Abstract
Aims
Little is known about the effects of phytochemicals against Borrelia sp. causing Lyme disease. Current therapeutic approach to this disease is limited to antibiotics. This study examined the anti‐borreliae efficacy of several plant‐derived compounds and micronutrients.
Methods and Results
We tested the efficacy of 15 phytochemicals and micronutrients against three morphological forms of Borrelia burgdoferi and Borrelia garinii: spirochetes, latent rounded forms and biofilm. The results showed that the most potent substances against the spirochete and rounded forms of B. burgdorferi and B. garinii were cis‐2‐decenoic acid, baicalein, monolaurin and kelp (iodine); whereas, only baicalein and monolaurin revealed significant activity against the biofilm. Moreover, cis‐2‐decenoic acid, baicalein and monolaurin did not cause statistically significant cytotoxicity to human HepG2 cells up to 125 μg ml−1 and kelp up to 20 μg ml−1.
Conclusions
The most effective antimicrobial compounds against all morphological forms of the two tested Borrelia sp. were baicalein and monolaurin. This might indicate that the presence of fatty acid and phenyl groups is important for comprehensive antibacterial activity.
Significance and Impact of the Study
This study reveals the potential of phytochemicals as an important tool in the fight against the species of Borrelia causing Lyme disease.
Keywords: biofilm, Borrelia sp., cysts, Lyme disease, phytochemicals, spirochetes
Introduction
Lyme disease (LM, Lyme borreliosis) is a zoonotic infectious disease that has become a worldwide threat for humans and animals (Burgdorfer et al. 1982; Dryden and Hodgkins 2010; Johnson et al. 2014). This disease is transmitted by ticks of the genus Ixodes (Benach et al. 1983; Steere et al. 1983), which harbor the Borrelia sp. bacteria and spread them when feeding on reservoir animals such as reptiles, birds, and small and large mammals (Benach et al. 1983; Salkeld and Lane 2010; Richter et al. 2011; Stricker and Johnson 2011; Norte et al. 2013). These pathogens are now documented in the northeastern, mid‐Atlantic, north‐central and western Pacific coasts of the United States, as well as in Europe, Asia, Africa, and Australia (Burgdorfer and Keirans 1983; Bacon et al. 2008; Hubalek 2009). People of all ages and both genders are equally at risk, although the highest rates of infection have been reported in children ages 10–14 and in adults ages 45 and older (Strle et al. 2013; Robinson 2014). The newest estimates, which are calculated based on diagnostic test results and insurance records, indicate that the number of Lyme disease cases in the United States alone reaches 300 000 each year. Based on physician reporting, it is estimated to be ca. 30 000 new cases per year. There are unreported cases, however, that are not reflected in the statistics (Johnson et al. 2014; Stricker and Johnson 2014).
The causative agent of LM was established by Willy Burgdorfer who identified Borrelia burgdorferi as a pathogenic factor (Burgdorfer 1991; Stricker and Johnson 2014). Today we know that two more species, Borrelia afzelii and Borerlia garinii, are involved as well (Lovrich et al. 1994; Rudenko et al. 2011). Borrelia are micro‐aerophilic and slow‐growing spirochetal bacteria classified in 36 known species with unknown numbers of genomic strains. Although the clinical symptoms of infection with different species may vary, common indicators have been identified (Vanousova and Hercogova 2008; Murray and Shapiro 2010). Early signs of LM occur within a month after tick's bite and are indicated by skin lesion (described by some as a redness or rash with a bull's eye pattern). This lesion called erythema migrans is one of the hallmarks of Lyme disease. It is often accompanied by fever, fatigue, body aches and headache (Steere 1989; Burgdorfer 1991; Shapiro 2014). Approximately 4–6 weeks or months after the first symptoms appear, the systemic signs may surface and are much more severe with musculoskeletal indications, neurologic problems, cardiac abnormalities, and eye and liver inflammation (Burgdorfer 1991; Stanek et al. 2012; Shapiro 2014). Interestingly, not all Lyme patients have all the symptoms. Asymptomatic infection has been observed, but it occurs in <10% of patients in the United States (Steere et al. 2003; Stricker and Phillips 2003).
A few antimicrobial compounds (i.e. mainly synthetic antibiotics) have been scientifically examined against Borrelia sp. (Brorson and Brorson 2006; Brorson et al. 2009; Sapi et al. 2011; Kadam et al. 2014). To date, there are several FDA‐approved antibiotic‐related treatments used as a primary approach (guideline) for patients (Klempner et al. 2013; Cameron et al. 2014; Shapiro 2014). For early stages of Lyme disease, administration of doxycycline is generally the first choice; however, this antibiotic cannot be taken by children and pregnant or breastfeeding women (Loewen et al. 1999; Hansmann 2009). Other alternatives include amoxicillin, and cefuroxime (Loewen et al. 1999; Stricker et al. 2004; Larkin 2008; Hansmann 2009; Shapiro 2014), and for late stages of the disease ceftriaxone or cefotaxime (Maraspin et al. 2011; Delong et al. 2012) are administrated. Unfortunately, continued antibiotic treatment is not recommended as its long‐term effectiveness has not been observed or proven (Delong et al. 2012; Stanek et al. 2012). The increasing trend with new and relapsing Lyme disease cases was noted and attributed mainly to inadequate prevention, ineffective therapy and/or bacterial persistency (Straubinger et al. 1997; Bockenstedt et al. 2002; Peltomaa et al. 2003; Hodzic et al. 2008; Feng et al. 2014).
The efficacy of natural plant‐derived chemicals and micronutrients, including a variety of essential oils, vitamins, and plant metabolites, as anti‐borreliaea agents is still not well known. (Zhang et al. 2013; Morrison and Hergenrother 2014). To date, only a few plant metabolites have undergone extensive scientific evaluation for antimicrobial activity against Borrelia sp., e.g. grape seed extract and teasel root extract (Brorson and Brorson 2007; Liebold et al. 2011). There is an enormous potential in exploring anti‐borreliae properties of natural substances as they are generally ascribed as safe with promising outcomes, and they might be effective as a substitution or adjunct treatment to the standard antibiotic based therapies (Patil and Saraogi 2014; Takeuchi et al. 2014).
In this study, we tested the efficacy of 15 phytochemicals and micronutrients against different forms of B. burgdorferi and B. garinii (i.e. spirochetes, rounded forms and biofilm). These compounds were selected from naturally occurring (nonsynthetic) and plant‐derived substances with potential anti‐bacterial properties that have not yet been scientifically evaluated against Borrelia sp. Also, the selection was limited to compounds with validated safety in in vivo studies and with chemical structure suggesting effectiveness against all morphological forms of Borrrelia sp. To our knowledge this is the first attempt to provide such a comprehensive evaluation of naturally occurring compounds against Borrelia sp., which could advance our knowledge about their antibacterial properties, and help in the development of new approaches or improve already existing treatments for Lyme disease.
Materials and methods
Test compounds
The following compounds, with purity between 90–98% according to the manufacturer, were obtained from Sigma (St. Louis, MO): hydroxytyrosol, baicalein, cis‐2‐decenoic acid, morin, oenin, vitamin D3 (1,25‐dihydroxycholecalciferol) and vitamin C (ascorbic acid). The following compounds, with purity between 97–99% according to the manufacturer, were purchased from Tocris Bioscience (Bristol, UK): rosmarinic acid, kaempferol, piceatannol, rottlerin, luteolin and fisetin. Other reagents used in this study were organic kelp with standardized iodine content (i.e. 150 μg ml−1 as 100% Daily Value) purchased from World Organic Ltd. (Auckland, New Zealand), and monolaurin (Lauricidin®) purchased from Med‐Chem Laboratories, Inc., (Goodyear, AZ) as a pure sn‐1 monolaurin (glycerol monolaurate) derived from coconut oil.
Preparation of test compounds for susceptibility testing
A stock solution (50–100 mg ml−1) of all compounds (depending on solubility of each substance) was prepared by suspending each test compound in absolute ethanol and sterilizing it by 0·22 μm syringe filtration. All stock solutions were stored in aluminium foil‐wrapped tubes at −20°C. As ethanol could be bactericidal, the amount of ethanol added to growth medium was kept as low as possible. A preliminary experiment determined that its content should not exceed 0·5% (v/v) (data not shown). Therefore, in the experiments the final concentration of ethanol in growth medium was kept below 0·4% (v/v). The appropriate amounts of each stock solution were added to 1·8 ml sterile screw‐cap test tubes containing 1 ml of BSH complete medium to yield final concentrations of 50–1000 μg ml−1 for all compounds. Ethanol at 0·1–0·4% (v/v) was applied as negative control. Doxycycline at 5–500 μg ml−1 concentration range was used as positive control.
Test micro‐organisms
Two Borrelia species, B. burgdorferi and B. garinii, were tested in their three morphological forms: spirochetes, rounded forms, and biofilm. As Borrelia sp. are aero‐tolerant anaerobes, they were cultured stationary in the presence of 5% CO2 in screw‐capped tubes. Low passage isolates of the B31 strain of B. burgdorferi and CIP103362 strain of B. garinii were obtained from the American Type Culture Collection (Manassas, VA). Stocks of both species were cultured in commonly applied conditions, i.e. Barbour‐Stoner‐Kelly H (BSK‐H) medium, supplemented with 6% rabbit serum (Sigma, St. Louis, MO) without antibiotics at 33°C with 5% CO2, in sterile screw‐capped 15 ml polypropylene test tubes with or without gentle shaking. B31 strain is an isolate from Ixodes dammini, whereas CIP103362 strain is an isolate from Ixodes ricinus.
Preparation of test micro‐organisms for susceptibility testing
Borrelia burgdorferi and B. garinii strains were prepared for testing as described by Sapi, et al. (Sapi et al. 2011). Briefly, the strains were activated from original cryobank vials and inoculated into 10 ml BKS‐H complete medium, and maintained at 33°C. For generation of homogeneous cultures (i.e. having only spirochetal form) of B. burgdorferi or B. garinii, spirochetes were inoculated and maintained at 33°C in a shaking incubator at 250 rev min−1, so there was no biofilm formation (Sapi et al. 2011). To generate biofilm‐like colonies of B. burgdorferi or B. garinii, the spirochetes were inoculated in four‐well chambers (BD Biosciences, Sparks, MD) coated with rat‐tail collagen type I and incubated for 1 week without shaking.
Evaluation of bacteriostatic effect of test compounds on test micro‐organisms
Growth of B. burgdorferi and B. garinii was tested using a macro‐dilution method according to Sapi, et al. (Sapi et al. 2011). Briefly, 1·8 ml sterile screw‐capped test tubes containing 1 ml BSK‐H medium, supplemented with the test compound of interest were inoculated with 2 × 106 spirochetes ml−1 of the homogenous bacterial suspension. The tubes were then incubated at 33°C and growth was monitored at regular intervals for up to 72 h. The entire experiment was repeated three times for each bacteria strain and each concentration of the tested compounds. Control cultures were treated with ethanol (0·1–0·4 v/v) alone or doxycycline (5–500 μg ml−1). Bacterial growth was assessed by a bacterial Petroff‐Hausser counting chamber for up to 72 h of incubation using dark field microscopy (direct cell counting) as a standard procedure.
Evaluation of bactericidal effect of test compounds on test micro‐organisms
Bactericidal effect of tested compounds was examined using a fluorescence method according to Sapi, et al. (Sapi et al. 2011). Briefly, 2 × 106 spirochetes ml−1 of the homogenous bacterial suspension was inoculated into each 1·8 ml sterile screw‐capped test tube containing 1 ml BSK‐H medium, supplemented with the test compound of interest. Control cultures were treated with ethanol (0·1–0·4 v/v) alone or doxycycline (5–500 μg ml−1). The tubes were then incubated at 33°C and viability was monitored at regular intervals for up to 72 h. The entire experiment was repeated three times for each strain and each concentration. The susceptibility of rounded forms to the test compound was then assessed up to 72 h by LIVE/DEAD® BacLight™ Bacterial Viability Assay using a fluorescence microscope (Nikon, Eclipse E600, Melville, NY) and the percentage of live (green) and dead (red) B. burgdorferi and B. garinii rounded forms was calculated.
Evaluation of test compounds on bacterial biofilm
The qualitative effect of the test compounds against biofilm‐like colonies of B. burgdorferi and B. garinii was evaluated using the commonly used crystal violet (CV) staining method, according to Sapi, et al. (Sapi et al. 2011). Briefly, 1 × 107 spirochetes ml−1 of homogeneous bacterial suspension were inoculated into collagen type I‐coated four‐well chambers and incubated for 1 week. Subsequently, biofilm‐like colonies were treated with various concentrations of the test compounds. Control wells were treated with ethanol (0·1–0·4 v/v) alone or doxycycline (5–500 μg ml−1). All chambers were then incubated at 33°C for up to 72 h. Next, for quantitative analysis, all wells were fixed with 500 μl of cold methanol‐formalin (1 : 1) for 30 min. and stained with 1 ml of CV (0·1%) for 10 min. The biofilms were washed carefully three times with 1× PBS (phosphate‐buffered saline), and 1 ml of methanol was added to each well to extract a dye which was measured at 595 nm (Molecular Device, Spectra Max 340, Sunnyvale, CA). Each experiment was repeated three times for each strain and each compound concentration. For qualitative analysis, all wells were fixed with 500 μl of cold formalin‐acetic acid mixture for 20 min. followed by staining with 200 μl of 2× BacLight staining mixture for 15 min. in the dark, according to the manufacturer's recommendation. Pictures were immediately taken from untreated (control) and treated mounted slides using a fluorescence microscope (Nikon, Eclipse E600). Immunofluorescence staining with primary antibodies against B. burgdorferi (Abcam, Cambridge, MA) and B. garinii (Santa Cruz, Santa Cruz, CA) with AlexaFluor‐488 as a secondary antibody was performed accordingly to manufactures' recommendation respectively.
Evaluation of cytotoxicity of tested compounds on human cells
Cells viability was assessed using MTT assay according to the manufacturer's protocol. Briefly, human HepG2 cell line was plated in 48‐well plates at 1 × 105 cells per well in the DMEM containing 10% FBS. After 24 h, the medium was replaced with the same medium supplemented with the following compounds: cis‐2‐decenoic acid (125–1000 μg ml−1), monolaurin (125–1000 μg ml−1), baicalein (125–1000 μg ml−1), and kelp (iodine) (5–50 μg ml−1). After 72 h of treatment, cells viability was measured at 570 nm, using an ELISA reader (Molecular Device, Spectra Max 340). The experiment was repeated four times for each strain and each compound concentration.
Statistical analysis
All data are presented as means ± SD (n = 3). The Student's two‐tailed t test was used to determine statistically significant differences set at 0·05 levels. Statistical analysis was performed using graphpad software (La Jolla, CA).
Results
Effect of tested compounds on spirochetes of Borrelia sp
The effect of 15 selected natural compounds on the growth of spirochete forms of B. burgdorferi (B31 strain) and B. garinii (CIP103362 strain) was examined and presented as the minimal inhibitory (MIC) and the minimal bactericidal (MBC) concentrations in Table 1. The results show that all compounds expressed anti‐spirochetal activity with MIC values ranging from 50 to 250 μg ml−1 for phytochemicals, and from 0·0005 to 35 μg ml−1 for the micronutrients. The MBC values oscillated between 200 and 500 μg ml−1 for phytochemicals, and from 0·002 to 88 μg ml−1 for the micronutrients. The MIC values for fisetin, kaemferol, rosmarinic acid, baicalein, monolaurin, morin, piceatannol, rottlerin, vitamin D3 and kelp (iodine) revealed their anti‐spirochetal potential at the same concentrations in both tested species of Borreli; whereas, other substances such as hydroxytyrosol, oenin, cis‐2‐decenoic acid, luteolin and vitamin C were effective at lower concentrations against B. burgdorferi and higher concentrations against B. garinii. The MBC values of all tested compounds corresponded to each other for both tested Borrelia sp. Antibiotic doxycycline was used as positive control with the MIC value established as 25 μg ml−1 and the MBC value as 200 μg ml−1 for B. burgdorferi and 250 μg ml−1 for B. garinii. These values were in agreement with those reported by Sapi, et al. (Sapi et al. 2011). Kinetic growth and viability of untreated spirochetes of both tested species of Borrelia was not affected up to 72 h (Fig. 1).
Table 1.
Antibacterial activity of 15 tested compounds against spirochete form of Borrelia burgdorferi B31 and Borrelia garinii CIP103362
| Tested compound | B. burgdorferi | B. garinii | ||
|---|---|---|---|---|
| MIC (μg ml−1) | MBC (μg ml−1) | MIC (μg ml−1) | MBC (μg ml−1) | |
| Hydroxytyrosol | 50 | 250 | 100 | 250 |
| Fisetin | 125 | 250 | 125 | 250 |
| Kaemferol | 200 | 300 | 200 | 300 |
| Oenin | 75 | 200 | 100 | 200 |
| Cis‐2‐decenoic acid | 125 | 250 | 250 | 250 |
| Rosmarinic acid | 150 | 250 | 150 | 250 |
| Luteolin | 125 | 250 | 150 | 250 |
| Baicalein | 150 | 250 | 150 | 250 |
| Monolaurin | 100 | 250 | 100 | 250 |
| Morin | 100 | 250 | 100 | 250 |
| Piceatannol | 250 | 500 | 250 | 500 |
| Rottlerin | 125 | 250 | 125 | 250 |
| Vitamin D3 | 0·0005 | 0·001 | 0·0005 | 0·001 |
| Vitamin C | 35 | 88 | 88 | 88 |
| Kelp (Iodine) | 5 | 15 | 5 | 15 |
| Doxycycline | 25 | 200 | 25 | 250 |
MIC, minimal inhibitory concentration; MBC, minimal bactericidal concentration.
Figure 1.
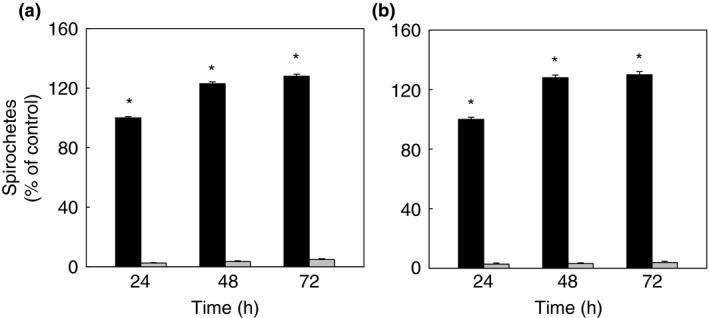
Kinetic evaluation of growth of live/dead untreated spirochetes of Borrelia burgdorferi B31 strain (a) and Borrelia garinii CIP103362 strain (b) monitored up to 72 h. Live spirochetes (■), dead spirochetes ( ), *&!blank;P ≤ 0·001.
), *&!blank;P ≤ 0·001.
Effect of tested compounds on rounded forms of Borrelia sp
The bactericidal effects of 15 selected natural compounds on latent rounded forms of B. burgdorferi (B31 strain) and B. garinii (CIP103362 strain) were examined and presented as LD50 values in Table 2. The results show that none of the tested compounds could eliminate rounded forms in 90–99%, up to their maximum tested concentration (i.e. 1000 μg ml−1). The MBC determination method is a very rigorous requirement even for many antibiotics. However, compounds such as hydroxytyrosol, cis‐2‐decenoic acid, baicalein and monolaurin were able to induce dead of latent rounded forms at LD50 values between 300 and 500 μg ml−1, and at 20 μg ml−1 for kelp (iodine). Moreover, their bactericidal potential towards rounded forms was observed at the same concentrations for both tested species of Borrelia. Doxycycline was used as positive control in this experiment. This antibiotic could not eliminate rounded forms in 90–99% or achieve LD50 level, even at the maximum tested concentration of 500 μg ml−1. These values stay in agreement with those reported by Sapi, et al. (Sapi et al. 2011).
Table 2.
Antibacterial activity of 15 tested compounds against latent rounded forms of Borrelia burgdorferi B31 and Borrelia garinii CIP103362
| Tested compound | B. burgdorferi | B. garinii |
|---|---|---|
| LD50 (μg ml−1) | LD50 (μg ml−1) | |
| Hydroxytyrosol | 300 | 300 |
| Fisetin | NS | NS |
| Kaemferol | NS | NS |
| Oenin | NS | NS |
| Cis‐2‐decenoic acid | 500 | 500 |
| Rosmarinic acid | NS | NS |
| Luteolin | 200 | NS |
| Baicalein | 350 | 350 |
| Monolaurin | 300 | 300 |
| Morin | NS | NS |
| Piceatannol | NS | NS |
| Rottlerin | NS | NS |
| Vitamin D3 | NS | NS |
| Vitamin C | NS | NS |
| Kelp (Iodine) | 20 | 20 |
| Doxycycline | NS | NS |
LD50, lethal dose causing 50% of killing; NS, not susceptible at the maximal tested concentration.
Antibacterial potential of tested compounds on Borrelia sp. biofilm
The effect of 15 selected natural compounds on biofilm formed by B. burgdorferi (B31 strain) and B. garinii (CIP103362 strain) are presented on Fig. 2. The results show that five compounds such as baicalein (≥500 μg ml−1), luteolin (≥200 μg ml−1), monolaurin (≥500 μg ml−1), cis‐2‐decenoic acid (≥500 μg ml−1) and kelp (iodine) (≥20 μg ml−1), could reduce biofilm‐like colonies formed by B. burgdorferi (B31 strain). Only two compounds, baicalein (≥500 μg ml−1) and monolaurin, (≥500 μg ml−1), were effective against biofilm formed by B. garinii (CIP103362 strain). Baicalein and monolaurin reduced B. burgdorferi biofilm‐like colonies by approx. 30–60% and B. garinii by approx. 40–60%. In addition, kelp (iodine), cis‐2‐decenoic acid and luteolin significantly reduced biofilm‐like colonies formed by B. burgdorferi, but displayed only a decreasing tendency towards biofilm formed by B. garinii. We observed that biofilm‐like colonies formed by B. burgdorferi appeared as loose in consistency and smaller compared to those seen in control. However, biofilm formed by B. garinii treated with monolaurin or baicalein looked similar to the control except it was more detachable (Fig. 3). The antibiotic doxycycline (positive control) reduced biofilms formed by both tested Borrelia sp. by about 40%. These results were in agreement with those reported by Sapi, et al. (Sapi et al. 2011).
Figure 2.
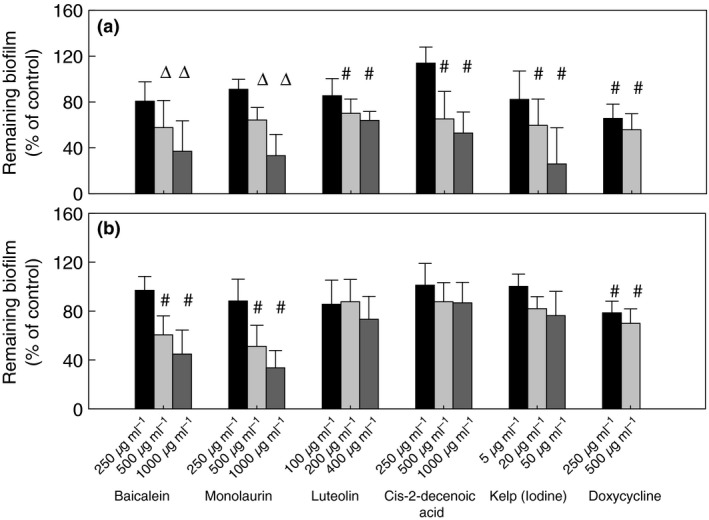
Dose‐dependent estimation of remaining biofilm of Borrelia burgdorferi B31 strain (a) and Borrelia garinii CIP103362 strain (b) after 72 h of post‐treatment with phytochemicals and antibiotic doxycycline presented as a quantitative examination; #P ≤ 0·05, ∆P ≤ 0·01.
Figure 3.
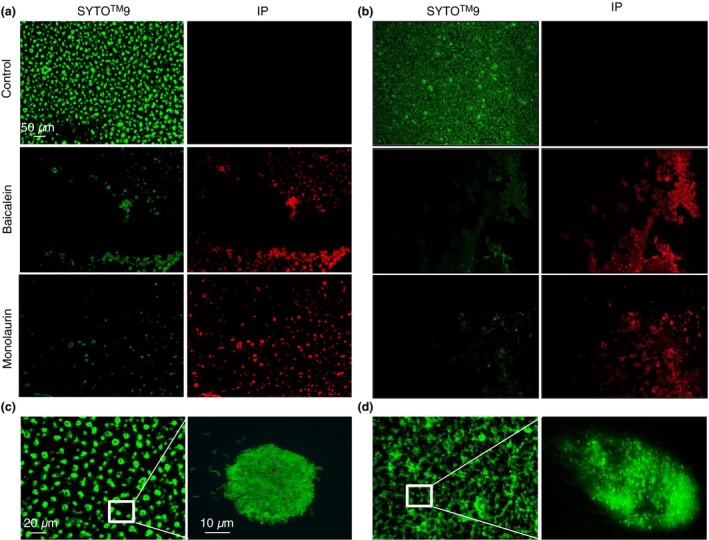
Evaluation of untreated (control) and treated with Baicalein (500 μg ml−1) or Monolaurin (500 μg ml−1) biofilm of Borrelia burgdorferi B31 strain (a) and Borrelia garinii CIP103362 strain (b) after 72 h as a qualitative measurement using LIVE/DEAD BacLight staining; green – live biofilm, red – dead biofilm, images taken at 4 × magnification. Immunofluorescence staining with primary antibodies against untreated B. burgdorferi B31 strain (c) and B. garinii CIP103362 strain (d) after 72 h using AlexaFluor‐488 as a secondary antibody respectively; images taken at 10× (right) and 63× (left) magnification.
Bactericidal kinetic of tested compounds
We evaluated the kinetic of the bactericidal effect of cis‐2‐decenoic acid, baicalein, monolaurin and kelp (iodine) as these compounds were most effective in eliminating spirochetes and latent rounded forms of both tested Borrelia sp. The results presented in Fig. 4a,b show that these phytochemicals acted in a time‐dependent manner in decreasing spirochete forms of B. burgdorferi and B. garinii. A time‐dependent killing effect on their latent rounded forms was also observed that oscillated between 20–25% after 24 h, 30–40% after 48 h and 50–55% after 72 h of exposure, compared to controls (Fig. 4c,d).
Figure 4.
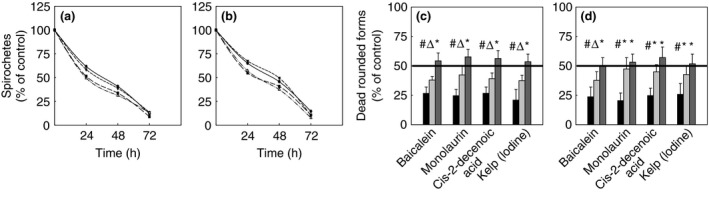
Kinetic evaluation of bactericidal effect of phytochemicals against the spirochetes (a and b) and rounded forms (c and d) of Borrelia burgdorferi B31 strain (a and c) and Borrelia garinii CIP103362 strain (b and d) monitored up to 72 h. (a and b) All data are statistically significant; tested compounds: 250 μg ml−1 Baicalein (▼), 250 μg ml−1 Monolaurin (∆), 250 μg ml−1 Cis‐2‐decenoic acid (●), 20 μg ml−1 Kelp (Iodine) (□). (c and d) LD50 marked with the solid line; #P ≤ 0·05, ∆P ≤ 0·01, *P ≤ 0·001; tested compounds: 350 μg ml−1 Baicalein, 300 μg ml−1 Monolaurin, 500 μg ml−1 Cis‐2‐decenoic acid, 20 μg ml−1 Kelp (Iodine); 24 h (■), 48 h ( ), 72 h (
), 72 h ( ).
).
Cytotoxicity
Cytotoxic evaluation results presented in Fig. 5 show that cis‐2‐decenoic acid, baicalein and monolaurin did not display significant cytotoxicity towards human HepG2 cells up to 125 μg ml−1 and caused about 30% decrease in cells survival at 250 μg ml−1. Their 50% of cytotoxic concentration (CC50) was found to be near 500 μg ml−1. Kelp (iodine) was nontoxic at 20 μg ml−1 and 50% of cytotoxic concentration (CC50) at 50 μg ml−1 (Fig. 5). For comparison, baicalein and monolaurin have shown to be effective against all morphological forms of both tested Borrelia sp. (Fig. 6).
Figure 5.
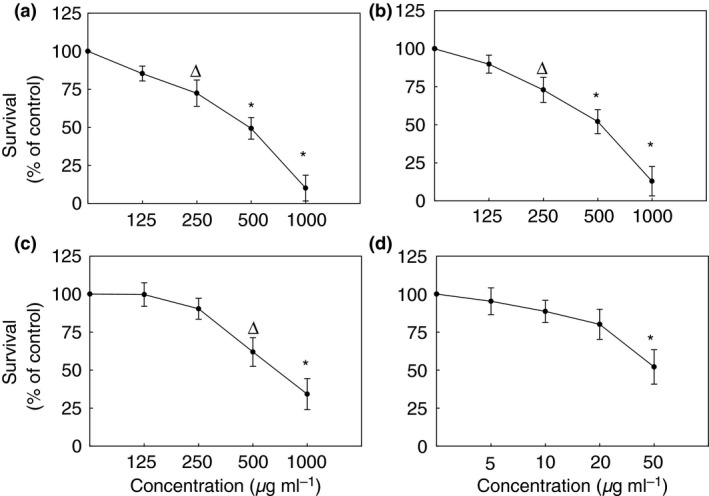
Dose‐dependent cytotoxic effect of phytochemicals on HepG2 cells assessed by MTT assay after 72 h; #P ≤ 0·05, ∆P ≤ 0·01, *P ≤ 0·001. Tested compounds: 125–1000 μg ml−1 Baicalein (a), 125–1000 μg ml−1 Monolaurin (b), 125–1000 μg ml−1 Cis‐2‐decenoic acid (c), 5–50 μg ml−1 Kelp (Iodine) (d).
Figure 6.
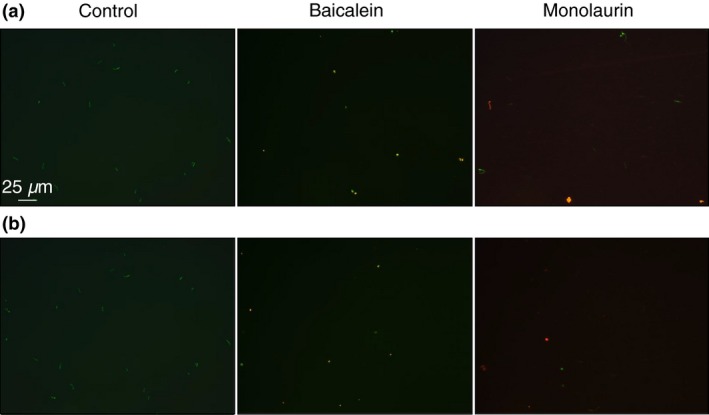
Evaluation of untreated (control) and treated with Baicalein (350 μg ml−1) or Monolaurin (300 μg ml−1) Borrelia burgdorferi B31 strain (a) and Borrelia garinii CIP103362 strain (b) after 72 h as a qualitative measurement by fluorescence microscope using LIVE/DEAD BacLight staining; green fluorescence – live organisms (spirochetes and rounded forms), red fluorescence – dead organisms (spirochetes and rounded forms). Merged images taken at 20× magnification.
Discussion
Our study demonstrates the in vitro susceptibility of different morphological forms of B. burgdorferi (prevalent in North America), as well as B. garinii (prevalent in Europe), to various nonsynthetic plant‐derived compounds and micronutrients. It has been suggested that to successfully eliminate Borrelia sp., the antimicrobial agents ought to be effective against their vegetative and viable atypical (latent) forms (i.e. rounded forms and biofilm) (Sapi et al. 2011). The vegetative form of Borrelia sp. are spirochetes that are active and motile and can survive viscous conditions in human and animal bloodstreams and penetrate tissues and cells (Groshong and Blevins 2014; Miller et al. 2014). Any hostile conditions, such as changes in temperature, pH, starvation, antibiotic exposure or threats from the host's immune system, can alter their structure converting them to the latent rounded forms (i.e. cysts, granular forms and spheroplats/CDW forms) and/or biofilm. Presence of these atypical forms might attribute to persistency of Borrelia sp. in the human body even for decades (Brorson and Brorson 1997; Alban et al. 2000; Gruntar et al. 2001; Stewart and Costerton 2001; Jefferson 2004; Murgia and Cinco 2004), although further studies are warranted. The detection of only rounded forms of B. burgdorferi in human specimens indicates the need for further studies assessing a relationship between the presence of atypical forms of Borrelia sp. and Lyme disease, in its both typical and persistent clinical forms (Lantos et al. 2014).
Our study has shown that the most promising compounds effective against spirochete and latent rounded forms of both tested Borrelia sp. were cis‐2‐decenoic acid, baicalein, monolaurin and kelp (iodine). They demonstrated bacteriostatic and bactericidal effects in a time‐dependent manner against spiral and rounded forms. We are aware that fluorescence staining used in our study may not reveal few persisting rounded forms that may grow out from a population treated with the ‘drug of choice’; however, this would be relatively a small population. At the same time, these compounds, except kelp (iodine), revealed mild or moderate cytotoxic effect on human HepG2 cell line at their MBCs concentrations. However, no significant cytotoxic effects were observed at their MICs concentrations. In addition, while cis‐2‐decenoic acid, luteolin, baicalein, monolaurin and kelp (iodine) were effective against biofilm‐like colonies of B. burgdorferi, only baicalein and monolaurin showed significant reduction in biofilm of B. garinii, although at 1·5–2 times higher concentrations than those needed to induce bactericidal effect. This could be due to different biofilm morphologies, as B. burgdorferi forms more a colony‐like and scattered structure and B. garinii forms a layered and very condense assembly. These morphological differences might affect biofilm penetration by various compounds or signal different pathophysiological effects. Interestingly, doxycycline was effective against the spirochete form of both tested Borrelia sp. and displayed moderate effect against biofilm, but not against dormant rounded forms. These results correspond to findings reported by Sapi, et al. (Sapi et al. 2011).
Our results corroborate other findings on general antibacterial activity of the tested compounds and expand knowledge on their efficacy specifically against the Borrelia sp., for which there are only limited data. Several studies have shown that fatty acids and/or their derivatives, such as cis‐2‐decenoic acid and monolaurin, may play important antibacterial roles. Cis‐2‐decenoic acid is a short chain fatty acid belonging to a class of signaling molecules targeting cell membranes causing their disassembly and increased permeability. It was found to be bacteriostatic at 125–500 μg ml−1 against methicillin‐resistant Staphylococcus aureus and its biofilm, without significant cytotoxicity on human dermal fibroblasts (Jennings et al. 2012). In separate studies, Davies and Marques observed dispersion of biofilms formed by Pseudomonas aeruginosa PAO1 by using cis‐2‐decenoic acid at a native concentration of 2·5 nmol l−1. A similar effect was observed on biofilms formed by other species, including Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Streptococcus pyogenes, Bacillus subtilis, Staph. aureus and Candida albicans (Davies and Marques 2009). Another compound, monolaurin, has been shown to inhibit growth and induce biofilm dispersion of a broad spectrum of Gram‐positive and Gram‐negative bacteria at concentrations of 30–500 μg ml−1 (Schlievert et al. 1992) without cytotoxic effects on human umbilical vein endothelial cells (HUVECs) when used up to 100 μg ml−1 over a 2‐week period. Also, a study carried out by Batovska, et al., revealed antibacterial activity of monolaurin against several Gram‐positive strains such as Staphylococcus sp., Corynebacterium sp., Bacillus sp., Listeria sp. and Streptococcus sp. (Batovska et al. 2009). Monolaurin is the ester of glycerol and dodecanoic acid, a medium chain fatty acid. As a lipophilic compound it may disrupt cell membranes, and thus similar to cis‐2‐decenoic acid, it may act as a destabilizer of the biofilm matrix (Kabara et al. 1973).
Baicalein, another compound tested in our study, belongs to flavonoids with phenolic groups in their structure. Yun, et al., have indicated that baicalein's antibacterial activity against Staph. aureus may include increasing penetrability of bacterial membranes, inhibiting protein synthesis and affecting activities of succinate dehydrogenase (SDH), malate dehydrogenase (MDH) and DNA topoisomerase I and II (Yun et al. 2012). Others have demonstrated that baicalein could reverse the ciprofloxacin resistance of methicillin‐resistant Staph. aureus by possibly causing ATP deficiency (Chan et al. 2011). It can act either alone (at a concentration of 256 μg ml−1) or synergistically (at a concentration of 32 μg ml−1) with gentamicin against vancomycin‐resistant Enterococcus isolates (Chang et al. 2007). In addition, Moghaddam et al., noticed that baicalein had no cytotoxic effect on Vero cells up to 62 μg ml−1, and at 125–250 μg ml−1, it decreased the survival of these cells by 20–25% (Moghaddam et al. 2014). In additionWang et al. (2014), observed no effect of baicalein below 100 μmol l−1 on the viability of HeLa cells.
One of the best antibacterial, antifungal and antiviral agents still considered is iodine, due to its rapid penetration of phospholipid membranes and its oxidative damage to bacterial proteins, nucleotides and fatty acids (McDonnell and Russell 1999). Iodine is also reported as a relatively safe compound (York et al. 1988; Slots 2002). The study of Wichelhaus, et al., has shown the antibacterial effects of povidone‐iodine at concentrations between 1–10% against highly resistant Gram‐positive bacteria (Wichelhaus et al. 1998). Soares, et al., established the MICs for povidone‐iodine against dermatophytes isolated from patients with tinea pedis that ranged from 4 to 128 μg ml−1 (Soares and Cury 2001). In addition, Kaspar, et al. reported that a povidone‐iodine concentration below 0·2% had no significant effect on proteoglycan and DNA synthesis of chondrocytes as well as viability and proliferation of BALB3T3 fibroblasts (Kaspar et al. 2006).
In summary, this study fills the gap in scientific data regarding the efficacy of select phytochemicals against active and latent forms of B. burgdorferi and B. garinii. This comprehensive evaluation of 15 natural compounds shows that baicalein and monolaurin were effective against all morphological forms of both tested species of Borrelia, which might indicate the importance of phenyl groups and fatty acids in the anti‐borelieae activity. Their effective concentrations were within the same ranges as reported earlier for other bacterial species and showed no or moderate cytotoxicity against human HepG2 cells.
Conflict of Interest
No conflict of interest declared.
Acknowledgements
The authors thank Ms. Cathy Flowers and Waldemar Sumera for valuable input during preparation of the manuscript. Funds were provided by the nonprofit Dr. Rath Health Foundation, a separate entity from the Dr. Rath Research Institute BV. All authors are not hired by the Dr. Rath Health Foundation and the funders had no role in the study design, performance, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Alban, P.S. , Johnson, P.W. and Nelson, D.R. (2000) Serum‐starvation‐induced changes in protein synthesis and morphology of Borrelia burgdorferi . Microbiology 146, 119–127. [DOI] [PubMed] [Google Scholar]
- Bacon, R.M. , Kugeler, K.J. , Mead, P.S. and Centers for Disease, C. and Prevention (2008) Surveillance for Lyme disease–United States, 1992‐2006. MMWR Surveill Summ 57, 1–9. [PubMed] [Google Scholar]
- Batovska, D.I. , Todorova, I.T. , Tsvetkova, I.V. and Najdenski, H.M. (2009) Antibacterial study of the medium chain fatty acids and their 1‐monoglycerides: individual effects and synergistic relationships. Pol J Microbiol 58, 43–47. [PubMed] [Google Scholar]
- Benach, J.L. , Bosler, E.M. , Hanrahan, J.P. , Coleman, J.L. , Habicht, G.S. , Bast, T.F. , Cameron, D.J. , Ziegler, J.L. et al (1983) Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med 308, 740–742. [DOI] [PubMed] [Google Scholar]
- Bockenstedt, L.K. , Mao, J. , Hodzic, E. , Barthold, S.W. and Fish, D. (2002) Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi‐infected mice after antibiotic treatment. J Infect Dis 186, 1430–1437. [DOI] [PubMed] [Google Scholar]
- Brorson, O. and Brorson, S.H. (1997) Transformation of cystic forms of Borrelia burgdorferi to normal, mobile spirochetes. Infection 25, 240–246. [DOI] [PubMed] [Google Scholar]
- Brorson, O. and Brorson, S.H. (2006) An in vitro study of the activity of telithromycin against mobile and cystic forms of Borrelia afzelii . Infection 34, 26–28. [DOI] [PubMed] [Google Scholar]
- Brorson, O. and Brorson, S.H. (2007) Grapefruit seed extract is a powerful in vitro agent against motile and cystic forms of Borrelia burgdorferi sensu lato. Infection 35, 206–208. [DOI] [PubMed] [Google Scholar]
- Brorson, O. , Brorson, S.H. , Scythes, J. , MacAllister, J. , Wier, A. and Margulis, L. (2009) Destruction of spirochete Borrelia burgdorferi round‐body propagules (RBs) by the antibiotic tigecycline. Proc Natl Acad Sci USA 106, 18656–18661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer, W. (1991) Lyme borreliosis: ten years after discovery of the etiologic agent, Borrelia burgdorferi . Infection 19, 257–262. [DOI] [PubMed] [Google Scholar]
- Burgdorfer, W. and Keirans, J.E. (1983) Ticks and Lyme disease in the United States. Ann Intern Med 99, 121. [DOI] [PubMed] [Google Scholar]
- Burgdorfer, W. , Barbour, A.G. , Hayes, S.F. , Benach, J.L. , Grunwaldt, E. and Davis, J.P. (1982) Lyme disease‐a tick‐borne spirochetosis? Science 216, 1317–1319. [DOI] [PubMed] [Google Scholar]
- Cameron, D.J. , Johnson, L.B. and Maloney, E.L. (2014) Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev Anti Infect Ther 12, 1103–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, B.C. , Ip, M. , Lau, C.B. , Lui, S.L. , Jolivalt, C. , Ganem‐Elbaz, C. , Litaudon, M. , Reiner, N.E. et al (2011) Synergistic effects of baicalein with ciprofloxacin against NorA over‐expressed methicillin‐resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J Ethnopharmacol 137, 767–773. [DOI] [PubMed] [Google Scholar]
- Chang, P.C. , Li, H.Y. , Tang, H.J. , Liu, J.W. , Wang, J.J. and Chuang, Y.C. (2007) In vitro synergy of baicalein and gentamicin against vancomycin‐resistant Enterococcus. J Microbiol Immunol Infect 40, 56–61. [PubMed] [Google Scholar]
- Davies, D.G. and Marques, C.N. (2009) A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191, 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delong, A.K. , Blossom, B. , Maloney, E.L. and Phillips, S.E. (2012) Antibiotic retreatment of Lyme disease in patients with persistent symptoms: a biostatistical review of randomized, placebo‐controlled, clinical trials. Contemp Clin Trials 33, 1132–1142. [DOI] [PubMed] [Google Scholar]
- Dryden, M.W. and Hodgkins, E. (2010) Vector‐borne diseases in pets: the stealth health threat. Compend Contin Educ Vet 32, E1–E4. [PubMed] [Google Scholar]
- Feng, J. , Wang, T. , Shi, W. , Zhang, S. , Sullivan, D. , Auwaerter, P.G. and Zhang, Y. (2014) Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg Microbes Infect 3, e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groshong, A.M. and Blevins, J.S. (2014) Insights into the biology of Borrelia burgdorferi gained through the application of molecular genetics. Adv Appl Microbiol 86, 41–143. [DOI] [PubMed] [Google Scholar]
- Gruntar, I. , Malovrh, T. , Murgia, R. and Cinco, M. (2001) Conversion of Borrelia garinii cystic forms to motile spirochetes in vivo. APMIS 109, 383–388. [DOI] [PubMed] [Google Scholar]
- Hansmann, Y. (2009) Treatment and prevention of Lyme disease. Curr Probl Dermatol 37, 111–129. [DOI] [PubMed] [Google Scholar]
- Hodzic, E. , Feng, S. , Holden, K. , Freet, K.J. and Barthold, S.W. (2008) Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother 52, 1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubalek, Z. (2009) Epidemiology of lyme borreliosi. Curr Probl Dermatol 37, 31–50. [DOI] [PubMed] [Google Scholar]
- Jefferson, K.K. (2004) What drives bacteria to produce a biofilm? FEMS Microbiol Lett 236, 163–173. [DOI] [PubMed] [Google Scholar]
- Jennings, J.A. , Courtney, H.S. and Haggard, W.O. (2012) Cis‐2‐decenoic acid inhibits S. aureus growth and biofilm in vitro: a pilot study. Clin Orthop Relat Res 470, 2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L. , Wilcox, S. , Mankoff, J. and Stricker, R.B. (2014) Severity of chronic Lyme disease compared to other chronic conditions: a quality of life survey. PeerJ 2, e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabara, J.J. , Conley, A.J. and Swieczkowski, D.M. (1973) Antimicrobial action of isomeric fatty acids on group A Streptococcus. J Med Chem 16, 1060–1063. [DOI] [PubMed] [Google Scholar]
- Kadam, P. , Gregory, N.A. , Zelger, B. and Carlson, J.A. (2014) Delayed onset of the jarisch‐herxheimer reaction in doxycycline‐treated disease: a case report and review of its histopathology and implications for pathogenesis. Am J Dermatopathol 37, e68–e74. [DOI] [PubMed] [Google Scholar]
- Kaspar, D. , Schwarz, W. , Claes, L. and Ignatius, A. (2006) Study of the toxicity of povidone‐lodine for fibroblast‐like cells (BALB‐3T3) and primary human chondrocytes. Arzneimittelforschung 56, 605–611. [DOI] [PubMed] [Google Scholar]
- Klempner, M.S. , Baker, P.J. , Shapiro, E.D. , Marques, A. , Dattwyler, R.J. , Halperin, J.J. and Wormser, G.P. (2013) Treatment trials for post‐Lyme disease symptoms revisited. Am J Med 126, 665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos, P.M. , Auwaerter, P.G. and Wormser, G.P. (2014) A systematic review of Borrelia burgdorferi morphologic variants does not support a role in chronic lyme disease. Clin Infect Dis 58, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, J.M. (2008) Lyme disease in children and pregnant women. Med Health R I 91, 212. [PubMed] [Google Scholar]
- Liebold, T. , Straubinger, R.K. and Rauwald, H.W. (2011) Growth inhibiting activity of lipophilic extracts from Dipsacus sylvestris Huds. roots against Borrelia burgdorferi s. s. in vitro. Pharmazie 66, 628–630. [PubMed] [Google Scholar]
- Loewen, P.S. , Marra, C.A. and Marra, F. (1999) Systematic review of the treatment of early Lyme disease. Drugs 57, 157–173. [DOI] [PubMed] [Google Scholar]
- Lovrich, S.D. , Callister, S.M. , Lim, L.C. , DuChateau, B.K. and Schell, R.F. (1994) Seroprotective groups of Lyme borreliosis spirochetes from North America and Europe. J Infect Dis 170, 115–121. [DOI] [PubMed] [Google Scholar]
- Maraspin, V. , Ruzic‐Sabljic, E. , Pleterski‐Rigler, D. and Strle, F. (2011) Pregnant women with erythema migrans and isolation of borreliae from blood: course and outcome after treatment with ceftriaxone. Diagn Microbiol Infect Dis 71, 446–448. [DOI] [PubMed] [Google Scholar]
- McDonnell, G. and Russell, A.D. (1999) Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12, 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, K.A. , Motaleb, M.A. , Liu, J. , Hu, B. , Caimano, M.J. , Miller, M.R. and Charon, N.W. (2014) Initial characterization of the FlgE hook high molecular weight complex of Borrelia burgdorferi . PLoS ONE 9, e98338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam, E. , Teoh, B.T. , Sam, S.S. , Lani, R. , Hassandarvish, P. , Chik, Z. , Yueh, A. , Abubakar, S. et al (2014) Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci Rep 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, K.C. and Hergenrother, P.J. (2014) Natural products as starting points for the synthesis of complex and diverse compounds. Nat Prod Rep 31, 6–14. [DOI] [PubMed] [Google Scholar]
- Murgia, R. and Cinco, M. (2004) Induction of cystic forms by different stress conditions in Borrelia burgdorferi . APMIS 112, 57–62. [DOI] [PubMed] [Google Scholar]
- Murray, T.S. and Shapiro, E.D. (2010) Lyme disease. Clin Lab Med 30, 311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norte, A.C. , Lobato, D.N. , Braga, E.M. , Antonini, Y. , Lacorte, G. , Goncalves, M. , Lopes de Carvalho, I. , Gern, L. et al (2013) Do ticks and Borrelia burgdorferi s.l. constitute a burden to birds? Parasitol Res 112, 1903–1912. [DOI] [PubMed] [Google Scholar]
- Patil, U.K. and Saraogi, R. (2014) Natural products as potential drug permeation enhancer in transdermal drug delivery system. Arch Dermatol Res 306, 419–426. [DOI] [PubMed] [Google Scholar]
- Peltomaa, M. , McHugh, G. and Steere, A.C. (2003) Persistence of the antibody response to the VlsE sixth invariant region (IR6) peptide of Borrelia burgdorferi after successful antibiotic treatment of Lyme disease. J Infect Dis 187, 1178–1186. [DOI] [PubMed] [Google Scholar]
- Richter, D. , Schlee, D.B. and Matuschka, F.R. (2011) Reservoir competence of various rodents for the lyme disease Spirochete Borrelia spielmanii . Appl Environ Microbiol 77, 3565–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, S. (2014) Lyme disease in maine: a comparison of NEDSS surveillance data and maine health data organization hospital discharge data. Online J Public Health Inform 5, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko, N. , Golovchenko, M. , Grubhoffer, L. and Oliver, J.H. Jr (2011) Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis 2, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkeld, D.J. and Lane, R.S. (2010) Community ecology and disease risk: lizards, squirrels, and the Lyme disease spirochete in California, USA. Ecology 91, 293–298. [DOI] [PubMed] [Google Scholar]
- Sapi, E. , Kaur, N. , Anyanwu, S. , Luecke, D.F. , Datar, A. , Patel, S. , Rossi, M. and Stricker, R.B. (2011) Evaluation of in‐vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi . Infect Drug Resist 4, 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert, P.M. , Deringer, J.R. , Kim, M.H. , Projan, S.J. and Novick, R.P. (1992) Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob Agents Chemother 36, 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, E.D. (2014) Clinical practice. Lyme disease. N Engl J Med 370, 1724–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots, J. (2002) Selection of antimicrobial agents in periodontal therapy. J Periodontal Res 37, 389–398. [DOI] [PubMed] [Google Scholar]
- Soares, M.M.S.R. and Cury, A.E. (2001) In vitro activity of antifungal and antiseptic agents against dermatophyte isolates from patients with tinea pedis. Braz J Microbiol 32, 130–134. [Google Scholar]
- Stanek, G. , Wormser, G.P. , Gray, J. and Strle, F. (2012) Lyme borreliosis. Lancet 379, 461–473. [DOI] [PubMed] [Google Scholar]
- Steere, A.C. (1989) Lyme disease. N Engl J Med 321, 586–596. [DOI] [PubMed] [Google Scholar]
- Steere, A.C. , Grodzicki, R.L. , Kornblatt, A.N. , Craft, J.E. , Barbour, A.G. , Burgdorfer, W. , Schmid, G.P. , Johnson, E. et al (1983) The spirochetal etiology of Lyme disease. N Engl J Med 308, 733–740. [DOI] [PubMed] [Google Scholar]
- Steere, A.C. , Sikand, V.K. , Schoen, R.T. and Nowakowski, J. (2003) Asymptomatic infection with Borrelia burgdorferi . Clin Infect Dis 37, 528–532. [DOI] [PubMed] [Google Scholar]
- Stewart, P.S. and Costerton, J.W. (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358, 135–138. [DOI] [PubMed] [Google Scholar]
- Straubinger, R.K. , Summers, B.A. , Chang, Y.F. and Appel, M.J. (1997) Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Clin Microbiol 35, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker, R.B. and Johnson, L. (2011) Lyme disease: the next decade. Infect Drug Resist 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker, R.B. and Johnson, L. (2014) Lyme disease: call for a “Manhattan Project” to combat the epidemic. PLoS Pathog 10, e1003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker, R.B. and Phillips, S.E. (2003) Lyme disease without erythema migrans: cause for concern? Am J Med 115, 72–73; author reply 73‐74. [DOI] [PubMed] [Google Scholar]
- Stricker, R.B. , Gaito, A. , Harris, N.S. and Burrascano, J.J. (2004) Treatment of early Lyme disease. Ann Intern Med 140, 577; author reply 577‐578. [DOI] [PubMed] [Google Scholar]
- Strle, F. , Wormser, G.P. , Mead, P. , Dhaduvai, K. , Longo, M.V. , Adenikinju, O. , Soman, S. , Tefera, Y. et al (2013) Gender disparity between cutaneous and non‐cutaneous manifestations of Lyme borreliosis. PLoS ONE 8, e64110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, H. , Trang, V.T. , Morimoto, N. , Nishida, Y. , Matsumura, Y. and Sugiura, T. (2014) Natural products and food components with anti‐Helicobacter pylori activities. World J Gastroenterol 20, 8971–8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanousova, D. and Hercogova, J. (2008) Lyme borreliosis treatment. Dermatol Ther 21, 101–109. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Wang, Q. , Li, G. , Wu, J. and Tao, L. (2014) Baicalein enhances cisplatin cytotoxicity via increasing gap junction intercellular communication in Cx32 transfected hela cells. J Sun Yat‐Sen Univ 35, 641–649. [Google Scholar]
- Wichelhaus, T.A. , Schafer, V. , Hunfeld, K.P. , Reimer, K. , Fleischer, W. and Brade, V. (1998) Antibacterial effectiveness of povidone‐iodine (Betaisodona) against highly resistance gram positive organisms. Zentralbl Hyg Umweltmed 200, 435–442. [PubMed] [Google Scholar]
- York, K.K. , Miller, S. , Gaster, R.N. and Burstein, N.L. (1988) Polyvinylpyrrolidone iodine: corneal toxicology and epithelial healing in a rabbit model. J Ocul Pharmacol 4, 351–358. [DOI] [PubMed] [Google Scholar]
- Yun, B.Y. , Zhou, L. , Xie, K.P. , Wang, Y.J. and Xie, M.J. (2012) Antibacterial activity and mechanism of baicalein. Yao Xue Xue Bao 47, 1587–1592. [PubMed] [Google Scholar]
- Zhang, L. , Ravipati, A.S. , Koyyalamudi, S.R. , Jeong, S.C. , Reddy, N. , Bartlett, J. , Smith, P.T. , de la Cruz, M. et al (2013) Anti‐fungal and anti‐bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac J Trop Med 6, 673–681. [DOI] [PubMed] [Google Scholar]


