Abstract
Objectives
Since 1985, Malawi has experienced a dual epidemic of HIV and tuberculosis (TB) which has been moderated recently by the advent of antiretroviral therapy (ART). The aim of this study was to describe the association over several decades between HIV/AIDS, the scale‐up of ART and TB case notifications.
Methods
Aggregate data were extracted from annual reports of the National TB Control Programme, the Ministry of Health HIV Department and the National Statistics Office. ART coverage was calculated using the total HIV population as denominator (derived from UNAIDS Spectrum software).
Results
In 1970, there were no HIV‐infected persons but numbers had increased to a maximum of 1.18 million by 2014. HIV prevalence reached a maximum of 10.8% in 2000, thereafter decreasing to 7.5% by 2014. Numbers alive on ART increased from 2586 in 2003 to 536 527 (coverage 45.3%) by 2014. In 1985, there were 5286 TB cases which reached a maximum of 28 234 in 2003 and then decreased to 17 723 by 2014 (37% decline from 2003). There were increases in all types of new TB between 1998–2003 which then declined by 30% for extrapulmonary TB, by 37% for new smear‐positive PTB and by 50% for smear‐negative PTB. Previously treated TB cases reached a maximum of 3443 in 2003 and then declined by 42% by 2014.
Conclusion
The rise and fall of TB in Malawi between 1985 and 2014 was strongly associated with HIV infection and ART scale‐up; this has implications for ending the TB epidemic in high HIV–TB burden countries.
Keywords: antiretroviral therapy, tuberculosis, Malawi, HIV/AIDS, recurrent TB, operational research
Abstract
Objectifs
Depuis 1985, le Malawi a connu une double épidémie de VIH et de tuberculose (TB) qui a été récemment modérée par l'avènement de la thérapie antirétrovirale (ART). Le but de cette étude était de décrire l'association au cours de plusieurs décennies entre le VIH/SIDA, le déploiement de l’ART et La notifications des cas de TB.
Méthodes
Les données agrégées ont été extraites des rapports annuels du Programme National de Lutte Contre la Tuberculose, du Département VIH du Ministère de la Santé et du Bureau National des Statistiques. La couverture de l’ART a été calculée en utilisant la population VIH totale comme dénominateur (dérivée du logiciel Spectrum ONUSIDA).
Résultats
En 1970, il n'y avait aucun cas d'infection par le VIH, mais le nombre a augmenté jusqu’à un maximum de 1,18 million en 2014. La prévalence du VIH a atteint un maximum de 10,8% en 2000, puis a baissé à 7,5% en 2014. Le nombre de personnes vivantes sous ART a augmenté de 2 586 en 2003 à 536.527 (couverture de 45,3%) en 2014. En 1985, il y avait 5.286 cas de TB qui ont atteint un maximum de 28.234 en 2003, puis a baissé à 17.723 en 2014 (37% de déclin à partir de 2003). Il y avait des augmentations dans tous les types de nouveaux cas de TB entre 1998 et 2003, qui ont ensuite diminué de 30% pour la TB extrapulmonaire, de 37% pour les nouveaux cas de TB pulmonaire à frottis positif et de 50% pour les cas de TB pulmonaire à frottis négatif. Les cas de TB précédemment traitée ont atteint un maximum de 3.443 en 2003, puis ont diminué de 42% en 2014.
Conclusion
L'ascension et la chute de la TB au Malawi entre 1985 et 2014 ont été fortement associées à l'infection au VIH et au déploiement de l’ART; cela a des implications pour mettre fin à l’épidémie de TB dans les pays fortement touchés par le VIH‐TB.
Keywords: thérapie antirétrovirale, tuberculose, Malawi, VIH/SIDA, TB récurrente, recherche opérationnelle
Abstract
Objetivos
Desde 1985, Malawi ha experimentado una epidemia dual de VIH y tuberculosis (TB) la cual ha sido recientemente moderada por la llegada de la terapia antirretroviral (TAR). El objetivo de este estudio era describir la asociación a lo largo de varias décadas entre el VIH/SIDA, la distribución masiva del TAR y la notificación de casos de TB.
Métodos
Se extrajeron datos agregados de los informes anuales del Programa Nacional de Control de la TB, del Departamento para VIH del Ministerio de Salud y de la Oficina Nacional de Estadísticas. La cobertura de TAR se calculó utilizando la población total con VIH como denominador (derivados del software Spectrum de ONUSIDA).
Resultados
En 1970 no había personas infectadas con VIH, pero los números han ido aumentado hasta un máximo de 1.18 millones en el 2014. La prevalencia de VIH alcanzó un máximo del 10.8% en el 2000 y después disminuyó hasta el 7.5% en el 2014. El número de personas vivas recibiendo TAR aumentó de 2586 en el 2003 a 536,527 (cobertura 45.3%) en el 2014. En 1985 había 5,286 casos de TB, que alcanzaron un máximo de 28,234 en el 2003 y después disminuyeron a 17,723 en el 2014 (una disminución del 37% desde el 2003). Hubo un aumento en todos los tipos de nuevos casos de TB entre 1998‐2003, que luego disminuyeron en un 30% para TB extra pulmonar, en 37% para nuevos casos de TB pulmonar con baciloscopia positiva y en un 50% para los casos de TB pulmonar con baciloscopia negativa. Los casos de TB previamente tratados alcanzaron un máximo de 3,443 en el 2003 y luego disminuyeron en un 42% en el 2014.
Conclusión
El aumento y la caída de la TB en Malawi entre 1985 y 2014 estaba fuertemente asociada con la infección por VIH y la llevada a gran escala del TAR; esto tiene implicaciones para las políticas que buscan poner fin a la epidemia de TB en países con una alta carga de VIH‐TB.
Introduction
Malawi's National Tuberculosis (TB) Control Programme (NTP) was established in 1964, shortly after the country's independence. In 1969, a TB control unit was established at the Ministry of Health's headquarters, responsible for coordinating district activities and monitoring TB cases in the country. There was a gradual increase in new notified TB cases from 3492 in 1970 to 4115 in 1979 to 5286 in 1985 – an increase of just over 50% during the 15‐year period 1. In 1984, under guidance from the International Union Against Tuberculosis and Lung Disease, the country became one of the first to implement what later became known as the World Health Organization's ‘DOTS’ framework for TB control 2. By 1985, this standardised strategy had been rolled out countrywide with good TB case detection, high rates of smear‐positive tuberculosis and excellent treatment outcomes.
This was not to last. The first case of AIDS was reported in Blantyre, southern Malawi, in December 1985, and within 10 years, HIV prevalence in the adult population had soared to above 10% 3. The effect of this HIV epidemic on the Malawi NTP was devastating. Annual TB case notifications spiralled out of control increasing to 500% or more of the 1985 figures. Hospital‐based, district‐based and national surveys showed rapidly increasing HIV‐TB co‐infection rates that rose from 26% in 1986 to 77% in 1995 4, 5, 6, 7, 8. Case fatality rates soared and treatment success plummeted to below 70% 1.
The advent of antiretroviral therapy (ART) in 2004, with rapid national scale‐up of treatment thereafter, reaching over 500 000 people living with HIV (PLHIV) by 2014, started to turn the tide; TB case notification rates have stabilised and are beginning to fall 9. Malawi's comprehensive national recording and reporting system for TB, the national epidemiological projections of HIV prevalence using the Estimation and Projection package and Spectrum software from UNAIDS 10, and the excellent recording and reporting of HIV‐infected persons on ART, provide an opportunity to review the association between HIV and TB at a national level over a 30‐year period. The aim of this study was to describe the association between HIV/AIDS, the scale‐up of ART and TB case notifications, stratified by type and category of disease, between 1985 and 2014.
Methods
Study design and setting
This was a retrospective descriptive study using national aggregate data in Malawi, a poor country in central‐southern Africa with a population of 17 million and an estimated one million PLHIV. The scale‐up of ART started in 2004, with numbers of patients alive and retained on treatment being reported quarterly from the HIV Department, Ministry of Health. PLHIV are currently eligible for ART if they are pregnant/breast feeding, have WHO clinical stage 3 or 4 disease or a CD4 count below the nationally agreed threshold (this was ≤250 cells/μl up until 2010, ≤350 cells/μl up to 2014 and ≤500 cells/μl thereafter) in accordance with national and international guidelines 11, 12. In the first years of scale‐up, first‐line treatment was mainly with a fixed‐dose combination of stavudine–lamivudine–nevirapine; from 2011, there has been a gradual change to tenofovir–lamivudine–efavirenz.
Malawi has had a well‐respected ‘DOTS’ national TB control programme since 1985, with case finding, diagnosis, registration, treatment and treatment outcomes following agreed international guidelines 13. Patients with TB are classified according to new and previously treated disease and divided into smear‐positive pulmonary TB (PTB), smear‐negative PTB and extra‐pulmonary TB (EPTB) 13. Patients with previously treated TB are classified as relapse smear‐positive PTB and ‘other’ which includes smear‐positive failure cases, smear‐positive treatment after lost‐to‐follow‐up cases and other retreatment cases with smear‐negative or extra‐pulmonary disease.
Study population
The study population included estimated annual numbers of PLHIV in the country from 1970 to 2014, the number of PLHIV (adults and children) recorded alive and retained on ART at the end of each year from 2004 to 2014 and all adults and children registered nationally each year with TB under the Malawi DOTS TB Programme from 1985 to 2014.
Data variables and sources of data
Sources of aggregate data were national annual reports from the National TB Control Programme and HIV Department, Ministry of Health, Malawi. Given the change in national ART eligibility criteria during the study period, ART coverage was calculated using the total HIV population as the denominator (derived from national epidemiological projections using the Estimation and Projection package and Spectrum software from UNAIDS 10 and based on national population estimates obtained from the Malawi National Statistics Office). Annual TB case notifications were tabulated and TB case rates per 100 000 were derived from national population estimates. Patients with TB included registered cases, stratified by new and previously treated disease and types of TB.
Analysis and statistics
Data were analysed descriptively, and comparisons between highest and lowest annual numbers of patients with TB were made with chi‐square tests. Levels of significance were set at 5%.
Ethics approval
Ethics approval for the study was obtained from the Malawi National Health Science Research Committee. The Ethics Advisory Group of the Union, Paris, France, waived the need for further international ethics approval. As records and reports were used, informed patient consent was not necessary.
Results
Figure 1a,b shows HIV estimates (numbers of persons infected with HIV and HIV prevalence) and numbers alive and retained on ART between 1970 and 2014. In 1970, there were no persons infected with HIV but numbers increased to 228 by the end of 1971 and increased to a maximum of 1 184 103 by the end of 2014. HIV prevalence increased from zero in 1970 to a maximum of 10.8% in 2000 and thereafter prevalence decreased to 7.5% by 2014. The numbers alive and on ART increased from 2586 in 2003 to 536 527 by the end of 2014.
Figure 1.
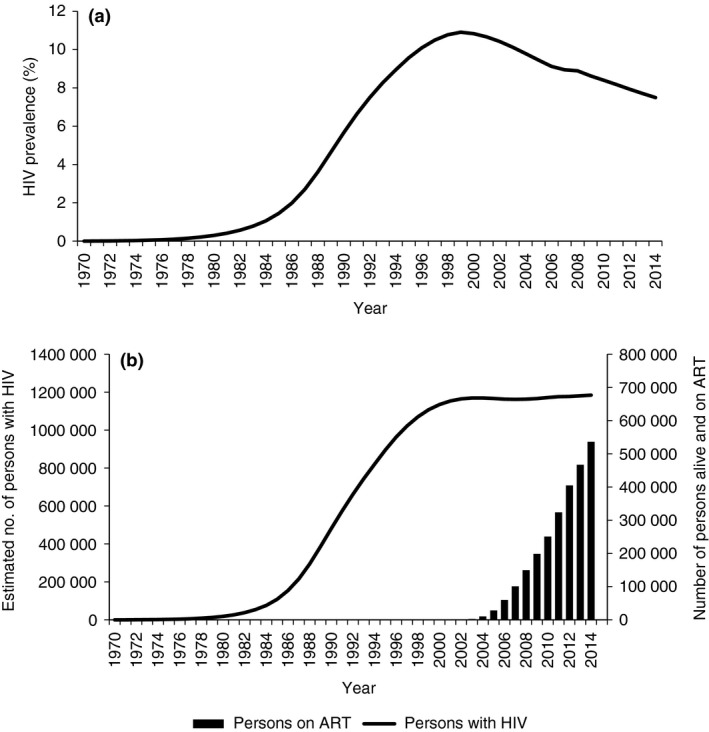
Estimated HIV trends and numbers cumulatively on antiretroviral treatment in Malawi, 1970–2014. (a) Estimated HIV prevalence. HIV, Human immunodeficiency virus. (b) Estimated number of persons with HIV and number cumulatively recorded as alive and on antiretroviral treatment. HIV, Human immunodeficiency virus; ART, antiretroviral treatment.
Figure 2a–c shows TB case notifications, stratified by type and category of TB, between 1985 and 2014. In 1985, there were 5286 TB cases registered in the country which increased to a maximum of 28 234 by 2003 (530% increase from 1985) and then decreased to 17 723 by 2014 (37% decline from 2003) (Figure 2a).
Figure 2.
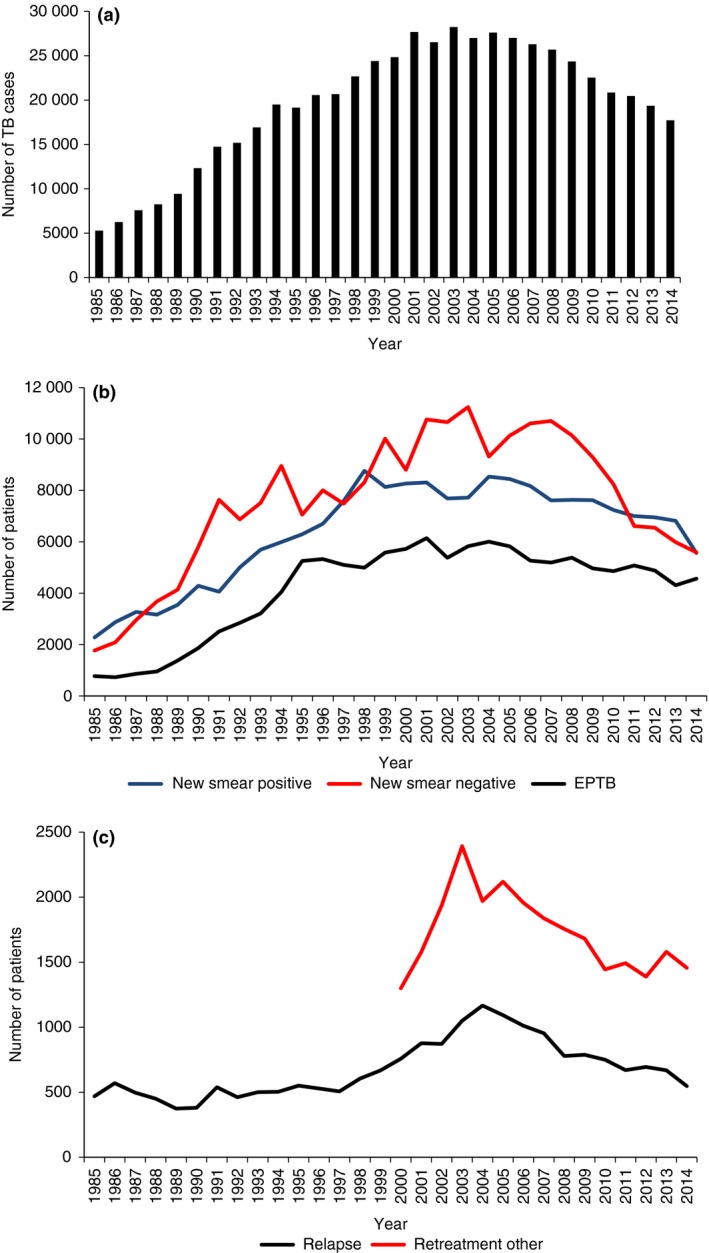
Tuberculosis case notifications in Malawi, 1970–2014. (a) Number of new and previously treated tuberculosis cases registered per annum. TB, Tuberculosis. (b) Number of new tuberculosis cases per annum stratified by type. TB, Tuberculosis; EPTB, extrapulmonary tuberculosis. (c). Number of previously treated tuberculosis cases per annum stratified by relapse and ‘retreatment other’ cases.
There were increases in all types of new TB from 1985 which reached a maximum of 8765 for new smear‐positive PTB in 1998, a maximum of 6145 for new EPTB in 2001 and a maximum of 11 246 for smear‐negative PTB in 2003 (Figure 2b). The numbers then declined to reach 5564 for new smear‐positive PTB in 2014 (37% decline), 4308 for new EPTB in 2013 (30% decline) and 5589 for new smear‐negative PTB in 2014 (50% decline). The differences in declines between new EPTB and new smear‐negative PTB compared with new smear‐positive PTB were significant.
For categories of TB, new TB cases increased to a maximum of 24 791 in 2003 and then declined to 15 720 in 2014 (37% decline) while previously treated TB cases increased to a maximum of 3443 in 2003 and thereafter declined to 2003 in 2014 (42% decline) – these differences in decline were significant. Changes in relapse TB and ‘other’ categories of previously treated TB are shown in Figure 2c. The category of ‘other’ TB was only reported from 2000. Relapse TB cases declined from 1167 in 2004 to 547 in 2014 (53% decline) which was significantly higher than the decline shown with ‘other TB’ which was 2393 in 2005 and 1389 in 2012 (34% decline; P < 0.001).
The correlation between coverage of ART and TB case notification rates per 100 000 between 2000 and 2014 is shown in Figure 3. A steady decline in TB case notification rates occurred from 2005 when ART coverage was still below 5% to 2014 when ART coverage was 45.3%.
Figure 3.
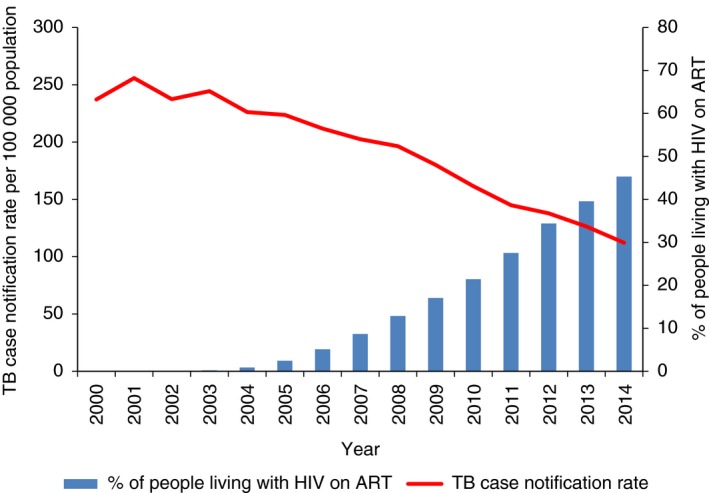
Correlation between tuberculosis case notification rates and coverage of antiretroviral therapy in Malawi, 2000–2014. TB, Tuberculosis; ART, antiretroviral therapy.
Discussion
This study shows the rise and fall of TB in Malawi over the 30‐year period from 1985 to 2014. The rise of TB observed between 1985 and 2003 (for all types and categories of TB) has been well‐documented in many other sub‐Saharan African countries faced with large and growing HIV epidemics 14. The start of the continuous decline in TB case notifications in Malawi began in 2005, 1 year after national scale‐up of ART had started. Swaziland has also observed and reported a decline in all TB case notifications in the last few years, although this trend started later in the ART scale‐up phase compared with Malawi 15. Similar to that observed in Malawi, the decline in cases in Swaziland was particularly noted for smear‐negative PTB. Interestingly, there was no significant reduction of relapse TB in Swaziland, while a marked decline for this category of TB was seen in Malawi.
The strengths of this study are the comprehensive national TB reports extending back to 1985, the well‐accepted use of Spectrum software to derive national HIV epidemiological projections and the excellent and well‐recognised quality of data of ART scale‐up in the country 16. However, there were several limitations. First, although we had projected HIV data from 1970, we did not use TB data prior to 1985 as this was before the roll‐out of DOTS in the country and we therefore cannot vouch for the reliability or accuracy of TB case notifications at that time. Second, the study was dependent on TB case notifications and these may have been affected by laboratory performance of smear microscopy over the 30 years as well as the recognised difficulties in a resource‐poor country like Malawi in establishing accurate diagnoses of smear‐negative PTB and EPTB 17, 18. Third, Malawi's ART programme extends access to treatment to all Malawian and other nationals living in the country, while the NTP only includes Malawians living in the country (foreigners are recorded in separate registers and not reported on). That being the case, foreigners reportedly account for only 1% of TB cases in one study 19. Finally, the increase and decrease in TB case notifications over the 30 years may have occurred as a result of other factors such as socio‐economic status of the population, the quality of TB diagnosis or isoniazid preventive therapy. However, although there have been plans to do so, there has been no significant scale‐up of isoniazid preventive therapy over the last decade.
While causality cannot be proven in an ecological study such as this, the decline in the burden of TB is likely to be due to the protective effects of ART against TB. A systematic review and meta‐analysis of 11 studies involving cohorts of PLHIV from around the world has shown that ART significantly reduces rates of TB across a broad range of CD4 strata by a factor of 65%, this being most noticeable in patients with advanced HIV disease or low CD4 counts 20. In sub‐Saharan Africa, smear‐negative PTB is particularly associated with HIV infection and advanced immune suppression, and it is with this type of disease in Malawi that the most marked changes have occurred over the last 30 years. Recurrent TB is also a feature of HIV co‐infection, with a decline in cases also observed in a rural district of Malawi several years ago in association with the scale‐up of ART 21. Recurrent TB may be due to reactivation of Mycobacterium tuberculosis which has not been cleared by the previous treatment or due to a new infection with another strain of Mycobacterium tuberculosis as a result of new exposure and transmission in the community. The immune status of the host, and particularly the CD4 cell count, is crucial for maintaining protection against TB whether this is due to preventing reactivation or preventing progression from new infection to active disease. Given the rationale for how ART works by immune reconstitution with elevation of CD4 cell counts, the effects of ART in reducing recurrent TB are therefore not surprising as over the last 10 years the proportion of HIV‐infected TB patients started on ART at the start of TB treatment has gradually increased to exceed 80% 14.
There are a number of important programmatic implications from this study. First, Malawi needs to continue with scaling up ART to reach 100% of its HIV‐infected population not only as a means of controlling the HIV epidemic but also the TB epidemic. The joint United Nations Programme on HIV/AIDS (UNAIDS) launch of the new 90‐90‐90 treatment targets for HIV should provide countries like Malawi with the necessary impetus to move in this direction 22. These targets specify that by 2020, 90% of individuals living with HIV will be diagnosed and know their HIV status, 90% of people with diagnosed HIV infection will receive sustained ART, and 90% of those on ART will be virally suppressed. These targets are important because they focus on the majority of PLHIV learning their HIV status and they advocate for early treatment by indicating that the majority of those starting ART are alive and retained on therapy sometime in the future. In this regard, Malawi pioneered the Option B+ approach in 2011 whereby HIV‐infected women who are pregnant or breastfeeding can start ART for life regardless of CD4 cell count or WHO clinical stage 23, and, in line with recent WHO guidance on when to start ART 24, the country is preparing to start all HIV‐infected people on ART in the near future. Recent studies have highlighted the TB preventive benefits of early start of ART, emphasising that delays in ART initiation when CD4 cell counts have dropped <200 cells/μl may result in long‐term immune dysfunction and persistent increased risk for TB 25, 26.
Second, in terms of TB prevention, ART is not enough on its own; in high HIV–TB burden settings, the risk of tuberculosis in HIV–TB‐infected individuals on ART never decreases to a level seen in patients without HIV infection 27. Thus, other interventions may be necessary such as isoniazid preventive therapy. Observational studies from Brazil 28, South Africa 29 and a trial in Botswana 30, all suggest that sequential or concurrent use of ART and isoniazid preventive therapy have additive effects in reducing the risk of active TB. A randomised placebo‐controlled study in South Africa showed that isoniazid preventive therapy given for 12 months to PLHIV and on ART significantly reduced the risk of active TB by 37% 31, and results from the TEMPRANO study in Cote d'Ivoire showed similar effects of isoniazid preventive therapy when added to ART 32. Malawi needs to take these findings into consideration and think seriously about the benefits and risks of including isoniazid preventive therapy into its ART scale‐up programme.
Third, attention needs to be paid to effective implementation of currently available strategies and tools for TB control: prompt and accurate diagnosis, effective treatment started immediately after diagnosis and good infection control practices in health facilities and wherever people with HIV and TB congregate 33. WHO has launched a post‐2015 bold new patient‐centred strategy that if followed by countries could result in elimination of TB by 2050 34.
Finally, while this study shows the strong association between HIV and TB, it is important to note the growing association and important links between these two communicable diseases and non‐communicable diseases such as diabetes mellitus and hypertension 35. Communicable and non‐communicable diseases occur within the same patient populations, and as we move forward in 2016 to the new era of the Sustainable Development Goals 36, it will be vital to secure the political and programmatic commitment at international and national levels for integrated and effective action to address them.
In conclusion, this paper reports on the rise and fall of TB in Malawi between 1985 and 2014, notes the strong association between HIV infection and the scale‐up of ART and highlights ways forward to end the epidemic.
References
- 1. Communicable Diseases Cluster, WHO . TB Research: putting research into policy and practice. The experience of the Malawi National Tuberculosis Control Programme. Geneva, Switzerland: World Health Organization, 1999. WHO/CDS/CPC/TB/99.268. [Google Scholar]
- 2. World Health Organization . WHO Tuberculosis Programme. Framework for effective tuberculosis control. Geneva, Switzerland: WHO, 1994. WHO/TB/94.179. Geneva, Switzerland. [Google Scholar]
- 3. National AIDS Commission . Malawi National HIV/AIDS estimates. Technical Report 2014. Image Printing Works, Lilongwe, Malawi, 2014.
- 4. Kool HE, Bloemkolk D, Reeve PA, Danner SA. HIV seropositivity and tuberculosis in a large general hospital in Malawi. Trop Geogr Med 1990: 42: 128–132. [PubMed] [Google Scholar]
- 5. Kelly P, Burnham G, Radford C. HIV seropositivity and tuberculosis in a rural Malawi hospital. Trans R Soc Trop Med Hyg 1990: 84: 725–727. [DOI] [PubMed] [Google Scholar]
- 6. Harries AD, Maher D, Mvula B, Nyangulu D. An audit of HIV testing and HIV serostatus in tuberculosis patients, Blantyre, Malawi. Tuber Lung Dis 1995: 76: 413–417. [DOI] [PubMed] [Google Scholar]
- 7. Harries AD, Nyangulu DS, Kang'ombe C et al Treatment outcome in an unselected cohort of tuberculosis patients in relation to human immunodeficiency virus serostatus in Zomba hospital, Malawi. Trans R Soc Trop Med Hyg 1998; 92: 343–347. [DOI] [PubMed] [Google Scholar]
- 8. Kwanjana JK, Harries AD, Gausi F, Nyanagulu DS, Salaniponi FM. TB‐HIV seroprevalence in patients with tuberculosis in Malawi. Malawi Med J 2001: 13: 7–10. [Google Scholar]
- 9. Kanyerere H, Mganga A, Harries AD et al Decline in national tuberculosis notifications with national scale‐up of antiretroviral therapy in Malawi. Public Health Action 2014: 4: 113–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghys PD, Brown T, Grassly NC et al The UNAIDS Estimation and Projection Package: a software package to estimate and project national HIV epidemics. Sex Transm Infect 2004: 80(Suppl 1): i5–i9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ministry of Health . 2014 Clinical Management of HIV in Children and Adults (2nd edn), February 2014. Malawi. (Available from: www.hivunitmohmw.org) [23 June 2015]. [Google Scholar]
- 12. World Health Organization . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV infection. Recommendations for a public health approach. June 2013. [PubMed]
- 13. World Health Organization . Treatment of Tuberculosis Guidelines (4th edn), Geneva, Switzerland: WHO, 2010. WHO/HTM/TB.2009.420. [Google Scholar]
- 14. World Health Organization . Global Tuberculosis Report 2014. Geneva, Switzerland: WHO, 2014. WHO/HTM/TB/2014.08. [Google Scholar]
- 15. Haumba S, Dlamini T, Calnan M et al Declining tuberculosis notification trend associated with strengthened and expanded HIV care in Swaziland. Public Health Action 2015: 5: 103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The Global Fund for AIDS, TB and Malaria . Report for the Data Quality Audit for HIV/AIDS in Malawi (MLW‐1‐2‐G01‐H, MLW‐506‐G‐3‐H, MLW‐708‐G07‐H). Final Audit Report, October 26, 2010 (Amended April 5, 2011). Geneva, Switzerland, 2010. [Google Scholar]
- 17. Harries AD, Hargreaves NJ, Kwanjana JH, Salaniponi FM. Clinical diagnosis of smear‐negative pulmonary tuberculosis: an audit of diagnostic practice in hospitals in Malawi. Int J Tuberc Lung Dis 2011: 5: 1143–1147. [PubMed] [Google Scholar]
- 18. Harries AD, Hargreaves NJ, Kwanjana JH, Salaniponi FM. The diagnosis of extrapulmonary tuberculosis in Malawi. Trop Doct 2003: 33: 7–11. [DOI] [PubMed] [Google Scholar]
- 19. Salaniponi FM, Gausi FK, Chimzizi RB, Harries AD. The missing cases of tuberculosis in Malawi: the contribution from cross‐border registrations. Trans R Soc Trop Med Hyg 2004: 98: 251–254. [DOI] [PubMed] [Google Scholar]
- 20. Suthar AB, Lawn SD, del Amo J et al Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta‐analysis. PLoS Med 2012: 9: e1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zachariah R, Bemelmans M, Akesson A et al Reduced tuberculosis case notification associated with scaling up antiretroviral treatment in rural Malawi. Int J Tuberc Lung Dis 2011: 15: 933–937. [DOI] [PubMed] [Google Scholar]
- 22. UNAIDS . 90‐90‐90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 23. Schouten EJ, Jahn A, Midiani D et al Prevention of mother‐to‐child transmission of HIV and the health‐related Millennium Development Goals: time for a public health approach. Lancet 2011: 378: 282–284. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization . Guideline on When to Start Antiretroviral Therapy and on Pre‐Exposure Prophylaxis for HIV. Geneva, Switzerland: WHO, September 2015. [PubMed] [Google Scholar]
- 25. Grinsztejn B, Hosseinipour MC, Ribaudo HJ et al: Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV‐1 infection: results from the phase3 HPTN 052 randomised controlled trial. Lancet Infect Dis 2014; 14: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collins SE, Jean Juste MA, Koenig SP et al CD4 deficit and tuberculosis risk persist with delayed antiretroviral therapy: 5‐year data from CIPRA HT‐001. Int J Tuberc Lung Dis 2015: 19: 50–57. [DOI] [PubMed] [Google Scholar]
- 27. Gupta A, Wood R, Kaplan R, Bekker L‐G, Lawn SD. Tuberculosis incidence rates during 8 years of follow‐up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One 2012: 7: e34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Golub JE, Saraceni V, Cavalcante SC et al The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV‐infected patients in Rio de Janeiro, Brazil. AIDS 2007: 21: 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Golub JE, Pronyk P, Mohapi L et al Isoniazid preventive therapy, HAART and tuberculosis risk in HIV‐infected adults in South Africa: a prospective cohort. AIDS 2009: 23: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Samandari T, Agizew TB, Nyirenda S et al 6‐month versus 36‐month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double‐blind, placebo‐controlled trial. Lancet 2011: 377: 1588–1598. [DOI] [PubMed] [Google Scholar]
- 31. Rangaka MX, Wilkinson RJ, Boulle A et al Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double‐blind, placebo‐controlled trial. Lancet 2014: 384: 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The Temprano ANRS 12136 Study Group . A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015: 373: 808–822. [DOI] [PubMed] [Google Scholar]
- 33. Frieden TR, Brudney KF, Harries AD. Global tuberculosis: perspectives, prospects, and priorities. JAMA 2014: 312: 1393–1394. [DOI] [PubMed] [Google Scholar]
- 34. World Health Organization . The END TB Strategy. Global Strategy and Targets for Tuberculosis Prevention, Care and Control after 2015. Geneva, Switzerland: WHO, 2014. (Available from: www.who.int/tb/post2015_TBstrategy.pdf) [27 June 2015]. [Google Scholar]
- 35. Harries AD, Kumar AMV, Satyanarayana S et al Communicable and non‐communicable diseases: connections, synergies and benefits of integrating care. Public Health Action 2015: 5: 156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. United Nations . Transforming our World: the 2030 Agenda for Sustainable Development, 2015. (Available from: http://sustainabledevelopment.un.org/post2015/transformingourworld) [20 October 2015].


