy.
Abbreviations: cDDD: cumulative defined daily dose; H2RAs: histamine‐2 receptor antagonists; HBV: hepatitis B virus; HCV: hepatitis C virus; GERD: gastroesophageal reflux disease; NAFLD: nonalcoholic fatty liver disease; NSAID: nonsteroidal anti‐inflammatory drug; OR: odds ratio; PUD: peptic ulcer disease.
Discussion
This population‐based nested case–control study found an association between PPI use and increased risk of periampullary cancers. Subgroup analysis showed similar trends when focusing on patients without H. pylori eradication therapy. Two sensitivity analyses were performed using: (i) longer washout period to reduce a carryover effect of drugs and (ii) lung cancer as a negative cancer case to rule out unobserved confounding bias additionally confirmed the initial findings. Periampullary cancers are relatively rare compared with the other gastrointestinal cancers; however, over 60% of the patients died within a year, once diagnosed in this cohort. Thus, identifying the potential risk of the cancers is significantly important.
Long‐term PPI use and carcinoma has remained questionable for years. The first published report involved endochromaffin cell‐derived (ECL cell‐derived) neuroendocrine carcinoma. The authors postulated whether carcinoma was secondary to hypergastrinemia due to PPI use over a 15‐year course.25 As found in this study, long‐term use of PPI was associated with the risk of periampullary cancers. There are two possible mechanisms that contribute or explain our finding. First, pronounced acid suppression has been shown to cause elevated serum gastrin levels in individuals. Prolonged and increased gastrin can stimulate an increase in intermediates known to have trophic effects on normal gastrointestinal mucosa and can stimulate carcinogenesis. Numerous in vitro and in vivo studies have explored trophic effects of gastrin on numerous cancers including pancreatic,26 liver,27 esophageal28 and colon.29 It is therefore conceivable that the trophic effects of gastrin allow sporadic mutations to ultimately proliferate and progress to neoplastic precursors.
Second, it is hypothesized that chronic use of PPI might induce a metaplasia‐dysplasia‐carcinoma sequence. If a PPI fails to suppress gastric acid, then unconjugated bile salts may diffuse into the epithelial cells and cause mucosal metaplasia. Pharmacologically, hypochlorhydria induced by daily PPI use produces periods during the day in which pH of the gastric juice is at or near a neutral pH levels.30 A study by Shindo et al. showed that hypochlorhydria can induce major changes in the gastric flora and affect the pH of small bowel fluid to allow bacterial overgrowth.30 Moreover, the Shindo et al.'s study revealed that PPI treatment in patients with gastric ulcer resulted in bacterial overgrowth of gastrointestinal tract. These bacteria alter the metabolism of bile acids through increased deconjugation and fat malabsorption, as evidenced by glycine‐1‐14C‐labeled glycocholate and breathe analysis studies.31, 32 A substantial body of evidence suggests that bile acids play a role in the development of intestinal tumors. Evidence reviewed by Bernstein et al. suggests that bile acids act as carcinogens in human gastrointestinal cancers.15 Most benign and malignant tumors of the small intestine and extrahepatic bile ducts arise in the region of the Papilla of Vater. Long‐term exposure to bile acids has been known to activate prosurvival stress‐response pathways and modulate numerous genes/proteins associated with chromosome maintenance and mitosis.33 Therefore, a likely mechanism by which hydrophobic bile acids can induce periampullary cancers involves bile acid induction of reactive oxygen species (ROS), reactive nitrogen species (RNS) and DNA damage in cells of the gastrointestinal tract. These stresses, if too much, can overwhelm cellular defenses resulting in cell death.34
Our results show that patients, who received a large amount of PPI for reasons unrelated to H pylori eradication therapy, were more likely to have periampullary cancers. Previous studies have demonstrated that PPI treatment has a prominent acid‐suppressive effect in H. pylori‐positive patients compared to H. pylori‐negative patients.35 Furthermore, studies have also reported better symptomatic control and enhanced healing of mucosal lesions with PPI in patients with H. pylori and GERD versus H. pylori‐negative patients. One randomized double‐blind trial showed that a total of 44% of H pylori‐negative subjects with PPI therapy group developed dyspepsia in comparison with 9% in the placebo group (p < 0.01).36 This suggests a correlation between symptoms and acid‐rebound hypersecretion. Patients, who develop H. pylori infection and undergo PPI treatment, have been shown to have gastritis affecting the antrum and fundus regions of the stomach.37 Many have proposed that the enhanced acid‐secretory potency of PPI in H. pylori patients may be due to this gastritis.38 Although an earlier study5 suggested that inflammation of mucosa in fundal region is associated with gastric cancer, it is generally agreed that patients with H. pylori infection should take PPI for H. pylori eradication.
By using a dose–response curve to track the relative risk of periampullary cancers as a function of PPI use (measured in cDDD), we found that the risk of periampullary cancers peaked at cDDD levels near 180 days and decreased as cDDD levels increased. We believe this decrease in periampullary cancer risk as cDDD levels increase beyond 180 days is partly attributed to mechanism of PPI tolerance. Studies on H2RAs (another medication commonly used for GERD and PUD) have found that tolerance of the drug decreases after previous drug treatments, especially intravenous medication.39, 40 It is possible that patients on long‐term PPI use encounter similar tolerance problems with PPI just as patients experience with H2RAs. Further investigations are needed to confirm this unproven mechanism.
The cancer risk of PPI use has been reported in many observational studies. However, consensus remains elusive. For example, a study in UK found that long‐term PPI therapy at a regular dose was not associated with colorectal cancer41; however, another study in Taiwan found that PPI use had a 2.5‐fold association risk of colorectal cancer.42 Authors of the Taiwan study concluded that patients with occult colorectal cancer had longstanding reflux or upper gastrointestinal symptoms and concomitantly received PPI treatment. The UK researchers found that PPIs/H2RAs use was not associated with pancreatic cancer risk,43 which is consistent with our finding when limited to the cancer site into pancreatic cancer (Supporting Information Table 2). However, we did not restrict our sample to those with pancreatic cancer as it is difficult to clinically distinguish it from periampullary cancers.18 Besides to further explore the risk of PPI on gastrointestinal tract cancers, we additionally selected the gastric cancer as a case cancer. We found that PPI was associated with the risk of gastric cancers in the body of stomach (Supporting Information Table 3). Long‐term PPI use has been reported to be associated with an increase in gastritis and even the development of gastric atrophy in the body portion of the stomach.5 Histologic studies in combination with culture confirmed that PPI use is associated with a change in the distribution of gastritis with histological improvement in antrum and worsening of the gastritis in the body portion of the stomach.44 These changes have been attributed to alteration in local pH as H. pylori are killed at pHs below 4 and above 8, are able to survive but not replicate at pHs between 4 and 6 and only replicate at pHs between 6 and 8.45 Kuipers et al. also suggested that use of PPI was associated with an increased risk of development of atrophic gastritis, the known primary risk factor for development of gastric adenocarcinoma.46
Our study possesses a number of strengths, including a large sample size and homogeneity of the study population. First, the nested case–control design with appropriate matching cases and controls on the observational period is an appropriate design for evaluating drug effect. Second, multiple regression analyses were performed to adjust the potential confounding bias. Third, two sensitivity analyses were used to increase the validity of our main findings.
However, several limitations remain. First, the study was based on the population‐based claims that did not have information on risk behaviors, such as smoking and alcoholic consumption. Second, the use of PPI was measured by prescribed claims; thus, we were unable to obtain data on OTC PPI or patient's medication compliance. Third, the healthcare and prescription data we used did not provide the indication for an exposed drug and the severity of comorbidities; therefore we can only adjust the presence of drug use and disease but not for the medication indication and severity. Confounding by indication and disease severity is a bias frequently encountered in observational epidemiologic studies of drug effects.47 Because the allocation of treatment in observational studies is not randomized and the indication for treatment may be related to the risk of future health outcomes, the resulting imbalance in the underlying risk profile between case and comparison groups can generate biased results. Besides, in case–control studies, if exposure influences the diagnosis of the disease, detection bias occurs.48 For example, PPI users might be more likely to visit their healthcare providers compared to non‐PPI users and therefore increased the probability to detect their cancer. Moreover, this study only considered the presence of disease that occurred in the diagnostic claims; however, the time of disease starts to the time of disease is identified or diagnosed is usually lagged. The latency time windows of disease diagnosis might induce confounding of the association with cancer incidence by failure to account for disease duration. Therefore, the results should be interpreted cautiously. Finally, this study was conducted in the Pacific‐Asian region, where prevalence of PPI use is relatively low; thus, our results might not be generalized to other populations.
In conclusion, all pharmacological agents (including PPI) carry the risk of potential adverse effects. In this study, we observed an increased risk of periampullary cancers among long‐term PPI users. Our findings highlight what may be an underappreciated adverse effect of PPI therapy. Future research is needed to validate and further characterize our findings. In the interim, we suggest physicians weigh potential risks and therapeutic benefits of long‐term PPI use.
Supporting information
Supporting Information
Conflict of interest: Nothing to report
References
- 1. Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008;135:1383–91.e5. [DOI] [PubMed] [Google Scholar]
- 2. Thomson ABR, Sauve MD, Kassam N, et al. Safety of the long‐term use of proton pump inhibitors. World J Gastroenterol 2010;16:2323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. Ambulatory Setting, 2002–2009. PLoS One 2013;8:e56060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zink DA, Pohlman M, Barnes M, et al. Long‐term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005;21:1203–9. [DOI] [PubMed] [Google Scholar]
- 5. Graham DY, Genta RM. Long term proton pump inhibitor use and gastrointestinal cancer. Curr Gastroenterol Rep 2008;10:543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang YX, Lewis JD, Epstein S, et al. Long‐term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006;296:2947–53. [DOI] [PubMed] [Google Scholar]
- 7. Sarkar M, Hennessy S, Yang Y‐X. Proton‐pump inhibitor use and the risk for community‐acquired pneumonia. Ann Intern Med 2008;149:391–8. [DOI] [PubMed] [Google Scholar]
- 8. Blank ML, Parkin L, Paul C, et al. A nationwide nested case‐control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int 2014;86:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zipursky J, Macdonald EM, Hollands S, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population‐based case‐control study. PLoS Med 2014;11:e1001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tennant SM, Hartland EL, Phumoonna T, et al. Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infect Immun 2008;76:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alper SL. Genetic diseases of acid‐based transporters. Annu Rev Physiol 2002;64:899–923. [DOI] [PubMed] [Google Scholar]
- 12. Ohnishi S, Ma N, Thanan R, et al. DNA damage in inflammation‐related carcinogenesis and cancer stem cells. Oxid Med Cell Long 2013;2013:387014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tselepis C, Morris CD, Wakelin D, et al. Upregulation of the oncogene c‐myc in Barrett's adenocarcinoma: induction of c‐myc by acidified bile acid in vitro. Gut 2003;52:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miner PB, Jr. Review article: physiologic and clinical effects of proton pump inhibitors on non‐acidic and acidic gastro‐oesophageal reflux. Aliment Pharmacol Ther 2006;23:25–32. [DOI] [PubMed] [Google Scholar]
- 15. Bernstein H, Bernstein C, Payne CM, et al. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res 2005;589:47–65. [DOI] [PubMed] [Google Scholar]
- 16. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol 2003;158:915–20. [DOI] [PubMed] [Google Scholar]
- 17. McMahon AD, MacDonald TM. Design issues for drug epidemiology. Br J Clin Pharmacol 2000;50:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarmiento JM, Nagorney DM, Sarr MG, et al. Periampullary cancers: are there differences? Surg Clin North Am 2001;81:543–55. [DOI] [PubMed] [Google Scholar]
- 19. Suissa S, Azoulay L. Metformin and the risk of cancer: time‐related biases in observational studies. Diabetes Care 2012;35:2665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Welzel TM, Graubard BI, El‐Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population‐based case‐control study. Clin Gastroenterol Hepatol 2007;5:1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bragazzi MC, Cardinale V, Carpino G, et al. Cholangiocarcinoma: epidemiology and risk factors. Transl Gastrointest Cancer 2011;1:21–32. [Google Scholar]
- 22. Chey WD, Wong BCY. American College of Gastroenterology Guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007;102:1808–25. [DOI] [PubMed] [Google Scholar]
- 23. Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richardson D. An incidence density sampling program for nested case‐control analyses. Occup Environ Med 2004;61:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jianu CS, Lange OJ, Viset T, et al. Gastric neuroendocrine carcinoma after long‐term use of proton pump inhibitor. Scand J Gastroenterol 2012;47:64–7. [DOI] [PubMed] [Google Scholar]
- 26. Guo YS, Townsend CM, Jr. Roles of gastrointestinal hormones in pancreatic cancer. J Hepato‐Biliary‐Pancreatic Surg 2000;7:276–85. [DOI] [PubMed] [Google Scholar]
- 27. Caplin M, Khan K, Grimes S, et al. Effect of gastrin and anti‐gastrin antibodies on proliferation of hepatocyte cell lines. Dig Dis Sci 2001;46:1356–66. [DOI] [PubMed] [Google Scholar]
- 28. Abdalla SI, Lao‐Sirieix P, Novelli MR, et al. Gastrin‐induced cyclooxygenase‐2 expression in Barrett's carcinogenesis. Clin Cancer Res 2004;10:4784–92. [DOI] [PubMed] [Google Scholar]
- 29. Baldwin GS, Shulkes A. Gastrin as an autocrine growth factor in colorectal carcinoma: implications for therapy. World J Gastroenterol 1998;4:461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shindo K, Machida M, Fukumura M, et al. Omeprazole induces altered bile acid metabolism. Gut 1998;42:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sherr HP, Sasaki Y, Newman A, et al. Detection of bacterial deconjugation of bile salts by a convenient breath‐analysis technic. N Engl J Med 1971;285:656–61. [DOI] [PubMed] [Google Scholar]
- 32. Fromm H, Hofmann AF. Breath test for altered bile‐acid metabolism. Lancet 1971;2:621–5. [DOI] [PubMed] [Google Scholar]
- 33. Payne CM, Bernstein C, Dvorak K, et al. Hydrophobic bile acids, genomic instability, Darwinian selection, and colon carcinogenesis. Clin Exp Gastroenterol 2008;1:19–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Komichi D, Tazuma S, Nishioka T, et al. Glycochenodeoxycholate plays a carcinogenic role in immortalized mouse cholangiocytes via oxidative DNA damage. Free Radic Biol Med 2005;39:1418–27. [DOI] [PubMed] [Google Scholar]
- 35. Verdu EF, Armstrong D, Idstrom JP, et al. Effect of curing Helicobacter pylori infection on intragastric pH during treatment with omeprazole. Gut 1995;37:743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Niklasson A, Lindstrom L, Simren M, et al. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: a double‐blind placebo‐controlled trial. Am J Gastroenterol 2010;105:1531. [DOI] [PubMed] [Google Scholar]
- 37. Eissele R, Brunner G, Simon B, et al. Gastric mucosa during treatment with lansoprazole: Helicobacter pylori is a risk factor for argyrophil cell hyperplasia. Gastroenterology 1997;112:707–17. [DOI] [PubMed] [Google Scholar]
- 38. Gillen D, Wirz AA, Ardill JE, et al. Rebound hypersecretion after omeprazole and its relation to on‐treatment acid suppression and Helicobacter pylori status. Gastroenterology 1999;116:239–47. [DOI] [PubMed] [Google Scholar]
- 39. Gillen D, McColl KE. Problems associated with the clinical use of proton pump inhibitors. Pharmacol Toxicol 2001;89:281–6. [DOI] [PubMed] [Google Scholar]
- 40. Qvigstad G, Waldum H. Rebound hypersecretion after inhibition of gastric acid secretion. Basic Clin Pharmacol Toxicol 2004;94:202–8. [DOI] [PubMed] [Google Scholar]
- 41. Yang YX, Hennessy S, Propert K, et al Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Gastroenterology 2007;133:748–54. [DOI] [PubMed] [Google Scholar]
- 42. Lai S‐W, Liao K‐F, Lai H‐C, et al. Use of proton pump inhibitors correlates with increased risk of colorectal cancer in Taiwan. Asia‐Pac J Clin Oncol 2013;9:192–3. [DOI] [PubMed] [Google Scholar]
- 43. Bradley MC, Murray LJ, Cantwell MM, et al. Proton pump inhibitors and histamine‐2‐receptor antagonists and pancreatic cancer risk: a nested case‐control study. Br J Cancer 2012;106:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Graham DY, Opekun AR, Yamaoka Y, et al. Early events in proton pump inhibitor‐associated exacerbation of corpus gastritis. Aliment Pharmacol Ther 2003;17:193–200. [DOI] [PubMed] [Google Scholar]
- 45. Scott D, Weeks D, Melchers K, et al. The life and death of Helicobacter pylori . Gut 1998;43(Suppl 1):S56–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuipers EJ, Lee A, Klinkenberg‐Knol EC, et al. Review article: the development of atrophic gastritis–Helicobacter pylori and the effects of acid suppressive therapy. Aliment Pharmacol Ther 1995;9:331–40. [DOI] [PubMed] [Google Scholar]
- 47. Salas M, Hofman A, Stricker BH. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am J Epidemiol 1999;149:981–3. [DOI] [PubMed] [Google Scholar]
- 48. Delgado‐Rodríguez M, Llorca J. Bias. J Epidemiol Commun Health 2004;58:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


