Summary
Background
Apixaban is a direct factor Xa inhibitor approved for the treatment and prevention of thromboembolic disease. There is a lack of data regarding its reversal in cases of acute bleeding or prior to emergency surgery that needs addressing.
Objectives
This study assessed whether a four‐factor prothrombin complex concentrate (4F‐PCC; Beriplex®/Kcentra®, CSL Behring) can effectively reverse apixaban‐associated bleeding in an in vivo rabbit model and evaluated the correlations between in vivo hemostasis and in vitro coagulation parameters.
Methods
For dose‐finding purposes, anesthetized rabbits were treated with a single intravenous dose of apixaban (800–1600 μg kg−1) and, following a standardized kidney incision, volume of blood loss and time to hemostasis were measured. In a subsequent study phase, anesthetized rabbits were treated with apixaban 1200 μg kg−1 followed by 4F‐PCC (6.25–100 IU kg−1), and the effects on the same bleeding parameters were assessed. In parallel, coagulation parameters were monitored.
Results
Dose‐dependent increases in time to hemostasis and total blood loss were observed post apixaban administration. Preincision treatment with 4F‐PCC resulted in a statistically significant reversal in bleeding time (all doses) and volume (doses ≥ 12.5 IU kg−1). Of the coagulation parameters measured, thrombin generation initiated using the RD reagent (phospholipids only) was the most sensitive to in vivo measures of 4F‐PCC's hemostatic efficacy, although some correlations were also observed for prothrombin time and whole blood clotting time.
Conclusions
In this rabbit model of acute hemorrhage, 4F‐PCC showed potential for reversing the bleeding effects of apixaban. Clinical data in apixaban‐treated patients are needed to confirm these results.
Keywords: anticoagulants, apixaban, hemorrhage, preclinical study, drug evaluation, prothrombin complex concentrates
Introduction
Apixaban (Eliquis®; Bristol‐Myers Squibb, Princeton, NJ, USA) is one of several oral direct factor Xa (FXa) inhibitors and is approved in several countries, including the United States and Europe, for the prevention and treatment of various thrombotic conditions 1, 2.
As with vitamin K antagonists (VKAs), reversal of anticoagulation induced by newer oral anticoagulants (NOACs) such as apixaban may be necessary in bleeding patients or in those requiring urgent surgery. Guidelines suggest the use of recombinant activated FVII (rFVIIa) or activated/non‐activated prothrombin complex concentrates (aPCCs/PCCs) 3, 4, 5. However, validated NOAC reversal strategies are lacking, although antidotes for FXa inhibitors are currently in development 6. Though there have been several studies of NOAC reversal in healthy volunteers 7, 8, 9, real‐world data regarding management of NOAC‐related major bleeding are scarce. Observations suggest that traditional coagulation assays may not be predictive of the ability of prohemostatic agents to stop bleeding 10, adding to the complexity of patient management in time‐critical situations.
Reversal of the anticoagulant effects of dabigatran, edoxaban, and rivaroxaban by a non‐activated four‐factor PCC (4F‐PCC) has been demonstrated in an in vivo rabbit model of acute bleeding 11, 12, 13. The aims of this study are to evaluate the ability of the same 4F‐PCC to reverse the anticoagulant effects of apixaban in this model and to investigate correlations between in vitro coagulation parameters and in vivo measures of hemostasis.
Materials and methods
Study design
This open‐label study was conducted using a previously described rabbit model 11, 12, 13; a brief description of study design and methods is provided next. Study animals received care in compliance with the European Convention on Animal Care, and procedures were approved by the local animal welfare authority.
The primary endpoint was the ability of 4F‐PCC to reverse the effects of apixaban on time to hemostasis and volume of blood loss. Coagulation parameters were also assessed, including prothrombin time (PT), activated partial thromboplastin time (aPTT), whole blood clotting time (WBCT) 14, and thrombin generation (TG), following either extrinsic activation using phospholipids and tissue factor (5 pmol L–1), or intrinsic activation using phospholipids only (RD reagent). Apixaban plasma levels were measured via the FXa inhibition assay, as previously described 12, 13.
Study agents
4F‐PCC (Beriplex®/Kcentra®; CSL Behring GmbH, Marburg, Germany), containing FII, FVII, FIX, FX, and coagulation proteins C and S 15, was reconstituted as per label. Apixaban (kindly provided by Bristol‐Myers Squibb, Plainsboro, NJ, USA) was reconstituted in a vehicle solution of dimethylacetamide:propanediol:water (10%:20%:70%) to a concentration of 0.5 mg mL−1.
Treatment
Treatment group assignments are shown in Table 1. In the dose‐finding part of the study, anesthetized animals received a single intravenous (i.v.) administration of either reconstituted apixaban or vehicle solution followed 3 min later by an i.v. bolus of 0.9% (w/v) isotonic saline.
Table 1.
Treatment group assignments
| Apixaban dose (μg kg−1) | 4F‐PCC dose (IU kg−1) | Animals (n) |
|---|---|---|
| Dose‐finding | ||
| 0* | 0† | 3 |
| 800 | 0† | 5 |
| 1000 | 0† | 5 |
| 1200 | 0† | 5 |
| 1600 | 0† | 5 |
| Apixaban reversal with 4F‐PCC | ||
| 1200 | 6.25 | 5 |
| 1200 | 12.5 | 5 |
| 1200 | 25 | 5 |
| 1200 | 50 | 5 |
| 1200 | 75 | 5 |
| 1200 | 100 | 5 |
4F‐PCC, four‐factor prothrombin complex concentrate; IU, international unit. *Vehicle (dimethylacetamide:propanediol:water, 10%:20%:70%) administered. †Isotonic saline 0.9% w/v administered; apixaban/vehicle was administered at t = 0 min, 4F‐PCC/saline was administered at t = 3 min.
For the subsequent reversal study phase, anesthetized animals were randomized to receive an i.v. administration of reconstituted apixaban (1200 μg kg−1; t = 0) followed by an i.v. bolus of 4F‐PCC (6.25, 12.5, 25, 50, 75, or 100 IU kg−1; t = 3 min). Sample sizes were based on previous experience of NOAC reversal in this rabbit model.
In both parts of the study, at t = 8 min a standardized kidney incision was created as described previously 12. The 30‐min observation period for the assessment of blood loss and time to hemostasis began immediately after the incision. Blood loss was measured as the volume of blood collected by syringe from the kidney incision site; time to hemostasis was defined as the time elapsed between kidney incision and cessation of observable bleeding or oozing.
Blood samples for determination of apixaban plasma levels and coagulation parameters were collected at baseline, just before 4F‐PCC administration (t = 3 min), immediately before kidney incision (t = 8 min), and at the end of the observation period (t = 40 min). Coagulation parameters were assessed as described previously 12.
Statistical analysis
Mean (SD) or median (range) are reported. Time to hemostasis and blood loss were compared between 4F‐PCC–treated and untreated groups using the log‐rank test. In vitro coagulation parameters were compared between 4F‐PCC–treated and untreated groups in analysis of covariance models. Treatment group was input as the fixed factor, while the value of the coagulation marker at t = 3 min (immediately before 4F‐PCC treatment) was the covariate.
Results and discussion
Dose‐finding study
In the vehicle control group, a standardized kidney incision led to a median (range) time to hemostasis and total blood loss of 3 (3–5) min and 2 (2–3) mL, respectively. Following i.v. administration of apixaban (800–1600 μg kg−1), significant increases were seen in both bleeding endpoints (Fig. 1A). Maximum bleeding signals were seen at 1200 μg kg−1. For this reason, the 1200 μg kg−1 apixaban dose was used to investigate the potential of 4F‐PCC to reverse the anticoagulant effects of apixaban. This represents a supratherapeutic dose of apixaban, with the maximal plasma levels, seen at time of first monitoring (3 min, 1379 ng mL−1, Fig. 1B), corresponding to approximately 4 times the Cmax seen in healthy volunteers after twice‐daily dosing with apixaban 10 mg for 7 days 16, 17.
Figure 1.
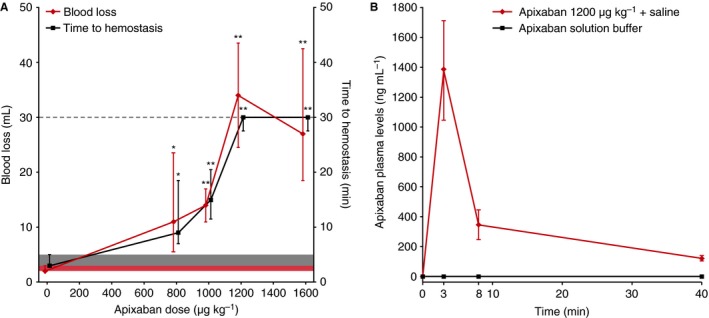
(A) Effects of i.v. administration of apixaban (800–1600 μg kg−1) on time to hemostasis and total blood loss after standardized kidney incision and (B) apixaban plasma levels over time following administration of apixaban 1200 μg kg−1 or buffer. Data are median ± IQR. (A) Gray shaded area denotes baseline range for time to hemostasis (3–5 min) in vehicle‐treated animals; time to hemostasis > 30 min was not measured and the maximum observation time is denoted by the horizontal dashed line; red shaded area denotes baseline range for blood loss (2–3 mL) in vehicle‐treated animals. Animals in the control group received vehicle solution instead of apixaban. * and ** indicate statistical significance at the P < 0.05 and P < 0.01 levels, respectively, compared with vehicle‐treated animals. IQR, interquartile range.
Effect of 4F‐PCC on apixaban‐associated bleeding
Treatment with 4F‐PCC at 3 min post apixaban administration led to statistically significant reductions in time to hemostasis compared with saline‐treated animals for all doses tested. Significant reductions in total blood loss were also observed for 4F‐PCC doses ≥ 12.5 IU kg−1 (Fig. 2).
Figure 2.
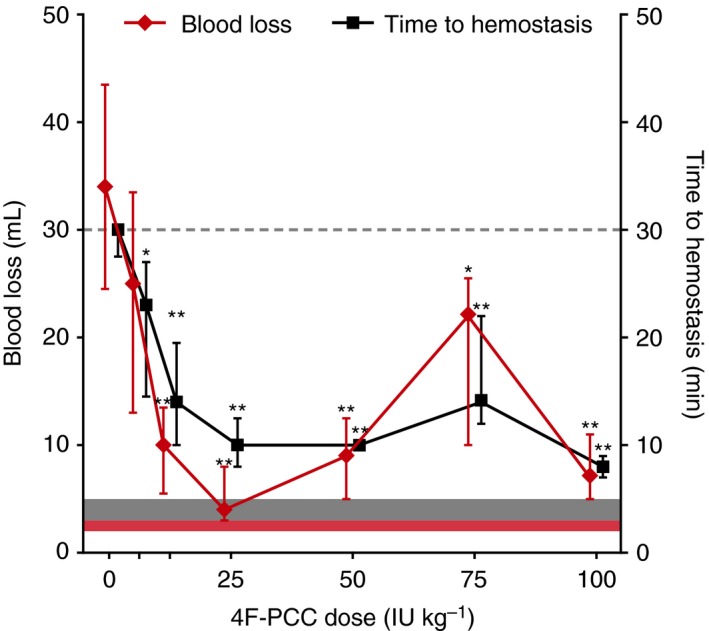
Reversal of apixaban (1200 μg kg−1) effects on time to hemostasis and blood loss with 4F‐PCC. Data are median ± IQR. Gray shaded area denotes baseline range for time to hemostasis (3–5 min) in vehicle‐treated animals; time to hemostasis > 30 min was not measured and the maximum observation time is denoted by the horizontal dashed line; red shaded area denotes baseline range for blood loss (2–3 mL) in vehicle‐treated animals. * and ** indicate statistical significance at the P < 0.05 and P < 0.01 levels, respectively, compared with animals that received apixaban plus saline. 4F‐PCC, four‐factor prothrombin complex concentrate; IQR, interquartile range; IU, international units.
Effects of apixaban and 4F‐PCC on biomarkers of hemostasis
Modest but dose‐dependent increases in PT (1.7‐ to 2.2‐fold), aPTT (2.1‐ to 2.7‐fold), and WBCT (2.1‐ to 2.7‐fold) were observed at 3 min post apixaban treatment (data not shown). Reversal of apixaban‐induced PT prolongation with 4F‐PCC was partial and significant only with 4F‐PCC 12.5, 75, and 100 IU kg−1 (Fig. 3A), with a maximum reduction of 1.2‐fold compared with saline‐treated animals seen with 4F‐PCC 100 IU kg−1. The reversal of apixaban‐induced WBCT prolongation was also only partial but could be seen for all 4F‐PCC doses ≥ 25 IU kg−1 (Fig. 3B). Compared with saline‐treated animals, 4F‐PCC 100 IU kg−1 reduced WBCT levels by 1.2‐fold. No reversal of apixaban‐induced aPTT prolongation was observed following 4F‐PCC treatment (data not shown).
Figure 3.
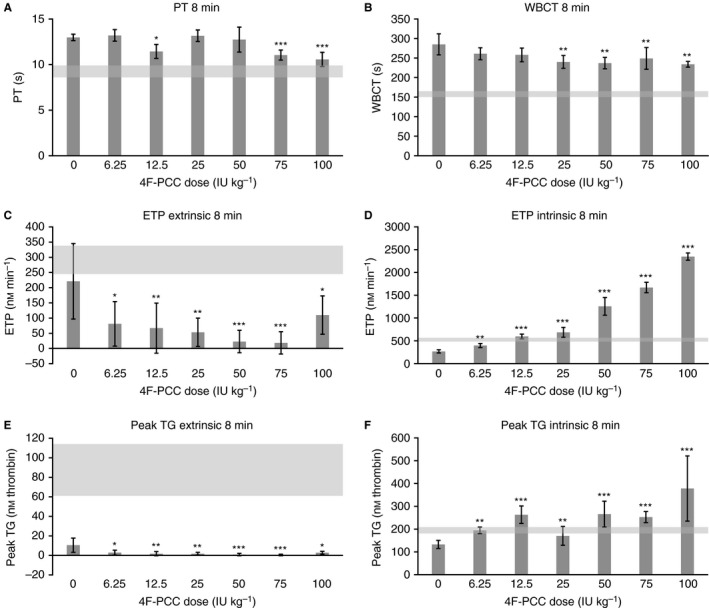
Effects of 4F‐PCC (6.25–100 IU kg−1, i.v.) on (A) PT, (B) WBCT, (C) ETP (extrinsic activation), (D) ETP (intrinsic activation), (E) peak TG (extrinsic activation), and (F) peak TG (intrinsic activation) following apixaban treatment (1200 μg kg−1). PT, WBCT, ETP, and peak TG measured at t = 8 min (5 min after 4F‐PCC administration). Data are mean ± SEM. (A) Gray shaded area denotes baseline ranges for PT (8.6–9.9 s) in vehicle‐treated animals; (B) gray shaded area denotes baseline ranges for WBCT (150–165 s) in vehicle‐treated animals; (C) gray shaded area denotes baseline range for ETP (245–338 nm min−1) following extrinsic activation in vehicle‐treated animals; (D) gray shaded area denotes baseline range for ETP (482–566 nm min−1) following intrinsic activation in vehicle‐treated animals; (E) gray shaded area denotes baseline range for peak TG (61–114 nm thrombin) following extrinsic activation in vehicle‐treated animals; (F) gray shaded area denotes baseline range for peak TG (181–210 nm thrombin) following intrinsic activation in vehicle‐treated animals. *, **, and *** indicate statistical significance at the P < 0.05, P < 0.01, and P < 0.001 levels, respectively, compared with animals that received apixaban plus saline. 4F‐PCC, four‐factor prothrombin complex concentrate; ETP, endogenous thrombin potential; IU, international units; PT, prothrombin time; SEM, standard error of the mean; TG, thrombin generation; WBCT, whole blood clotting time.
Apixaban administration led to nearly full inhibition of extrinsic TG, as indicated by reductions of 42–100% for endogenous thrombin potential (ETP) and 74–99% for peak TG. Subsequent administration of 4F‐PCC did not restore either parameter to baseline levels within the dose range tested (Fig. 3C, E). Conversely, apixaban‐induced reductions of TG parameters following intrinsic activation, while less pronounced (17–62% for ETP, 29–47% for peak TG, at t = 3 min), were fully reversed by 4F‐PCC. Intrinsic TG was the most sensitive parameter to 4F‐PCC–mediated effects on bleeding diathesis, showing statistically significant increases in ETP and peak TG at all 4F‐PCC doses tested (Fig. 3D, F). At 5 min post 4F‐PCC dosing, ETP and peak TG were restored to baseline values using 4F‐PCC doses as low as 12.5 IU kg−1 (Fig. 3D) and 6.25 IU kg−1 (Fig. 3F), respectively.
This study evaluated the potential of 4F‐PCC to reverse apixaban‐associated anticoagulation in a rabbit model. Blood loss and time to hemostasis following a standardized kidney incision were both markedly increased by i.v. administration of 1200 μg kg−1 apixaban, relative to baseline. Administration of 4F‐PCC shortly after apixaban dosing significantly reduced both bleeding time and total blood loss post incision. Notably, these effects were observed at 4F‐PCC doses as low as 6.25 IU kg−1 for bleeding time and 12.5 IU kg−1 for blood loss, lower than those required to reverse effects of other NOACs on hemostasis in this model 11, 12, 13. Reasons underlying this observation, and its clinical relevance, remain unclear.
In the only other published preclinical study of apixaban‐associated bleeding reversal, administration of 4F‐PCC (Kanokad®, LFB, Les Ullis, France) did not reduce hepatosplenic blood loss but restored ETP and thromboelastography parameters in apixaban‐treated rabbits 18. However, baseline bleeding signals in apixaban‐treated animals were small (~1.4‐fold increase compared with control rabbits) 18, which may have limited the possibility of detecting the efficacy of potential reversal agents in this model.
Owing to the predictable anticoagulant effects of NOACs, routine anticoagulation monitoring in NOAC‐treated patients is generally not required 5, but validated coagulation assays may still be useful in emergency situations. Changes in PT, aPTT, WBCT, and TG were seen following apixaban dosing, in agreement with in vitro studies of apixaban in human plasma 19 and in vivo studies in healthy volunteers 20, 21. However, there was a lack of robust correlation with 4F‐PCC reversal of bleeding diathesis in vivo in this study. Apixaban‐induced aPTT prolongation was not reversed by 4F‐PCC, and apixaban‐induced increases in PT and WBCT were only partially reversed. This disconnect between coagulation parameters and bleeding cessation extends to reversal of other NOACs in preclinical models 11, 12, 13, 18, 22 and clinical situations 10. Appropriate assays for the monitoring of NOAC reversal therefore still need to be validated.
In this study, the most sensitive in vitro marker of 4F‐PCC–mediated reversal of apixaban‐associated bleeding was intrinsic TG. Baseline levels were achieved at 4F‐PCC doses as low as 12.5 IU kg−1 for ETP and 6.25 IU kg−1 for peak TG, correlating well with the observed reversal of apixaban effects on bleeding time and volume. Similar findings were reported for reversal of rivaroxaban in this animal model 10. Recent studies of edoxaban reversal in this model 12 and in healthy volunteers using a punch biopsy 23 also confirmed that ETP was a good surrogate biomarker of 4F‐PCC's hemostatic efficacy. However, extrinsic TG was used for the edoxaban reversal studies, indicating differential assay responses to 4F‐PCC for each FXa inhibitor.
In the current study, overcorrection of ETP was observed when higher doses of 4F‐PCC were administered following intrinsic, but not extrinsic, activation. Therefore, the clinical relevance of this overcorrection regarding a possible thromboembolic risk associated with 4F‐PCC use for NOAC reversal remains unclear, and future studies of the safety of PCC treatment for NOAC reversal are warranted.
The strengths and limitations of this study should be mentioned. Similar study designs allow comparison of anticoagulant and reversal effects between NOACs using the same 4F‐PCC 11, 12, 13 in a model of pharmacologic relevance, owing to the similarity of NOAC anticoagulant effects in rabbits and humans 24, 25, 26, 27. However, the results of this study following supratherapeutic doses of apixaban may differ to the effects of clinical doses in humans, and our simplified model of a single standardized incision wound may not be reflective of all situations where anticoagulant reversal is required. Furthermore, this preclinical rabbit model may not fully emulate human anticoagulation reversal, and results therefore need to be confirmed in a clinical context. Finally, this study evaluated an off‐label use of this 4F‐PCC (currently indicated in the European Union for treatment and perioperative prophylaxis of bleeding in acquired deficiency of the prothrombin complex factors or congenital deficiency of any of the vitamin K–dependent coagulation factors 28).
In conclusion, this 4F‐PCC effectively reduced apixaban‐associated bleeding in a rabbit model of acute hemorrhage. These results are broadly consistent with those obtained previously for other NOACs 11, 12, 13. TG initiated using the RD reagent was the most sensitive assay to 4F‐PCC–mediated reversal of apixaban effects on bleeding time and volume. However, in light of the variable correlation of laboratory parameters with in vivo measures of hemostasis, there is a clear need for validated assays to guide NOAC anticoagulation reversal. The agreement of the results from this study with those seen for other NOACs in the same model indicates that this 4F‐PCC may be an option for the urgent reversal of the anticoagulant effect of NOACs, but clinical data in human patients are required to confirm these results.
Addendum
E. Herzog designed the study, analyzed and interpreted the data and critically reviewed the manuscript. F. Kaspereit contributed to the study design, conducted the experimental work, and critically reviewed the manuscript. W. Krege contributed to the study design, conducted the experimental work, and critically reviewed the manuscript. J. Mueller‐Cohrs conducted the statistical analysis and critically reviewed the manuscript. B. Doerr conducted the experimental work and critically reviewed the manuscript. P. Niebl conducted the experimental work and critically reviewed the manuscript. G. Dickneite designed the study, analyzed and interpreted the data, and critically reviewed the manuscript.
Disclosure of Conflict of Interests
All authors report personal fees from CSL Behring GmbH, during the conduct of the study.
Acknowledgements
Medical writing assistance was provided by Margarita Lens of Fishawack Communications Ltd. and funded by CSL Behring GmbH. Pancras Wong of Bristol‐Myers Squibb kindly provided input to the study design and provided apixaban study material.
Herzog E, Kaspereit F, Krege W, Mueller‐Cohrs J, Doerr B, Niebl P, Dickneite G. Four‐factor prothrombin complex concentrate reverses apixaban‐associated bleeding in a rabbit model of acute hemorrhage. J Thromb Haemost 2015; 13: 2220–6.
Manuscript handled by: M. Levi
Final decision: P. H. Reitsma, 23 September 2015
References
- 1. Bristol‐Myers Squibb Company . Eliquis highlights of prescribing information. 2014. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed 12 June 2015.
- 2. Bristol‐Myers Squibb/Pfizer EEIG . Eliquis summary of product characteristics. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf. Accessed 12 June 2015.
- 3. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012; 141: e44S–88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez‐Mondejar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Rossaint R. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care 2013; 17: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P. EHRA practical guide on the use of new oral anticoagulants in patients with non‐valvular atrial fibrillation: executive summary. European Heart J 2013; 34: 2094–106. [DOI] [PubMed] [Google Scholar]
- 6. Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, Luan P, Hutchaleelaha A, Inagaki M, Conley PB, Phillips DR, Sinha U. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19: 446–51. [DOI] [PubMed] [Google Scholar]
- 7. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, crossover study in healthy subjects. Circulation 2011; 124: 1573–9. [DOI] [PubMed] [Google Scholar]
- 8. Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non‐specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 2012; 108: 217–24. [DOI] [PubMed] [Google Scholar]
- 9. Levi M, Moore KT, Castillejos CF, Kubitza D, Berkowitz SD, Goldhaber SZ, Raghoebar M, Patel MR, Weitz JI, Levy JH. Comparison of three‐factor and four‐factor prothrombin complex concentrates regarding reversal of the anticoagulant effects of rivaroxaban in healthy volunteers. J Thromb Haemost 2014; 12: 1428–36. [DOI] [PubMed] [Google Scholar]
- 10. Masotti L, Lorenzini G, Seravalle C, Panigada G, Landini G, Cappelli R, Schulman S. Management of new oral anticoagulants related life threatening or major bleedings in real life: a brief report. J Thromb Thrombolysis 2015; 39: 427–33. [DOI] [PubMed] [Google Scholar]
- 11. Pragst I, Zeitler SH, Doerr B, Kaspereit FJ, Herzog E, Dickneite G, van Ryn J. Reversal of dabigatran anticoagulation by prothrombin complex concentrate (Beriplex P/N) in a rabbit model. J Thromb Haemost 2012; 10: 1841–8. [DOI] [PubMed] [Google Scholar]
- 12. Herzog E, Kaspereit F, Krege W, Doerr B, Mueller‐Cohrs J, Pragst I, Morishima Y, Dickneite G. Effective reversal of edoxaban‐associated bleeding with four‐factor prothrombin complex concentrate in a rabbit model of acute hemorrhage. Anesthesiology 2015; 122: 387–98. [DOI] [PubMed] [Google Scholar]
- 13. Herzog E, Kaspereit F, Krege W, Mueller‐Cohrs J, Doerr B, Niebl P, Dickneite G. Correlation of coagulation markers and 4F‐PCC‐mediated reversal of rivaroxaban in a rabbit model of acute bleeding. Thromb Res 2015; 135: 554–60. [DOI] [PubMed] [Google Scholar]
- 14. Gouin‐Thibault I, Flaujac C, Delavenne X, Quenet S, Horellou MH, Laporte S, Siguret V, Lecompte T. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti‐Xa assays. A multicentre French GEHT study. Thromb Haemost 2014; 111: 240–8. [DOI] [PubMed] [Google Scholar]
- 15. CSL Behring . Kcentra® highlights of prescribing information. 2013. http://labeling.cslbehring.com/PI/US/Kcentra/EN/Kcentra-Prescribing-Information.pdf. Accessed 12 June 2015.
- 16. Cui Y, Song Y, Wang J, Yu Z, Schuster A, Barrett YC, Frost C. Single‐ and multiple‐dose pharmacokinetics, pharmacodynamics, and safety of apixaban in healthy Chinese subjects. Clin Pharmacol 2013; 5: 177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamahira N, Frost C, Fukase H, Yu Z, Wang J, Pursley J, LaCreta F, Hiraoka M. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple doses of apixaban in healthy Japanese male subjects. Int J Clin Pharmacol Ther 2014; 52: 564–73. [DOI] [PubMed] [Google Scholar]
- 18. Martin AC, Le Bonniec B, Fischer AM, Marchand‐Leroux C, Gaussem P, Samama CM, Godier A. Evaluation of recombinant activated factor VII, prothrombin complex concentrate, and fibrinogen concentrate to reverse apixaban in a rabbit model of bleeding and thrombosis. Int J Cardiol 2013; 168: 4228–33. [DOI] [PubMed] [Google Scholar]
- 19. Wong PC, Crain EJ, Xin B, Wexler RR, Lam PY, Pinto DJ, Luettgen JM, Knabb RM. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost 2008; 6: 820–9. [DOI] [PubMed] [Google Scholar]
- 20. Frost C, Yu Z, Moore K, Nepal S, Barrett Y, Mosqueda‐Garcia R, Shenker A. An oral direct factor Xa inhibitor: multiple‐dose safety, pharmacokinetics, and pharmacodynamics in healthy subjects. J Thromb Haemost 2007; 5: P‐M‐664. [Google Scholar]
- 21. Frost C, Yu Z, Nepal S, Mosqueda‐Garcia R, Shenker A. Factor Xa inhibitor: single‐dose safety, pharmacokinetics and pharmacodynamics in healthy subjects. J Thromb Haemost 2007; 5: P‐M‐665. [Google Scholar]
- 22. Zhou W, Zorn M, Nawroth P, Butehorn U, Perzborn E, Heitmeier S, Veltkamp R. Hemostatic therapy in experimental intracerebral hemorrhage associated with rivaroxaban. Stroke 2013; 44: 771–8. [DOI] [PubMed] [Google Scholar]
- 23. Zahir H, Brown KS, Vandell AG, Desai M, Maa JF, Dishy V, Lomeli B, Feussner A, Feng W, He L, Grosso MA, Lanz HJ, Antman EM. Edoxaban effects on bleeding following punch biopsy and reversal by a 4‐factor prothrombin complex concentrate. Circulation 2015; 131: 82–90. [DOI] [PubMed] [Google Scholar]
- 24. Wong PC, Pinto DJ, Zhang D. Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis 2011; 31: 478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Furugohri T, Isobe K, Honda Y, Kamisato‐Matsumoto C, Sugiyama N, Nagahara T, Morishima Y, Shibano T. DU‐176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profiles. J Thromb Haemost 2008; 6: 1542–9. [DOI] [PubMed] [Google Scholar]
- 26. Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, Straub A. In vitro and in vivo studies of the novel antithrombotic agent BAY 59‐7939 – an oral, direct Factor Xa inhibitor. J Thromb Haemost 2005; 3: 514–21. [DOI] [PubMed] [Google Scholar]
- 27. Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. In‐vitro profile and ex‐vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost 2007; 98: 155–62. [PubMed] [Google Scholar]
- 28. CSL Behring . Beriplex P/N 1000 IU summary of product characteristics. 2014. http://www.medicines.org.uk/emc/medicine/27570. Accessed 12 June 2015.


