Abstract
Objective
Aims of this consensus panel were to determine (1) an optimal symptom‐based method for assessing opioid‐induced constipation in clinical practice and (2) a threshold of symptom severity to prompt consideration of prescription therapy.
Methods
A multidisciplinary panel of 10 experts with extensive knowledge/experience with opioid‐associated adverse events convened to discuss the literature on assessment methods used for opioid‐induced constipation and reach consensus on each objective using the nominal group technique.
Results
Five validated assessment tools were evaluated: the Patient Assessment of Constipation–Symptoms (PAC‐SYM), Patient Assessment of Constipation–Quality of Life (PAC‐QOL), Stool Symptom Screener (SSS), Bowel Function Index (BFI), and Bowel Function Diary (BF‐Diary). The 3‐item BFI and 4‐item SSS, both clinician administered, are the shortest tools. In published trials, the BFI and 12‐item PAC‐SYM are most commonly used. The 11‐item BF‐Diary is highly relevant in opioid‐induced constipation and was developed and validated in accordance with US Food and Drug Administration guidelines. However, the panel believes that the complex scoring for this tool and the SSS, PAC‐SYM, and 28‐item PAC‐QOL may be unfeasible for clinical practice. The BFI is psychometrically validated and responsive to changes in symptom severity; scores range from 0 to 100, with higher scores indicating greater severity and scores >28.8 points indicating constipation.
Conclusions
The BFI is a simple assessment tool with a validated threshold of clinically significant constipation. Prescription treatments for opioid‐induced constipation should be considered for patients who have a BFI score of ≥30 points and an inadequate response to first‐line interventions.
Keywords: Chronic Pain, Bowel Function Index, PAMORAs, Methylnaltrexone, Naloxegol, Lubiprostone
Introduction
Strategies for the management of pain commonly involve the use of opioid analgesics coupled with appropriate vigilance in patient selection and monitoring 1. The analgesic efficacy of many opioids is attributable to actions exerted by these agents via µ‐opioid receptors in the central nervous system 2. Because µ‐opioid receptors are also expressed throughout body tissues including the gastrointestinal tract 2, opioid analgesics are often associated with various types of opioid bowel dysfunction (OBD) 3, 4. Opioid‐induced constipation (OIC) is the most prevalent OBD and is caused by opioid‐mediated reductions in small intestinal and colonic transit, increased fluid absorption, inhibition of gastrointestinal chloride secretion, and stimulation or decreased relaxation of the pyloric and internal anal sphincters 4, 5, 6, 7. The condition has been reported in up to 47% of opioid‐treated patients and may occur at a higher incidence in women and with increasing age; the greatest risk factor is a longer duration of opioid therapy 8. Furthermore, OIC imposes a substantial burden on quality of life (QOL), reduces work productivity, impairs effectiveness of pain management, and can lead to clinically significant physical sequelae such as those related to bowel obstruction and fecal impaction 9, 10.
Although the current Rome III diagnostic criteria provide a multifaceted definition of functional constipation 11, this condition is not opioid related, and a standardized OIC‐focused definition is needed 12. In 2014, a multidisciplinary consensus group proposed defining OIC as a change from baseline bowel habits upon initiation of opioids that is characterized by any of the following symptoms: (1) reduced bowel movement (BM) frequency; (2) development or worsening of straining to pass stool; (3) a sense of incomplete rectal evacuation; or (4) harder stool consistency 3. In a 2015 systematic review, outcomes including BM frequency, stool consistency, straining, and QOL were suggested for use in OIC clinical trials 12. However, the appropriateness of using these or other outcomes for assessing OIC in clinical practice has not been determined.
In view of the lack of a standardized definition for OIC, robust research guiding therapy for this condition is limited 13. To date, OIC has been predominantly managed with nonspecific laxative regimens 14, 15. Existing treatment standards for OIC suggest that opioid rotation, increased fluid and fiber intake, exercise, and over‐the‐counter (OTC) stool softeners, natural dietary supplements, and laxatives should be considered before evaluating a patient's need for prescription medications 16, 17, 18, 19. Most of these options are well‐tolerated, readily available, and therefore recommended as first‐line treatments; however, first‐line agents are neither sufficiently supported by high‐quality evidence nor associated with specific targeting of the opioid receptor–mediated mechanism of OIC 4, 13, 15. Survey results have shown that only 46% of laxative‐treated patients with OIC achieve their desired treatment outcomes frequently (i.e., >50% of the time) 15. A 2013 systematic review concluded that large, well‐designed studies of laxative efficacy in the treatment of OIC are needed 13.
Several prescription treatments used for OIC have been evaluated in large, multicenter, randomized, controlled trials. Despite the availability of these agents for the treatment of OIC, no guidelines published to date have provided a specific threshold for initiating pharmacologic prescription therapy. Two peripherally acting µ‐opioid receptor antagonists (PAMORAs), methylnaltrexone (RELISTOR®; Salix Pharmaceuticals, Inc., Raleigh, NC, and Progenics Pharmaceuticals, Inc., Tarrytown, NY) and naloxegol (MOVANTIK™; AstraZeneca Pharmaceuticals LP, Wilmington, DE), are approved for the treatment of OIC 20, 21. Lubiprostone (AMITIZA®; Sucampo Pharma Americas, LLC, Bethesda, MD, and Takeda Pharmaceuticals America, Inc., Deerfield, IL) is a locally acting chloride channel activator that is also approved for the treatment of OIC, as well as for irritable bowel syndrome with constipation (IBS‐C) in women and chronic idiopathic constipation (CIC) 22. Although the high‐affinity serotonin type‐4 receptor agonist prucalopride (RESOLOR®; Shire Pharmaceuticals Ireland Ltd., Dublin, Ireland; Sanico NV, Turnhout, Belgium; and Janssen‐Cilag SpA, Borgo San Michele, Italy) is not approved for OIC, this agent has been evaluated in OIC 23 and is approved in several countries (but not in the United States) for the treatment of chronic constipation 24, 25. Additional OIC‐targeted prescription treatments are in development, including other PAMORAs and linaclotide (LINZESS®; Ironwood Pharmaceuticals, Inc., Cambridge, MA, and Actavis, Parsippany, NJ), a locally acting guanylate cyclase‐C receptor agonist currently approved by the US Food and Drug Administration (FDA) for the treatment of CIC and IBS‐C 3, 26, 27, 28, 29.
Appropriate selection of OIC prescription treatments may not be clear to prescribers because of the absence of guidelines or treatment algorithms that formally characterize patients who could benefit from these therapies. Thus, the purpose of this article is to present the views and recommendations of a multidisciplinary consensus panel regarding (1) the most effective method for assessing OIC and (2) the threshold in OIC symptom severity at which to consider initiation of OIC‐targeted prescription medications in clinical practice.
Methods
On March 18, 2015, an OIC consensus panel meeting was held in Washington, DC. The panel comprised a multidisciplinary group of experts in pain, addiction medicine, neurology, palliative medicine, physiatry, anesthesiology, geriatrics, pharmacy, family practice, and gastroenterology. Prior to the meeting, panel members participated in a series of preparative conference calls and reviewed the literature on outcome measures and assessment tools used in OIC. Tools validated in OIC were identified by searching the PubMed database for articles published through 2014 using various combinations of the terms opioid‐induced constipation, opioids, constipation, instrument, assessment, tool, valid, validation, and validity. Other measures and tools used (but not necessarily validated) in OIC trials were identified in a 2015 systematic literature review 12.
The nominal group technique 30, 31 was used to reach consensus. This well‐established and validated technique was selected on the basis of time availability and cost‐effectiveness and to ensure the ability of all panel members to contribute to the final outcomes. The consensus meeting was standardized and followed several steps. First, a balanced review of the key literature identified prior to the meeting was presented by three panel members. During this presentation, three potential definitions were considered to help guide assessment method selection 3, 11, 12. The 2014 definition for OIC proposed by Camilleri et al. 3 was selected on the basis of succinctness and the inclusion of both baseline bowel habits (i.e., BM frequency, straining, incomplete evacuation, and stool consistency) and a change in these habits upon initiation of opioid therapy. The presentation was followed by a description of the nominal group technique by the meeting facilitator.
During the remainder of the meeting, the panel addressed assessment methods followed by thresholds that may prompt consideration of prescription therapy in relation to each assessment method. Panel members independently completed worksheets to generate options based on the available evidence and practicality of application for each assessment method. Each panel member then shared his or her options, one at a time, while the facilitator recorded them on a computer screen projected to all participants. In a discussion session, the rationale for each option was elaborated on, supported, and/or defended. The panel then collectively produced a list of the most viable assessment options and recorded them on a priority sheet. Individual voting was completed by assigning a ranking to each option on the sheet, with higher rankings indicating greater importance. Voting outcomes were shared by the facilitator and further discussed by the group. Revisions and repeat voting were permitted but proved to be unnecessary.
Outcome Measures
Outcome measures may be either objectively measured or directly reported by patients. Objective outcome measures can be collected by clinicians as well as by patients and typically provide numerical values for stool frequency, time‐based outcomes (e.g., time to laxation, transit time [measured using the lactulose hydrogen breath test]), Bristol Stool Form Scale (BSFS) score (i.e., stool consistency) 12, and use of rescue therapy 23, 32, 33, 34, 35, 36. Patient‐reported outcome (PRO) measures are direct reports from patients about how they feel or function with regard to a condition and its therapy, without interpretation by others 37. In OIC, PROs include constipation intensity/severity, ease/difficulty of defecation, incomplete evacuation, straining, discomfort, constipation distress, and satisfaction (i.e., satisfaction with BMs or treatment) 12, 35, 38, 39, 40, 41, 42, 43, 44, 45.
Objective Outcome Measures
The definition of OIC proposed by Camilleri et al. 3 includes two elements that are objectively measured—BM frequency and stool consistency, the latter commonly assessed using the BSFS 12, 33. Improvements in these outcomes have been identified as important to patients with OIC 9, 18, 46, 47, and BM frequency is the central element used in most OIC clinical trials 12. However, the degree of change in this outcome that is considered meaningful may vary among patients 18, 47. One longitudinal study found that patients with OIC would like to have ≥1 BM per day 18, whereas a separate study involving an online patient survey demonstrated that only 1 additional BM per week was viewed as a meaningful improvement 47. Stool frequency and consistency are important to patients but may not sufficiently portray the severity of OIC 46, 48 or the discomfort and other bothersome OIC symptoms 49.
Patient‐Reported Outcome Measures
Compared with objective measures, PRO measures are more effective in capturing patient perceptions of constipation severity and patient experience 49. The PROs of straining and incomplete rectal evacuation, both included in the proposed consensus definition of OIC 3, are common and highly bothersome among patients with the condition 9, 46. Other key PROs include pain/discomfort, bloating, fatigue, and fear or distress 9, 44, 47. Many patients who experience these symptoms in the context of OIC would consider themselves constipated, regardless of stool frequency. However, there are no studies validating specific outcome measures. In addition, different scales are being used for the same outcome across trials (often inconsistently) 12. Thus, in order to identify a comprehensive and practical method for assessing bowel function and determining changes in OIC severity, an analysis of formally validated PRO assessment tools is required.
Patient‐Reported Outcome Assessment Tools
To identify patients with OIC in need of treatment, PRO assessment tools are commonly advocated 46. The five PRO tools identified (Table 1) 3, 12, 46, 48, 50, 51, 52, 53, 54, 55, 56, 57, 58 each capture several PRO measures 59 and may also provide information on BM frequency 53, 57. Two Patient Assessment of Constipation (PAC) tools, the PAC‐Symptoms (PAC‐SYM) and the PAC‐Quality of Life (PAC‐QOL), were developed to address the need for standardized, constipation‐specific PRO instruments 50, 53, 54, 60. The PAC‐SYM stool symptoms domain was adapted by an expert advisory panel for pivotal OIC studies of naloxegol, which resulted in the shorter Stool Symptom Screener (SSS) 52, 58. Two PRO tools were developed specifically for OIC—the Bowel Function Diary (BF‐Diary) 57 and the Bowel Function Index (BFI) (Figure 1) 48. The BFI was designed for use in OIC studies to evaluate the effects of oxycodone prolonged‐release (PR)/naloxone PR (TARGINIQ®; Purdue Pharma L.P., Stamford, CT) 49, an opioid agonist/antagonist combination for chronic pain 49, 61.
Table 1.
PRO assessment tools
| Tool | Length | Symptoms/Outcomes Evaluateda | Scores | Administration | Recall Period | No. of OIC Clinical Trial Publications 12 | No. of OIC Validation Publications | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | Subscores | Total | Content Validity | Construct Validity | ||||||
| PAC‐SYM 50, 51 | 12 items (3 domains) | I. Abdominal (i.e., discomfort, pain, bloating, cramping) II. Rectal (i.e., pain, burning, bleeding/tearing) III. Stool (i.e., incomplete BMs, consistency, size, straining, inability to defecate/false alarm) | 0–4 | Average of items 1–4; 5–7; and 8–12 | Average of all 12 items | Patient administered | 14 days | 5b | 0 | 1 |
| SSS 52 | 4 items | Four of the 5 items from the PAC‐SYM stool symptoms domain: I. Incomplete BMs II. Stool consistency III. Straining IV. Inability to defecate/false alarm | 0–4 | N/A | N/A | Clinician administered | 14 days | 0c | 1 | 0 |
| PAC‐QOL 53, 54 | 28 items (4 subscales) | I. Physical discomfort (e.g., bloating) II. Psychosocial discomfort (e.g., embarrassment, decreased appetite) III. Worries and concerns (e.g., anxiety, fear) IV. Satisfaction (e.g., with BM frequency) | 0–4 |
Average of items 1–4; 5–12; 13–23; and 24–28 |
Average of all 28 items | Patient administered | 14 days | 3 | 0 | 0 |
| BFI 46, 48, 55, 56 | 3 items | I. Ease of defecation II. Incomplete evacuation III. Patients' judgment of constipation | 0 to 100 | N/A | Average of all 3 items | Clinician administered | 7 days | 7 | 0 | 3 |
| BF‐Diary 57 | 11 itemsd (3 modules) | I. BM assessment (e.g., straining, incomplete evacuation, rectal pain, stool consistency) II. Daily/symptom assessment (e.g., inability to defecate, bloating, abdominal pain, bothersome gas, lack of appetite) III. Treatments used (e.g., fiber, laxatives) Composite items: SBMs/wk, SCBMs/wk | Varies by item | N/A | N/A | Patient administered | After each BM and daily | 1 | 1 | 1 |
Abbreviations: BF‐Diary, Bowel Function Diary; BFI, Bowel Function Index; BM, bowel movement; N/A, not applicable; OIC, opioid‐induced constipation; PAC‐QOL, Patient Assessment of Constipation–Quality of Life; PAC‐SYM, Patient Assessment of Constipation–Symptoms; SBM, spontaneous bowel movement; SCBM, spontaneous complete bowel movement; SSS, Stool Symptom Screener.
Symptoms/outcomes included in the proposed OIC definition 3 are in bold.
One additional OIC clinical trial publication used the sum score of rectal and stool symptom domains of the PAC‐SYM.
Although the primary publication for the SSS indicates that this tool was adapted from the PAC‐SYM for use in the naloxegol clinical program, published phase 3 naloxegol studies do not include any details on the use or results of this tool [52,58].
Includes two composite items.
Figure 1.
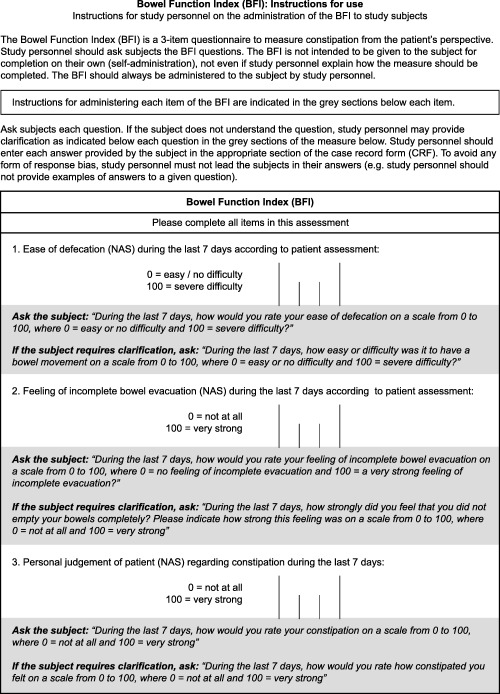
The BFI assessment tool and instructions for use. Abbreviation: BFI, Bowel Function Index. Reproduced with permission from: Rentz AM, Yu R, Müller‐Lissner S, Leyendecker P. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid‐induced constipation. J Med Econ 2009;12:371–83. Copyright 2009 Informa Healthcare.
Relevance in OIC
Items included in the five OIC assessment tools are somewhat varied. The BF‐Diary includes items for assessing all four elements of the proposed OIC definition and a module to identify use of first‐line interventions 3, 57. The PAC‐SYM and SSS include items for assessing all but the BM frequency component of the proposed OIC definition 3, 51, 52; the BFI and PAC‐QOL each assess 1 component of the proposed definition—sense of incomplete rectal evacuation in the BFI and BM frequency in the PAC‐QOL 46, 54. The BFI also includes two relatively general items, ease of defecation and personal judgment of the patient regarding constipation (Figure 1) 48, that may capture other OIC definition elements or key outcomes important in assessing OIC severity, such as bloating and pain 9, 46, 47, 52. These symptoms are also addressed by the BF‐Diary and PAC tools 51, 54, 57.
Clinical Application
The clinical practicality of an assessment tool may be related to its length and ease of use and scoring. With only three items, the BFI is the shortest PRO assessment tool 46. Each item is scored using a numerical analog scale from 0 to 100 points, which is similar to some scales used by patients with chronic pain in clinical trials 46, 48, 49. Furthermore, clinicians can quickly assess OIC severity by calculating the total BFI index score using the average score of the three items 46. Although the 4‐item SSS is also relatively short, no information on calculating a total score is provided 52. Similarly, the 3‐module BF‐Diary does not have a validated combined index score, and the 11 items (including two composite items) must be independently assessed 57. Thus, this method was believed to be time‐consuming, cumbersome, and inconvenient for most clinicians. The 12‐item, 3‐domain PAC‐SYM 51, 60 and the 28‐item, 4‐subscale PAC‐QOL 54 have also been described as too cumbersome for use in a clinical setting 48.
Tool Administration
The PAC‐SYM, PAC‐QOL, and BF‐Diary are self‐administered by patients 50, 53, 57, whereas the BFI and SSS require that clinicians collect the PRO information 48, 52. Involvement of the clinician may help to minimize inaccurate patient interpretations of instructions 46. For self‐administered tools, issues with a patient's reading or comprehension ability could result in failure to complete the assessment 46. In contrast, patient administration limits the potential for clinician bias. Although the standardized BFI instructions (Figure 1) deter clinicians from leading the subjects in their answers 48, the risk of response bias may be inconsistent with the definition of a PRO, which specifies that the outcome should represent a report directly from the patient without interpretation by others 37. Nevertheless, the clinician‐administered BFI is practical in assessing OIC across a broad range of patients, including those with reading or comprehension challenges 44.
Recall Period
Recall periods for the five tools range from 1 to 14 days. For the PAC‐SYM, the selection of a 14‐day recall period was aimed at minimizing patient recall burden while ensuring sufficient time for a symptom complex characterized by relatively infrequent BMs 60. During interviews on the SSS, 95.5% of patients reported they could remember their constipation symptoms “easily” or “very well” with a 14‐day recall period 52. However, authors of the validation article for the daily BF‐Diary expressed concern that a 14‐day recall period may be susceptible to error or bias 57. The 7‐day recall period for the BFI has been described as sufficiently short for appropriate recall but sufficiently long for ensuring clinical relevance with regard to BMs that may not occur on a daily basis 46.
Clinical Trials in OIC
The BFI and PAC‐SYM are the most commonly used tools in published OIC clinical trials (Table 1). The BFI has been used as a primary or coprimary end point in seven published OIC trials reporting the efficacy and safety of oxycodone PR/naloxone PR 39, 62, 63, 64, 65, 66, 67. The PAC‐SYM has been used in five published OIC studies, including two studies of oxycodone PR/naloxone PR 39, 64, one study of methylnaltrexone 68, one study of prucalopride 23, and one study of the µ‐opioid receptor antagonist alvimopan (ENTEREG®; Cubist Pharmaceuticals U.S., Lexington, MA) 35, 69. The PAC‐QOL was used in prucalopride, alvimopan, and methylnaltrexone studies 23, 34, 35. The BF‐Diary was used in a study designed to evaluate effects of the analgesic agent tapentadol IR (NUCYNTA®; Depomed Inc., Newark, CA), a combined µ‐opioid receptor agonist and norepinephrine reuptake inhibitor, on bowel function and gastrointestinal tolerability 70, 71. All of these drugs showed significant improvements (relative to placebo or active comparators) 23, 34, 35, 39, 64, 65, 71, which supports the responsiveness of the assessment tools in patients receiving efficacious treatments for OIC.
Development and Validation of Assessment Tools in OIC
During deliberations, the OIC consensus panel considered 2009 FDA guidance on the development, documentation, and validation (i.e., content and construct validity) of PRO assessment tools 59. In the literature, the BFI has been validated in OIC most frequently, with three publications collectively showing reliability and internal consistency, convergent and known‐groups validity, and a clinically meaningful BFI score change of ≥12 points 48, 55, 56. The validation program also confirmed the ability of BFI values from 27 to 29 points to differentiate patients with constipation from those without constipation 55. These scores support previous findings by Ueberall et al. 49 that defined a reference range of BFI scores reflecting nonconstipation in chronic pain; this range had a 28.8‐point upper limit and included 95% of the nonconstipated population (Figure 2). However, content validation of the BFI has not been documented. For the BF‐Diary, a single validation publication has documented both content and construct validity 57, consistent with FDA guidelines 59. The PAC‐SYM has shown concurrent validity in patients with OIC 51, and the SSS has shown adequate content validity 52. No content or construct validation articles in OIC have been published for the PAC‐QOL.
Figure 2.
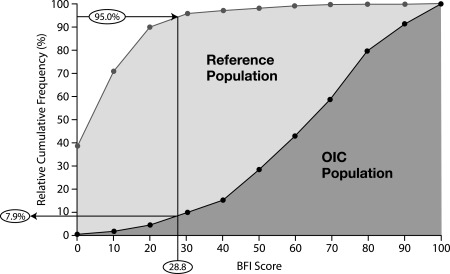
Relative cumulative frequencies of BFI scores for the reference and OIC populations. The reference population comprised nonconstipated patients with chronic pain who were treated with WHO step I and II analgesics; the OIC population comprised patients with chronic pain who had been pretreated with WHO step III opioids and laxative regimens and who reported constipation caused or aggravated by an opioid; 95% of the reference population and 7.9% of the OIC population had a BFI score ≤28.8 points. Abbreviations: BFI, Bowel Function Index; OIC, opioid‐induced constipation; WHO, World Health Organization. Adapted with permission from: Ueberall MA, Müller‐Lissner S, Buschmann‐Kramm C, Bosse B. The Bowel Function Index for evaluating constipation in pain patients: definition of a reference range for a non‐constipated population of pain patients. J Int Med Res 2011;39:41–50. Copyright 2011 SAGE Publications.
Discussion and Recommendations
Initial Clinical Considerations Related to Diagnosis and Prophylactic and First‐Line Interventions
Although this consensus article is not intended to provide specific treatment recommendations, we highlight the need to consider selected factors before evaluating whether treatment with OIC prescription medication is warranted. In anticipation of potential OIC development with long‐term opioid use, treatment guidelines recommend initiation of a prophylactic bowel regimen that may involve increased fluid and fiber intake, stool softeners, and/or laxatives 16, 17, 72. When a diagnosis of OIC is suspected despite prophylactic treatment, clinicians should confirm that initiation of opioid therapy has led to a change from baseline in the patient's typical bowel habits, as defined by Camilleri et al. 3, before consideration of further or alternative interventions. First‐line approaches to intervention also include dietary changes and OTC treatments as well as exercise 16, 17. However, prophylactic and first‐line OTC therapies for OIC may not consistently and predictably lead to desired treatment responses 15, 46, and the number and quality of OIC studies of such therapies are inadequate to determine their efficacy and tolerability 13. Nevertheless, the panel believes that the accessibility and relatively low risk of dietary and OTC options justify their prophylactic and first‐line use for OIC.
Enemas/rectal suppositories and manual evacuation, which are sometimes recommended for patients with constipation 72, are associated with invasiveness, discomfort, embarrassment, and health care burdens. Rectal procedures have been described as painful and traumatic for patients and may lead to complications such as rectal bleeding or bowel perforation, especially in patients who have thrombocytopenia or are receiving anticoagulation therapy 19, 73, 74. For manual evacuation, competent practitioners should be involved in cautious execution and only when absolutely necessary 19, 73, 75, 76 after OTC and prescription therapies have been exhausted. Particular caution is required to avoid invasive options in patients who are immunocompromised because of the risk of producing a systemic infection 19, 73, 75. The health care burden and costs of enemas and manual evacuation are reflected in results from a palliative care study showing that the total health care staff time spent on these processes was higher than time spent on most other tasks related to the management of constipation (e.g., oral laxative administration, discussions of bowel care) 76.
The panel maintains that initial consideration of first‐line OTC interventions for OIC is essential because these approaches may be effective for some patients; however, failure of these options to provide adequate relief should be determined quickly to facilitate consideration of further intervention with PAMORAs (i.e., methylnaltrexone and naloxegol) or other newer prescription medications (e.g., lubiprostone), consistent with the literature 72.
The Panel Recommends the Bowel Function Index for Assessing OIC
To ensure rapid and reliable assessment of OIC by clinicians, a simple and easy‐to‐use method was preferred by the panel. Practicality is critical in selecting a validated assessment tool for universal application across clinical settings. The BFI is a simple, clinically responsive, and validated tool with a clear published threshold for constipation 49, 55. On the basis of the panel members' clinical experience, patients with OIC rarely feel satisfied with the completeness of their BM evacuations, thereby supporting the relevance of the second BFI item (i.e., incomplete evacuation) to OIC. The other two items (i.e., ease of defecation, personal judgment of constipation) are general but may indirectly provide insight into information that is important to each patient (e.g., straining, changes in BM frequency, abdominal symptoms). Perceptions of these general and subjective items may vary among patients. Significant correlations demonstrated between the BFI and both the PAC‐SYM and PAC‐QOL 55, 56 provide reassurance that the BFI captures the symptoms relevant in OIC. Although shorter recall periods are generally preferred for PRO assessments, the 7‐day recall period of the BFI may be appropriate given the general nature of its items. The requirement for a clinician to administer the BFI is acceptable but may represent a limitation of this PRO assessment tool.
Other options proposed by the panel, which would require formal validation, involved use of the BFI in combination with BM frequency or a BF‐Diary module. Stool frequency is perceived as highly important to patients 18, 47, is included in the proposed OIC definition 3, and can be easily tracked in OIC trials. However, in clinical practice, some patients may have difficulty in accurately recalling this outcome. With regard to BF‐Diary modules, which capture OIC definition elements 3 and the use of other interventions, index scoring has not been validated 57. The panel concluded that the complexity involved in individually assessing each BF‐Diary item in a module might be too cumbersome for universal clinical application.
The Panel Recommends a Score of ≥30 Points on the Bowel Function Index for Consideration of Prescription Medications in Patients With Previous or Current Use of First‐line Interventions
A score of ≥30 points on the BFI was ultimately selected on the basis of a study conducted by Ueberall et al. 49 that identified a reference range of 0–28.8 points for most (i.e., 95%) nonconstipated patients with chronic pain. The selected threshold was also based on the general belief that patients should not be denied consideration for further therapy if they surpass the range of nonconstipation, provided that they have also shown inadequate responses to first‐line options. The panel recommends rounding the upper 95% limit of the nonconstipated range on the BFI (i.e., 28.8 points) to 30 points in order to provide a simpler, slightly more conservative, and memorable minimum threshold for application in clinical settings. This threshold includes the scores documented for approximately 90% of the OIC cohort evaluated by Ueberall et al. (Figure 2) 49. This OIC cohort is relevant, as it was derived from a pooled analysis of two studies in which patients, most of whom had been pretreated with World Health Organization step III opioids and laxative regimens, reported constipation (i.e., <3 complete spontaneous BMs per week, without the need to strain) that was caused or aggravated by an opioid 49, 77. Thus, the panel determined that the selected threshold is generalizable to the overall OIC population.
Higher cutoffs of ≥40 or ≥50 points represent values that incorporate both the upper 95% limit of the nonconstipated range defined by Ueberall et al. 49 (i.e., ≤28.8 points) and a clinically meaningful increase of ≥12 points identified by Rentz et al. 48. However, for theoretical cutoff scores, higher magnitudes of increase beyond 28.8 points would result in the exclusion of higher percentages of patients who could otherwise benefit from prescription therapy (Figure 2) 49. For example, a ≥50‐point theoretical threshold would include approximately 70% of the OIC cohort in the Ueberall et al. study but would also exclude up to 30% of these OIC patients from potentially receiving further interventions (Figure 2) 49.
Supplementary Assessments for OIC
The panel recognizes that the BFI may be insufficient in some clinical settings. As such, the BFI may be supplemented with additional outcome measures as necessary on the basis of clinical judgment and individual patient needs. Changes from baseline in BM frequency may be relevant for patients who are able to accurately recall this information. The BSFS may be helpful as a patient education tool and in patients who require a visual method of communication, such as those with cognitive impairment, advanced or serious illness, or other challenges including verbal difficulties and low literacy level.
Proposed Future Efforts in the Assessment of OIC
The panel acknowledges that the BFI may not precisely capture the symptoms and outcomes relevant in OIC. Therefore, development and validation of additional assessment tools for OIC that have comparable or better practicality and ease of use are warranted. For optimal efficiency, future tools might comprise a single question that incorporates the most important aspects of OIC (e.g., “While on opioid therapy, do you have painful or difficult BMs that have not been relieved by laxatives?”). A tool that has the flexibility to be either patient or clinician administered may minimize the potential for clinician bias while maintaining the option, if needed, to avoid patient miscommunication or misinterpretation. Additional clinical studies are needed to help improve understanding of baseline bowel habits and the effectiveness of OTC therapies in OIC.
Conclusions
OIC is common in patients with chronic pain who are receiving opioid therapy, and the condition may have a substantial impact on QOL. The BFI is a practical, validated, and responsive assessment tool that is clinically relevant in OIC. After prophylactic and first‐line interventions have been evaluated, a BFI score of ≥30 points should prompt consideration of prescription OIC medications such as currently available PAMORAs (i.e., methylnaltrexone and naloxegol) or lubiprostone. If necessary, BFI results may be supplemented with assessments that are appropriate on the basis of the clinical setting and individual patient needs, as judged by the clinician. Development and validation of additional OIC assessment tools are warranted.
Author Contributions: Charles E. Argoff, MD, contributed to the comprehensive review of the published literature; consensus meeting facilitation and participation; analysis and interpretation of the data; drafting of the manuscript; critical revision of the manuscript for scientific soundness and intellectual content; approval of the final manuscript; and general supervision. Michael J. Brennan, MD, contributed to the comprehensive review of the published literature; consensus meeting participation; analysis and interpretation of the data; drafting of the manuscript; critical revision of the manuscript for scientific soundness and intellectual content; and approval of the final manuscript. Michael Camilleri, MD, contributed to the comprehensive review of the published literature; consensus meeting participation; analysis and interpretation of the data; drafting of the manuscript; critical revision of the manuscript for scientific soundness and intellectual content; and approval of the final manuscript. Andrew Davies, FRCP, contributed to the comprehensive review of the published literature; consensus meeting participation; analysis and interpretation of the data; drafting of the manuscript; critical revision of the manuscript for scientific soundness and intellectual content; and approval of the final manuscript. Jeffrey Fudin, PharmD, contributed to the comprehensive review of the published literature; consensus meeting participation; analysis and interpretation of the data; drafting of the manuscript; critical revision of the manuscript for scientific soundness and intellectual content; and approval of the final manuscript. Katherine E. Galluzzi, DO, contributed to the comprehensive review of the published literature; consensus meeting participation; analysis and interpretation of the data; drafting of the manuscript; critical revision of the manuscript for scientific soundness and intellectual content; and approval of the final manuscript. Jeffrey Gudin, MD, contributed to the comprehensive review of the published literature; consensus meeting presentation of the literature; consensus meeting participation; analysis and interpretation of the data; drafting of the manuscript; critical revision of the manuscript for scientific soundness and intellectual content; and approval of the final manuscript. Anthony Lembo, MD, contributed to the comprehensive review of the published literature; consensus meeting presentation of the literature; consensus meeting participation; analysis and interpretation of the data; drafting of the manuscript; critical revision of the manuscript for scientific soundness and intellectual content; and approval of the final manuscript. Steven P. Stanos, DO, contributed to the comprehensive review of the published literature; consensus meeting presentation of the literature; consensus meeting participation; analysis and interpretation of the data; drafting of the manuscript; critical revision of the manuscript for scientific soundness and intellectual content; and approval of the final manuscript. Lynn R. Webster, MD, contributed to the comprehensive review of the published literature; consensus meeting facilitation and participation; analysis and interpretation of the data; drafting of the manuscript; critical revision of the manuscript for scientific soundness and intellectual content; approval of the final manuscript; and general supervision.
Acknowledgments
Financial support for this document was provided by educational grants from AstraZeneca Pharmaceuticals LP; Salix Pharmaceuticals, Inc.; Takeda Pharmaceuticals International, Inc., U.S. Region/Sucampo Pharmaceuticals, Inc.; and Shionogi Inc. This document was sponsored by the American Academy of Pain Medicine, with technical and editorial support from Stefanie Dorlas, BMath, of MedLogix Communications, LLC, and was developed on the basis of published literature as well as discussions and voting outcomes from a panel of experts who attended a consensus meeting on March 18, 2015, in Washington, DC. All authors participated in the meeting, preparation of the manuscript, critical revision, and final approval for submission. The authors did not receive an honorarium to participate.
These consensus recommendations of the American Academy of Pain Medicine on initiating prescription therapies for opioid‐induced constipation have been endorsed by the American Gastroenterological Association.
Disclosure and Conflicts of Interest: Financial support for this document was provided by educational grants from AstraZeneca Pharmaceuticals LP; Salix Pharmaceuticals, Inc.; Takeda Pharmaceuticals U.S.A., Inc., and Sucampo; and Shionogi Inc. This document was sponsored by the American Academy of Pain Medicine and included technical and editorial support from MedLogix Communications, LLC. The authors are guarantors of this document, which expresses the opinions and conclusions of the authors and not those of their corresponding affiliations. The authors actively participated in the preparation of this document in accordance with the principles of the Uniform Requirements of the International Committee of Medical Journal Editors (ICMJE). The authors did not receive an honorarium to participate. The authors disclose the following: Charles E. Argoff, MD, has received consulting and speaker fees from AstraZeneca Pharmaceuticals LP and Depomed Inc.; Michael J Brennan, MD, has received consulting and speaker fees from Purdue Pharma L.P. and speaker fees from AstraZeneca Pharmaceuticals LP, Depomed Inc., and Johnson & Johnson; Michael Camilleri, MD, has received research grants or consulting fees (paid to the Mayo Clinic College of Medicine) from AstraZeneca Pharmaceuticals LP, Cubist Pharmaceuticals U.S., Ironwood Pharmaceuticals, Inc., Salix Pharmaceuticals, Inc., Shire Pharmaceuticals Ireland Ltd., and Sucampo Pharma Americas, LLC; Andrew Davies, FRCP, has received consulting and speaker fees from AstraZeneca Pharmaceuticals LP and Wyeth Pharmaceuticals; Jeffrey Fudin, PharmD, has received advisory board and speaker fees from AstraZeneca Pharmaceuticals LP and advisory board fees from Depomed Inc.; Katherine E. Galluzzi, DO, has nothing to disclose; Jeffrey Gudin, MD, has received consulting and speaker fees from AstraZeneca Pharmaceuticals LP, Purdue Pharma L.P., and Salix Pharmaceuticals, Inc.; Anthony Lembo, MD, has received consulting and advisory board fees from Actavis, Ironwood Pharmaceuticals, Inc., Progenics Pharmaceuticals, Inc., and Salix Pharmaceuticals, Inc., and consulting fees from AstraZeneca Pharmaceuticals LP; Steven P. Stanos, DO, has received consulting fees from AstraZeneca Pharmaceuticals LP; Lynn R. Webster, MD, has received consulting and travel fees from AstraZeneca Pharmaceuticals LP and advisory board and travel fees from Depomed Inc.
The copyright line for this article was changed on 13 January after original online publication
References
- 1. Warner EA. Opioids for the treatment of chronic noncancer pain. Am J Med 2012;125:1155–61. [DOI] [PubMed] [Google Scholar]
- 2. Kurz A, Sessler DI. Opioid‐induced bowel dysfunction: Pathophysiology and potential new therapies. Drugs 2003;63:649–71. [DOI] [PubMed] [Google Scholar]
- 3. Camilleri M, Drossman DA, Becker G, et al. Emerging treatments in neurogastroenterology: A multidisciplinary working group consensus statement on opioid‐induced constipation. Neurogastroenterol Motil 2014;26:1386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorn S, Lembo A, Cremonini F. Opioid‐induced bowel dysfunction: Epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol 2014;2:31–7. [DOI] [PubMed] [Google Scholar]
- 5. Camilleri M. Opioid‐induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol 2011;106:835–42; quiz 43. [DOI] [PubMed] [Google Scholar]
- 6. De Schepper HU, Cremonini F, Park MI, Camilleri M. Opioids and the gut: Pharmacology and current clinical experience. Neurogastroenterol Motil 2004;16:383–94. [DOI] [PubMed] [Google Scholar]
- 7. Ketwaroo GA, Cheng V, Lembo A. Opioid‐induced bowel dysfunction. Curr Gastroenterol Rep 2013;15:344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid‐induced bowel disorders and narcotic bowel syndrome in patients with chronic non‐cancer pain. Neurogastroenterol Motil 2010;22:424–30, e96. [DOI] [PubMed] [Google Scholar]
- 9. Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid‐induced bowel dysfunction: Results of a US and European patient survey (PROBE 1). Pain Med 2009;10:35–42. [DOI] [PubMed] [Google Scholar]
- 10. Glare P, Lickiss JN. Unrecognized constipation in patients with advanced cancer: A recipe for therapeutic disaster. J Pain Symptom Manage 1992;7:369–71. [DOI] [PubMed] [Google Scholar]
- 11. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130:1480–91. [DOI] [PubMed] [Google Scholar]
- 12. Gaertner J, Siemens W, Camilleri M, et al. Definitions and outcome measures of clinical trials regarding opioid‐induced constipation: A systematic review. J Clin Gastroenterol 2015;49:9–16. [DOI] [PubMed] [Google Scholar]
- 13. Ruston T, Hunter K, Cummings G, Lazarescu A. Efficacy and side‐effect profiles of lactulose, docusate sodium, and sennosides compared to PEG in opioid‐induced constipation: A systematic review. Can Oncol Nurs J 2013;23:236–46. [DOI] [PubMed] [Google Scholar]
- 14. Coyne KS, Margolis MK, Yeomans K, et al. Opioid‐induced constipation among patients with chronic noncancer pain in the United States, Canada, Germany, and the United Kingdom: Laxative use, response, and symptom burden over time. Pain Med 2015;16:1551–65. [DOI] [PubMed] [Google Scholar]
- 15. Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg 2001;182:11S–8S. [DOI] [PubMed] [Google Scholar]
- 16. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: Evidence‐based recommendations from the EAPC. Lancet Oncol 2012;13:e58–68. [DOI] [PubMed] [Google Scholar]
- 17. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009;10:113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coyne KS, LoCasale RJ, Datto CJ, et al. Opioid‐induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: Descriptive analysis of baseline patient‐reported outcomes and retrospective chart review. Clinicoecon Outcomes Res 2014;6:269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thorpe DM. Management of opioid‐induced constipation. Curr Pain Headache Rep 2001;5:237–40. [DOI] [PubMed] [Google Scholar]
- 20. RELISTOR [package insert]. Raleigh, NC, and Tarrytown, NY: Salix Pharmaceuticals, Inc., and Progenics Pharmaceuticals, Inc; September 2014. http://shared.salix.com/shared/pi/relistor-pi.pdf?id=915545. Accessed October 13, 2015. [Google Scholar]
- 21. MOVANTIK [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; January 2015. http://www.azpicentral.com/movantik/movantik.pdf. Accessed October 13, 2015. [Google Scholar]
- 22. AMITIZA [package insert]. Bethesda, MD, and Deerfield, IL: Sucampo Pharma Americas, LLC, and Takeda Pharmaceuticals America, Inc; April 2013. http://www.amitiza.com. Accessed October 13, 2015. [Google Scholar]
- 23. Sloots CE, Rykx A, Cools M, Kerstens R, De Pauw M. Efficacy and safety of prucalopride in patients with chronic noncancer pain suffering from opioid‐induced constipation. Dig Dis Sci 2010;55:2912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. RESOLOR [summary of product characteristics]. Dublin, Ireland, Turnhout, Belgium, Borgo San Michele, Italy: Shire Pharmaceuticals Ireland Ltd., Sanico NV, and Janssen‐Cilag SpA; June 2015. http://www.medicines.org.uk/emc/print-document?documentId=23204. Accessed October 13, 2015. [Google Scholar]
- 25.Shire. RESOLOR® Available at: http://www.shire.com/shireplc/en/products/gastrointestinal/resolor (accessed March 6, 2015).
- 26. Salix Pharmaceuticals . Our drug research and development pipeline. Available at: http://www.salix.com/about-us/pharmaceutical-research-development/drug-pipeline/ (accessed February 17, 2015).
- 27. Ironwood Pharmaceuticals . Ironwood Pharmaceuticals announces initiation of phase II trial of linaclotide in adult patients with opioid‐induced constipation [press release]. Available at: http://news.ironwoodpharma.com/Press-Releases/Ironwood-Pharmaceuticals-Announces-Initiation-of-Phase-II-Trial-of-Linaclotide-in-Adult-Patients-wit-10b.aspx. Published October 16, 2014 (accessed March 5, 2015).
- 28. Synergy Pharmaceuticals . Synergy's proprietary platform. Available at: http://www.synergypharma.com/drugpipeline/sp-333 (accessed February 17, 2015).
- 29. Burness CB, Keating GM. Oxycodone/Naloxone prolonged‐release: A review of its use in the management of chronic pain while counteracting opioid‐induced constipation. Drugs 2014;74:353–75. [DOI] [PubMed] [Google Scholar]
- 30. Gallagher M, Hares T, Spencer J, Bradshaw C, Webb I. The nominal group technique: A research tool for general practice? Fam Pract 1993;10:76–81. [DOI] [PubMed] [Google Scholar]
- 31. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anissian L, Schwartz HW, Vincent K, et al. Subcutaneous methylnaltrexone for treatment of acute opioid‐induced constipation: Phase 2 study in rehabilitation after orthopedic surgery. J Hosp Med 2012;7:67–72. [DOI] [PubMed] [Google Scholar]
- 33. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 34. Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for treatment of opioid‐induced constipation in patients with chronic, nonmalignant pain: A randomized controlled study. J Pain 2011;12:554–62. [DOI] [PubMed] [Google Scholar]
- 35. Webster L, Jansen JP, Peppin J, et al. Alvimopan, a peripherally acting mu‐opioid receptor (PAM‐OR) antagonist for the treatment of opioid‐induced bowel dysfunction: Results from a randomized, double‐blind, placebo‐controlled, dose‐finding study in subjects taking opioids for chronic non‐cancer pain. Pain 2008;137:428–40. [DOI] [PubMed] [Google Scholar]
- 36. Yuan CS, Foss JF, O'Connor M, et al. Effects of intravenous methylnaltrexone on opioid‐induced gut motility and transit time changes in subjects receiving chronic methadone therapy: A pilot study. Pain 1999;83:631–5. [DOI] [PubMed] [Google Scholar]
- 37. Patrick DL, Guyatt GH, Acquadro C. Chapter 17: Patient‐reported outcomes. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, UK: The Cochrane Collaboration. Available at: http://handbook.cochrane.org/. Updated March 2011 (accessed May 1, 2015).
- 38. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: A quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- 39. Ahmedzai SH, Nauck F, Bar‐Sela G, et al. A randomized, double‐blind, active‐controlled, double‐dummy, parallel‐group study to determine the safety and efficacy of oxycodone/naloxone prolonged‐release tablets in patients with moderate/severe, chronic cancer pain. Palliat Med 2012;26:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chamberlain BH, Cross K, Winston JL, et al. Methylnaltrexone treatment of opioid‐induced constipation in patients with advanced illness. J Pain Symptom Manage 2009;38:683–90. [DOI] [PubMed] [Google Scholar]
- 41. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- 42. Irving G, Pénzes J, Ramjattan B, et al. A randomized, placebo‐controlled phase 3 trial (Study SB‐767905/013) of alvimopan for opioid‐induced bowel dysfunction in patients with non‐cancer pain. J Pain 2011;12:175–84. [DOI] [PubMed] [Google Scholar]
- 43. Jansen JP, Lorch D, Langan J, et al. A randomized, placebo‐controlled phase 3 trial (Study SB‐767905/012) of alvimopan for opioid‐induced bowel dysfunction in patients with non‐cancer pain. J Pain 2011;12:185–93. [DOI] [PubMed] [Google Scholar]
- 44. Portenoy RK, Thomas J, Moehl Boatwright ML, et al. Subcutaneous methylnaltrexone for the treatment of opioid‐induced constipation in patients with advanced illness: A double‐blind, randomized, parallel group, dose‐ranging study. J Pain Symptom Manage 2008;35:458–68. [DOI] [PubMed] [Google Scholar]
- 45. Twycross RG, McNamara P, Schuijt C, Kamm MA, Jordan C. Sodium picosulfate in opioid‐induced constipation: Results of an open‐label, prospective, dose‐ranging study. Palliat Med 2006;20:419–23. [DOI] [PubMed] [Google Scholar]
- 46. Ducrotté P, Caussé C. The Bowel Function Index: A new validated scale for assessing opioid‐induced constipation. Curr Med Res Opin 2012;28:457–66. [DOI] [PubMed] [Google Scholar]
- 47. Epstein RS, Cimen A, Benenson H, et al. Patient preferences for change in symptoms associated with opioid‐induced constipation. Adv Ther 2014;31:1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rentz AM, Yu R, Müller‐Lissner S, Leyendecker P. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid‐induced constipation. J Med Econ 2009;12:371–83. [DOI] [PubMed] [Google Scholar]
- 49. Ueberall MA, Müller‐Lissner S, Buschmann‐Kramm C, Bosse B. The Bowel Function Index for evaluating constipation in pain patients: Definition of a reference range for a non‐constipated population of pain patients. J Int Med Res 2011;39:41–50. [DOI] [PubMed] [Google Scholar]
- 50. Mapi Research Trust . PROQOLID Database: Patient Assessment of Constipation ‐ Symptoms (PAC‐SYM). Available at: http://www.proqolid.org/instruments/patient_assessment_of_constipation_symptoms_pac_sym. Published September 1999. Updated January 2015 (accessed April 22, 2015).
- 51. Slappendel R, Simpson K, Dubois D, Keininger DL. Validation of the PAC‐SYM questionnaire for opioid‐induced constipation in patients with chronic low back pain. Eur J Pain 2006;10:209–17. [DOI] [PubMed] [Google Scholar]
- 52. Coyne KS, Currie BM, Holmes WC, Crawley JA. Assessment of a stool symptom screener and understanding the opioid‐induced constipation symptom experience. Patient 2015;8:317–27. [DOI] [PubMed] [Google Scholar]
- 53. Mapi Research Trust . PROQOLID Database: Patient Assessment of Constipation ‐ Quality of Life (PAC‐QOL). Available at: http://proqolid.org/instruments/patient_assessment_of_constipation_quality_of_life_questionnaire_pac_qol. Published May 2005. Updated January 2015 (accessed April 22, 2015).
- 54. Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol 2005;40:540–51. [DOI] [PubMed] [Google Scholar]
- 55. Abramowitz L, Béziaud N, Caussé C, et al. Further validation of the psychometric properties of the Bowel Function Index for evaluating opioid‐induced constipation (OIC). J Med Econ 2013;16:1434–41. [DOI] [PubMed] [Google Scholar]
- 56. Rentz AM, van Hanswijck de Jonge P, Leyendecker P, Hopp M. Observational, nonintervention, multicenter study for validation of the Bowel Function Index for constipation in European countries. Curr Med Res Opin 2011;27:35–44. [DOI] [PubMed] [Google Scholar]
- 57. Camilleri M, Rothman M, Ho KF, Etropolski M. Validation of a bowel function diary for assessing opioid‐induced constipation. Am J Gastroenterol 2011;106:497–506. [DOI] [PubMed] [Google Scholar]
- 58. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid‐induced constipation in patients with noncancer pain. N Engl J Med 2014;370:2387–96. [DOI] [PubMed] [Google Scholar]
- 59. US Food and Drug Administration . Guidance for Industry: Patient‐Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Available at: http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. Published December 2009 (accessed February 20, 2015).
- 60. Frank L, Kleinman L, Farup C, Taylor L, Miner PJ. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol 1999;34:870–7. [DOI] [PubMed] [Google Scholar]
- 61. TARGINIQ [package insert]. Stamford, CT: Purdue Pharma L.P; July 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205777lbl.pdf. Accessed October 13, 2015. [Google Scholar]
- 62. Clemens K, Quednau I, Klaschik E. Analgesic efficacy and improved bowel function during a pain therapy with a combination of oxycodone/naloxone prolonged‐release tablets in geriatric patients [World Congress‐World Institute of Pain abstract PB155]. Pain Pract. 2009;9:1–168. [Google Scholar]
- 63. Clemens KE, Quednau I, Klaschik E. Bowel function during pain therapy with oxycodone/naloxone prolonged‐release tablets in patients with advanced cancer. Int J Clin Pract 2011;65:472–8. [DOI] [PubMed] [Google Scholar]
- 64. Löwenstein O, Leyendecker P, Hopp M, et al. Combined prolonged‐release oxycodone and naloxone improves bowel function in patients receiving opioids for moderate‐to‐severe non‐malignant chronic pain: A randomised controlled trial. Expert Opin Pharmacother 2009;10:531–43. [DOI] [PubMed] [Google Scholar]
- 65. Meissner W, Leyendecker P, Mueller‐Lissner S, et al. A randomised controlled trial with prolonged‐release oral oxycodone and naloxone to prevent and reverse opioid‐induced constipation. Eur J Pain 2009;13:56–64. [DOI] [PubMed] [Google Scholar]
- 66. Sandner‐Kiesling A, Leyendecker P, Hopp M, et al. Long‐term efficacy and safety of combined prolonged‐release oxycodone and naloxone in the management of non‐cancer chronic pain. Int J Clin Pract 2010;64:763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Simpson K, Leyendecker P, Hopp M, et al. Fixed‐ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid‐induced constipation in moderate‐to‐severe noncancer pain. Curr Med Res Opin 2008;24:3503–12. [DOI] [PubMed] [Google Scholar]
- 68. Iyer SS, Randazzo BP, Tzanis EL, et al. Effect of subcutaneous methylnaltrexone on patient‐reported constipation symptoms. Value Health 2011;14:177–83. [DOI] [PubMed] [Google Scholar]
- 69. ENTEREG [package insert]. Whitehouse Station, NJ: Merck & Co., Inc; August 2015. http://www.merck.com/product/usa/pi_circulars/e/entereg/entereg_pi.pdf. Accessed October 13, 2015. [Google Scholar]
- 70. NUCYNTA [package insert]. Newark, CA: Depomed Inc; September 2013. http://www.nucynta.com/_assets/pdf/nucynta-pi.pdf. Accessed October 13, 2015. [Google Scholar]
- 71. Etropolski M, Kelly K, Okamoto A, Rauschkolb C. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Adv Ther 2011;28:401–17. [DOI] [PubMed] [Google Scholar]
- 72. Larkin PJ, Sykes NP, Centeno C, et al. The management of constipation in palliative care: Clinical practice recommendations. Palliat Med 2008;22:796–807. [DOI] [PubMed] [Google Scholar]
- 73. Leppert W. The role of opioid receptor antagonists in the treatment of opioid‐induced constipation: A review. Adv Ther 2010;27:714–30. [DOI] [PubMed] [Google Scholar]
- 74. Vilke GM, DeMers G, Patel N, Castillo EM. Safety and efficacy of milk and molasses enemas in the emergency department. J Emerg Med 2015;48:667–70. [DOI] [PubMed] [Google Scholar]
- 75. Kyle G. Constipation and palliative care ‐ where are we now? Int J Palliat Nurs 2007;13:6–16. [DOI] [PubMed] [Google Scholar]
- 76. Wee B, Adams A, Thompson K, et al. How much does it cost a specialist palliative care unit to manage constipation in patients receiving opioid therapy? J Pain Symptom Manage 2010;39:644–54. [DOI] [PubMed] [Google Scholar]
- 77. Löwenstein O, Leyendecker P, Lux EA, et al. Efficacy and safety of combined prolonged‐release oxycodone and naloxone in the management of moderate/severe chronic non‐malignant pain: Results of a prospectively designed pooled analysis of two randomised, double‐blind clinical trials. BMC Clin Pharmacol 2010;10:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


