Abstract
Antifibrinolytic drugs are routinely used worldwide to reduce the bleeding that results from a wide range of hemorrhagic conditions. The most commonly used antifibrinolytic drug, tranexamic acid, is associated with an increased incidence of postoperative seizures. The reported increase in the frequency of seizures is alarming, as these events are associated with adverse neurological outcomes, longer hospital stays, and increased in‐hospital mortality. However, many clinicians are unaware that tranexamic acid causes seizures. The goal of this review is to summarize the incidence, risk factors, and clinical features of these seizures. This review also highlights several clinical and preclinical studies that offer mechanistic insights into the potential causes of and treatments for tranexamic acid–associated seizures. This review will aid the medical community by increasing awareness about tranexamic acid–associated seizures and by translating scientific findings into therapeutic interventions for patients. ANN NEUROL 2016;79:18–26
Antifibrinolytic drugs are used worldwide to decrease the requirement for blood transfusions, reduce the risk of reoperation for bleeding, and lower mortality associated with hemorrhage following major trauma.1, 2, 3 The most commonly used antifibrinolytic drugs include tranexamic acid (TXA), ɛ‐aminocaproic acid (EACA), and aprotinin.1 TXA and EACA are synthetic derivatives of the amino acid lysine that exert their hemostatic effects by binding to plasminogen.4, 5, 6 This binding prevents the conversion of plasminogen to plasmin and reduces the degradation of fibrin‐containing blood clots.4, 5, 6 Aprotinin, conversely, is a serine protease inhibitor that binds directly to plasmin and inhibits its function.7
Antifibrinolytic agents are considered to be safe and affordable drugs with few serious adverse effects.1 However, observational clinical trials and case reports have shown that TXA, and to a lesser extent EACA, but not aprotinin, are associated with seizures.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 Most TXA‐associated seizures occur in patients who have undergone cardiac procedures.16, 17, 18, 20, 21, 24, 25, 26, 30, 31, 32, 34 However, several case reports indicate that TXA‐associated seizures also occur in nonsurgical patients.10, 22, 27 Seizures in postoperative cardiac surgery patients have been reported to be associated with a 2‐fold increase in hospital length of stay and a 2.5‐fold higher mortality rate.26 An increase in the incidence of delirium and stroke, and a reduced quality of life have also been reported.35
The goal of this review is to increase awareness about seizures associated with antifibrinolytic drugs and provide mechanistic‐based prevention and treatment recommendations. The review focuses on TXA, the most commonly used and widely studied antifibrinolytic drug. First, the incidence, risk factors, and clinical features of TXA‐associated seizures are summarized. Next, preclinical and clinical studies that offer insights into the underlying causes of seizures are reviewed. In particular, a study that measured the concentration of TXA in the cerebral spinal fluid (CSF) of patients undergoing major cardiovascular surgery is considered. The study then compared TXA concentrations in the CSF to TXA concentrations that modulate the activity of neurotransmitter receptors in the brain in vitro. Based on these findings, treatment strategies to mitigate TXA‐associated seizures in patients are proposed.
Clinical Indications, Incidence, and Risk Factors
TXA was originally approved by the US Food and Drug Administration for the treatment of patients with hemophilia undergoing dental surgery and for women suffering from heavy menstrual bleeding.36, 37 The clinical indications of TXA have rapidly expanded and now include multiple “off‐label” uses, including cardiac, gastrointestinal, and orthopedic surgery as well as treatment of postpartum hemorrhage.38, 39, 40, 41 The World Health Organization (WHO) recently included TXA in its “Model List of Essential Medicines.”42 The WHO recommended that TXA be used to reduce blood loss in patients undergoing cardiopulmonary bypass procedures, in trauma patients with significant hemorrhage, and in patients with postpartum hemorrhage.42
The broad introduction of TXA into surgical care has resulted in an increased reported incidence of seizures, particularly during the early postoperative period after cardiac surgery.21 Retrospective analyses have shown that the incidence of seizures in postoperative cardiac patients has increased from 0.5–1.0% to 6.4–7.3% with the use of higher doses of TXA.8, 17 Additionally, several multicenter retrospective studies confirm increased seizures in postoperative patients who received TXA, with an incidence ranging from 0.9% to 2.5%.16, 18, 20, 26 A single prospective trial found that seizures occurred in 3% (3 of 100 patients) of post–cardiac surgical patients treated with TXA.34 Although the incidence of TXA‐associated seizures after cardiac surgery varies between studies, treatment with TXA was a strong independent predictor of seizure.16, 20, 26
Retrospective studies have identified several risk factors for TXA‐associated seizures. These include higher doses of TXA, such as those recommended in the BART study (Blood conservation using Antifibrinolytics in a Randomized Trial; ∼80–100mg/kg total dose).16, 20, 26, 43 Female gender, increased age, and poor overall health also predispose patients to seizures.16, 17, 20, 26 Seizures are observed more frequently in patients older than 70 years, and those with a high disease severity score, as measured by an APACHE II index (Acute Physiology and Chronic Health Evaluation II) > 20.16, 20 Patients with renal dysfunction or prior neurological and cardiovascular disorders are also at increased risk.16, 17, 20, 26 Other important risk factors for TXA‐associated seizures include the type and duration of surgery. Most seizures are reported in patients undergoing “open chamber surgery” (eg, aortic valve replacement).16, 17, 18, 20 The risk is also increased in patients with deep hypothermic circulatory arrest, long cardiopulmonary bypass time, or prolonged aortic cross‐clamp time.16, 18, 20, 26
Several case reports indicate that seizures are not restricted to cardiac surgery patients. For example, a patient with chronic kidney failure who was treated with TXA experienced a convulsive seizure.10 Another patient who underwent a craniotomy for meningioma had tonic–clonic convulsions after the administration of TXA.22 A third patient who was admitted for hemoptysis had a focal seizure after TXA treatment, which progressed to a generalized seizure.27 None of these patients had a history of seizure disorders and no abnormalities were detected on subsequent electroencephalography (EEG) or computed tomographic scans.10, 22, 27 Collectively, these case studies indicate that a wide range of patients may be vulnerable to TXA‐associated seizures. Increasing global “off‐label” use of TXA may further increase the incidence of TXA‐associated seizures.
Clinical Features and Diagnosis
A clear understanding of the clinical features of TXA‐associated seizures will aid in their diagnosis. Reports of patients who received an accidental intrathecal injection of TXA have offered rare insights into the clinical manifestation of TXA‐associated seizures.11, 13, 15, 19, 23, 29 These patients experienced severe back pain that radiated below the waist, with burning pain in the lower limbs and gluteal region.11, 13, 15, 19, 23, 29 Involuntary motor activity, such as a “jerking” of the lower extremities (referred to as myoclonic movements) and twitching of facial muscles, was also observed.11, 12, 13, 15, 19, 23 These abnormal movements rapidly progressed to generalized tonic–clonic seizures.11, 12, 13, 15, 19, 23 Myoclonic movements may serve as a warning sign of impending seizures.
In postoperative cardiac surgery patients, TXA‐associated seizures are typically generalized tonic–clonic events, although focal and mixed seizures also occur.20, 24, 26 Approximately 20% of these seizure patients experience myoclonic activity.24 Seizures usually occur within the first 5 to 8 hours after surgery, during the period of weaning from intravenous sedation in the intensive care unit.17, 20, 24, 26 Seizure events typically persist for a few minutes17, 20 and do not progress into status epilepticus.34 About 30 to 60% of patients have recurrent episodes during the first 24 to 48 hours after surgery.16, 17, 20
The diagnosis of TXA‐associated seizures may be facilitated by EEG monitoring in the early postoperative period. EEG monitoring could help distinguish between shivering, myoclonic movements, and seizures, and thereby prevent a misdiagnosis.17, 18, 26 EEG monitoring may also detect subclinical seizures that are not apparent by observing sedated patients. Continuous EEG monitoring following cardiac surgery identified 1 patient with EEG evidence of seizure who exhibited no convulsive behaviour.34 Finally, EEG monitoring may be particularly useful for the diagnosis of TXA‐associated seizures in patients cotreated with a neuromuscular blocker agent such as rocuronium. These drugs inhibit motor activity and mask the behavioral correlates of network hyperexcitability. In the absence of EEG monitoring, the incidence of TXA‐associated seizures may be underestimated.
TXA Concentrations in the Central Nervous System of Patients
The proconvulsant properties of TXA likely result from direct effects on the central nervous system (CNS), as application of TXA to the cortex or injection into the cisterna magna in experimental animals causes generalized seizures.44, 45, 46 In an effort to identify the mechanism underlying TXA‐associated seizures, the concentration of TXA in the CNS of patients was measured. One study took advantage of a unique clinical scenario where CSF was intermittently sampled during surgery.47 Specifically, an indwelling catheter was inserted into the lumbar intrathecal space of patients undergoing repair of thoracoabdominal aneurysms to allow the CSF to be intermittently drained. The purpose of this procedure is to prevent spinal cord ischemia by decreasing the volume of CSF and reducing intrathecal pressure.48
Measurements of TXA levels in the CSF from these patients produced unexpected results. After infusion of the drug was discontinued, the concentration of TXA in the CSF failed to decline and in some cases continued to increase, reaching peak concentration of about 200 µM (Fig 1A).47 In contrast, TXA levels in the serum peaked following cardiopulmonary bypass, then rapidly declined after the drug infusion was terminated.47 The peak serum concentration of TXA (2 mM) was about 10 times higher than the concentration of TXA in the CSF (200 µM). Notably, 1 patient with a high TXA concentration in the CSF experienced postoperative seizures. The time course of TXA concentrations in the CSF and serum from this patient is illustrated in the top half of Figure 1A. The average concentrations from the CSF and serum of 4 patients are shown in the bottom half of Figure 1A. These results suggest that seizures could arise due to persistently high concentrations of TXA in the brain during the early postoperative period.
Figure 1.
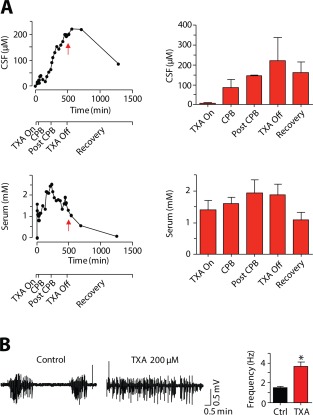
Tranexamic acid (TXA) concentrations measured in the cerebral spinal fluid (CSF) and serum of patients cause hyperexcitability in vitro. (A) The time course of TXA levels in the CSF and serum of 1 patient who experienced a seizure is shown on the left. The decline of TXA levels in the brain lags behind that in the blood. The timeline at the bottom of each figure indicates key surgical events during cardiopulmonary bypass (CPB). The red arrow highlights the concentrations when TXA administration was terminated. On the right are the summarized data of TXA concentrations in the CSF and serum during key surgical events (n = 4). TXA levels in the serum (2mM) are 10‐fold higher than those in the CSF (200 µM). (B) Clinically relevant concentration of TXA (200 µM) causes hyperexcitability by increasing the frequency of seizure‐like events in neocortical slices. *P < 0.05.
Molecular Mechanism of TXA‐Associated Seizures
Studies of animal models have offered insights into the molecular mechanisms underlying TXA‐associated seizures. Application of a clinically relevant concentration of TXA (200 μM) to slices of neocortex markedly increased field responses to excitatory stimuli.47 TXA also increased the frequency of spontaneous epileptiform field potentials or “seizurelike events” (see Fig 1B).47 Another study showed that application of TXA (1mM) to mouse amygdala slices caused widespread neuronal depolarization.49 Collectively, these studies show that TXA directly increases the excitability of neuronal networks. Increasing evidence suggests that this hyperexcitability produced by TXA results from reduced inhibitory neurotransmission or “disinhibition.” γ‐Aminobutyric acid type A (GABAA) receptors and glycine receptors are major mediators of inhibition in the CNS.50, 51 These transmitter‐gated anion channels, which are well‐known targets for a variety of proconvulsant and anticonvulsant agents, are plausible targets for TXA.52, 53, 54, 55, 56
The effects of TXA on GABAA receptors were examined first by Furtmuller and colleagues.57 They showed that TXA is a competitive antagonist of GABAA receptors and that it inhibits recombinant GABAA receptors (α1β2γ2) with a half‐maximal inhibitory concentration (IC50) of 7mM.57 Other investigators showed that TXA inhibits native GABAA receptors in cortical and spinal cord neurons (IC50 = 1.5 and 1mM, respectively).47 Collectively, these results demonstrated that TXA inhibits GABAA receptors, but only at concentrations that are higher than the concentration detected in the CSF of patients (200 μM). GABAA receptors generate 2 distinct forms of inhibition, synaptic and tonic, which could exhibit different sensitivities to TXA.58, 59, 60, 61 Synaptic currents are fast, transient events that are activated by near‐saturating concentrations of agonist.62 In contrast, tonic currents are generated by low, ambient concentrations of transmitter.62 Synaptic and tonic currents are mediated by different receptor subtypes that often exhibit different pharmacological properties.60 Surprisingly, the potency of TXA for synaptic currents (IC50 = 0.8mM) and tonic inhibitory currents (IC50 = 1mM) was similar.47, 49 Thus, although inhibition of GABAA receptors may contribute to TXA‐associated seizures, higher affinity target receptors are likely to exist in the CNS.
Our research group searched for novel receptors that are sensitive to clinically relevant concentrations of TXA. Because TXA is a structural analogue of glycine, we hypothesized that TXA competitively inhibits glycine receptors (Fig 2A), and this action contributes to seizures. In support of this hypothesis, glycine receptor antagonists, such as strychnine, cause myoclonic movements and twitching, particularly in the lower limbs, as well as muscle spasms and convulsions.55, 56, 63, 64 Interestingly, the pattern of twitching, myoclonus, and seizures observed in patients treated with TXA is similar to the pattern of the proconvulsant effects of strychnine.11, 22, 27 We found that TXA acts as a competitive antagonist of glycine receptors, with an IC50 of 1mM (see Fig 2B).47 Similar to GABAA receptors, glycine receptors generate both synaptic currents and tonic inhibitory currents (Fig 3A).61 Thus, we compared the potency of TXA for inhibition of spontaneous miniature inhibitory postsynaptic currents and a tonic current in spinal cord neurons (see Fig 3C).47 Tonic glycine current was found to be 10‐fold more sensitive to TXA (IC50 = 90 µM) than synaptic currents.47 Therefore, the potency of TXA is greatest for glycine receptors that generate a tonic inhibitory current (see Fig 3B).
Figure 2.
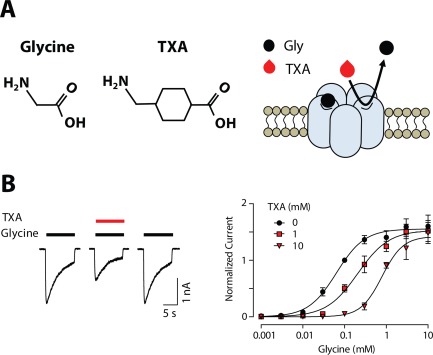
Tranexamic acid (TXA) is a competitive antagonist of glycine (Gly) receptors. (A) Glycine and TXA are structural analogues, suggesting that TXA competes with glycine at the agonist binding site of glycine receptors. (B) TXA (1mM) inhibits glycine (100 µM)‐activated currents in cortical neurons. The concentration–response plots for glycine current recorded in the absence and presence of TXA are shown. The results indicate that TXA is a competitive antagonist of glycine receptors.
Figure 3.
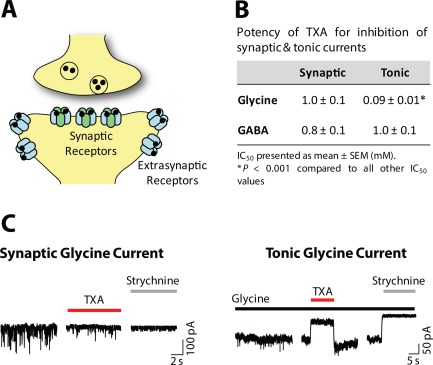
Tonic glycine current is highly sensitive to tranexamic acid (TXA) inhibition. (A) Inhibitory receptors are expressed in synaptic and extrasynaptic regions of the neuron. These receptors are composed of different subunits and have distinct pharmacological properties. Extrasynaptic receptors mediate a tonic inhibitory conductance. (B) Summary table of the half‐maximal inhibitory concentration (IC50) values for TXA inhibition of synaptic and tonic currents mediated by glycine and γ‐aminobutyric acid type A (GABA) receptors. (C) TXA (1mM) inhibits synaptic and tonic glycine currents in a similar manner as the competitive glycine antagonist, strychnine. Synaptic currents were studied by recording miniature inhibitory postsynaptic currents. Tonic currents were evoked by applying a low concentration of glycine (10 µM), similar to the ambient concentration present in the extracellular fluid, to the bath solution. SEM = standard error of the mean.
Finally, others have studied the effects of TXA on the activity of excitatory amino acid receptors.49, 57 Binding assays and electrophysiological studies show that TXA (5mM) does not directly inhibit the N‐methyl‐D‐aspartate57 or α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid subtypes of glutamate receptors.49 Thus, the proconvulsant properties of TXA are likely mediated by disinhibition of tonic glycine current.
Anesthetics Reverse TXA Inhibition of Glycine Receptors
Because the tonic current generated by glycine receptors is highly sensitive to TXA inhibition, drugs that reverse this inhibitory effect may mitigate TXA‐associated hyperexcitability. There are no commonly used selective glycine receptor agonists that can be administered intravenously to patients in the intensive care unit. However, several general anesthetics, including the inhalational agents isoflurane, sevoflurane, and desflurane and the intravenous anesthetic propofol, act as positive allosteric modulators of glycine receptors.65, 66 Whole‐cell recordings of currents in spinal cord neurons showed that clinically relevant concentrations of isoflurane (150 and 250 µM) and propofol (3 µM) fully reversed TXA inhibition of tonic glycine current.47 In addition, field recordings from slices of mouse cortex showed that isoflurane (250 µM) and propofol (1 µM) completely reversed the hyperexcitability produced by TXA.47 Therefore, isoflurane and propofol, as well as other anesthetics that increase glycine receptor function, might be effective either for treating or for preventing TXA‐associated seizures (Fig 4).
Figure 4.
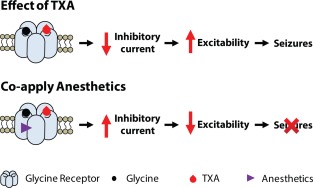
The molecular mechanism underlying tranexamic acid (TXA)‐associated seizures and the reversal of TXA‐mediated inhibition by anesthetics. TXA binds to the glycine receptors, resulting in a decrease in inhibitory current. This reduction in anion conduction increases excitability, which gives rise to seizures. Anesthetics reverse the effect of TXA by increasing glycine receptor function and thereby prevent or reverse TXA‐induced seizures.
Prevention and Treatment of TXA‐Associated Seizures
Currently, there are no recommended treatments for TXA‐associated seizures. Given the low incidence and variable clinical manifestations of TXA‐associated seizures, randomized controlled clinical trials that compare the efficacy of various anticonvulsants treatments are likely to be impractical. Nevertheless, results from animal studies have shown that TXA inhibition of tonic glycine current is rapidly and completely reversed by the general anesthetics isoflurane or propofol.47 These results suggest that general anesthetics may be useful to consider for the first‐line treatment for TXA‐associated seizures in patients. For example, TXA‐associated seizures could be prevented by simply prolonging the delivery of anesthetics during the early postoperative period. Notably, patients experience seizures most often in the first few hours after admission to the intensive care unit. At this time, TXA levels are either peaking or declining slowly.47 In contrast, anesthetic levels are declining rapidly in the CNS, as drug delivery has been terminated.67 Thus, the anesthetic is no longer available to provide anticonvulsant effects. Consistent with this notion that anesthetics protect against TXA‐induced seizures, many patients first develop seizures during emergence from propofol sedation.17, 24 Also, case reports indicate that propofol is effective for treating seizures in patients who inadvertently received an intrathecal injection of TXA.15, 23 Although treatment with a general anesthetic is likely to be effective, these drugs should only be administered under conditions that allow their safe use. This review does not provide specific recommendations regarding doses of anesthetics, as treatment of patients during the early postoperative period in the intensive care unit is highly complex. Treatment must be guided by the judgment and skill of the care providers.
If the use of propofol or other anesthetics is deemed to be unsafe or if these drugs are unavailable, alternative therapies can be considered. A second‐line treatment for TXA‐associated seizures includes compounds that increase GABAA receptor activity, which may compensate for a reduction in glycinergic inhibition. Benzodiazepines (lorazepam, midazolam, diazepam, and clonazepam), which do not modify glycine receptors but rather upregulate GABAA receptor function,68, 69 have been used to treat seizures following inadvertent intrathecal injection of TXA27, 28, 29 or after cardiac surgery.16, 26 Lorazepam may be considered for the treatment of seizures, rather than other benzodiazepines that have shorter duration of action.
Finally, reducing the dose of TXA during surgery may be the simplest and most practical strategy to prevent TXA‐associated seizures. TXA is a competitive antagonist of glycine and GABAA receptors. Thus, a lower dose of TXA is less likely to cause seizures, as lower concentrations would be “outcompeted” by endogenous neurotransmitters at the agonist‐binding sites of glycine and GABAA receptors. The notion that higher doses of TXA increase the risk of seizures is supported by animal45 and human studies.16, 20, 26 Specifically, in cardiac surgery patients, the use of higher doses of TXA drastically increased the incidence of seizures.16, 20, 26
Lowered TXA dosing should also be considered for patients with renal dysfunction, as renal excretion is the major route of TXA elimination. Case reports indicate that patients treated with TXA while undergoing dialysis experience generalized seizures and myoclonic movements.10, 70 Interestingly, TXA administered to cardiac surgery patients at doses recommended in the BART Trial resulted in higher than expected plasma concentrations, which exceeded the recommended therapeutic levels.71, 72 Consistent with these findings, lowering TXA doses reduced the frequency of postoperative seizures.20 Therefore, decreasing the dose of TXA is likely the simplest and most effective strategy to reduce the incidence and/or severity of postoperative seizures. However, the benefits of reducing TXA dose need to be balanced against the possibility of reducing the drug's antifibrinolytic effects.
Summary and Outstanding Questions
In summary, TXA‐associated seizures occur most frequently during the early postoperative period after cardiac surgery but also occur in patients undergoing noncardiac surgery and other medical treatments. To reduce the risk of seizures, the lowest effective TXA dose should be considered and dosing should be adjusted for clinical conditions such as renal dysfunction. A high index of suspicion is required to detect seizures, and EEG monitoring may be considered for patients who experience myoclonic movements or twitching or show evidence of focal seizures. Based on results from preclinical studies, general anesthetics including propofol and isoflurane may be considered as the first line for prevention and/or treatment. In high‐risk patients, terminating the TXA infusion early and/or prolonging the administration of anesthetics may prevent seizures.
Although progress has been made in our understanding of the causes underlying TXA‐associated seizures, many questions remain unanswered. First, it is uncertain why cardiac surgery patients are more vulnerable to TXA‐associated seizures. One potential factor is the high doses of TXA administered during cardiac surgery.73 Also, cardiac surgery can cause intensive systemic inflammation that increases the permeability of the blood–brain barrier.74 A jeopardized blood–brain barrier could facilitate the entry of TXA into the CNS. Second, it is important to understand the mechanism by which TXA gains entry into the CNS, as such knowledge could aid in the development of neuroprotective strategies that reduce TXA penetration. Third, it is of interest to know whether TXA dosing should be reduced or avoided in patients with a previous history of a seizure disorder or those with clinical conditions, such as traumatic brain injury, that damage the blood–brain barrier and predispose to seizures. Also, antibiotics such as penicillins and cephalosporins inhibit GABAA receptors and it is unknown whether these drugs exacerbate the proconvulsant properties of TXA.
Finally, future studies are needed to determine whether antifibrinolytic drugs other than TXA also cause seizures. Interestingly, EACA is a structural analogue of the amino acid glycine and case reports show that EACA causes seizures.33 Our electrophysiological studies demonstrated that EACA acts as a competitive antagonist of glycine receptors (IC50 = 12mM) in mouse neurons.47 The potency of EACA for glycine receptors is 10‐fold lower than that of TXA; however, EACA is often administered at 10‐fold higher doses than TXA to patients.75, 76 Aprotinin is structurally distinct from TXA and EACA. We observed that aprotinin does not inhibit glycine currents, even at a very high concentration (10mM).47
Antifibrinolytic drugs remain an important and effective, low‐cost intervention that reduces blood loss, morbidity, and mortality. Understanding the cause of TXA‐associated seizures, recognizing the early warning signs of impending seizures, and using anesthetics may reduce the incidence and severity of seizures and lead to better patient outcomes.
Author Contributions
C.D.M. proposed the concept for the review and contributed to the writing. I.L. wrote the review and created the figures. D.‐S.W., P.D.W, S.A., and B.A.O. helped structure and edit the review. All authors read and approved the final manuscript.
Potential Conflicts of Interest
Nothing to report.
Acknowledgment
This work was supported by operating grants from the Canadian Institute of Health Research (MOP‐38028, MOP‐79428), a Canada Research Chair (B.A.O.), a grant from the Canadian Anaesthesiologists' Society (B.A.O.), the CIHR Frederick Banting and Charles Best Doctoral Award (I.L.), a CIHR Fellowship (S.A.), a Clinician Scientist Transition Award from the Department of Anesthesia, University of Toronto (S.A.), a Mentored Research Award from the International Anesthesia Research Society (S.A.), and a Merit Award from the Department of Anesthesia, University of Toronto (C.D.M.).
References
- 1. Henry DA, Carless PA, Moxey AJ, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 2007;(3):CD001886. [DOI] [PubMed] [Google Scholar]
- 2. Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs 1999;57:1005–1032. [DOI] [PubMed] [Google Scholar]
- 3. Roberts I, Shakur H, Afolabi A, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH‐2 randomised controlled trial. Lancet 2011;377:1096–1101. [DOI] [PubMed] [Google Scholar]
- 4. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta 1981;673:75–85. [PubMed] [Google Scholar]
- 5. Iwamoto M. Plasminogen‐plasmin system IX. Specific binding of tranexamic acid to plasmin. Thromb Diath Haemorrh 1975;33:573–585. [PubMed] [Google Scholar]
- 6. Thorsen S. Differences in the binding to fibrin of native plasminogen and plasminogen modified by proteolytic degradation. Influence of omega‐aminocarboxylic acids. Biochim Biophys Acta 1975;393:55–65. [DOI] [PubMed] [Google Scholar]
- 7. McEvoy MD, Reeves ST, Reves JG, et al. Aprotinin in cardiac surgery: a review of conventional and novel mechanisms of action. Anesth Analg 2007;105:949–962. [DOI] [PubMed] [Google Scholar]
- 8. Berman M, Cardone D, Sharples L, et al. Safety and efficacy of aprotinin and tranexamic acid in pulmonary endarterectomy surgery with hypothermia: review of 200 patients. Ann Thorac Surg 2010;90:1432–1436. [DOI] [PubMed] [Google Scholar]
- 9. Bertholini DM, Butler CS. Severe hyponatraemia secondary to desmopressin therapy in von Willebrand's disease. Anaesth Intensive Care 2000;28:199–201. [DOI] [PubMed] [Google Scholar]
- 10. Bhat A, Bhowmik DM, Vibha D, et al. Tranexamic acid overdosage‐induced generalized seizure in renal failure. Saudi J Kidney Dis Transpl 2014;25:130–132. [DOI] [PubMed] [Google Scholar]
- 11. Butala BP, Shah VR, Bhosale GP, et al. Medication error: subarachnoid injection of tranexamic acid. Indian J Anaesth 2012;56:168–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Leede‐van der Maarl MG, Hilkens P, Bosch F. The epileptogenic effect of tranexamic acid. J Neurol 1999;246:843. [DOI] [PubMed] [Google Scholar]
- 13. Garcha PS, Mohan CV, Sharma RM. Death after an inadvertent intrathecal injection of tranexamic acid. Anesth Analg 2007;104:241–242. [DOI] [PubMed] [Google Scholar]
- 14. Ichikawa J, Kodaka M, Nishiyama K, et al. A case of postoperative convulsive seizure following tranexamic acid infusion during aortic valve replacement. Masui 2013;62:186–189. [PubMed] [Google Scholar]
- 15. Kaabachi O, Eddhif M, Rais K, et al. Inadvertent intrathecal injection of tranexamic acid. Saudi J Anaesth 2011;5:90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalavrouziotis D, Voisine P, Mohammadi S, et al. High‐dose tranexamic acid is an independent predictor of early seizure after cardiopulmonary bypass. Ann Thorac Surg 2012;93:148–154. [DOI] [PubMed] [Google Scholar]
- 17. Keyl C, Uhl R, Beyersdorf F, et al. High‐dose tranexamic acid is related to increased risk of generalized seizures after aortic valve replacement. Eur J Cardiothorac Surg 2011;39:114–121. [DOI] [PubMed] [Google Scholar]
- 18. Koster A, Borgermann J, Zittermann A, et al. Moderate dosage of tranexamic acid during cardiac surgery with cardiopulmonary bypass and convulsive seizures: incidence and clinical outcome. Br J Anaesth 2013;110:34–40. [DOI] [PubMed] [Google Scholar]
- 19. Mahmoud K, Ammar A. Accidental intrathecal injection of tranexamic acid. Case Rep Anesthesiol 2012;2012:646028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manji RA, Grocott HP, Leake J, et al. Seizures following cardiac surgery: the impact of tranexamic acid and other risk factors. Can J Anaesth 2012;59:6–13. [DOI] [PubMed] [Google Scholar]
- 21. Martin K, Wiesner G, Breuer T, et al. The risks of aprotinin and tranexamic acid in cardiac surgery: a one‐year follow‐up of 1188 consecutive patients. Anesth Analg 2008;107:1783–1790. [DOI] [PubMed] [Google Scholar]
- 22. Merriman B, Mayson K, Sawka A, et al. Postoperative seizure in a neurosurgical patient: should tranexamic acid be on the differential? Can J Anaesth 2013;60:506–507. [DOI] [PubMed] [Google Scholar]
- 23. Mohseni K, Jafari A, Nobahar MR, et al. Polymyoclonus seizure resulting from accidental injection of tranexamic acid in spinal anesthesia. Anesth Analg 2009;108:1984–1986. [DOI] [PubMed] [Google Scholar]
- 24. Murkin JM, Falter F, Granton J, et al. High‐dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg 2010;110:350–353. [DOI] [PubMed] [Google Scholar]
- 25. Sander M, Spies CD, Martiny V, et al. Mortality associated with administration of high‐dose tranexamic acid and aprotinin in primary open‐heart procedures: a retrospective analysis. Crit Care 2010;14:R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharma V, Katznelson R, Jerath A, et al. The association between tranexamic acid and convulsive seizures after cardiac surgery: a multivariate analysis in 11 529 patients. Anaesthesia 2014;69:124–130. [DOI] [PubMed] [Google Scholar]
- 27. Wang CS, Yang CJ, Chen SC, et al. Generalized convulsion resulted in hyperammonemia during treatment with tranexamic acid for hemoptysis. Ir J Med Sci 2011;180:761–763. [DOI] [PubMed] [Google Scholar]
- 28. Wong JO, Yang SF, Tsai MH. Accidental injection of tranexamic acid (Transamin) during spinal anesthesia. Ma Zui Xue Za Zhi 1988;26:249–252. [PubMed] [Google Scholar]
- 29. Yeh HM, Lau HP, Lin PL, et al. Convulsions and refractory ventricular fibrillation after intrathecal injection of a massive dose of tranexamic acid. Anesthesiology 2003;98:270–272. [DOI] [PubMed] [Google Scholar]
- 30. Casati V, Romano A, Novelli E, et al. Tranexamic acid for trauma. Lancet 2010;376:1049–1050. [DOI] [PubMed] [Google Scholar]
- 31. Jimenez JJ, Iribarren JL, Brouard M, et al. Safety and effectiveness of two treatment regimes with tranexamic acid to minimize inflammatory response in elective cardiopulmonary bypass patients: a randomized double‐blind, dose‐dependent, phase IV clinical trial. J Cardiothorac Surg 2011;6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Makhija N, Sarupria A, Kumar Choudhary S, et al. Comparison of epsilon aminocaproic acid and tranexamic acid in thoracic aortic surgery: clinical efficacy and safety. J Cardiothorac Vasc Anesth 2013;27:1201–1207. [DOI] [PubMed] [Google Scholar]
- 33. Rabinovici R, Heyman A, Kluger Y, et al. Convulsions induced by aminocaproic acid infusion. DICP 1989;23:780–781. [DOI] [PubMed] [Google Scholar]
- 34. Gofton TE, Chu MW, Norton L, et al. A prospective observational study of seizures after cardiac surgery using continuous EEG monitoring. Neurocrit Care 2014;21:220–227. [DOI] [PubMed] [Google Scholar]
- 35. Hunter GR, Young GB. Seizures after cardiac surgery. J Cardiothorac Vasc Anesth 2011;25:299–305. [DOI] [PubMed] [Google Scholar]
- 36. Sindet‐Pedersen S, Stenbjerg S. Effect of local antifibrinolytic treatment with tranexamic acid in hemophiliacs undergoing oral surgery. J Oral Maxillofac Surg 1986;44:703–707. [DOI] [PubMed] [Google Scholar]
- 37. Wellington K, Wagstaff AJ. Tranexamic acid: a review of its use in the management of menorrhagia. Drugs 2003;63:1417–1433. [DOI] [PubMed] [Google Scholar]
- 38. Ducloy‐Bouthors AS, Jude B, Duhamel A, et al. High‐dose tranexamic acid reduces blood loss in postpartum haemorrhage. Crit Care 2011;15:R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rahman Z, Hoque R, Ali A, et al. Blood conservation strategies for reducing peri‐operative blood loss in open heart surgery. Mymensingh Med J 2011;20:45–53. [PubMed] [Google Scholar]
- 40. Sabovic M, Lavre J, Vujkovac B. Tranexamic acid is beneficial as adjunctive therapy in treating major upper gastrointestinal bleeding in dialysis patients. Nephrol Dial Transplant 2003;18:1388–1391. [DOI] [PubMed] [Google Scholar]
- 41. Zufferey PJ, Miquet M, Quenet S, et al. Tranexamic acid in hip fracture surgery: a randomized controlled trial. Br J Anaesth 2010;104:23–30. [DOI] [PubMed] [Google Scholar]
- 42. Roberts I, Kawahara T. Proposal for the inclusion of tranexamic acid (anti‐fibrinolytic‐lysine analogue) in the WHO model list of essential medicines. Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- 43. Fergusson DA, Hebert PC, Mazer CD, et al. A comparison of aprotinin and lysine analogues in high‐risk cardiac surgery. N Engl J Med 2008;358:2319–2331. [DOI] [PubMed] [Google Scholar]
- 44. Pellegrini A, Giaretta D, Chemello R, et al. Feline generalized epilepsy induced by tranexamic acid (AMCA). Epilepsia 1982;23:35–45. [DOI] [PubMed] [Google Scholar]
- 45. Schlag MG, Hopf R, Zifko U, et al. Epileptic seizures following cortical application of fibrin sealants containing tranexamic acid in rats. Acta Neurochir (Wien) 2002;144:63–69. [DOI] [PubMed] [Google Scholar]
- 46. Yamaura A, Nakamura T, Makino H, et al. Cerebral complication of antifibrinolytic therapy in the treatment of ruptured intracranial aneurysm. Animal experiment and a review of literature. Eur Neurol 1980;19:77–84. [DOI] [PubMed] [Google Scholar]
- 47. Lecker I, Wang DS, Romaschin AD, et al. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest 2012;122:4654–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Estrera AL, Sheinbaum R, Miller CC, et al. Cerebrospinal fluid drainage during thoracic aortic repair: safety and current management. Ann Thorac Surg 2009;88:9–15. [DOI] [PubMed] [Google Scholar]
- 49. Kratzer S, Irl H, Mattusch C, et al. Tranexamic acid impairs γ‐aminobutyric acid receptor type A‐mediated synaptic transmission in the murine amygdala: a potential mechanism for drug‐induced seizures? Anesthesiology 2014;120:639–649. [DOI] [PubMed] [Google Scholar]
- 50. Olsen RW, Tobin AJ. Molecular biology of GABAA receptors. FASEB J 1990;4:1469–1480. [DOI] [PubMed] [Google Scholar]
- 51. Sigel E, Steinmann ME. Structure, function, and modulation of GABAA receptors. J Biol Chem 2012;287:40224–40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Greenfield LJ Jr. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure 2013;22:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Henschel O, Gipson KE, Bordey A. GABAA receptors, anesthetics and anticonvulsants in brain development. CNS Neurol Disord Drug Targets 2008;7:211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sillito AM. The effectiveness of bicuculline as an antagonist of GABA and visually evoked inhibition in the cat's striate cortex. J Physiol 1975;250:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Karkar KM, Thio LL, Yamada KA. Effects of seven clinically important antiepileptic drugs on inhibitory glycine receptor currents in hippocampal neurons. Epilepsy Res 2004;58:27–35. [DOI] [PubMed] [Google Scholar]
- 56. Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev 2004;84:1051–1095. [DOI] [PubMed] [Google Scholar]
- 57. Furtmuller R, Schlag MG, Berger M, et al. Tranexamic acid, a widely used antifibrinolytic agent, causes convulsions by a γ‐aminobutyric acid A receptor antagonistic effect. J Pharmacol Exp Ther 2002;301:168–173. [DOI] [PubMed] [Google Scholar]
- 58. Chebib M, Johnston GA. GABA‐activated ligand gated ion channels: medicinal chemistry and molecular biology. J Med Chem 2000;43:1427–1447. [DOI] [PubMed] [Google Scholar]
- 59. Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci 1994;17:569–602. [DOI] [PubMed] [Google Scholar]
- 60. Mody I. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res 2001;26:907–913. [DOI] [PubMed] [Google Scholar]
- 61. Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology 2009;56:303–309. [DOI] [PubMed] [Google Scholar]
- 62. Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 2005;6:215–229. [DOI] [PubMed] [Google Scholar]
- 63. Parker AJ, Lee JB, Redman J, et al. Strychnine poisoning: gone but not forgotten. Emerg Med J 2011;28:84. [DOI] [PubMed] [Google Scholar]
- 64. Young AB, Snyder SH. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A 1973;70:2832–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Downie DL, Hall AC, Lieb WR, et al. Effects of inhalational general anaesthetics on native glycine receptors in rat medullary neurones and recombinant glycine receptors in Xenopus oocytes. Br J Pharmacol 1996;118:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol 1991;104:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Belelli D, Callachan H, Hill‐Venning C, et al. Interaction of positive allosteric modulators with human and Drosophila recombinant GABA receptors expressed in Xenopus laevis oocytes. Br J Pharmacol 1996;118:563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tan KR, Rudolph U, Luscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci 2011;34:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Curtis DR, Game CJ, Lodge D. Benzodiazepines and central glycine receptors. Br J Pharmacol 1976;56:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hui AC, Wong TY, Chow KM, et al. Multifocal myoclonus secondary to tranexamic acid. J Neurol Neurosurg Psychiatry 2003;74:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bojko B, Vuckovic D, Cudjoe E, et al. Determination of tranexamic acid concentration by solid phase microextraction and liquid chromatography‐tandem mass spectrometry: first step to in vivo analysis. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:3781–3787. [DOI] [PubMed] [Google Scholar]
- 72. Sharma V, Fan J, Jerath A, et al. Pharmacokinetics of tranexamic acid in patients undergoing cardiac surgery with use of cardiopulmonary bypass. Anaesthesia 2012;67:1242–1250. [DOI] [PubMed] [Google Scholar]
- 73. Fox MA. Tranexamic acid: how much is enough? Anesth Analg 2010;111:580–581. [DOI] [PubMed] [Google Scholar]
- 74. Merino JG, Latour LL, Tso A, et al. Blood‐brain barrier disruption after cardiac surgery. Am J Neuroradiol 2013;34:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dubber AH, McNicol GP, Douglas AS. Amino methyl cyclohexane carboxylic acid (AMCHA), a new synthetic fibrinolytic inhibitor. Br J Haematol 1965;11:237–245. [DOI] [PubMed] [Google Scholar]
- 76. Dubber AH, McNicol GP, Douglas AS, et al. Some properties of the antifibrinolytically active isomer of amino‐methylcyclohexane carboxylic acid. Lancet 1964;2:1317–1319. [DOI] [PubMed] [Google Scholar]


