Summary
Background
Vonoprazan is a novel potassium‐competitive acid blocker which may provide clinical benefit in acid‐related disorders.
Aim
To verify the non‐inferiority of vonoprazan vs. lansoprazole in patients with erosive oesophagitis (EE), and to establish its long‐term safety and efficacy as maintenance therapy.
Methods
In this multicentre, randomised, double‐blind, parallel‐group comparison study, patients with endoscopically confirmed EE (LA Classification Grades A–D) were randomly allocated to receive vonoprazan 20 mg or lansoprazole 30 mg once daily after breakfast. The primary endpoint was the proportion of patients with healed EE confirmed by endoscopy up to week 8. In addition, subjects who achieved healed EE in the comparison study were re‐randomised into a long‐term study to investigate the safety and efficacy of vonoprazan 10 or 20 mg as maintenance therapy for 52 weeks.
Results
Of the 409 eligible subjects randomised, 401 completed the comparison study, and 305 entered the long‐term maintenance study. The proportion of patients with healed EE up to week 8 was 99.0% for vonoprazan (203/205) and 95.5% for lansoprazole (190/199), thus verifying the non‐inferiority of vonoprazan (P < 0.0001). Vonoprazan was also effective in patients with more severe EE (LA Classification Grades C/D) and CYP2C19 extensive metabolisers. In the long‐term maintenance study, there were few recurrences (<10%) of EE in patients treated with vonoprazan 10 or 20 mg. Overall, vonoprazan was well‐tolerated.
Conclusions
The non‐inferiority of vonoprazan to lansoprazole in EE was verified in the comparison study, and vonoprazan was well‐tolerated and effective during the long‐term maintenance study.
Introduction
Gastro‐oesophageal reflux disease (GERD) is a common disorder characterised by heartburn and/or acid regurgitation as a result of reflux of the stomach contents.1 It is the most common out‐patient diagnosis in gastroenterology in the USA and affects about 20% of the adult population weekly and 7% daily.2, 3, 4 In East Asia, the prevalence ranges from 2.5% to 7.8%.5, 6 The symptomatic nature of the disease and its high prevalence not only impacts the well‐being and quality of life of the patient but it also places a large burden on healthcare systems in terms of time and costs.7
Patients with GERD fall into two broad categories: the large majority of patients do not develop oesophageal lesions and have non‐erosive reflux disease (NERD) while a smaller number of patients develop erosive oesophagitis (EE), which is characterised by mucosal damage and symptoms of reflux.1, 7 The main goals of EE treatment are to relieve symptoms, heal and maintain remission of EE, prevent complications and improve health‐related quality of life.
Gastric acid suppression is the principle aim of treatment for patients with GERD, and proton pump inhibitors (PPIs) are the current gold standard in the clinical setting for reducing gastric acidity and producing symptomatic relief and mucosal healing in patients with reflux oesophagitis.4, 8 However, for patients receiving PPI therapy, oesophageal mucosal healing is much more predictable than resolution of symptoms.9
Vonoprazan is a novel oral potassium‐competitive acid blocker (P‐CAB) discovered and developed by Takeda Pharmaceutical Company Ltd., Japan.10 Like PPIs, the P‐CABs inhibit gastric H+, K+‐ATPase, an enzyme that catalyses the final step in the gastric acid secretion pathway. However, unlike the PPIs, they inhibit the enzyme in a K+‐competitive and reversible manner.11 Furthermore, the inhibitory effect of vonoprazan (pKa 9.4) on gastric acid secretion is largely unaffected by ambient pH and it has been shown to accumulate in parietal cells under acidic and neutral conditions.12, 13
In preclinical studies, vonoprazan produced more potent and more sustained suppression of gastric acid secretion than lansoprazole.11, 12, 13 These effects appear to be related to greater accumulation of vonoprazan into, and its subsequent slower clearance from, gastric glands.12 In healthy volunteers, single doses of vonoprazan 1–120 mg were well‐tolerated and produced a rapid, profound and dose‐related suppression of 24‐h gastric acid secretion.14 These effects were maintained with multiple dosing (10–40 mg once daily) over 7 days.15 In a phase II dose‐ranging study, the proportion of patients with healed EE confirmed by endoscopy was comparable for vonoprazan (5–40 mg once daily) and lansoprazole (30 mg once daily) over an 8‐week period.16 Vonoprazan 20 mg once daily produced the optimal balance between rapid healing of EE and good tolerability.
Since the acid‐inhibitory effects of vonoprazan are much more potent than those of lansoprazole, it is expected to be at least as effective when used in the treatment of patients with EE. Therefore, the objective of these studies was to verify the non‐inferiority of vonoprazan with lansoprazole when used as first‐line therapy for patients with EE and to establish its long‐term safety and efficacy over a 52‐week maintenance period, in subjects who achieved healed EE by week 8, confirmed by endoscopy.
Methods
Study design
The initial study was an 8‐week randomised, double‐blind, multicentre, parallel‐group, active‐controlled comparison study designed to verify the non‐inferiority of vonoprazan 20 mg to lansoprazole 30 mg in patients with endoscopically confirmed EE [Los Angeles (LA) Classification Grades A–D]. Patients who achieved endoscopically healed EE by week 8 in the comparison study were re‐randomised into a second sequential randomised, single‐blind, multicentre, parallel‐group, long‐term maintenance study to evaluate the safety and efficacy of vonoprazan 10 or 20 mg in patients with healed EE.
The comparison study was conducted in 39 sites in Japan between October 2011 and August 2012, and the sequential long‐term maintenance study was conducted at the same sites between November 2011 and July 2013. The studies were approved by the Institutional Review Board at each study site. They were conducted in accordance with the Declaration of Helsinki and the ICH Harmonised Tripartite Guideline for Good Clinical Practice, and all patients provided written informed consent for the comparison study as well as the long‐term maintenance study. The studies were registered at ClinicalTrials.gov with the identifiers NCT01452698 and NCT01452776.
In the comparison study, following a 3–7 day screening period, patients were randomised to treatment with vonoprazan 20 mg or lansoprazole 30 mg, both administered orally once daily after breakfast, for 2, 4 or 8 weeks. Subjects completed double‐blind treatment after 2, 4 or 8 weeks when healing was endoscopically confirmed. If the EE was not healed after completing the 8‐week treatment period, the patient received vonoprazan 40 mg for 4 or 8 weeks in a non‐blinded manner.
Subjects with healed EE at week 2, 4 or 8 in the comparison study were randomised 1:1 to receive vonoprazan 10 or 20 mg orally once daily after breakfast for 52 weeks in the sequential long‐term maintenance study after providing additional informed consent.
Patients
Male or female out‐patients aged ≥20 years with endoscopically confirmed EE LA Classification Grades A–D were eligible for inclusion in the comparison study. The enrolment of patients with LA Classification Grade A or B EE was limited to a maximum of 280 to ensure that more than 30% of the total number had more severe LA Classification Grade C or D EE. The main exclusion criteria included complications associated with the oesophagus; acute upper gastrointestinal bleeding; the presence of or a history of hypersecretion disorders; a history of surgery or treatment affecting GERD; serious neurological, cardiovascular, pulmonary, hepatic, renal, metabolic, gastrointestinal, urologic, endocrinological or haematological disorders; need for surgery requiring hospitalisation; history or complication of drug (including alcohol) abuse; patients with AIDS or hepatitis; history of malignancy; any of the following abnormal laboratory test values at the start of the screening period [serum creatinine level, >2 mg/dL; alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level, 2.5× upper limit of normal (ULN), or total bilirubin level, >2× ULN] and female subjects who were pregnant or lactating. Any female of child‐bearing potential who was sexually active was required to use adequate contraceptive measures.
Patients were not allowed to concomitantly use any medications which would affect the efficacy evaluation of the study. These included PPIs, histamine type 2 receptor antagonists, muscarinic M3 receptor antagonists, gastrointestinal motility stimulants, anticholinergic drugs, prostaglandins, acid suppressants, anti‐gastrin drugs, mucosal protective agents, Helicobacter pylori eradication therapies, atazanavir sulphate and other investigational products.
Treatment, randomisation and blinding
In the comparison study, patients were randomised to receive treatment with vonoprazan or lansoprazole. Independent randomisation personnel designated by the sponsor generated the randomisation table. LA Classification Grades (A/B or C/D) at the start of the treatment period were used to stratify patients based on their disease severity to ensure that the two treatment groups were well‐balanced in this respect. All randomisation information was securely stored in an area accessible only by authorised personnel. A double‐dummy method, using matching vonoprazan placebo tablets and lansoprazole placebo capsules, was employed to ensure that the study was double‐blind to avoid possible bias. All medications were provided in sealed boxes and supplied by the site study medication supervisor to ensure blinded allocation.
In the sequential long‐term maintenance study, subjects were allocated to receive vonoprazan 10 or 20 mg using a randomisation table generated by randomisation personnel designated by the sponsor, which incorporated LA Classification Grades (A/B or C/D) as a stratification factor at the start of the screening period in the preceding comparison study. The study was maintained double‐blind, using indistinguishable tablets, until the database lock at the last patient at week 24 when the sponsor was unblinded for an interim data submission to the regulatory authority. The study then continued until week 52 in a single‐blind manner.
Procedures
At the start of the screening period (3–7 days before randomisation) for the comparison study, patient demographics and other baseline characteristics were recorded, including medical history, concurrent medical conditions, medication history, concomitant medications and pre‐treatment adverse events and patient eligibility was confirmed. In addition, the following were monitored at the start of screening period: clinical laboratory tests (haematology, serum chemistry and urinalysis), vital signs, physical examination, serum gastrin/pepsinogen I/II levels, pregnancy test, electrocardiogram (ECG) and anti‐H. pylori IgG antibody measurement. CYP2C19 genotyping was performed at 2 weeks after the start of treatment. Throughout the study [weeks 2, 4, and 8 (or upon early termination)], the following were monitored: physical examination, vital signs (including ECG), clinical laboratory tests, pregnancy test, serum gastrin/pepsinogen I/II levels, adverse events, concomitant medication, patients' diary and treatment compliance.
Endoscopy was performed at the start of the screening period, weeks 2, 4 and 8 (or upon early termination) under fasted conditions and was classified in terms of Barrett's mucosa (present ≥3 cm, present <3 cm, absent, or unknown for the screening period and increased, unchanged, reduced, disappeared or unknown for other time points), hiatal hernia (present ≥2 cm, present <2 cm, absent, unknown) and LA Classification Grades (A to D or no mucosal breaks).
Gastric biopsy (at the designated study sites only) was performed at the start of the screening period and at the end of the treatment period (weeks 2, 4, 8 or upon early termination). The biopsy fragment was taken from the greater curvature of the upper corpus of the stomach during endoscopic procedure.
All subjects maintained daily diaries in which they recorded erosive oesophageal symptoms such as heartburn and regurgitation (daytime and night‐time), and compliance with treatment.
During the long‐term maintenance study visits were scheduled at weeks 4, 12, 24, 36 and 52 (and upon early termination), and the following were monitored throughout: physical examination, vital signs, clinical laboratory tests, pregnancy test, serum gastrin/pepsinogen I/II levels, subjective symptoms of EE, adverse events, concomitant medications and treatment compliance. ECGs and endoscopy were performed at weeks 12, 24, 36 and 52 or upon early termination. Gastric biopsy (at the same designated study sites only) was performed at weeks 24 and 52 or upon early termination.
A central adjudication committee (CAC) was established to assess all endoscopic images at the start of the screening period in the comparison study, and at the study visit when the recurrence was endoscopically confirmed in the long‐term maintenance study. The committee was responsible for standardised reviews of endoscopic EE grading by the investigators. However, the investigators made decisions on eligibility of subjects for study entry, and study completion due to recurrence in the long‐term maintenance study, regardless of the CAC's assessment. Similarly, an independent assessment committee was set up to assess gastric mucosa histopathology specimens.
Adverse events (including frequency, severity, investigator‐assessed causality and seriousness) and concomitant medications were monitored throughout the study. Treatment‐emergent adverse events (TEAEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA). All TEAEs were descriptively summarised and categorised in terms of Preferred Term (PT) by treatment group. While vonoprazan has shown no signs or symptoms of liver function test abnormalities, drug‐related hepatic changes have been reported for drugs in this class.17 Consequently, liver function abnormalities were classified as a special interest TEAE and monitored throughout the study.
The primary endpoint of the comparison study was the proportion of healed EE patients up to week 8. The secondary endpoints were the proportions of healed EE patients at week 2 and up to week 4. EE healing was endoscopically confirmed by the investigators. Other endpoints included subjective symptoms of EE (daytime and night‐time heartburn and regurgitation as reported in patient diaries) and safety endpoints.
In the long‐term maintenance study, the primary endpoint was the incidence of TEAEs. The secondary endpoints were clinical laboratory test values, ECG findings, vital signs and serum gastrin and pepsinogen I/II levels. An additional secondary endpoint was the proportion of patients with EE recurrence (defined as endoscopically confirmed EE of LA Classification Grades A–D during maintenance treatment).
Statistical analyses
Based upon the results of a Phase II study in which the proportion of patients with healed EE over 8 weeks was 96.5% for vonoprazan 20 mg and 95.5% for lansoprazole 30 mg, a sample size of 111 subjects per treatment group would have >90% power to detect a non‐inferiority difference between treatments with a non‐inferiority margin of 10% utilising a two‐sided 95% confidence interval (CI). Since this study was to continue as a long‐term safety study requiring at least 300 subjects, it was decided to randomise 200 patients into each treatment group to ensure enough patients entered the long‐term safety study to meet exposure requirements.
For the primary endpoint in the comparison study, the proportion of healed EE patients up to week 8, frequency, and point estimates and two‐sided 95% CIs were calculated by treatment group in the full analysis set (FAS: all randomised patients who received at least one dose of study drug). Point estimates and two‐sided 95% CIs of the difference in the proportion of healed EE patients up to week 8 between the treatment groups (vonoprazan group – lansoprazole group) were also calculated. In addition, non‐inferiority of the vonoprazan group to the lansoprazole group was tested using the Farrington and Manning test with a non‐inferiority margin of 10%.18
The same analyses were performed for the secondary endpoints in the comparison study. The Fisher's exact test was also performed as a post hoc analysis of superiority for the primary and secondary endpoints. The proportion of patients with healed EE at week 2 and up to week 4 was analysed to assess the onset of EE healing and, in addition, point estimates and two‐sided 95% CIs of the difference between the proportion of healed EE patients after 4 weeks' treatment with vonoprazan and 8 weeks' treatment with lansoprazole (vonoprazan group – lansoprazole group) were calculated.
During the long‐term maintenance study the proportion of patients with EE recurrence at each time‐point was summarised by treatment group in the FAS and the frequency, point estimates and two‐sided 95% CIs were calculated. In both studies, the EE grading by the investigator was included in the analyses. TEAEs were coded using MedDRA and descriptively summarised in both studies.
Results
Baseline characteristics
A total of 508 subjects signed the informed consent form and 409 eligible subjects (mean ± s.d. age 57.9 ± 13.5 years and 71.1% males) were randomly allocated to receive treatment with vonoprazan 20 mg (n = 207) or lansoprazole 30 mg (n = 202) (Figure 1). A total of 401 subjects completed the study (203 and 198 in the vonoprazan and lansoprazole groups, respectively). The two groups were comparable at randomisation with no obvious difference in the baseline characteristics (Table 1). This was particularly true for the proportions of patients assessed by the principal investigator to have LA Classification Grades A/B or C/D EE: 63.8/36.2% and 63.9/36.1% in the vonoprazan and lansoprazole groups, respectively (Table 1). The proportions of patients assessed by the CAC to have LA Classification Grades A/B or C/D EE were 66.2/27.1% and 58.9/30.7% in the vonoprazan and lansoprazole groups, respectively; 6.8% and 10.4%, respectively, were considered to have no mucosal breaks at baseline (Table 1). The two groups were also comparable with respect to the percentage of subjects with any medical history, concurrent medical conditions, medication history and concomitant medications (data not shown). Mean treatment compliance was 98.9% in the vonoprazan group and 97.9% in the lansoprazole group, and 95.6% of subjects took ≥90% of their medications (96.6% in the vonoprazan group and 94.6% in the lansoprazole group).
Figure 1.
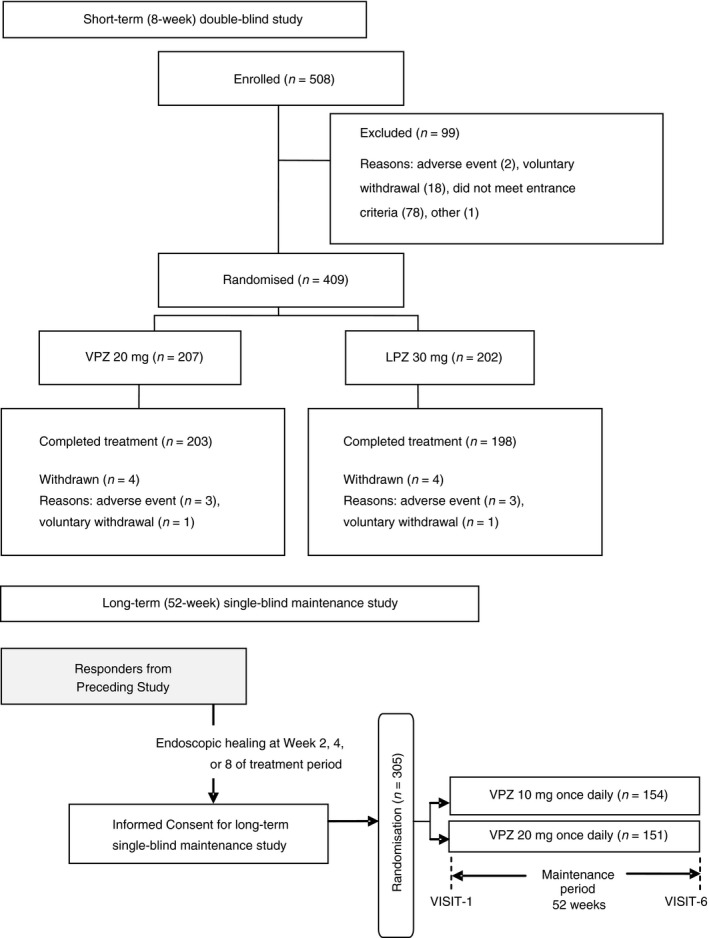
Patient disposition during a short‐term (8‐week) double‐blind comparison of vonoprazan (VPZ) and lansoprazole (LPZ) and long‐term (52‐week) single‐blind maintenance study with VPZ 10 or 20 mg in patients with erosive oesophagitis (EE)
Table 1.
Baseline and other demographical characteristics for patients treated with vonoprazan or lansoprazole for erosive oesophagitis (EE) during a short‐term (8 week) double‐blind comparison and patients treated with vonoprazan 10 or 20 mg during a long‐term (52 week) single‐blind maintenance study
| Short‐term, double‐blind study | Long‐term, single‐blind study | |||||
|---|---|---|---|---|---|---|
| VPZ 20 mg | LPZ 30 mg | Total | VPZ 10 mg | VPZ 20 mg | Total | |
| Number of subjects | 207 | 202 | 409 | 154 | 151 | 305 |
| Age (years), mean ± s.d. | 58.3 ± 13.8 | 57.4 ± 13.2 | 57.9 ± 13.5 | 58.8 ± 13.0 | 56.4 ± 13.7 | 57.6 ± 13.4 |
| Gender Male, n (%) | 137 (66.2) | 154 (76.2) | 291 (71.1) | 111 (72.1) | 108 (71.5) | 219 (71.8) |
| Gender Female, n (%) | 70 (33.8) | 48 (23.8) | 118 (28.9) | 43 (27.9) | 43 (28.5) | 86 (28.2) |
| Height (cm) mean ± s.d. | 163.3 ± 10.9 | 165.0 ± 10.1 | 164.1 ± 10.6 | 164.1 ± 10.6 | 164.7 ± 10.7 | 164.4 ± 10.7 |
| Weight (kg), mean ± s.d. | 64.7 ± 12.7 | 68.3 ± 13.1 | 66.5 ± 13.0 | 66.3 ± 13.4 | 66.7 ± 12.8 | 66.5 ± 13.1 |
| BMI (kg/m2), mean ± s.d. | 24.1 ± 3.4 | 24.9 ± 3.4 | 24.5 ± 3.4 | 24.5 ± 3.4 | 24.4 ± 3.2 | 24.4 ± 3.3 |
| Baseline LA Classification Grades A/B (by PI), n (%) | 132 (63.8) | 129 (63.9) | 261 (63.8) | N/Aa | N/Aa | N/Aa |
| Baseline LA Classification Grades C/D (by PI), n (%) | 75 (36.2) | 73 (36.1) | 148 (36.2) | N/Aa | N/Aa | N/Aa |
| No mucosal breaks at baseline (by CAC), n (%) | 14 (6.8) | 21 (10.4) | 35 (8.6) | N/Aa | N/Aa | N/Aa |
| Baseline LA Classification Grades A/B (by CAC), n (%) | 137 (66.2) | 119 (58.9) | 256 (62.6) | N/Aa | N/Aa | N/Aa |
| Baseline LA Classification Grades C/D (by CAC), n (%) | 56 (27.1) | 62 (30.7) | 118 (28.9) | N/Aa | N/Aa | N/Aa |
| Oesophageal hiatal hernia ≥2 cm, n (%) | 51 (24.6) | 45 (22.3) | 96 (23.5) | 36 (23.4) | 33 (21.9) | 69 (22.6) |
| Oesophageal hiatal hernia <2 cm, n (%) | 96 (46.4) | 98 (48.5) | 194 (47.4) | 71 (46.1) | 70 (46.4) | 141 (46.2) |
| Oesophageal hiatal hernia none, n (%) | 60 (29.0) | 59 (29.2) | 119 (29.1) | 46 (29.9) | 48 (31.8) | 94 (30.8) |
| Helicobacter pylori infection status Positive, n (%) | 34 (16.4) | 18 (8.9) | 52 (12.7) | 27 (17.5) | 15 (9.9) | 42 (13.8) |
| Helicobacter pylori infection status Negative, n (%) | 173 (83.6) | 184 (91.1) | 357 (87.3) | 127 (82.5) | 136 (90.1) | 263 (86.2) |
| CYP2C19 Genotype test Extensive Metaboliser, n (%) | 183 (88.4) | 167 (82.7) | 350 (85.6) | 128 (83.1) | 129 (85.4) | 257 (84.3) |
| CYP2C19 Genotype test Poor Metaboliser, n (%) | 24 (11.6) | 35 (17.3) | 59 (14.4) | 26 (16.9) | 22 (14.6) | 48 (15.7) |
BMI, body mass index; CAC, central adjudication committee; LA, Los Angeles; LPZ, lansoprazole; PI, principal investigator; VPZ, vonoprazan.
Not applicable since all patients were required not to have endoscopically confirmed mucosal breaks by the LA Classification Grades to enter the long‐term maintenance study.
Efficacy analysis
The proportion of patients with healed EE up to week 8 of the treatment period was 99.0% in the vonoprazan group and 95.5% in the lansoprazole group (Table 2). The difference between the two groups was 3.5% (95% CI: 0.362–6.732), thus confirming the non‐inferiority of vonoprazan vs. lansoprazole (P < 0.0001). Furthermore, the lower limit of the 95% CI exceeded 0, indicating that the proportion of patients with healed EE in the vonoprazan group was statistically different to that produced by lansoprazole (P = 0.0337; Fisher's exact test, post hoc analysis). A similar result was observed for the week 2 assessment, with vonoprazan being non‐inferior to lansoprazole (P < 0.0001). The difference between the two groups was 8.8% (95% CI: 2.105–15.448) and once again the lower limit of the 95% CI exceeded 0, indicating that the proportion of healed EE patients with vonoprazan was statistically different to that produced by lansoprazole (P = 0.0132; Fisher's exact test, post hoc analysis). The week 4 result also confirmed the non‐inferiority of vonoprazan vs. lansoprazole (P < 0.0001), but in the post hoc analysis, the difference between the two treatments did not achieve statistical significance (P = 0.0806).
Table 2.
Proportion of patients with healed erosive oesophagitis (EE) up to week 8 (primary endpoint), and at week 2 and up to week 4 (secondary endpoints) following treatment with vonoprazan (VPZ) or lansoprazole (LPZ)
| Time point | Treatment | % Pts healed (n/N) [95% CIs] | Difference [95% CIs] (VPZ – LPZ) | P‐value non‐inferioritya | P‐value Fisher's exact testb |
|---|---|---|---|---|---|
| Week 8 | VPZ 20 mg | 99.0 (203/205) [96.520–99.882] | 3.5 [0.362–6.732] | <0.0001 | 0.0337 |
| LPZ 30 mg | 95.5 (190/199) [91.589–97.911] | ||||
| Week 2 | VPZ 20 mg | 90.7 (185/204) [85.838–94.299] | 8.8 [2.105–15.448] | <0.0001 | 0.0132 |
| LPZ 30 mg | 81.9 (163/199) [75.846–86.996] | ||||
| Week 4 | VPZ 20 mg | 96.6 (198/205) [93.091–98.616] | 4.1 [−0.308–8.554] | <0.0001 | 0.0806 |
| LPZ 30 mg | 92.5 (184/199) [87.872–95.720] |
Farrington and Manning test with a non‐inferiority margin of 10%.
Post‐hoc analysis.
Interestingly, the proportion of patients with healed EE was 96.6% at week 4 for vonoprazan compared with 95.5% at week 8 for lansoprazole; there was a small difference in favour of vonoprazan [1.1% (95% CI: −2.702 to 4.918)]; the lower limit of the 95% CI exceeded −10% (the non‐inferiority margin).
The proportions of patients with healed EE at week 2, and up to weeks 4 and 8 were analysed in subgroups stratified according to age, gender, baseline LA Classification Grades, serological determination of H. pylori, and CYP2C19 genotype. For every subgroup, the proportion of patients with healed EE tended to be higher in the vonoprazan group at all timepoints. Some of the more notable differences in which the proportion of healed patients tended to be higher for vonoprazan compared with lansoprazole included patients with more severe EE (LA Classification Grades C/D) as assessed by the principal investigator, and those classified as extensive metabolisers of CYP2C19 (Table 3).
Table 3.
Proportion of patients with healed erosive oesophagitis (EE) in various subgroups up to week 8 (a) (primary endpoint), and at week 2 (b) and up to week 4 (c) (secondary endpoints) sub grouped according to LA Classification Grades [A/B and C/D] and CYP2C19 metaboliser status following treatment with vonoprazan (VPZ) or lansoprazole (LPZ)
| VPZ 20 mg | LPZ 30 mg | P‐valuea | |||
|---|---|---|---|---|---|
| N | Healed EE, n (%) | N | Healed EE, n (%) | ||
| (a) Week 8 primary endpoint | |||||
| Baseline LA Classification Grade by principal investigator | |||||
| A/B | 130 | 129 (99.2) | 127 | 127 (100.0) | 1.0000 |
| C/D | 75 | 75 (98.7) | 72 | 63 (87.5) | 0.0082 |
| CYP2C19 genotype test | |||||
| Extensive metaboliser | 181 | 179 (98.9) | 164 | 155 (94.5) | 0.0290 |
| Poor metaboliser | 24 | 24 (100.0) | 35 | 35 (100.0) | N/A |
| (b) Week 2 Secondary endpoint | |||||
| Baseline LA Classification Grade by principal investigator | |||||
| A/B | 129 | 119 (92.2) | 127 | 117 (92.1) | 1.0000 |
| C/D | 75 | 66 (88.0) | 72 | 46 (63.9) | 0.0008 |
| CYP2C19 genotype test | |||||
| Extensive metaboliser | 180 | 162 (90.0) | 164 | 130 (79.3) | 0.0065 |
| Poor metaboliser | 24 | 23 (95.8) | 35 | 33 (94.3) | 1.0000 |
| (c) Week 4 Secondary endpoint | |||||
| Baseline LA Classification Grade by principal investigator | |||||
| A/B | 130 | 126 (96.9) | 127 | 126 (99.2) | 0.3703 |
| C/D | 75 | 72 (96.0) | 72 | 58 (80.6) | 0.0040 |
| CYP2C19 genotype test | |||||
| Extensive metaboliser | 181 | 174 (96.1) | 164 | 149 (90.9) | 0.0496 |
| Poor metaboliser | 24 | 24 (100.0) | 35 | 35 (100.0) | N/A |
Fisher exact test (post‐hoc analysis).
Only eight subjects (seven previously treated with lansoprazole and one with vonoprazan) received an additional 4 or 8 weeks of treatment with vonoprazan 40 mg once daily. All eight subjects completed the additional treatment period, and six of them achieved healing of EE (all previously treated with lansoprazole).
Erosive oesophagitis symptoms of heartburn and regurgitation recorded in patient diaries tended to be improved in both the vonoprazan and lansoprazole groups, with no notable differences between them (data not shown).
Tolerability and safety
Overall, both treatments were well‐tolerated during the comparison study and the incidences of TEAEs, drug‐related TEAEs and TEAEs leading to study drug discontinuation were similar between the vonoprazan and lansoprazole treatment groups (Table 4). The majority of TEAEs were mild in intensity (114 of 123) and there were two moderate TEAEs in the vonoprazan group, and six moderate TEAEs and one severe TEAE in the lansoprazole group. Nasopharyngitis was the most frequently reported TEAE in both groups and was the only TEAE with an incidence of ≥2% in the vonoprazan group during the comparison study. One TEAE of special interest occurred in each group (abnormal liver function test result); the one reported in the vonoprazan group was considered to be unrelated to the study medication by the investigator while the one reported in the lansoprazole group was considered to be related to study medication by the investigator.
Table 4.
Treatment‐emergent adverse events (TEAEs) during short‐term treatment with vonoprazan (VPZ) 20 mg or lansoprazole (LPZ) 30 mg for erosive oesophagitis (EE)
| VPZ (N = 207) | LPZ (N = 202) | |
|---|---|---|
| Any TEAEa | 46 (22.2)/59 | 45 (22.3)/64 |
| Drug‐related TEAEa | 14 (6.8)/18 | 12 (5.9)/17 |
| TEAE leading to study drug discontinuationa | 2 (1.0)/4 | 3 (1.5)/4 |
| Any serious TEAEa | 0 (0.0)/0 | 3 (1.5)/4 |
| Deatha | 0 (0.0)/0 | 0 (0.0)/0 |
| TEAEs occurring in at least 2% of patients in any treatment group by system organ class and preferred term | ||
|
SOC PT | ||
| Infections and infestationsb | 13 (6.3) | 15 (7.4) |
| Nasopharyngitis | 7 (3.4) | 8 (4.0) |
| Respiratory, thoracic and mediastinal disordersb | 2 (1.0) | 5 (2.5) |
| Upper respiratory tract infection | 1 (0.5) | 4 (2.0) |
The data in this table includes findings observed in the additional 4 or 8 weeks of treatment with vonoprazan 40 mg once daily for eight patients whose EE was not healed during the treatment period. These are hepatic steatosis in one subject in vonoprazan group and hepatic function abnormal, protein urine present and blood glucose increased in one subject in lansoprazole group.
Number of subjects (% subjects)/Number of events.
n (%).
The mean levels of serum gastrin, pepsinogen I and pepsinogen II increased after administration of vonoprazan and lansoprazole, with greater increases in the vonoprazan group (Figure 2). There was no marked change in the mean ± s.d. pepsinogen I/II ratio between the vonoprazan (baseline 6.71 ± 2.135 to 5.31 ± 1.421 at week 2) and lansoprazole (6.97 ± 2.065 to 7.53 ± 1.812) groups. Gastric mucosa histopathology, evaluated for subjects enrolled at the designated study sites only (n = 35 at baseline in each of the vonoprazan and lansoprazole treatment groups), revealed no remarkable effects of the study drugs on neuroendocrine cells. No clinically significant changes in laboratory test values, vital signs or ECG findings were observed during the comparison study.
Figure 2.
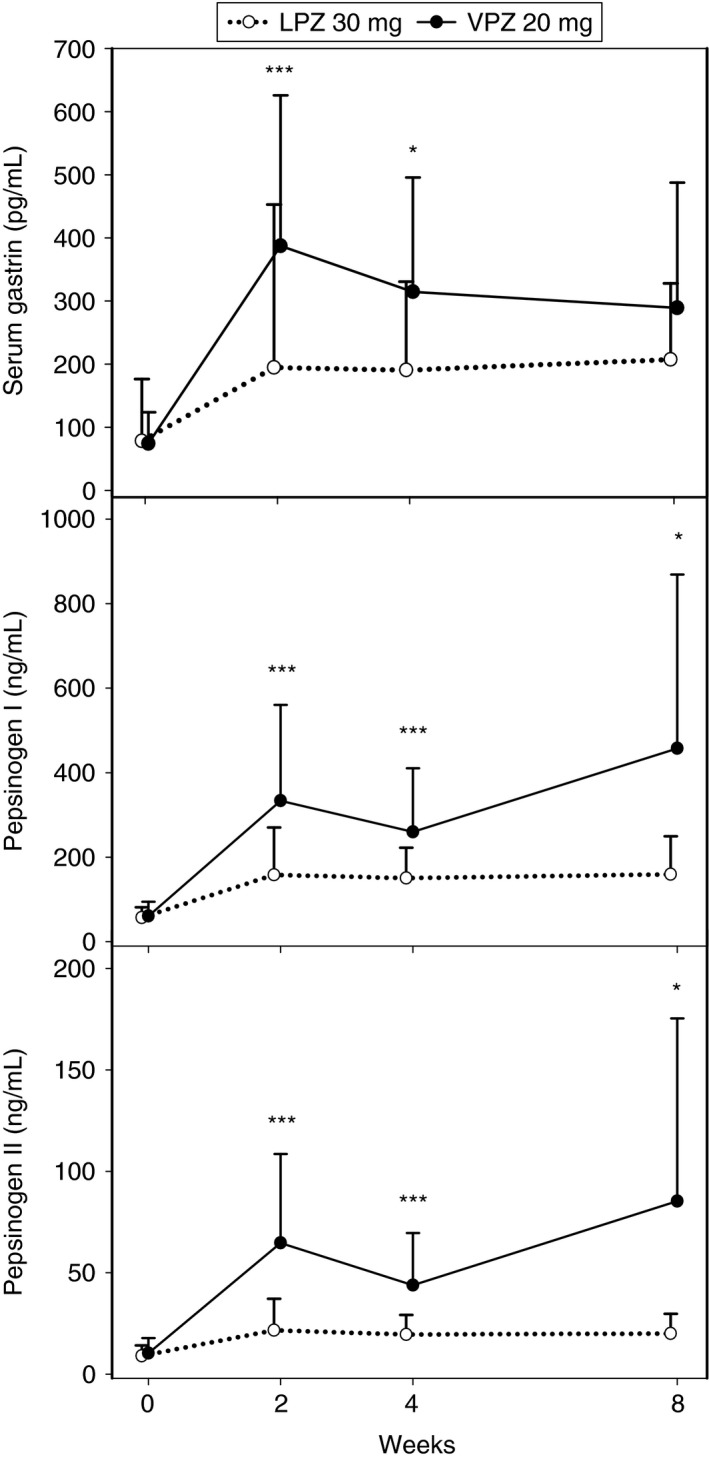
Arithmetic mean serum gastrin, pepsinogen I and pepsinogen II levels at baseline and after 2, 4 and 8 weeks' treatment with vonoprazan (VPZ) 20 mg or lansoprazole (LPZ) 30 mg once daily in patients with erosive oesophagitis (EE). Each bar shows the standard deviation. Difference between vonoprazan and lansoprazole groups: ***P < 0.0001; *P < 0.05.
Long‐term maintenance study
A total of 305 subjects who achieved endoscopically healed EE in the comparison study were randomised to vonoprazan 10 mg (n = 154) or 20 mg (n = 151) once daily in the long‐term maintenance study and 135 (87.7%) and 129 (85.4%), respectively completed the 52 weeks of treatment (Figure 1). There were no obvious differences between the two groups with respect to demographical or other baseline characteristics (Table 1). The main reasons for not completing 52 weeks of treatment in the 10 mg (n = 19) and 20 mg (n = 22) groups were: AEs (n = 8, 42.1% vs. n = 12, 54.5%); lost to follow‐up (n = 2, 10.5% vs. n = 1, 4.5%); and voluntary withdrawal (n = 5, 26.3% vs. n = 7, 31.8%).
The overall incidences of TEAEs (76.6% vs. 78.8%), drug‐related TEAEs (9.7% vs. 16.6%), TEAEs leading to study drug discontinuation (5.2% vs. 7.9%), moderate to severe TEAEs (12.3% vs. 11.3%), serious AEs (5.2% vs. 7.3%) and serious drug‐related AEs (0.6% vs. 1.3%) were comparable between the vonoprazan 10 and 20 mg groups during the long‐term maintenance study. The most common TEAEs (with an incidence ≥2%) by system organ class or PT are shown in Table 5. Most of the TEAEs were mild in intensity and there was no obvious trend towards an increase in incidence over time in the two treatment groups.
Table 5.
Most frequent treatment‐emergent adverse events (TEAEs) with an incidence of ≥2% in any treatment group by system organ class (SOC) and preferred term (PT) during long‐term maintenance treatment with vonoprazan (VPZ) 10 or 20 mg for erosive oesophagitis (EE)
| TEAE according to SOC and PT | VPZ 10 mg (N = 154), n (%) | VPZ 20 mg (N = 151), n (%) |
|---|---|---|
| Gastrointestinal disorders | 37 (24.0) | 38 (25.2) |
| Gastric polyps | 7 (4.5) | 7 (4.6) |
| Enterocolitis | 2 (1.3) | 5 (3.3) |
| Gastric erosive | 1 (0.6) | 6 (4.0) |
| Constipation | 4 (2.6) | 2 (1.3) |
| Diarrhoea | 3 (1.9) | 3 (2.0) |
| Gastritis | 4 (2.6) | 1 (0.7) |
| Dental caries | 1 (0.6) | 3 (2.0) |
| Nausea | 1 (0.6) | 3 (2.0) |
| Hepatobiliary disorders | 1 (0.6) | 5 (3.3) |
| Hepatic function abnormal | 1 (0.6) | 3 (2.0) |
| Immune system disorders | 2 (1.3) | 3 (2.0) |
| Seasonal allergy | 2 (1.3) | 3 (2.0) |
| Infections and Infestations | 64 (41.6) | 72 (47.7) |
| Nasopharyngitis | 33 (21.4) | 43 (28.5) |
| Gastroenteritis | 11 (7.1) | 9 (6.0) |
| Pharyngitis | 9 (5.8) | 4 (2.6) |
| Bronchitis | 3 (1.9) | 6 (4.0) |
| Tinea pedis | – | 3 (2.0) |
| Tonsillitis | – | 3 (2.0) |
| Injury, poisoning and procedural complications | 16 (10.4) | 12 (7.9) |
| Fall | 7 (4.5) | 4 (2.6) |
| Investigations | 13 (8.4) | 17 (11.3) |
| Blood creatine phosphokinase increased | 2 (1.3) | 7 (4.6) |
| Liver function test abnormal | – | 5 (3.3) |
| Musculoskeletal and connective tissue disorders | 26 (16.9) | 18 (11.9) |
| Back pain | 6 (3.9) | 4 (2.6) |
| Osteoarthritis | 6 (3.9) | – |
| Periarthritis | 3 (1.9) | 3 (2.0) |
| Psychiatric disorders | 7 (4.5) | 7 (4.6) |
| Insomnia | 5 (3.2) | 7 (4.6) |
| Respiratory, thoracic and mediastinal disorders | 12 (7.8) | 13 (8.6) |
| Upper respiratory tract inflammation | 7 (4.5) | 11 (7.3) |
| Skin and subcutaneous tissue disorders | 9 (5.8) | 16 (10.6) |
| Eczema | 1 (0.6) | 7 (4.6) |
| Dermatitis contact | – | 3 (2.0) |
| Vascular disorders | 5 (3.2) | 6 (4.0) |
| Hypertension | 3 (1.9) | 5 (3.3) |
The mean serum gastrin level increased over the 52‐week treatment period in both groups. The increase in mean ± s.d. serum gastrin level was greater in the vonoprazan 20 mg group (baseline of the long‐term maintenance study 317.5 ± 336.42 to 777.6 ± 678.64 pg/mL at week 52) than in the vonoprazan 10 mg group (291.0 ± 219.59 to 514.4 ± 435.53 pg/mL). No clinically significant changes were noted in the mean levels of pepsinogen I and II, or in the pepsinogen I/II ratio in the vonoprazan 10 or 20 mg groups after the start of the long‐term maintenance study. The increase in serum gastrin observed during the study was not associated with any clinically significant effects on gastric mucosal neuroendocrine cells as evidenced by histopathological testing of samples taken at weeks 24 and 52 during the long‐term maintenance study (Table 6). No clinically significant changes in clinical laboratory test values, vital signs and ECG findings were observed during the long‐term maintenance study.
Table 6.
Mean (s.d.) gastric cell density (/mm2) measured at baseline and after 24 and 52 weeks of treatment with vonoprazan (VPZ) 10 or 20 mg once daily
| Cell type | N | VPZ 10 mg Mean (s.d.) | N | VPZ 20 mg Mean (s.d.) |
|---|---|---|---|---|
| Epithelial cells (×103) | ||||
| Baseline | 32 | 1.70 (0.33) | 33 | 1.86 (0.30) |
| Week 24 | 32 | 1.58 (0.42) | 30 | 1.73 (0.33) |
| Week 52 | 27 | 1.64 (0.44) | 28 | 1.67 (0.47) |
| Grimelius‐positive cells (×102) | ||||
| Baseline | 32 | 0.62 (0.46) | 33 | 0.85 (0.48) |
| Week 24 | 32 | 0.80 (0.43) | 30 | 0.94 (0.31) |
| Week 52 | 27 | 1.03 (0.40) | 28 | 0.93 (0.31) |
| Chromogranin A‐positive cells (×102) | ||||
| Baseline | 32 | 1.12 (0.57) | 33 | 1.50 (0.70) |
| Week 24 | 32 | 0.92 (0.56) | 30 | 1.11 (0.47) |
| Week 52 | 27 | 1.20 (0.40) | 28 | 1.09 (0.34) |
| Synaptophysin‐positive cells (×102) | ||||
| Baseline | 32 | 1.65 (0.59) | 33 | 1.81 (0.75) |
| Week 24 | 32 | 1.35 (0.52) | 30 | 1.50 (0.34) |
| Week 52 | 27 | 1.39 (0.39) | 28 | 1.34 (0.41) |
| Ki‐67(MIB‐1)‐positive cells (×102) | ||||
| Baseline | 32 | 1.42 (1.13) | 33 | 1.54 (0.89) |
| Week 24 | 32 | 1.12 (0.48) | 30 | 0.91 (0.42) |
| Week 52 | 27 | 1.19 (0.50) | 28 | 1.05 (0.40) |
There were few recurrences of EE during the maintenance treatment: the proportion of patients with EE recurrence was 6.0% (9/149 subjects) at week 24 and 9.4% (14/149 subjects) at week 52 in the vonoprazan 10 mg group, and 4.1% (6/145 subjects) at week 24 and 9.0% (13/145 subjects) at week 52 in the vonoprazan 20 mg group.
Discussion
The results of the comparison study demonstrate non‐inferiority of vonoprazan 20 mg compared with lansoprazole 30 mg, both administered once daily, for up to 8 weeks, in patients with EE. The magnitude of the difference was 3.5% [95% CI: 0.362–6.732], and in a subsequent post hoc analysis, the proportion of patients with healed EE up to week 8 (primary endpoint) in the vonoprazan group was shown to be statistically different to that in the lansoprazole group (P < 0.0337). Vonoprazan was also demonstrated to be non‐inferior to lansoprazole for the proportion of patients with healed EE after 2 and 4 weeks' treatment.
Analysis of subgroups showed that the proportion of patients with healed EE was generally higher in the vonoprazan group. For example, the proportion of healed patients was higher for vonoprazan compared with lansoprazole (at week 2, and up to weeks 4 and 8) in patients with more severe EE (LA Classification Grades C/D) and in those classified as CYP2C19 extensive metabolisers. The most notable finding regarding EE healing in this study relates to the much higher percentage of patients healed at week 2 in the vonoprazan group vs. the lansoprazole group [90.7% vs. 81.9%; difference 8.8% (95% CI: 2.105–15.448, P < 0.0001 for the non‐inferiority test)]. This is indicative of a stronger and faster clinical effect with vonoprazan, which presumably results from its rapid and strong suppression of gastric acid secretion,14 and a similar finding has been observed in animal studies.19 These findings may also explain the comparable proportion of healed patients after 4 weeks' treatment with vonoprazan vs. 8 weeks with lansoprazole; the difference being 1.1% in favour of vonoprazan and the lower limit of the 95% CI exceeding −10%.
Our findings are consistent with previous studies, in particular a Phase II dose‐ranging study in patients with EE in which the proportion of healed EE patients with vonoprazan 20 mg once daily was 94.4% after 4 weeks' treatment.16 In this study, vonoprazan also produced more rapid healing than lansoprazole by week 2 and it was more effective in patients with more severe EE (LA Classification Grades C/D). Furthermore, in the long‐term maintenance study, vonoprazan 10 and 20 mg once daily were shown to result in a low EE recurrence rate (<10%) in patients who achieved EE healing in the comparison study by week 8, which was comparable to the reported recurrence rate (8.0–12.5%) during a 24‐week maintenance treatment with esomeprazole 10 or 20 mg for healed EE in Japan.20
Vonoprazan and lansoprazole were similarly well‐tolerated in the comparison study, with only two subjects in the vonoprazan group and three in the lansoprazole group discontinuing treatment as a result of a TEAE. A total of 114 of 123 reported TEAEs were of mild intensity and the one severe TEAE in the lansoprazole group was not considered to be related to study treatment. In the long‐term maintenance study 86.6% of subjects completed 52 weeks of treatment and, overall, vonoprazan 10 and 20 mg once daily were considered to be safe and well‐tolerated with few recurrences of EE in either group during the study. In both studies, there were no new safety signals and no significant changes in laboratory test values, vital signs or ECG findings other than an increase in gastrin levels. Gastric mucosa histopathology revealed no remarkable effects of the study drugs on neuroendocrine cells during the comparison study or up to 52 weeks in the long‐term maintenance study. However, these studies had relatively short periods of follow‐up and the influence of vonoprazan on the gastric mucosa will be continuously monitored during long‐term treatment (>1 year).
A limitation of the current comparison study is that the efficacy evaluation assessing the statistical significance of vonoprazan vs. lansoprazole was not pre‐planned, and therefore the findings should be viewed as post hoc. However, statistically significant differences were observed and were consistent across a number of subgroups, with evidence of a time‐dependent effect, thus providing further confirmation of the benefits of vonoprazan when compared with a PPI, lansoprazole.
In summary, this comparison study demonstrated the non‐inferiority of vonoprazan compared with lansoprazole, and demonstrated that the novel P‐CAB is highly effective for the treatment of patients with EE, more notably for patients with more severe EE (LA Classification Grades C/D) and CYP2C19 extensive metabolisers. During the long‐term maintenance (52 weeks) treatment, there were few cases (<10%) of recurrence with vonoprazan 10 or 20 mg once daily. Overall, vonoprazan was well‐tolerated for up to 52 weeks with few patients discontinuing treatment.
Authorship
Guarantor of the article: Kiyoshi Ashida.
Author contributions: Kiyoshi Ashida, Yuuichi Sakurai, Tetsuharu Hori, and Akira Nishimura were involved in study conception and design. Naoki Hiramatsu served as Medical Expert. Eiji Umegaki, Katsuhiko Iwakiri and Kiyoshi Ashida served as the Central Adjudication Committee. Kentarou Kudou conducted statistical analysis. All authors were involved in the interpretation of study results, and in the drafting and critical revision of the manuscript. All authors approved the final version of the manuscript, including the authorship list.
Acknowledgements
Declaration of personal interests: Kiyoshi Ashida, Naoki Hiramatsu, Eiji Umegaki, and Katsuhiko Iwakiri are all paid consultants to Takeda Pharmaceutical Company Ltd. Yuuichi Sakurai, Tetsuharu Hori, Kentarou Kudou and Akira Nishimura are employees of Takeda Pharmaceutical Company Ltd.
Declaration of funding interests: These studies (TAK‐438/CCT‐002 and TAK‐438/OCT‐001) were funded in full by Takeda Pharmaceutical Company Ltd. Writing support was provided by Steve Clissold, PhD, ContentEdNet, and was funded by Takeda Pharmaceutical Company Ltd., Japan. The authors thank Richard Jenkins and Göran Hasselgren from Takeda Development Centre, Europe for reviewing the manuscript. The results of TAK‐438/CCT‐002 study were previously presented at the Digestive Disease Week (DDW) 2014 and at the Japanese Digestive Disease Week (JDDW) 2014.
This article was accepted for publication after full peer‐review.
References
- 1. Maradey‐Romero C, Fass R. New and future drug development for gastroesophageal reflux disease. J Neurogastroenterol Motil 2014; 20: 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peery AF, Dellon ES, Lund J, et al Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012; 143: 1179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population‐based study in Olmsted County, Minnesota. Gastroenterology 1997; 112: 1448–56. [DOI] [PubMed] [Google Scholar]
- 4. Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta‐analysis. Gastroenterology 1997; 112: 1798–810. [DOI] [PubMed] [Google Scholar]
- 5. Dent J, El‐Serag HB, Wallander MA, Johansson S. Epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut 2005; 54: 710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El‐Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut 2014; 63: 871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altomare A, Guarino MPL, Cocca S, Emerenziani S, Cicala M. Gastroesophageal reflux disease: update on inflammation and symptom perception. World J Gastroenterol 2013; 19: 6523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Pinxteren B, Numans ME, Lau J, et al Short‐term treatment of gastroesophageal reflux disease. J Gen Intern Med 2003; 18: 755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fass R. Alternative therapeutic approaches to chronic proton pump inhibitor treatment. Clin Gastroenterol Hepatol 2012; 10: 338–45. [DOI] [PubMed] [Google Scholar]
- 10. Shin JM, Inatomi N, Munson K, et al Characterization of a novel potassium‐competitive acid blocker of the gastric H,K‐ATPase, 1‐[5‐(2‐fluorophenyl)‐1‐(pyridin‐3‐ylsulfonyl)‐1H‐pyrrol‐3‐yl]‐N‐methylmethanamine monofumarate (TAK‐438). J Pharmacol Exp Ther 2011; 339: 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hori Y, Imanishi A, Matsukawa J, et al 1‐[5‐(2‐fluorophenyl)‐1‐(pyridin‐3‐ylsulfonyl)‐1H‐pyrrol‐3‐yl]‐N‐methylmethanamine monofumarate (TAK‐438), a novel and potent potassium‐competitive acid blocker for the treatment of acid‐related diseases. J Pharmacol Exp Ther 2010; 335: 231–8. [DOI] [PubMed] [Google Scholar]
- 12. Matsukawa J, Hori Y, Nishida H, et al A comparative study on the modes of action of TAK‐438, a novel potassium‐competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol 2011; 81: 1145–51. [DOI] [PubMed] [Google Scholar]
- 13. Hori Y, Matsukawa J, Takeuchi T, et al A study comparing the antisecretory effect of TAK‐438, a novel potassium‐competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther 2011; 337: 797–804. [DOI] [PubMed] [Google Scholar]
- 14. Sakurai Y, Nishimura A, Kennedy G, et al Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK‐438 (Vonoprazan) doses in healthy male Japanese/non‐Japanese subjects. Clin Transl Gastroenterol 2015; 6: e94. doi:10.1038/ctg.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jenkins H, Sakurai Y, Nishimura A, et al Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK‐438 (vonoprazan), a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2015; 41: 636–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ashida K, Sakurai Y, Nishimura A, et al Randomised clinical trial: a dose‐ranging study of vonoprazan, a novel potassium‐competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther 2015; 42: 685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kahrilas PJ, Dent J, Lauritsen K, et al A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol 2007; 5: 1385–91. [DOI] [PubMed] [Google Scholar]
- 18. Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non‐zero risk difference or non‐unity relative risk. Stat Med 1990; 9: 1447–54. [DOI] [PubMed] [Google Scholar]
- 19. Andersson K, Carlsson E. Potassium‐competitive acid blockade: a new therapeutic strategy in acid‐related diseases. Pharmacol Ther 2005; 108: 294–307. [DOI] [PubMed] [Google Scholar]
- 20. Kinoshita Y, Miwa H, Kasugai K. Efficacy and safety of esomeprazole, compared with omeprazole, in maintenance therapy for reflux esophagitis – a phase III, multicenter, randomized, double‐blind trial. Nihon Shokakibyo Gakkai Zasshi 2013; 110: 1428–38. [PubMed] [Google Scholar]


