Abstract
Background
Clinical trials have not yet compared the efficacy of capsaicin 8% patch with current standard therapy in peripheral neuropathic pain (PNP).
Objectives
Head‐to‐head efficacy and safety trial comparing the capsaicin patch with pregabalin in PNP.
Methods
Open‐label, randomized, multicentre, non‐inferiority trial. Patients with PNP, aged 18–80 years, were randomly assigned to either the capsaicin 8% patch (n = 282) or an optimised dose of oral pregabalin (n = 277), and assessed for a ≥30% mean decrease in Numeric Pain Rating Scale (NPRS) score from baseline to Week 8. Secondary endpoints included optimal therapeutic effect (OTE), time‐to‐onset of pain relief and treatment satisfaction.
Results
The capsaicin 8% patch was non‐inferior to pregabalin in achievement of a ≥30% mean decrease in NPRS score from baseline to Week 8 (55.7% vs. 54.5%, respectively; Odds ratio: 1.03 [95% CI: 0.72, 1.50]). The proportion of patients achieving OTE at Week 8 was 52.1% for the capsaicin 8% patch versus 44.8% for pregabalin (difference: 7.3%; 95% CI: −0.9%, 15.6%). The median time‐to‐onset of pain relief was significantly shorter for capsaicin 8% patch versus pregabalin (7.5 vs. 36.0 days; Hazard ratio: 1.68 [95% CI: 1.35, 2.08]; p < 0.0001). Treatment satisfaction was also significantly greater with the capsaicin 8% patch versus pregabalin. TEAEs were mild‐to‐moderate in severity, and resulted in treatment discontinuation only with pregabalin (n = 24). Systemic adverse drug reactions ranged from 0 to 1.1% with capsaicin 8% patch and 2.5 to 18.4% with pregabalin.
Conclusions
The capsaicin 8% patch provided non‐inferior pain relief to an optimized dose of pregabalin in PNP, with a faster onset of action, fewer systemic side effects and greater treatment satisfaction.
1.
What's already known about this topic?
Pregabalin can be considered a standard of care in treating peripheral neuropathic pain.
However, side effects limit its usefulness.
What does this study add?
We provide a direct comparison of pregabalin with a topical capsaicin patch.
It demonstrates capsaicin 8% patch is non‐inferior to pregabalin in relieving pain in patients with PNP, with a more rapid onset of action, fewer systemic side effects and greater treatment satisfaction.
1. Introduction
Painful neuropathy or peripheral neuropathic pain (PNP) is a common neurological condition according to the revised International Association for the Study of Pain (IASP) definition (Treede et al., 2008), and is estimated to affect ~7–8% of the general population in Europe (Torrance et al., 2006; Bouhassira et al., 2008). Managing patients with PNP is challenging (Dworkin et al., 2007, 2010; Finnerup et al., 2010; Finnerup, Attal et al., 2015); it often becomes chronic, with marked long‐term reductions in health‐related quality of life (HRQOL) (O'Connor, 2009), decreased individual productivity and increased patient and healthcare expenditure (Rodríguez et al., 2007; Navarro et al., 2011). Aetiologies range from mechanical and inflammatory diseases to injury and nerve compression (Hall et al., 2006). A key pathophysiological mechanism is neuronal hyperexcitability, both within the axon and cell body as well as peripheral nociceptors (Truini et al., 2013). This allows for single or combined therapies targeting one or more sites within the neurone. For example, pregabalin reduces neuronal excitability in the central nervous system by reversibly binding alpha‐2‐delta subunits of the Ca++ channels, thus reducing synaptic neurotransmitter release (Taylor et al., 2007). It has established efficacy in treating PNP, with one of the highest pain reductions over placebo when compared with other oral drugs (Ney et al., 2013) and, along with tricyclic antidepressants such as amitriptyline, can be considered a standard of care for the treatment of PNP (Finnerup, Attal et al., 2015). However, its use is commonly associated with side effects, such as somnolence, dizziness, weight gain and peripheral oedema that may be severe enough to be either dose limiting or lead to its discontinuation in clinical practice (Freynhagen et al., 2015).
An example of a peripherally acting agent is capsaicin, a highly selective, potent and high‐affinity (in the low nanomolar range) exogenous agonist for the transient receptor potential cation channel subfamily V member 1 (TRPV1) receptors (Bley, 2013). A single 60‐min application of a capsaicin 8% patch (hereon referred to as the capsaicin patch) rapidly delivers capsaicin into the skin, directly targeting the nociceptor leading to its defunctionalization (Malmberg et al., 2004; Kennedy et al., 2010; Anand and Bley, 2011). The efficacy and safety of the capsaicin patch in the treatment of PNP is well established (Backonja et al., 2008; Simpson et al., 2008; Irving et al., 2011; Mou et al., 2013) and it is approved for the treatment of PNP in non‐diabetic adults in the EU (QUTENZA™ , SPC, 2014).
Due to differences in the design of controlled studies, the difficulty of selecting a blinded control for the capsaicin patch and use of an active control, numbers needed to treat (NNTs), the data suggest that efficacy is lower for the capsaicin patch than pregabalin (Moore et al., 2009; Derry et al., 2013).
Until now, no clinical trials have compared the efficacy of the capsaicin patch with that of pregabalin. Accordingly, this open‐label, randomised, multicentre, non‐inferiority study compared the efficacy and safety of a single application of the capsaicin 8% patch with oral pregabalin in patients with PNP over 8 weeks.
2. Methods
2.1. Patients
In this study (ELEVATE), patients were between 18 and 80 years of age and had a documented diagnosis of probable or definite PNP using the currently recognized grading system (Treede et al., 2008). Patients had PNP due to post‐herpetic neuralgia (PHN) (pain persisting for at least 6 months since shingles vesicle crusting), post‐traumatic nerve injury (PNI) (minimum of 3 months) or non‐diabetic painful peripheral polyneuropathy (minimum of 3 months), and had an average numeric pain rating scale (NPRS) (Farrar et al., 2001) score ≥4 over a period of at least 4 consecutive days. Patients had to be naïve to treatment with the capsaicin 8% patch and either naïve to treatment with pregabalin and gabapentin, or, in the opinion of the investigator, had not received adequate treatment with pregabalin or gabapentin (Dworkin et al., 2005). Women of childbearing age must have had a negative pregnancy test result and been using an effective method of contraception throughout the study period.
Patients were also excluded for the following reasons: significant ongoing or recurrent pain of aetiology other than PHN, PNI or non‐diabetic painful peripheral polyneuropathy; Complex Regional Pain Syndrome (CRPS, Type I or II); neuropathic pain related to previously administered radiotherapy, diabetes mellitus or HIV‐associated nephropathy or located only on the face, above the hairline of the scalp and/or in proximity to mucous membranes; severe loss of heat sensation in the painful area indicative of C‐fibre denervation; a daily pain score of 10 on the NPRS for ≥4 days during the screening period; past or current history of diabetes mellitus; unstable or poorly controlled hypertension or a recent history of a cardiovascular event which, in the opinion of the investigator, would put the subject at risk of adverse cardiovascular reactions related to the patch application procedure; creatinine clearance <60 mL/min according to the Cockcroft‐Gault formula; clinical anxiety, depression or evidence of cognitive impairment; planned elective surgery during the trial; changes to stable neuropathic pain background medication in the 4 weeks prior to the baseline visit; use of opioids exceeding a total daily dose of morphine of 200 mg/day or equivalent or any intravenous opioids or tapentadol, regardless of dose, within 7 days preceding the baseline visit; use of any topical pain medication, such as non‐steroidal anti‐inflammatory drugs, menthol, methyl salicylate, local anaesthetics (including patch containing lidocaine), steroids or capsaicin products on the painful areas to be treated within 7 days preceding the baseline visit; chemotherapy within 3 months of the baseline visit, except maintenance hormone treatment; use of any investigational agent within 30 days prior to baseline visit; active or chronic substance abuse or history of chronic substance abuse within 1 year prior to screening; or, in the opinion of the investigator, were not suitable for the study for any reason.
The study was approved by the institutional review board at each participating site, was done in accordance with the ethical principles of the Declaration of Helsinki (World Medical Association, 2013) and was consistent with good clinical practice guidelines and applicable regulatory requirements. Written, informed consent was obtained from all patients before initiating study‐related procedures.
2.2. Study design
Assessment of the primary endpoint was at Week 8, consistent with Phase III trials of the capsaicin patch conducted in PHN patients (Backonja et al., 2008). This assessment time point has also been used in a number of pregabalin trials (Dworkin et al., 2003; Sabatowski et al., 2004). The established primary efficacy endpoint of ≥30% pain relief, as measured by the NPRS score, was employed. Patients assigned to the capsaicin patch arm received topical anaesthetic on their painful affected area(s) prior to placement of capsaicin patches and could receive a short‐acting pain medication (including short‐acting opioids) up to 5 days to reduce patch‐related pain/discomfort. Short‐acting pain medication was allowed during patch application, or as needed for up to 5 days following patch application.
2.3. Procedures
The study comprised a baseline screening period of at least 7 days, a treatment day (Day 0) and a post‐treatment assessment period of 8 weeks, with clinic visits at 2, 4 and 8 weeks. Patients were randomly assigned 1:1 to a high‐concentration (640 μg/cm2 [8% weight for weight]) capsaicin patch (QUTENZA™, Astellas Pharma Europe Ltd, Chertsey, UK) or oral pregabalin (75 mg capsules) at an optimized dose to best match clinical practice in Europe. Randomization was coordinated centrally using an Interactive Voice Response System which randomized eligible patients to one of the two treatments arms, assigned patient numbers and managed the distribution of the investigational medicinal product. Patients were also randomized by gender and country. The Summary of Product Characteristics advises that the dose of pregabalin should be up‐titrated over a period of 10–14 days to reduce the occurrence of dose‐limiting AEs. In European clinical practice, up‐titration of the dose is often performed over a longer time period, using varying dose changes and frequency of up‐titration steps. This study was designed to reflect current clinical practice as much as possible and thus included an up‐ and down‐titration scheme for pregabalin performed over a period of 4 weeks. During this pregabalin titration period, the initial dose of 75 mg/day was increased by 75 mg every 3–4 days, up to the highest tolerated dose or 600 mg/day. One pregabalin down‐titration was allowed by Week 4. For each patient, the optimal dose arrived at in this fashion was chosen for the maintenance of pregabalin for the remainder of the study from Weeks 4–8. Pregabalin 150–600 mg/day was administered in two or three doses a day. This method was intended to provide a level of flexibility while minimizing variability and represented a compromise between the different clinical practices across Europe. A topical anaesthetic cream (e.g. 4% lidocaine cream) was applied for up to 60 min prior to the application of the capsaicin patch. Up to four patches were applied (1120 cm2) to the painful area(s) for 30 min to the feet or 60 min to any other part of the body. Assignment to the treatment groups was done using a randomization scheme stratified by country and gender. A study withdrawal was considered to be a patient who was enrolled in the study and who chose to withdraw from the study prior to completion of all study procedures. Patients were free to withdraw from the study treatment and/or study for any reason and at any time without giving reason for doing so and without penalty or prejudice.
2.4. Assessments
2.4.1. Primary assessment
The proportion of patients in each arm who achieved ≥30% decrease in the ‘average pain for the past 24 h’ NPRS score was assessed from baseline to Week 8. As in the capsaicin 8% patch clinical trials (Backonja et al., 2008; Simpson et al., 2008; Irving et al., 2011), a responder was defined as a patient who exhibited a ≥30% decrease in NPRS score from baseline. Baseline scores referred to the mean NPRS scores recorded during the screening period. Week 8 scores referred to the mean NPRS scores recorded in the 7 days up to and including the Week 8 visit.
2.4.2. Secondary assessments
The proportion of patients in each arm who achieved optimal therapeutic effect (OTE) was assessed. OTE was defined as: no change in background chronic pain medication (assessed by an Independent Data Review Board); no discontinuation of the study drug due to lack of efficacy or tolerability prior to Week 8; ≥30% reduction in the NPRS score over at least 4 consecutive days from baseline to Week 8; and no moderate or severe adverse drug reactions (ADRs) during the stable treatment period. For the capsaicin patch, the stable treatment period equated to weeks 1–8 of the treatment period. It excluded the first week of treatment to avoid confounding factors associated with the use of short‐acting oral analgesics during this period, and to allow for the onset of action of the capsaicin patch. For pregabalin the stable treatment period equated to the period over which the subject was dosed at the optimal maintenance dose, and was different for each subject.
Time‐to‐onset of pain relief (in days) as assessed by ≥30% reduction in the NPRS score. The median time‐to‐onset of pain relief was the first of 3 consecutive days where 50% of patients had a ≥30% reduction in NPRS score versus baseline.
Change from baseline NPRS for the past 24 h was assessed by the proportion of patients who achieved ≥30% and ≥50% decrease in the NPRS score from baseline to the mean of all scores recorded between Week 2 and Week 8, respectively; as well as the absolute and per cent change.
Pre‐specified analyses of the primary endpoint were performed according to patient subgroups based on patient age (<65, ≥65, <75 years), gender, time since diagnosis (<6 months, ≥6 months to ≤1 year, >1 year to ≤2 years, >2 years to ≤10 years, >10 years), maximum Neuropathic Pain Symptom Inventory (NPSI) subscales [burning (superficial) spontaneous pain, pressing (deep) spontaneous pain, paroxymal pain, evoked pain, paraesthesia/dysaesthesia], type of pain (PHN, PNI, non‐diabetic painful peripheral polyneuropathy), pain grading (probable neuropathic pain, definite neuropathic pain), baseline pain score (<7, ≥7) and previous use of pregabalin/gabapentin.
Treatment satisfaction, as assessed by the proportion of patients who discontinued study drug or withdrew from the study due to either a lack of efficacy or tolerability; willingness to continue treatment at Week 8; and the Treatment Satisfaction Questionnaire for Medication (TSQM) questionnaire at Week 4 and Week 8, with statistical comparisons of TSQM scores adjusted for country group and gender.
Other patient rated outcomes were assessed (EQ‐5D‐5L, Patient Global Impression of Change, Medical Outcomes Study 6‐Item Cognitive Functioning and Sleep) and will be reported separately.
Safety analyses were conducted on all patients who received study drug. A treatment‐emergent adverse event (TEAE) was defined as an AE that started or increased in severity after intake/application of the study drug. An ADR was defined as an AE with probable or possible relationship with the study drug.
2.5. Statistical analysis
The required sample size was calculated using a margin of non‐inferiority ∆ of −8.5% for the proportion of responders, based on a systematic FDA review of pregabalin. The primary analysis of efficacy was performed on both the full analysis set (FAS) and Per Protocol Set (PPS). The FAS included all patients as randomized who initiated study treatment. The PPS used a subset of patients of the FAS, selected to ensure sensitivity to differences in treatment effects. The analysis of the primary efficacy variable was performed using a generalized linear model (GLM) with logit link function, adjusted on country and gender and with baseline pain score as covariate. The null hypothesis was tested using an odds ratio (OR) which translated ∆ into a margin on the OR scale (∆′) of 0.693. The null hypothesis of inferiority was rejected if the two‐sided 95% confidence interval (CI) for the OR of the capsaicin patch versus pregabalin fell completely above 0.693. The difference of means between treatment groups, and corresponding 95% CI were analysed for continuous secondary endpoints (except time‐to‐onset of pain relief) using an analysis of covariance (ANCOVA) model adjusted for gender, baseline secondary endpoint and country. The 95% CI of differences in proportions between both groups were estimated using a large sample normal approximation. Time‐to‐onset of pain relief was analysed using Kaplan–Meier techniques; categorical endpoints were summarized by treatment and visit. For the primary and secondary endpoints, Baseline Observation Carried Forward (BOCF) was used in case of missing data at Week 8 except for TSQM where last observation carried forward (LOCF) was used.
All safety analyses were conducted on the Safety Analysis Set (SAS). This population included all patients who received study drug.
3. Results
3.1. Patients
Patients were enrolled at 92 sites in a total of 22 countries and regions: Armenia (4 sites), Austria (5 sites), Belarus (1 site), Belgium (5 sites), Bulgaria (5 sites), Czech Republic (2 sites), Finland (3 sites), France (7 sites), Germany (5 sites), Greece (3 sites), Hungary (1 site), Italy (10 sites), Poland (6 sites), Portugal (3 sites), Romania (5 sites), Russia (7 sites), Slovakia (3 sites), Slovenia (1 site), Spain (2 sites), Sweden (3 sites), Turkey (5 sites) and the United Kingdom (UK) (6 sites).
A total of 629 patients were enrolled in the study; 568 were randomized and 559 received study medication and were included in all safety and efficacy analyses. Of those patients receiving treatment, 282 were treated with the capsaicin patch and 277 received pregabalin (Fig. 1). Following randomization, nine patients did not receive treatment. Four patients randomized to the capsaicin patch group were not treated due to an administrative error: one patient reported a pain score of 10 for at least 4 days during screening; one patient experienced a severe loss of heat sensation in the painful area, indicative of C‐fibre denervation; one patient was not in good health and experienced high blood pressure during the baseline screening period; no information was available about the other patient. Five patients randomized to the pregabalin group were not treated: one patient did not take any pregabalin following randomization and withdrew after 6 days; three patients withdrew consent after randomization to pregabalin; no information was available about the other patient.
Figure 1.
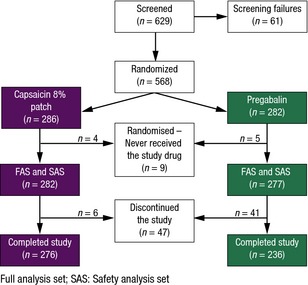
Disposition of patients.
In total, 47 patients discontinued from the study (Fig. 1), six in the capsaicin patch group and 41 in the pregabalin group; 24 due to AEs (all from the pregabalin group), 18 who chose to withdraw (four from the capsaicin patch group and 14 from the pregabalin group) and five who reported the reason to be lack of efficacy (two from the capsaicin patch group and three from the pregabalin group). The overall completion rate for the FAS was 96.5% for the capsaicin patch and 83.7% for pregabalin. Demographics, baseline pain scores, duration of PNP, time since diagnosis, pain grading, type of PNP, baseline NPRS scores and previous use of pregabalin/gabapentin were similar between treatment groups (Table 1). Over half [143 (50.7%)] of patients assigned the capsaicin patch were administered one patch only (mean number of patches was 1.383) and no patients were administered more than four patches, as per protocol. The mean (SD) daily dose of pregabalin was 182.7 mg (45.07 mg) from baseline to Week 2 (inclusive) and by Week 4 (inclusive), 85.2% of patients in the pregabalin arm reached their optimal dose. Between Week 4 and Week 8 (EoS), the mean (SD) daily dose of pregabalin was 364.4 mg (136.96 mg). By Week 8/EoS (last 7 days of study) it was 344.4 mg (140.53 mg) and a further 5.0% of patients reached an optimal pregabalin dose.
Table 1.
Baseline demographics (FAS)
| Parameter | Capsaicin 8% patch (n = 282) | Pregabalin (n = 277) |
|---|---|---|
| Gender, (n, %) | ||
| Male | 123 (43.6) | 122 (44.0) |
| Female | 159 (56.4) | 155 (56.0) |
| Ethnicity (n, %) | ||
| Caucasian | 278 (98.5) | 276 (99.6) |
| Asian | 1 (0.4) | 1 (0.4) |
| Other | 3 (1.1) | 0 (0.0) |
| Age (years) | ||
| Mean (SD) | 55.4 (14.0) | 56.3 (13.5) |
| Min–Max | 20–81 | 19–80 |
| Body Mass Index (kg/m2) | ||
| Mean (SD) | 27.0 (5.1) | 27.4 (5.2) |
| Min–Max | 17–58 | 18–49 |
| NPRS average score | ||
| Mean (SD) | 6.5 (1.2) | 6.7 (1.2) |
| <7 | 162 (57.4) | 150 (54.2) |
| ≥7 | 120 (42.6) | 127 (45.8) |
| Duration of neuropathic pain (years) | ||
| Mean (SD) | 2.58 (4.3) | 2.12 (2.9) |
| Min–Max | 0.0–36.2 | 0.0–19.3 |
| Type of neuropathic pain (n, %) | ||
| Post‐herpetic neuralgia | 63 (22.3) | 73 (26.3) |
| Post‐traumatic nerve injury | 146 (51.8) | 137 (49.5) |
| Non‐diabetic painful peripheral polyneuropathy | 73 (25.9) | 67 (24.2) |
| Time since diagnosis | ||
| <6 months | 69 (24.5) | 63 (22.7) |
| ≥6 months to ≤1 year | 61 (21.6) | 7 (27.8) |
| >1 year to ≤2 years | 60 (21.3) | 48 (17.3) |
| >2 years to ≤10 years | 76 (26.9) | 80 (28.9) |
| >10 years | 16 (5.7) | 9 (3.2) |
| Pain grading | ||
| Probable neuropathic pain | 154 (54.6) | 149 (53.8) |
| Definite neuropathic pain | 128 (45.4) | 128 (46.2) |
| Previous use of pregabalin/gabapentin | ||
| No | 224 (79.4) | 210 (75.8) |
| Yes | 58 (20.6) | 67 (24.2) |
FAS: Full analysis set; NPRS: Numeric Pain Rating Scale.
The results are reported using the CONSORT statement (Piaggio et al., 2006).
3.2. Primary efficacy endpoint
The proportion of patients in each group who achieved a ≥ 30% decrease in the mean NPRS score from baseline to Week 8 are shown in Fig. 2 (55.7% vs. 54.5% for the capsaicin patch and pregabalin, respectively). Patients with missing NPRS score at Week 8 were considered non‐responders to the primary endpoint (BOCF). Based on the primary analyses, the capsaicin patch was shown to be non‐inferior to pregabalin. The difference (capsaicin patch – pregabalin) in the proportion of responders was 1.2% for the FAS analysis and 0.3% for the PPS analysis OR 1.03 (95% CI: 0.71, 1.50) and 1.03 (95% CI 0.70, 1.52) for FAS and PPS respectively; both above 0.693.
Figure 2.
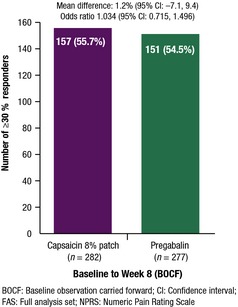
Achievement of a ≥ 30% decrease in NPRS score from baseline to Week 8 (FAS).
3.3. Secondary endpoints
3.3.1. Optimal therapeutic effect
There was no statistical difference between groups, although the proportion was numerically higher for the capsaicin patch group. The proportion of patients in the FAS who achieved OTE at Week 8 was numerically higher for the capsaicin patch [147 (52.1%) patients] than pregabalin [124 (44.8%) patients]; a non‐significant difference of 7.4% (95% CI −0.9%, 15.6%).
3.3.2. Time‐to‐onset of pain relief
The median time to pain relief (where 50% of patients had a 30% reduction in NPRS scores over 3 consecutive days) for the FAS was significantly shorter for the capsaicin patch [7.5 days (95% CI 6.0, 10.0) vs. 36.0 days (95% CI 22.0, 50.0) for pregabalin] (Fig. 3). The hazard ratio was 1.68 in favour of the capsaicin patch (95% CI 1.35, 2.08), p < 0.0001.
Figure 3.
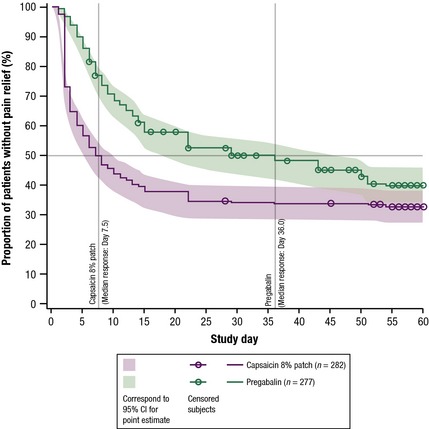
Kaplan–Meier curves for time‐to‐onset of pain relief (FAS).
3.3.3. Change from baseline NPRS for the past 24 h from baseline to Week 8
The absolute and per cent change in NPRS weekly scores from baseline to Week 8 reduced throughout the study in both treatment arms. The mean change from baseline in the NPRS score throughout the study (FAS) is presented in Fig. 4. A substantially greater decrease in mean NPRS scores was observed for the capsaicin patch versus pregabalin up to Week 3, indicating that average pain improvements were observed quicker with the capsaicin patch. From Week 4 to 8, the reduction in the mean NPRS score from baseline plateaued for both treatments and no notable numerical difference was observed between treatments. A greater decrease in mean NPRS scores was also observed from baseline to between Weeks 2‐8 for the capsaicin patch (−37.1%) vs. pregabalin (−27.5%).
Figure 4.
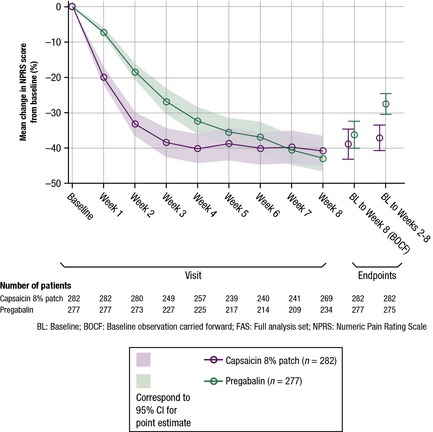
Mean percentage change from baseline in NPRS score throughout the study (FAS).
3.3.4. Subgroup analysis on the primary efficacy outcome
The proportion of patients in the FAS who achieved a ≥30% decrease in the mean NPRS score from baseline was examined according to the type of neuropathic pain: PHN, PNI or non‐diabetic painful peripheral polyneuropathy (Fig. 5). In patients with PNI, there was a higher proportion of responders in the capsaicin patch subgroup [78/146 patients (53.4%)] than in the pregabalin subgroup [56/137 patients (40.9%)] (difference: 12.5%, 95% CI: 1.0%, 24.1%). In patients with PHN, there were 45/63 patients (71.4%) who responded in the capsaicin patch subgroup compared with 56/73 patients (76.7%) in the pregabalin subgroup (difference: −5.3%, 95% CI: −20.1%, 9.5%). In patients with non‐diabetic painful peripheral polyneuropathy, there were 34/73 patients (46.6%) who responded in the capsaicin patch subgroup compared with 39/67 patients (58.2%) in the pregabalin subgroup (difference: −11.6%: 95% CI: −28.1%, 4.8%).
Figure 5.
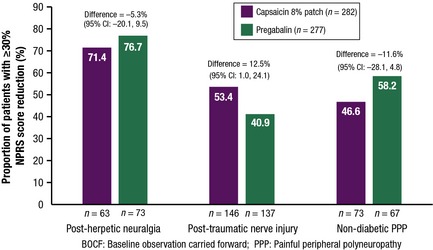
Proportion of patients achieving a ≥ 30% decrease in NPRS score from baseline to Week 8 (BOCF) by type of neuropathic pain (FAS).
Analyses of the primary endpoint in other subgroups found no substantial differences between capsaicin patch and pregabalin arms.
3.3.5. Treatment satisfaction
There was a significant difference in the proportion of patients who withdrew due to either lack of efficacy or tolerability in favour of the capsaicin patch [0.7% for the capsaicin patch vs. 9.7% for pregabalin: difference (capsaicin patch – pregabalin) −9.0%, 95% CI: −12.7%, −5.4%].
There was a significant difference in the proportion of patients willing to continue treatment at Week 8/EoS visit in favour of the capsaicin patch (78.4% for the capsaicin patch vs. 66.4% for pregabalin: difference 12.0%, 95% CI 4.6%, 19.3%).
Mean treatment satisfaction scores, as assessed by the TSQM scale, showed that there was a significant difference in patient perception of effectiveness in favour of the capsaicin patch at Week 8/EoS (LOCF); LS mean difference: 4.3 (95% CI 0.4, 8.1). This significant difference was reflected in the Week 8/EoS analysis but not at Week 4.
More patients given the capsaicin patch [233 (86.9%)] than in the pregabalin group [138 (57.7%)] considered that they had not had any side effects over the first 4 weeks of treatment. Scores were significantly higher for the capsaicin patch versus pregabalin; TSQM scores 95.6 versus 80.3; LS mean difference: 16.1 (95% CI 12.6, 19.7). At Week 8/EoS (LOCF), the difference in the LS means for side effects showed that there was a large and significant difference in patient perception of AEs in favour of the capsaicin patch; LS mean difference: 21.2 (95% CI 17.5, 24.9) (Fig. 6).
Figure 6.
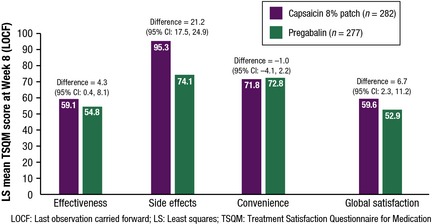
Treatment Satisfaction Questionnaire for Medication scores at Week 8 (LOCF).
There was a significant difference in favour of the capsaicin patch at Week 8/EoS (LOCF) for global satisfaction; LS means difference: 6.7 (95% CI 2.3, 11.2). Global satisfaction scores were similar for both treatment arms at Week 4 (60.7 and 58.5 for the capsaicin patch and pregabalin, respectively), but by Week 8/EoS (LOCF) there was a significant difference in global satisfaction in favour of the capsaicin patch; LS mean difference: 6.7 (95% CI 2.3, 11.2) (Fig. 6).
No significant differences were seen between treatment arms for LS mean TSQM convenience scores (71.8 for the capsaicin patch and 72.8 for pregabalin at Week 8/EoS) (Fig. 6).
3.3.6. Treatment‐emergent adverse events
The proportion of patients without TEAEs was higher for pregabalin versus the capsaicin patch (36.1% vs. 25.5%). The number of TEAEs was lower for the capsaicin patch (n = 667) versus pregabalin (n = 890). However, the proportion of patients without drug‐related TEAEs was similar for both treatment groups (capsaicin patch 38.7% vs. pregabalin 45.5%) (Table 2). The majority of TEAEs were mild or moderate in severity and the proportions of patients without ADRs or with severe TEAEs were comparable between the two treatment arms (87.6% and 87.7% for the capsaicin patch and pregabalin, respectively). The per cent days free from drug‐related TEAEs was higher for the capsaicin patch versus pregabalin (94.5% vs. 70.4%).
Table 2.
Treatment‐emergent adverse events (SAS)
| Capsaicin 8% patch (n = 282) | Pregabalin (n = 277) | |
|---|---|---|
| Patients with TEAEs (n, %) | 210 (74.5) | 177 (63.9) |
| Patients with drug‐related TEAEs (n, %) | 173 (61.3) | 151 (54.5) |
| Days free from TEAEs (%, SD) | 84.3 ± 26.5 | 64.5 ± 39.5 |
| Days free from drug‐related TEAEs (%, SD) | 94.5 ± 15.8 | 70.4 ± 37.9 |
SAS: Safety analysis set; SD: Standard deviation; TEAEs: Treatment‐emergent adverse events.
TEAEs leading to permanent discontinuation of the study drug were reported only for pregabalin (n = 24; 8.5%). There were no deaths and only three serious TEAEs were considered related to the study drug: one event of application site burn with the capsaicin patch, and one event each of cardiac failure and swollen tongue with pregabalin. Patients administered the capsaicin patch reported application site pain, burning sensation, erythema and application site erythema but lower proportions of patients on the capsaicin patch reported dizziness, somnolence, nausea, peripheral oedema and increased weight than with pregabalin. The profile for ADRs was similar to the profile for all TEAEs; the majority of application‐related TEAEs were drug‐related for patients in the capsaicin patch arm and largely systemic ADRs were reported by patients in the pregabalin arm (Table 3). Systemic ADRs ranged from 0–1.1% for the capsaicin patch and 2.5–18.4% for pregabalin. No clinically relevant findings in haematology, biochemistry or vital sign tests were reported.
Table 3.
Drug‐related treatment‐emergent adverse events (≥2.5%) (SAS)
| Capsaicin 8% patch (n = 282) | Pregabalin (n = 277) | |
|---|---|---|
| Overall (n, %) | 173 (61.3) | 151 (54.5) |
| Application site pain | 67 (23.8) | 0 (0.0) |
| Erythema | 59 (20.9) | 1 (0.4) |
| Burning sensation | 44 (15.6) | 0 (0.0) |
| Application site erythema | 25 (8.9) | 0 (0.0) |
| Pain | 15 (5.3) | 2 (0.7) |
| Headache | 3 (1.1) | 26 (9.4) |
| Abdominal pain upper | 2 (0.7) | 8 (2.9) |
| Nausea | 1 (0.4) | 30 (10.8) |
| Asthenia | 1 (0.4) | 9 (3.2) |
| Dizziness | 0 (0.0) | 51 (18.4) |
| Somnolence | 0 (0.0) | 43 (15.5) |
| Weight increased | 0 (0.0) | 17 (6.1) |
| Vertigo | 0 (0.0) | 14 (5.1) |
| Dry mouth | 0 (0.0) | 13 (4.7) |
| Fatigue | 0 (0.0) | 12 (4.3) |
| Constipation | 0 (0.0) | 12 (4.3) |
| Peripheral oedema | 0 (0.0) | 11 (4.0) |
| Disturbance in attention | 0 (0.0) | 8 (2.9) |
| Diarrhoea | 0 (0.0) | 7 (2.5) |
SAS: Safety analysis set.
4. Discussion
Randomized controlled studies have demonstrated efficacy and safety of the capsaicin patch in the treatment of PNP (Backonja et al., 2008; Simpson et al., 2008; Irving et al., 2011; Mou et al., 2013), and it is recommended as a second‐line treatment option for the condition, (Finnerup, Attal et al., 2015).
A Cochrane systematic review of eligible studies that compared the capsaicin patch with active control (0.04% capsaicin) found that the NNT for the capsaicin patch versus active control was relatively high: 8.8 (95% CI: 5.3 to 26) and 7.0 (95% CI: 4.6 to 15) in PHN at 8 and 12 weeks, respectively (Derry et al., 2013). However, the possibility of some therapeutic effect in the active control group cannot be ruled out and it is believed that this may have led to a possible underestimation of the efficacy of the study drug (Irving et al., 2011). AE‐related withdrawals were shown not to differ between capsaicin patch treatment and active control.
Topical application of 5% lidocaine medicated plaster was compared with pregabalin in patients with PHN in a randomised, open‐label, multicentre, non‐inferiority study (Baron et al., 2009). Lidocaine plaster was shown to be more efficacious, was associated with fewer AEs and related discontinuations, and had greater improvements in patient satisfaction and QOL. Unfortunately the therapeutic effects of lidocaine quickly wear off on removal of the patch. Since the therapeutic effect of capsaicin patch is long‐lasting after removal of the patch, there is a need for clinical studies that directly compare the capsaicin patch with alternative treatments for PNP.
This study is the first head‐to‐head randomized trial to directly compare the capsaicin 8% patch with an optimized dose of oral pregabalin in non‐diabetic patients with PNP, over a period of 8 weeks. The primary efficacy outcome measure in this study was similar to that used in previous RCTs, i.e. the proportion of patients in each arm who achieved ≥30% decrease in NPRS score over 8 weeks, which is considered a moderate minimal clinically important difference (MCID) (Dworkin et al., 2008). Justification for the use of any treatment for PNP that differs from current standard therapy requires evaluation of whether it is non‐inferior, equal or better than current standard therapy in terms of efficacy and/or safety. Our primary finding is that the capsaicin patch was non‐inferior to an optimised dose of oral pregabalin for pain relief in the PPS data set as well as FAS data set over a period of 8 weeks.
Most secondary outcomes were shown to be numerically similar between the two treatments. Of note, however, was the significantly shorter time‐to‐onset of pain relief, significantly greater treatment satisfaction, lower rate of systemic side effects, lower frequency of discontinuations due to TEAEs and greater decrease in NPRS scores observed from baseline to between Weeks 2‐8 with the capsaicin patch versus pregabalin.
Patient satisfaction has been shown to affect patients’ health‐related decisions and treatment‐related behaviours, which in turn, can substantially impact the success of treatment outcomes. In addition, patients’ satisfaction with their medication predicts continuance of pharmaceutical treatment, correct medication use and compliance with medication regimens (Atkinson et al., 2004). In this study there was a significant difference in patient perception of treatment effectiveness, a large and significant difference in patient perception of side effects and global satisfaction, all in favour of the capsaicin 8% patch.
The subgroup analysis of the primary endpoint in patients with PNI found that a higher proportion of the capsaicin patch versus pregabalin subgroups achieved a ≥30% reduction in pain. Although an interesting observation, this non‐inferiority study was not statistically powered to detect differences among subgroups and these results serve to suggest that patients with PNI may be a suitable group to assess in a further superiority study of the capsaicin patch versus pregabalin. The majority of RCTs in PNP have included patients with PHN or diabetic peripheral neuropathy (DPN) as experimental models, the results of which are translated to other types of PNP. This study highlights the subtle differences that can occur in response to treatment across different types of PNP and the need to personalize treatment to individual patient needs, as suggested by many theoretical studies (Truini et al., 2013).
In order to replicate real‐life clinical practice as much as possible and to try and minimize AEs and withdrawal due to TEAEs, the pregabalin dose was up‐ or down‐titrated to an optimized dose from baseline to Week 4. From baseline to Week 4 (inclusive), 85.2% of patients in the pregabalin arm reached their optimal dose. The mean (SD) pregabalin dose was 364.4 mg (136.96 mg) from Week 4 to Week 8/EoS and a numerically similar dose of 344.4 mg (140.53 mg) was used at Week 8/EoS, by which point 90.2% of the pregabalin arm had reached an optimal dose. Despite this, discontinuation of pregabalin due to TEAEs was still reported by 8.5% of patients and the limitations of pregabalin related to side effects previously reported in the Cochrane review were also seen: 15.5% of patients reporting somnolence and 19.5% reported dizziness. No withdrawals due to TEAEs were reported for the capsaicin patch, which was largely associated with application‐related ADRs that did not lead to study drug discontinuation. In fact, a systematic review (Moore et al., 2009) of pregabalin (600 mg) versus placebo found that the NNT was 3.9 (95% CI: 3.1‐5.1) in patients with PHN. However, the dose needed to achieve this level of efficacy is associated with a TEAE burden, with 15–25% of patients experiencing somnolence, 27–46% experiencing dizziness and 18‐28% discontinuing treatment due to TEAEs. The results of this study therefore confirm the currently recognized limitations of pregabalin in terms of its tolerability in patients with PNP.
The US FDA have suggested that analysis and presentation of pivotal RCTs for chronic pain conditions should consider patients who have dropped out as non‐responders. The BOCF analysis was employed because it is recognized as more conservative than LOCF in intent‐to‐treat (ITT) analyses if symptoms are expected to improve over the course of the study, as would be expected in the treatment of pain (Shao et al., 2009).
There were a number of limitations with this study. Recruitment of PNP patients was challenging as the majority are already prescribed pregabalin. This meant that the study had to include a broad range of countries in order to meet the required number of participants. The study could also not recruit patients with DPN because of the license restriction for the capsaicin patch. The study was conducted with an open‐label design. This was necessary because of the very different treatment procedures required for the capsaicin 8% patch and oral administration of pregabalin. The fact that the study outcome was limited to 8 weeks may also be considered a limitation. In clinical practice, progress is often evaluated based on longer term outcomes.
The results from this study provide new and clinically meaningful information regarding the treatment of PNP. The capsaicin patch is unique in terms of both its mechanism of action and in the potential response achieved from a single administration. To date, its use has been limited in the treatment of PNP, partly due to the lack of data versus other available oral therapies. Until now, no clinical trials have directly compared the capsaicin patch with alternative treatments for PNP. The outcomes of this head‐to‐head study show that the capsaicin 8% patch has non‐inferior efficacy to oral pregabalin, with a more rapid onset of action and lower rate of discontinuation due to TEAEs.
5. Conclusion
The capsaicin 8% patch was non‐inferior to an optimized dose of pregabalin in relieving pain in patients with PNP over 8 weeks. The capsaicin patch offered a faster onset of pain relief and an overall higher level of satisfaction versus pregabalin. The majority of TEAEs were mild or moderate in severity and, for the capsaicin patch, were largely application related. In contrast, pregabalin was associated with largely systemic TEAEs. TEAEs leading to permanent discontinuation of the study drug were reported only for pregabalin.
Author contributions
All authors discussed the results of the ELEVATE study and commented on the manuscript. M.H. contributed to acquisition, analysis and interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published. W.M. contributed to the analysis and interpretation of data, drafting the article and final approval of the version to be published. T.N. contributed to the study conception and design, analysis and interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published. G.C. contributed to the study conception and design, analysis and interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published. A.B. and D.A. made contributions to acquisition of study data, revision of the manuscript for important intellectual content and final approval of the version to be published. A.K.A. contributed to the study conception and design, analysis and interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published. E.E. and C.C. contributed to the analysis and interpretation of data, drafting the article and final approval of the version to be published.
Acknowledgements
The authors thank Dr Vanessa Lane of Ashley Communications, Hertfordshire County, UK for her medical writing assistance in the preparation of this manuscript, which was funded by Astellas Pharma Europe. Dr Tommaso Siciliano and Ms Sara Marques were instrumental in the implementation of the ELEVATE study while employed by Astellas Pharma Europe.
Funding sources This study was initiated & sponsored by Astellas Pharma Europe Ltd.
Conflicts of interest Professor Maija Haanpää was principal investigator for the ELEVATE study. She has received honoraria from Astellas for speaking at sponsored meetings. Dr William McBride as a member of the independent data review board received a fee for service from Astellas. He was a speaker at an Astellas sponsored symposium on 7th October 2014 at IASP. Professor Giorgio Cruccu received a fee for service from Astellas as member of the Independent Review Board for the ELEVATE study. He has worked with Astellas, Convergence, Lilly and Pfizer. Professor Turo Nurmikko has received fees for service from Astellas for speaking and acting as Chairman of the Independent Review Board for the ELEVATE study. Dr Bosilkov received financial remuneration from Astellas Pharma for participation in the ELEVATE study based on the study contract conditions. E Ernault, C Chambers, and A Abdulahad are employed by Astellas Pharma Europe.
References
- Anand, P. , Bley, K. (2011). Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high–concentration capsaicin 8% patch. Br J Anaesth 107, 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, M.J. , Sinha, A. , Hass, S.L. , Colman, S.S. , Kumar, R.N. , Brod, M. , Rowland, C.R. (2004). Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backonja, M. , Wallace, M.S. , Blonsky, E.R. , Cutler, B.J. , Malan, P. Jr. , Rauck, R. , Tobias, J. ; NGX‐4010 C116 Study Group . (2008) NGX–4010 C116 Study Group. NGX‐4010, a high‐concentration capsaicin patch, for the treatment of postherpetic neuralgia: A randomised, double‐blind study. Lancet Neurol 7, 1106–1112. [DOI] [PubMed] [Google Scholar]
- Baron, R. , Mayoral, V. , Leijon, G. , Binder, A. , Stergelwald, I., Serpell, M . (2009). 5% lidocaine medicated plaster versus pregabalin in postherpetic neuralgia and diabetic polyneuropathy: An open‐label, non‐inferiority two‐stage RCT study. Curr Med Res Opin 27, 1663–1676. [DOI] [PubMed] [Google Scholar]
- Bley, K. (2013) Effects of topical capsaicin on cutaneous innervation: Implications for pain management. Open Pain J, 6(Suppl 1: M9), 81–94. [Google Scholar]
- Bouhassira, D. , Lantéri‐Minet, M. , Attal, N. , Laurent, B. , Touboul, C. (2008). Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136, 380–387. [DOI] [PubMed] [Google Scholar]
- Derry, S. , Sven‐Rice, A. , Cole, P. , Tan, T. , Moore, R.A. (2013) Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2:CD007393. [DOI] [PubMed] [Google Scholar]
- Dworkin, R.H. , Corbin, A.E. , Young, J.P. Jr , Sharma, U. , LaMoreaux, L. , Bockbrader, H. , Garofalo, E.A. , Poole, R.M. (2003). Pregabalin for the treatment of postherpetic neuralgia: A randomized, placebo controlled trial. Neurology 60, 1274–1283. [DOI] [PubMed] [Google Scholar]
- Dworkin, R.H. , Turk, D.C. , Farrar, J.T. , Haythornthwaite, J.A. , Jensen, M.P. , Katz, N.P. , Kerns, R.D. , Stucki, G. , Allen, R.R. , Bellamy, N. , Carr, D.B. , Chandler, J. , Cowan, P. , Dionne, R. , Galer, B.S. , Hertz, S. , Jadad, A.R. , Kramer, L.D. , Manning, D.C. , Martin, S. , McCormick, C.G. , McDermott, M.P. , McGrath, P. , Quessy, S. , Rappaport, B.A. , Robbins, W. , Robinson, J.P. , Rothman, M. , Royal, M.A. , Simon, L. , Stauffer, J.W. , Stein, W. , Tollett, J. , Wernicke, J. , Witter, J. ; IMMPACT . (2005). Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113, 9–19. [DOI] [PubMed] [Google Scholar]
- Dworkin, R.H. , O'Connor, A.B. , Backonja, M. , Farrar, J.T. , Finnerup, N.B. , Jensen, T.S. , Kalso, E.A. , Loeser, J.D. , Miaskowski, C. , Nurmikko, T.J. , Portenoy, R.K. , Rice, A.S. , Stacey, B.R. , Treede, R.D. , Turk, D.C. , Wallace, M.S. (2007). Pharmacologic management of neuropathic pain: Evidence based recommendations. Pain 132, 237–251. [DOI] [PubMed] [Google Scholar]
- Dworkin, R.H. , Turk, D.C. , Wyrwich, K.W. , Beaton, D. , Cleeland, C.S. , Farrar, J.T. , Haythornthwaite, J.A. , Jensen, M.P. , Kerns, R.D. , Ader, D.N. , Brandenburg, N. , Burke, L.B. , Cella, D. , Chandler, J. , Cowan, P. , Dimitrova, R. , Dionne, R. , Hertz, S. , Jadad, A.R. , Katz, N.P. , Kehlet, H. , Kramer, L.D. , Manning, D.C. , McCormick, C. , McDermott, M.P. , McQuay, H.J. , Patel, S. , Porter, L. , Quessy, S. , Rappaport, B.A. , Rauschkolb, C. , Revicki, D.A. , Rothman, M. , Schmader, K.E. , Stacey, B.R. , Stauffer, J.W. , von Stein, T. , White, R.E. , Witter, J. , Zavisic, S. (2008). Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9, 105–21. [DOI] [PubMed] [Google Scholar]
- Dworkin, R.H. , O'Connor, A.B. , Audette, J. , Baron, R. , Gourlay, G.K. , Haanpää, M.L. , Kent, J.L. , Krane, E.J. , Lebel, A.A. , Levy, R.M. , Mackey, S.C. , Mayer, J. , Miaskowski, C. , Raja, S.N. , Rice, A.S. , Schmader, K.E. , Stacey, B. , Stanos, S. , Treede, R.D. , Turk, D.C. , Walco, G.A. , Wells, C.D. (2010). Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clin Proc 85(3 Suppl), S3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar, J.T. , Young, J.P. Jr , LaMoreaux, L. , Werth, J.L. , Poole, R.M. (2001). Clinical importance of changes in chronic pain intensity measured on an 11–point numerical pain rating scale. Pain 94, 149–158. [DOI] [PubMed] [Google Scholar]
- Finnerup, N.B. , Attal, N. , Haroutounian, S. , McNicol, E. , Baron, R. , Dworkin, R.H. , Gilron, I. , Haanpää, M. , Hansson, P. , Jensen, T.S. , Kamerman, P.R. , Lund, K. , Moore, A. , Raja, S.N. , Rice, A.S. , Rowbotham, M. , Sena, E. , Siddall, P. , Smith, B.H. , Wallace, M. (2015). Pharmacotherapy for neuropathic pain in adults: a systematic review and meta‐analysis. Lancet Neurol 14, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup, N.B. , Sindrup, S.H. , Jensen, T.S. (2010). The evidence for pharmacological treatment of neuropathic pain. Pain 150, 573–581. [DOI] [PubMed] [Google Scholar]
- Freynhagen, R. , Serpell, M. , Emir, B. , Whalen, E. , Parsons, B. , Clair, A. , Latymer, M. (2015) A comprehensive drug safety evaluation of pregabalin in peripheral neuropathic pain. Pain Pract, 15, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, G.C. , Carroll, D. , Parry, D. , McQuay, H.J. (2006). Epidemiology and treatment of neuropathic pain: The UK primary care perspective. Pain 122, 156–162. [DOI] [PubMed] [Google Scholar]
- Irving, G.A. , Backonja, M.M. , Dunteman, E. , Blonsky, E.R. , Vanhove, G.F. , Lu, S.P. , Tobias, J. ; NGX‐4010 C117 Study Group . (2011) A multicenter, randomized, double‐blind, controlled study of NGX‐4010, a high‐concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med 12, 99–109. [DOI] [PubMed] [Google Scholar]
- Kennedy, W.R. , Vanhove, G.F. , Lu, S.P. , Tobias, J. , Bley, K.R. , Walk, D. , Wendelschafer‐Crabb, G. , Simone, D.A. , Selim, M.M. (2010). A randomized, controlled, open–label study of the long–term effects of NGX–4010, a high concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J Pain 11, 579–587. [DOI] [PubMed] [Google Scholar]
- Malmberg, A.B. , Mizisin, A.P. , Calcutt, N.A. , von Stein, T. , Robbins, W.R. , Bley, K.R. (2004). Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high concentration capsaicin patch. Pain 4, 360–367. [DOI] [PubMed] [Google Scholar]
- Moore, R.A. , Straube, S. , Wiffen, P.J. , Derry, S. , McQuay, H.J. (2009) Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev 8:CD007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou, J. , Paillard, F. , Turnbull, B. , Trudeau, J. , Stoker, M. , Katz, N.P. (2013). Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: A meta‐analysis of the Qutenza Clinical Trials Database. Pain 154, 1632–1639. [DOI] [PubMed] [Google Scholar]
- Navarro, A. , Saldaña, M.T. , Pérez, C. , Torrades, S. , Rejas, J. (2011). A cost consequences analysis of the effect of pregabalin in the treatment of peripheral neuropathic pain in routine medical practice in primary care settings. BMC Neurol 11, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney, J.P. , Devine, E.B. , Watanabe, J.H. , Sullivan, S.D. (2013). Comparative efficacy of oral pharmaceuticals for the treatment of chronic peripheral neuropathic pain: Meta–analysis and indirect treatment comparisons. Pain Med 14, 706–719. [DOI] [PubMed] [Google Scholar]
- O'Connor, A. (2009). Neuropathic pain: Quality‐of‐life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 27, 95–112. [DOI] [PubMed] [Google Scholar]
- Piaggio, G. , Elbourne, D.R. , Altman, D.G. , Pocock, S.J. , Evans, S.J. , CONSORT Group . (2006) Reporting of noninferiority and equivalence randomized trials: An extension of the CONSORT statement. JAMA 295, 1152–1160. [DOI] [PubMed] [Google Scholar]
- QUTENZA™ . Summary of Product Characteristics. Available from: http://www.medicines.org.uk/emc/medicine/23156 (accessed 07 February 2014).
- Rodríguez, M.J. , Díaz, S. , Vera‐Llonch, M. , Dukes, E. , Rejas, J. (2007). Cost effectiveness analysis of pregabalin versus gabapentin in the management of neuropathic pain due to diabetic polyneuropathy or post‐herpetic neuralgia. Curr Med Res Opin 23, 2585–2596. [DOI] [PubMed] [Google Scholar]
- Sabatowski, R. , Gálvez, R. , Cherry, D.A. , Jacquot, F. , Vincent, E. , Maisonobe, P. , Versavel, M. ; 1008‐045 Study Group . (2004) Pregabalin reduces pain and improves sleep and mood disturbances in patients with post‐herpetic neuralgia: Results of a randomised, placebo–controlled clinical trial. Pain 109, 26–35. [DOI] [PubMed] [Google Scholar]
- Shao, J. , Jordan, D.C. , Pritchett, Y.L. (2009). Baseline observation carry forward: Reasoning, properties, and practical issues. J Biopharm Stat 19, 672–684. [DOI] [PubMed] [Google Scholar]
- Simpson, D.M. , Brown, S. , Tobias, J. (2008). Controlled trial of high‐concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology 70, 2305–2313. [DOI] [PubMed] [Google Scholar]
- Taylor, C.P. , Angelotti, T. , Fauman, E. (2007). Pharmacology and mechanism of action of pregabalin: The calcium channel alpha2‐delta (alpha2‐delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 73, 137–150. [DOI] [PubMed] [Google Scholar]
- Torrance, N. , Smith, B.H. , Bennett, M.I. , Lee, A.J. (2006). The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain 7, 281–289. [DOI] [PubMed] [Google Scholar]
- Treede, R.D. , Jensen, T.S. , Campbell, J.N. , Cruccu, G. , Dostrovsky, J.O. , Griffin, J.W. , Hansson, P. , Hughes, R. , Nurmikko, T. , Serra, J. (2008). Redefinition of neuropathic pain and a grading system for clinical use: Consensus statement on clinical and research diagnostic criteria. Neurology 70, 1630–1635. [DOI] [PubMed] [Google Scholar]
- Truini, A. , Garcia‐Larrea, L. , Cruccu, G. (2013). Reappraising neuropathic pain in humans – How symptoms help disclose mechanisms. Nat Rev Neurol 9, 572–82. [DOI] [PubMed] [Google Scholar]
- World Medical Association (2013). World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310, 2191–2194. [DOI] [PubMed] [Google Scholar]


