Summary
Pseudozyma species rarely cause invasive diseases in humans, which are usually isolated from plants. There have been anecdotal reports regarding Pseudozyma species infections in patients with underlying diseases or in neonates. However, clinical data and the pathogenicity in humans are still insufficient. We experienced a case of Pseudozyma aphidis fungaemia with invasive fungal pneumonia that developed during reinduction chemotherapy in a 51‐year‐old male with acute myeloid leukaemia (AML). P. aphidis was suspected based on the morphology of the yeast isolated from the blood and was confirmed via rDNA gene sequencing analysis. The patient successfully underwent stem cell transplantation with continuing antifungal treatment and finally completely recovered from both the AML and infectious complications. Here, we report a case of P. aphidis infection that developed during neutropenia in an AML patient and review the global literature.
Keywords: Acute myeloid leukaemia, fungaemia, neutropenia, pneumonia, Pseudozyma aphidis yeasts
Introduction
Rare fungi have recently been implicated in human infections ranging from colonisation to invasive fungal infections (IFIs) in immunocompromised patients, accounting for <10% of all isolated fungal pathogens.1 Pseudozyma species (spp.) are basidiomycetous yeast classified in the family Ustilaginaceae, and its members are close relatives of Ustilago maydis and other smut fungi. At least 20 Pseudozyma spp. are recognised, most of which are environmental pathogens.2 Pseudozyma spp. infections in humans have rarely been reported after the first description as a human pathogen in 2003.3 Data regarding the clinical characteristics and pathogenicity in humans remain insufficient. Recently, we experienced a case of Pseudozyma aphidis fungaemia with invasive fungal pneumonia after reinduction chemotherapy for acute myeloid leukaemia (AML). Here, we describe this case and review the global literature. The Institutional Review Board at Seoul St. Mary's Hospital approved this case report and waived the need for patient consent (No. KC15RISI0570).
Case report
A 51‐year‐old man who was diagnosed with AML and had not experienced remission after the first induction chemotherapy started reinduction chemotherapy with 100 mg m−2 cytarabine for 7 days and 90 mg m−2 daunorubicin for 3 days. On day seven after reinduction chemotherapy (D7), neutropenic fever (up to 38.0 °C) developed as measured using an axillary thermometer. No other symptoms were reported. Laboratory data included a white blood cell count of 220 μl−1 (absolute neutrophil count, 0 μl−1) and C‐reactive protein level of 8.60 mg dL−1. Empirical antibiotic therapy with ceftazidime (2 g twice a day) and isepamicin (400 mg once a day) was initiated after performing blood cultures. On D8, the results of the blood culture revealed presumptive growth of gram‐positive cocci (GPC). Teicoplanin (12 mg kg−1 a day after loading with 12 mg kg−1 every 12 h for three doses) was added based on the culture results.
Although the patient had no respiratory symptoms, infiltration was suspected in the right lower lung field on the chest radiograph on D9. Low‐dose computed tomography of the lung was performed, which showed consolidation with surrounding ground glass opacity at the medial segment of the right middle lobe (RML) (Fig. 1). The serum galactomannan assay performed twice a week was negative. The patient received fluconazole instead of posaconazole as the primary antifungal prophylaxis; because he had participated in a clinical trial using a thrombopoietin receptor agonist during the same period, a drug interaction was possible. Then, the antifungal prophylaxis was empirically changed to caspofungin (50 mg a day after the loading dose) for the possibility of fungal pneumonia according to the revised definition of IFI from the European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG).4
Figure 1.
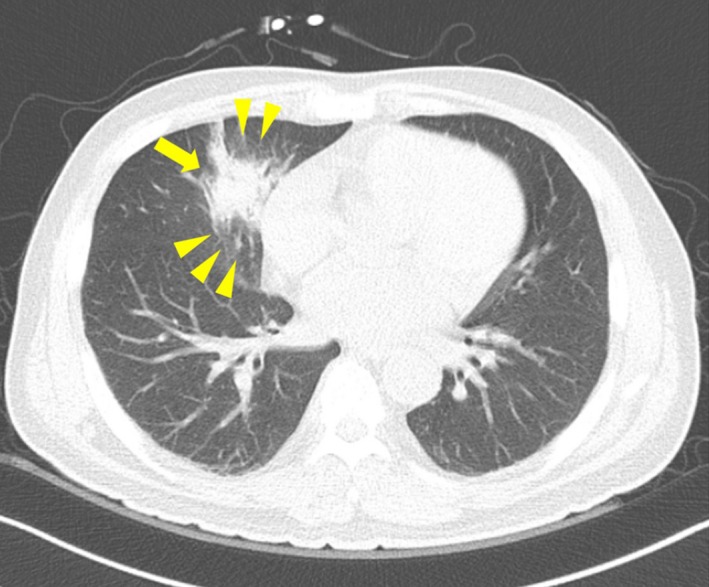
Low dose computed tomography of the lung. Consolidation with surrounding ground glass opacity at the medial segment of the right middle lobe.
The GPC from the blood culture was identified as vancomycin‐resistant Enterococcus faecium (3 of 4 bottles), using Vitek 2 (bioMérieux, Hazelwood, MO, USA). Teicoplanin was changed to linezolid (600 mg twice a day). The follow‐up blood culture performed on D11 revealed negative conversion of vancomycin‐resistant Enterococcus bacteraemia. However, the neutropenic fever persisted, and yeast‐like organisms were noted from central blood culture (1 of 2 bottles) performed on the same day (D11). This culture result was reported after 2 days of incubation, using BD BACTEC FX blood culture system (BD Diagnostics, Sparks, MD, USA).
Colonies observed on the blood agar plate were white, dry or wrinkled (Fig. 2a). Microscopically, they appeared as yeast with branching pseudohyphae (Fig. 2b). The species could not be identified using either Vitek 2 or API 20C (bioMérieux). Therefore, further identification was performed using rDNA gene sequencing analysis with the following primers: internally transcribed spacer (ITS)‐1 (forward primer [5′‐TCC GTA GGT GAA CCT GCG G‐3′] and reverse primer [5′‐GCT GCG TTC ATC GAT‐3′]), ITS‐2 (forward primer [5′‐GCA TCG ATG AAG AAC GCA‐3′] and reverse primer [5′‐TCC TCC GCT TAT TGA TAT‐3′]), and D1/D2 domain (forward primer [5′‐GCA TAT CAA TAA GCG GAG‐3′] and reverse primer [5′‐GGT CCG TGT TTC AAG ACG G‐3′]).5 The isolated gene sequence showed 100% concordance with the P. aphidis strain (GenBank accession number: KF443199.1 and KF443201.1). Because the fungaemia developed during the caspofungin therapy and pneumonia was aggravated with persistent neutropenic fever, caspofungin was changed to liposomal amphotericin B (5 mg kg−1 per day) on D15. The Hickman catheter was removed. The culture result of the tip of the removed Hickman catheter showed no growth.
Figure 2.
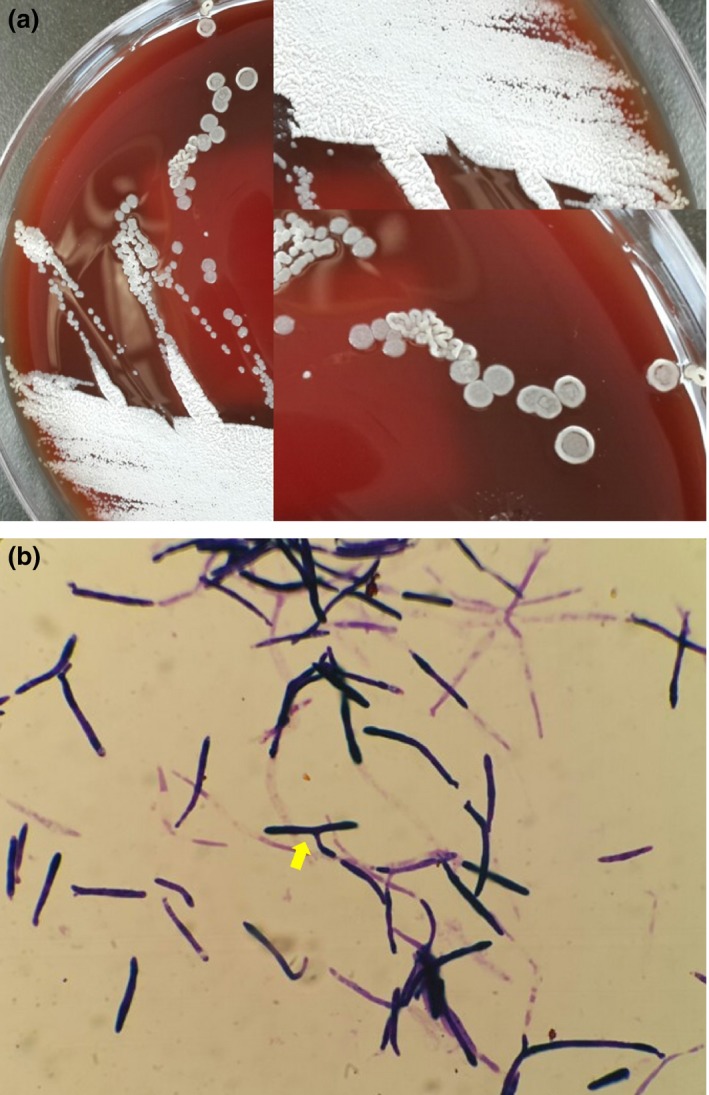
Colonies of yeast on blood agar and microscopic morphology. (a) Yeast‐like colonies that are dry, creamy, brightly coloured, and glabrous in texture; (b) Gram stain showing yeast with branching pseudohyphae (arrow).
The follow‐up blood culture performed on D14 showed no growth. However, pneumonia was aggravated despite the broad‐spectrum antifungal agent. The findings of the bronchoscopy performed on D23 showed no endobronchial lesion. Transbronchial lung biopsy and bronchial washing and brushing were performed at the RML bronchus to identify the pathogens of fungal pneumonia. Pathology specimens showed inflammatory changes with necrosis and dichotomous hyphae with a septum, suggestive of organising pneumonia with a fungal infection (Fig. 3). However, we could not identify the genus level due to tissue damage. Culture with bronchial washing fluid revealed no growth of fungal organisms. As the neutropenia recovered, the patient improved clinically and was discharged with itraconazole capsules (200 mg twice a day). At the outpatient clinic, chest radiography revealed little change in the RML consolidation despite 3 weeks of continued itraconazole. Considering the possibility of both proven invasive pulmonary aspergillosis and fungal pneumonia due to Pseudozyma spp., itraconazole was changed to voriconazole (4 mg kg−1 twice a day). The patient sequentially received consolidation chemotherapy and allogeneic stem cell transplantation (SCT) while continuing the voriconazole. During the voriconazole treatment, serum level of voriconazole was within the therapeutic range. The pneumonia resolved after 4 months of voriconazole treatment, with complete remission of AML after successful SCT.
Figure 3.
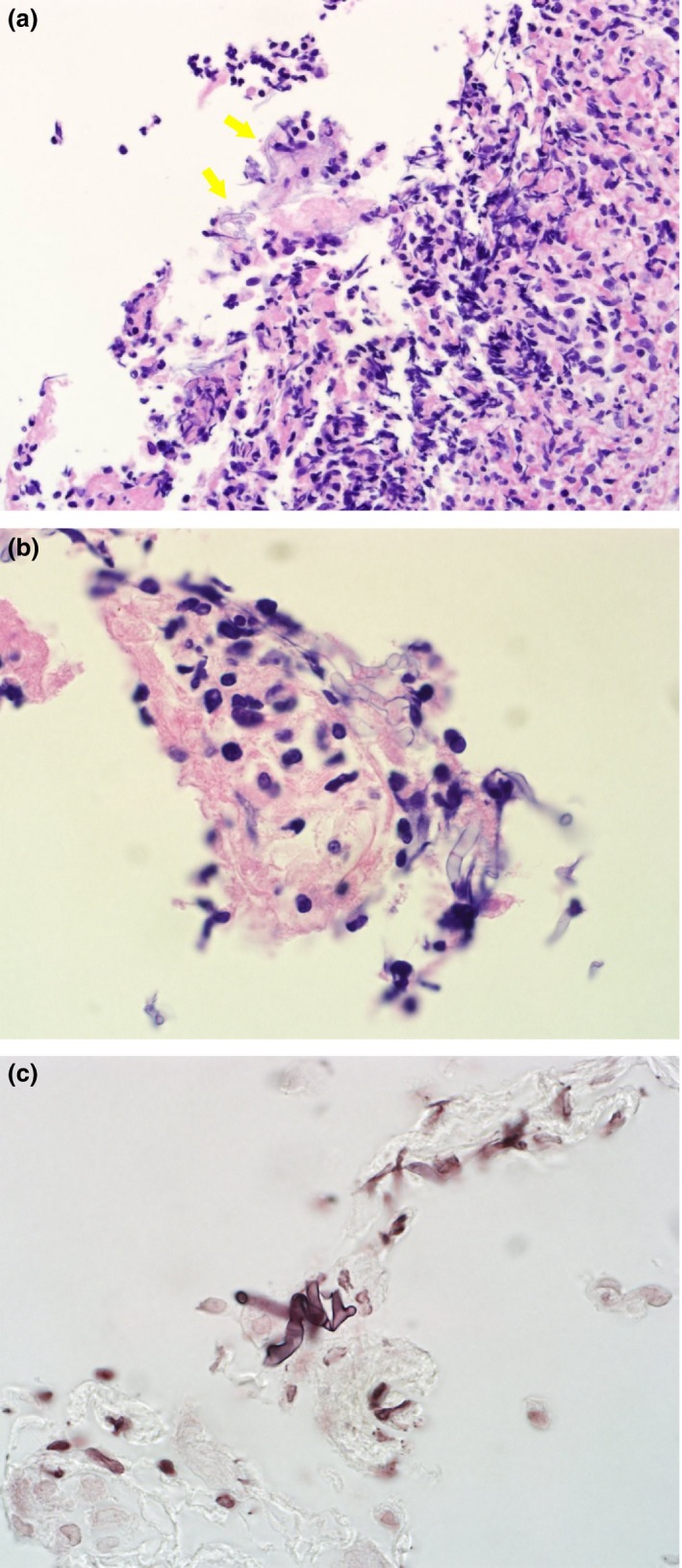
Histopathology of lung tissue from a transbronchial lung biopsy specimen. Fungal organisms on the background of necrosis and inflammatory infiltration. (a) Haematoxylin and eosin stain, ×100; (b) Haematoxylin and eosin stain, ×400; (c) Silver stain, ×400.
Discussion
Invasive fungal infections remain a major cause of significant morbidity and mortality in immunocompromised patients, especially those with hematologic malignancies. Of the IFIs, a rare fungus is difficult to treat in the aspects of the early diagnosis and appropriate treatment, which might be related to the severity of the patient's condition and often challenging intrinsic susceptibility pattern of the pathogens.6 Pseudozyma spp. was first described as a possible human pathogen in 2003, when it was identified from blood cultures from three Thai patients, as P. antarctica, P. parantarctica, and P. thailandica.3 The first case of P. aphidis human infection was reported in a 7‐year‐old girl with short gut syndrome in 2008.7
Pseudozyma spp. is classified under the family Ustilaginaceae, phylum Basidiomycota, subphylum Ustilaginomycotina, class Ustilaginomycetes, and order Ustilaginales.2, 8 Ustilaginales are plant pathogens that can infect corn plants to produce tumour‐like galls. Of the Pseudozyma spp., P. aphidis is known as the most common human pathogen. However, due to the rare isolation of P. aphidis in human infections, this rare fungus species cannot be identified using commercial systems that are available in routine diagnostic laboratories. However, sequencing and phylogenetic analysis can help with the direct detection of a fungus from blood or tissues because yeast taxonomy is continually evolving.
We retrospectively reviewed the global literature to identify the possible risk factors, clinical presentation, and optimal treatment strategies for Pseudozyma infection; in addition to the present case, 14 case reports were found (Table 1).3, 7, 8, 9, 10, 11, 12, 13, 14, 15 Since 2014 when previous literature review was reported by Prakash et al. 8, there were seven additional cases including our case. Therefore, we present a updated literature review with some modifications and adding recent cases. Of the 15 cases, seven were identified as P. aphidis, two were Pseudozyma spp. without spp. level identification, and the remaining six cases were non‐aphidis Pseudozyma isolates that were all different spp. from Thailand. Of the nine cases with reported underlying medical conditions, three had gastrointestinal (GI) tract problems such as short bowel syndrome or intestinal surgery,4, 9, 13 and three had neutropenia7, 14, 15 which can damage the gut mucosa. Of the seven patients for whom the presence or absence of a central venous catheter (CVC) was reported, six patients had a CVC. The risk factors are thought to be similar to those of other less common yeast infections including GI tract problems, the presence of a CVC, and neutropenia. We could not identify the exact social history or dietary history to determine potential exposure to plants or crops. However, crop exposure was identified in two cases: a farmer with mycetoma of the leg and a paediatric patient who had eaten corn chips.8, 10 In the present case, the possible port of entry for the P. aphidis fungaemia is uncertain. While the fungaemia met the definition of a catheter‐related bloodstream infection because P. aphidis was isolated only from central blood, the origin of P. aphidis is unclear.
Table 1.
Literature review of Pseudozyma species infections in human
| Case no. | Age/sex | Underlying condition | Predisposing factors | Clinical presentation | Isolated specimen | ||
|---|---|---|---|---|---|---|---|
| CVC | TPN | Plant/crop | |||||
| 1 | N/A | N/A | N/A | N/A | N/A | N/A | Blood |
| 2 | N/A | N/A | N/A | N/A | N/A | N/A | Blood |
| 3 | N/A | N/A | N/A | N/A | N/A | N/A | Blood |
| 4 | 7/F | Short gut syndrome | + | + | +a | Fever, chill, malaise, fatigue | Blood |
| 5 | 78/M | Astrocytoma | N/A | N/A | N/A | Fever after brain surgery | Abscess of brain |
| 6 | 51/M | Farmer, chronic leg swelling | − | − | + | Chronic mycetoma of leg | Legb |
| 7 | 17/M | Burkitt lymphoma, chemotherapy | + | N/A | N/A | Neutropenic fever, lung infiltrates | Pleural fluid |
| 8 | 0/M | Hemolytic jaundice | N/A | N/A | N/A | Lethargy, poor feeding | Blood |
| 9 | 52/F | Crohn's disease, total colectomy state | + | + | − | Fever, headache, weakness | Blood |
| 10 | N/A | N/A | N/A | N/A | N/A | N/A | Blood |
| 11 | N/A | N/A | N/A | N/A | N/A | N/A | Blood |
| 12 | N/A | N/A | N/A | N/A | N/A | N/A | Blood |
| 13 | 68/F | Adenocarcinoma of ampulla of Vater | + | N/A | N/A | Fever, chill | Blood |
| 14 | 6/F | Osteosarcoma with lung metastasis | + | N/A | N/A | Neutropenic fever | Blood |
| 15 | 51/M | AML, reinduction chemotherapy | + | + | − | Neutropenic fever, lung infiltratesc | Blood |
| Case no. | Pseudozyma spp. | MIC (mg dL− a) | Treatment | Outcome | Country, year [reference] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FLC | ITC | VRC | AMB | CAS | 5FC | Antifungal agent | Duration | ||||
| 1 | P. antarctica | N/A | N/A | N/A | N/A | N/A | Rd | N/A | – | N/A | Thai, 2003 3 |
| 2 | P. parantarctica | N/A | N/A | N/A | N/A | N/A | Rd | N/A | – | N/A | Thai, 2003 3 |
| 3 | P. thailandica | Rd | Rd | N/A | N/A | N/A | Rd | N/A | – | N/A | Thai, 2003 3 |
| 4 | P. aphidis | 4 | 0.125 | N/A | 0.25 | N/A | N/A | FLC → ITC | N/A | Improved | USA, 2008 8 |
| 5 | Pseudozyma spp. | N/A | N/A | N/A | N/A | N/A | N/A | N/A | – | Death | Korea, 2010 9 |
| 6 | P. aphidis | N/A | N/A | N/A | N/A | N/A | N/A | ITCe | 1 year | Improved | China, 2011 10 |
| 7 | P. aphidis | 4 | 0.25 | 0.03 | 0.25 | 4 | N/A | LAMB → VRC | 25 day | Improved | Brazil, 2013 11 |
| 8 | P. aphidis | 8 | 0.03 | 0.06 | 0.03 | 8 | >64 | AMB → VRC | 14 day | Improved | India, 2013 7 |
| 9 | Pseudozyma spp. | N/A | N/A | N/A | N/A | N/A | N/A | FLC → VRC | N/A | Improved | USA, 2014 12 |
| 10 | P. alboarmeniaca | 32 | 4 | 2 | 0.25 | >16f | >64 | N/A | – | N/A | Thai, 2014 13 |
| 11 | P. crassa | >64 | 4 | 2 | 0.25 | >16f | >64 | N/A | – | N/A | Thai, 2014 13 |
| 12 | P. siamensis | 32 | 4 | 2 | 0.125 | >16f | >64 | N/A | – | N/A | Thai, 2014 13 |
| 13 | P. aphidis | 16 | 0.19 | 0.032 | 0.19 | >32 | >32 | LAMB | 14 day | Improved | France, 2015 14 |
| 14 | P. aphidis | 2 | 0.03 | 0.03 | 0.13 | N/A | 128 | LAMB | 14 day | Improved | Argentina, 2015 15 |
| 15 | P. aphidis | N/A | N/A | N/A | N/A | N/A | N/A | LAMB → VRC | 4 month | Improved | [This case] |
AMB, amphotericin B; AML, acute myeloid leukaemia; CAS, caspofungin; CVC, central venous catheter; FLC, fluconazole; ITC, itraconazole; LAMB, liposomal amphotericin B; MIC, minimal inhibitory concentration; N/A, not available; TPN, total parenteral nutrition; VRC, voriconazole; 5FC, flucytosine.
The patient consumed large amounts of tortilla corn chips.
Histopathology of deep tissue from the foot showed grains surrounded by inflammatory cells, periodic acid‐Schiff stain showed clustered yeast‐like cells, and tissue cultures showed septate hyaline hyphae on a wet mount.
Histopathology of lung tissue showed necrosis, inflammatory cells, and septate dichotomous hyphae. However, a fungus culture or sequencing using the tissue was not performed.
Not reported MIC values.
In addition to repeated debridement.
MIC values for micafungin.
The major manifestation of Pseudozyma infection is fungaemia (12 of 15 cases, 80%); others (n = 3) have been isolated from a brain abscess that developed after brain surgery, deep biopsy of mycetoma of the leg, and pleural fluid.9, 10, 11 In our case, we proved P. aphidis fungaemia with fungal pneumonia from lung tissue. However, our case has a limitation, in that we could not identify the fungal pathogen from the lung tissue. Fungus culture with fresh lung tissue and sequencing of the tissue was not performed. Galactomannan assay using bronchial washing fluid was not performed either. Antifungal susceptibility testing was performed in 11 cases of human infections caused by Pseudozyma spp., P. aphidis was susceptible to itraconazole, voriconazole, and amphotericin B; had varying susceptibility to fluconazole; and was resistant to echinocandines and flucytosine.7, 8, 11, 14, 15 Non‐aphidis Pseudozyma spp. seemed to have susceptibility to amphotericin B only.13 In all reported cases, Pseudozyma spp. were resistant to echinocandins and flucytosine.
Here, we described a case of a 51‐year‐old male patient with AML who suffered from neutropenic fever during chemotherapy with a defined bacterial and fungal infection that was finally diagnosed as P. aphidis fungaemia and concurrent invasive fungal pneumonia without genus level identification. Many other fungal pathogens show subtle morphological differences between forms found in tissue and in culture. Further accumulation of data regarding rare fungi is needed. This case is worth reporting in the aspect that P. aphidis fungaemia developed during neutropenic fever with concurrent invasive fungal pneumonia in an AML patient.
Conflict of interest
We declare no conflicts of interest.
References
- 1. Azie N, Neofytos D, Pfaller M, Meier‐Kriesche HU, Quan SP, Horn D. The PATH (Prospective Antifungal Therapy) Alliance(R) registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis 2012; 73: 293–300. [DOI] [PubMed] [Google Scholar]
- 2. Howell SA, Hazen KC, Brandt ME. Candida, Cryptococcus, and other yeasts of medical importance In: Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (eds), Manual of Clinical Microbiology, 11th edn Washington, DC: ASM Press, 2015: 1984–2014. [Google Scholar]
- 3. Sugita T, Takashima M, Poonwan N et al The first isolation of ustilaginomycetous anamorphic yeasts, Pseudozyma species, from patients’ blood and a description of two new species: P. parantarctica and P. thailandica . Microbiol Immunol 2003; 47: 183–90. [DOI] [PubMed] [Google Scholar]
- 4. De Pauw B, Walsh TJ, Donnelly JP et al Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CLSI . Interpretive Criteria for Identification of Bacteria and Fungi by DNA Target Sequencing; Approved Guidelines. CLSI document MM18‐A. Wayne, PA: Clinical and Laboratory Standards Institute, 2008. [Google Scholar]
- 6. Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect 2014; 20(Suppl. 3): 76–98. [DOI] [PubMed] [Google Scholar]
- 7. Lin SS, Pranikoff T, Smith SF et al Central venous catheter infection associated with Pseudozyma aphidis in a child with short gut syndrome. J Med Microbiol 2008; 57: 516–8. [DOI] [PubMed] [Google Scholar]
- 8. Prakash A, Wankhede S, Singh PK et al First neonatal case of fungaemia due to Pseudozyma aphidis and a global literature review. Mycoses 2014; 57: 64–68. [DOI] [PubMed] [Google Scholar]
- 9. Hwang S, Kim J, Yoon S et al First report of brain abscess associated with Pseudozyma species in a patient with astrocytoma. Korean J Lab Med 2010; 30: 284–8. [DOI] [PubMed] [Google Scholar]
- 10. Chen B, Zhu LY, Xuan X et al Isolation of both Pseudozyma aphidis and Nocardia otitidiscaviarum from a mycetoma on the leg. Int J Dermatol 2011; 50: 714–9. [DOI] [PubMed] [Google Scholar]
- 11. Parahym AM, da Silva CM, Domingos Ide F et al Pulmonary infection due to Pseudozyma aphidis in a patient with Burkitt lymphoma: first case report. Diagn Microbiol Infect Dis 2013; 75: 104–6. [DOI] [PubMed] [Google Scholar]
- 12. Siddiqui W, Ahmed Y, Albrecht H, Weissman S. Pseudozyma spp catheter‐associated blood stream infection, an emerging pathogen and brief literature review. BMJ Case Rep 2014; 2014: doi: 10.1136/bcr‐2014‐206369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mekha N, Takashima M, Boon‐Long J, Cho O, Sugita T. Three new basidiomycetous yeasts, Pseudozyma alboarmeniaca sp. nov., Pseudozyma crassa sp. nov. and Pseudozyma siamensis sp. nov. isolated from Thai patients. Microbiol Immunol 2014; 58: 9–14. [DOI] [PubMed] [Google Scholar]
- 14. Herb A, Sabou M, Delhorme JB et al Pseudozyma aphidis fungemia after abdominal surgery: first adult case. Med Mycol Case Rep 2015; 8: 37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orecchini LA, Olmos E, Taverna CG et al First case of fungemia due to Pseudozyma aphidis in a pediatric patient with osteosarcoma in Latin America. J Clin Microbiol 2015; 53: 3691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


