Abstract
Background:
Androgenic alopecia (AGA) is a common cosmetically and psychosocially distressing condition. High androgen level contributes to the development of atherosclerosis, thrombosis leading to hypertension and hypercholesterolemia.
Objectives:
To study the clinico-epidemiological profile of AGA and the presence of metabolic syndrome (MetS) and carotid artery atherosclerosis in male patients with early onset AGA as compared to controls.
Materials and Methods:
In this case-control study, 100 male patients of age 18-35 years with AGA and an equal number of age-matched healthy controls attending skin and STD OPD were included. Assessment of the degree of hair loss, evaluation of MetS and carotid artery color Doppler for the atherosclerotic plaque was done in all patients.
Results:
Statistically significant number of patients with early onset AGA 22/100 (22%) (P < 0.05) fulfilled the criteria for MetS compared to 8/100 (8%) in the control group. There were statistically significant differences in mean values of waist circumference, serum triglycerides, serum cholesterol, systolic blood pressure, diastolic blood pressure, fasting glucose concentration, and very low-density lipoprotein (LDL). However, no significant differences were observed in the mean values of high-density lipoprotein cholesterol and LDL cholesterol. The atherosclerotic plaque was found in two patients of the study group, and no plaque was found in control patients.
Conclusion:
We suggest that all men with AGA should be thoroughly investigated, and lifestyle changes should be started in the early period of life so as to reduce the risk of various problems associated with MetS. AGA can be considered as an early marker for MetS.
Keywords: Androgenic alopecia, carotid artery atherosclerosis, metabolic syndrome
INTRODUCTION
Androgenic alopecia (AGA) is the most common hair loss condition in men and women with significant negative impact on their social and psychological wellbeing. AGA, a hereditary androgen-dependent disorder, is characterized by a progressive process that causes a gradual conversion of terminal hair into miniaturized hair defined by various patterns.[1] AGA developing before 36 years of age and reaching at least stage 3 of Hamilton-Norwood classification is termed as early onset AGA.[2] In many studies, AGA has been shown to be associated with several diseases, such as coronary heart disease,[3,4] insulin resistance (IR),[5] hypertension,[6] abnormal serum lipid profiles,[3,5,7] obesity,[8] prostate cancer,[9] benign prostatic hyperplasia,[10] and some environmental factors, such as smoking.[8,11] However, controversies also exist regarding the association of those diseases and environmental factors with AGA.[12,13,14,15,16]
Recently, the term “metabolic syndrome” (MetS), came into common usage, and its importance regarding increased risk of developing cardiovascular disease (CVD) and diabetes is being emphasized more and more. Coronary artery disease (CAD) is a major cause of death and disability worldwide.[17] Advanced obstructive CAD can exist in patients with minimal or no symptoms and can progress rapidly, so early detection is extremely important.[18] Until now, few studies[19,20,21,22,23,24,25,26] have reported on the association between AGA, MetS, and CAD.
The aim of this study was to analyze the association of the presence of MetS and carotid artery atherosclerosis with early onset AGA (age from 20 to 35 years). This age group was selected because MetS and coronary disease often does not occur in this age group and also, a risk factor in these persons may be present but are in apparent. So, early detection of the risk factor is useful for early intervention to decrease the incidence of MetS and CAD.
MATERIALS AND METHODS
Patients were selected from Dermatology Department, Government Medical College, district Amritsar, India from January 2013 to June 2014. The study was carried out on 100 male patients presenting with AGA between the age of 18 and 35 years and on 100 age-and-gender matched controls (for other minor skin problems) after obtaining informed witnessed consent. This study was assessed and approved by the Ethics Committee and Review Board of the Institution.
Patients suffering from another type of alopecia and alopecia associated with other dermatological conditions were excluded. The inclusion and exclusion criteria for the controls were the same as for the cases except that the control patients were not suffering from AGA.
Diagnosis of AGA was clinical. The degree of hair loss was assessed and classified according to the Hamilton and Norwood classification.[27,28] A photographic record of their hair status was kept.
After obtaining written consent, a detailed history regarding age of onset, history of smoking, CVD and consumption of drugs (antidiabetic agents, lipid-lowering, and antihypertensive agents), family history of AGA and CVD, hypertension, diabetes mellitus was obtained and every patient was subjected to thorough general physical, systemic, and dermatological examination. The severity of alopecia was assessed, and indices of obesity such as weight and height were recorded to calculate the body mass index (BMI). In addition, we recorded waist circumference (WC), waist to hip ratio (WHR), and blood pressure. Clinical examination of controls was also performed similarly.
We determined serum levels of glucose, triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR). Carotid ultrasound scans were performed to study the presence of atheromatous plaques of the common carotid arteries, carotid bulb, and internal carotid arteries.
The diagnosis MetS was based on criteria defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III, 2001),[29] which were modified to include the World Health Organization's proposed WC cut-off points for Asians.[30] Subjects were required to have three or more of the following: (i) WC >90 cm in men and >80 cm in women, (ii) serum TG ≥150 mg/dl (≥1.70 mmol/l), (iii) HDL-C levels <40 mg dl (<1.04 mmol/l) in men and <50 mg/dl) (<1.70 mmol/l) in women, (iv) impaired fasting glucose of ≥110 mg⁄dl (6.11 mmol/l) or use of medication for hyperglycaemia, and (v) blood pressure ≥130⁄85 mmHg or use of medication for hypertension.
Statistical evaluation was carried out using SPSS version 17.0 by IBM. The results were expressed as a mean ± standard error of the mean. ANOVA test applied for determining the significance of the difference between the cases and the controls for a different variable. P <0.05 were considered significant and P < 0.01 were taken as highly significant.
RESULTS
We studied 100 male patients with early onset AGA, 12% with type I, 25% with type II, 29% with type III, and 12% with type IV alopecia. Type V was present in 7% patients, type VI in 9%, whereas only 6% patients had most severe type VII alopecia according to the Hamilton and Norwood classification. The mean (standard deviation) age for both groups was similar, namely, 27.03 ± 5.36 years in the cases and 26.22 ± 5.10 years in the control (P = 0.478). The mean time since onset of alopecia was 40.55 ± 27.67 months. A positive family history of AGA, hypertension, and DM was found to be significantly more frequent in study cases than in the controls (P < 0.05) [Figure 1].
Figure 1.
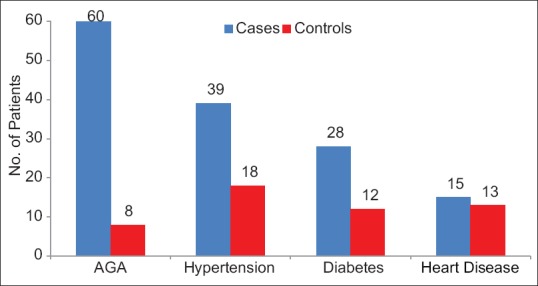
Family history in study group and control group
In our study, we found statistically highly significant difference in BMI (25.03 ± 4.35 kg/m2 vs. 22.34 ± 3.41 kg/m2, P < 0.05) and statistically significant difference in WC (87.8 ± 11.28 cm, P < 0.05) in study group patients as compared to patients in control group; however, the difference in WHR was statistically not significant (0.93 ± 0.06 vs. 0.90 ± 0.06, P > 0.05) [Table 1].
Table 1.
Anthropometric and specific biochemical profile of study group and control group
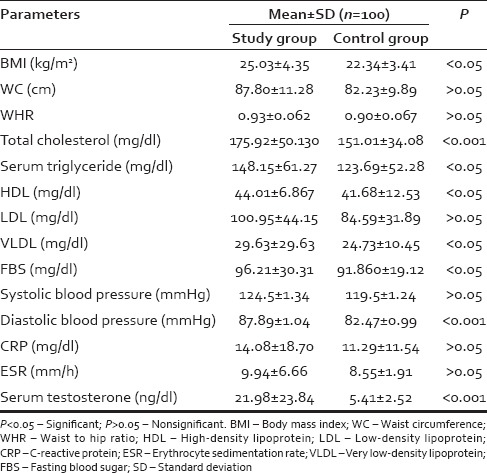
There were statistically significant differences in mean values of serum TGs (P < 0.05), serum cholesterol (P < 0.001), systolic blood pressure (P < 0.05), diastolic blood pressure (P < 0.05), fasting glucose concentration (P < 0.05), and very-LDL (P < 0.05). However, no significant differences were observed in the mean values of HDL-C (P > 0.05) and LDL-C (P > 0.05) [Table 1].
Of 100 AGA patients, 22 (22%) patients fulfilled 3 or more of the criteria for MetS compared with 8 (8%) of the controls, which was statistically significant (P < 0.05) [Table 2]. However, there was no relationship between severity of AGA and MetS [Table 3]. The differences between the cases and controls for all the parameters that make up the MetS are shown in [Table 4].
Table 2.
Comparative proportions of MetS and CAA plaque in AGA and control groups
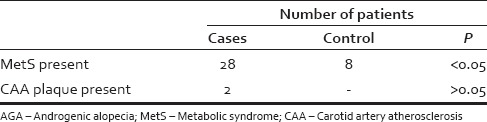
Table 3.
Comparative proportions of MetS in various grades of AGA
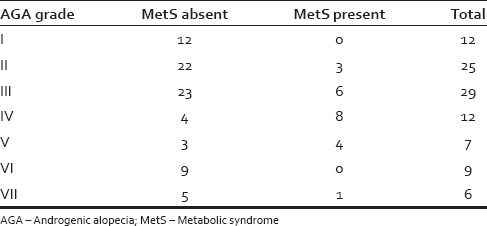
Table 4.
Abnormal components of MetS in study and control group
The atheromatous plaque was seen in 2% of cases however no atheromatous plaque was found in controls patients (P = 0.155) [Table 2].
There were no significant differences in mean values of ESR (9.94 ± 6.66 vs. 8.55 ± 1.91, >0.05) and CRP (14.08 ± 18.70 vs. 11.29 ± 11.54, P > 0.05) among study and control group. However CRP levels were increased in 48 (48%) patients in study group while in control group, levels were increased in 52 (52%) patients (P = 0.207) [Table 1]. There was statistically highly significant difference in the mean value for serum testosterone (21.98 ± 23.84 vs. 5.41 ± 2.52, P < 0.001) among study and control group [Table 1].
DISCUSSION
The results of this study confirm that male AGA is associated with a greater prevalence of cardiovascular risk factors included in the MetS and with an increase in risk of carotid atherosclerosis. Most of the studies have focused on the analysis of coronary disease (acute myocardial infarction or cardiac death) but only few authors have analyzed the association with cardiovascular risk factors, such as criteria for MetS or the presence of carotid atheroma.
The association between alopecia and CVD may have a genetic origin. Family history plays an important role in the development of alopecia. We found a very significant association between the presence of AGA and family history of this condition. In current study and as reported in similar studies,[31,17] family history of AGA and hypertension and diabetes mellitus were significantly high in study patients than controls. Genetic factors are said to play a role in causing AGA. Androgen receptor gene which is X-linked recessive has been mentioned to be the cardinal prerequisite for balding, but other genes are also involved.
There was a highly significant difference in BMI values of AGA patients and the controls in the present study [Table 1]. There was also a significant correlation between BMI and AGA grade. Hirsso et al.[32] found out statistically significant difference while Arias-Santiago et al.[25] and Ceydal et al.[33] could not find statistically significant difference in BMI values of AGA cases and controls.
In the present study, on comparing two groups, WC measurements of the AGA group were found to be higher than those of the control group (82.23 ± 9.89 cm). The difference was statistically significant (P < 0.05) [Table 1]. However, the difference between WHR values of the two groups was not found to be significant (P > 0.05) [Table 1]. Similarly, Arias-Santiago et al.[25] and Acibucu et al.[34] found statistically significant difference in WC of cases and controls (P = 0.032). In contrast, Ceydal.[33] could not find statistically significant difference between WCs of AGA patients and controls.
The abdominal fat tissue is associated with serious metabolic disorders such as hypertension, hyperinsulinemia, increased TG, glucose intolerance, and diabetes mellitus.[35] WC is associated with an increased risk for CAD.[36] Some studies have quoted abdominal fat tissue as an independent risk factor for CAD calculated by measuring the waist-hip proportion.[37,38] The International Diabetes Federation has emphasized the strong correlation between IR and abdominal obesity and has suggested that this criterion be made compulsory for MetS diagnosis.[39]
There was significant difference in levels of total cholesterol and TGs among cases and controls in the present study but statistically no significant difference in levels of HDL-cholesterol, LDL-C was observed [Tables 1 and 4] In 1997, Sasmaz et al.[3] also found meaningfully higher levels of serum TG and lipoprotein A in the AGA group.
Matilainen et al.[40] found higher LDL-C and TGs in men with AGA in patients who had undergone coronary artery bypass graft or percutaneous transluminal coronary angioplasty. Sadighha et al.[7] found out statistically significant higher TG and total cholesterol/HDL-C ratio, whereas HDL-cholesterol was lower in the study group (P < 0.01). In a study by Arias-Santiago et al.[25] men with AGA showed significant higher TGs, total cholesterol values, and LDL-C values. In contrast, Guzzo et al.[41] randomly compared lipid profile, but found no statistically meaningful differences in lipid indices between two groups. The elevated lipid values in AGA patients may contribute, alongside other mechanisms, to the development of CVD. A meta-analysis by Shepherd et al.[42] in 2003 stated that a 1 mmol/L increase in the TG value increases the possibility of CAD by 30% in men and by 69% in women. Sharrett et al.[43] studied that both high TG values and low HDL-C values were associated with the transition from atheroma to atherothrombosis, and therefore, control of both these cardiovascular risk factors is essential in patients with subclinical disease.
Blood sugar values were significantly higher in the study cases (96.21 ± 30.31 mg/dL vs. 91.860 ± 19.12 mg/dL; P < 0.05) [Tables 1, 4, and Figure 2]. Abnormal fasting glucose levels more than 100 mg/dL were present in 23% of the patients; however, in the control group, it was present in 12% patients only [Table 4]. One of the most notable observations in the study by Hirsso et al.[32] was that 21% of their patients with AGA and 12% of the control group had diabetes.
Figure 2.
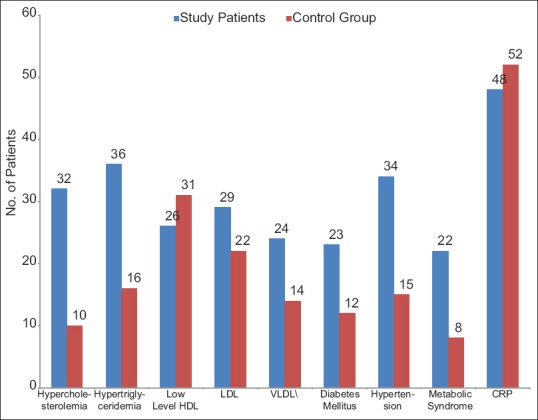
Abnormal biomedical parameters in study and control group
Mean systolic and diastolic blood pressure values were significantly higher in our cases. Hypertension was present in 34 (34%) patients in the study group as compared to 15 (15%) patients in the control group, and this result was statistically significant (P < 0.05) [Tables 1 and 4] Hirsso et al.[44] also found higher blood pressure values in patients with AGA than in a control group (65% vs. 45%), as well as a greater frequency of diabetes and hyperinsulinemia. However, a study published in 2007 by Hirsso et al.[32] did not find statistically significant differences for systolic or diastolic blood pressure levels in patients aged <35 years. The explanations offered for this association was that the androgens involved in the pathogenesis of AGA bind to vascular receptors and also cause hyperaldosteronism both which in turn cause hypertension.[43,45]
A comparison of the frequency of MetS revealed considerable differences between the groups. The prevalence rate of MetS as compared by NCEP ATP III criteria in the study group was 22% and in the control group was 8%. MetS was significantly associated with AGA (P < 0.05) in this study [Table 2] but there was no specific trend found in the prevalence rate of MetS among the different degrees of the AGA patient group [Table 3]. The association between CVD and MetS is well documented. Recent studies show that people who meet ATP III criteria are 2.59-3.5 times more likely to have a cardiovascular event during the next 10 years.[31,46] The prevalence of MetS reported for the general population ranges from 11.7%[47] to 30% in the population of Brazil,[48] with a mean value of 20% in some studies published in Spain.[49,50]
The results of our study were similar to the Acibucu et al.[34] and Arias-Santiago et al.[25] who found statistically significant (P < 0.05). MetS in 25% patients in the AGA group and 10.4% in the control group according to the NCEP ATP III 2001 diagnostic criteria. Su and Chen[19] reported a significant association between AGA and MetS in 670 Taiwanese men in a population-based cross-sectional survey with adjustment for age, family history, and smoking. In contrast, in a study by Ceydal et al.[33] no statistically significant difference of MetS was observed between the study and control groups.
We also measured acute phase reactants such as ESR and CRP in both groups. Mean values were not significantly higher for all reactants in the cases and controls. However, Hirsso et al.[32] reported high-sensitivity CRP values in patients aged <35 years who had moderate or severe alopecia. Chronic inflammation has been shown to play a key role in the presence of IR, endothelial dysfunction, and CVD.[51]
The hormone study performed in our patients revealed statistically significant higher levels of serum testosterone in the study group than in the control group (21.98 ± 23.84 ng/dl vs. 5.41 ± 2.52 ng/dl, P < 0.001) [Table 1]. Cipriani et al. (1983)[51] found that plasma testosterone levels were not raised in bald men. However, their salivary testosterone levels were significantly higher than in controls.
In 1972, it was first suggested that AGA may be a risk factor for CVD when Cotton et al.[52] demonstrated an association between the occurrence of CVD and hair loss. The second key area analyzed, in our study was the presence of carotid atheromatous plaques, as studied by Doppler ultrasound. The association between AGA and the presence of atheromatous plaque could be explained by a greater sensitivity of androgens in the scalp and in vascular smooth muscle, thus favoring miniaturization of the hair follicles in one case and the presence of atheromatous plaque in the other. Many studies assume that pathogenesis was similar for coronary atherosclerosis and carotid atherosclerosis and, therefore, that the presence of carotid atheroma also predicts coronary disease.[43] In our study, an atheromatous plaque was present in 2% of cases in contrast, no plaque was found in patients of control group, however, this result was not statistically significant [Table 2]. In addition, carotid intima-media thickness was greater in cases. Most studies on this topic do not analyze the presence of carotid atherosclerosis as a predisposing factor for CVD. Instead, they have directly analyzed cardiovascular events, generally myocardial infarction, although they restrict their analysis to patients who have survived coronary disease; therefore, they missed data on patients who die and did not take into account asymptomatic individuals with coronary disease. A study by Shahar et al.[13] concluded that male pattern baldness is not an important risk factor for myocardial infarction or asymptomatic atherosclerosis. They also did not find statistically significant differences in carotid intima-media thickness according to the degree of alopecia, despite studying a large number of patients. Dogramaci et al.[53] on the other hand, found an association between severe androgenetic alopecia and a higher carotid intima-media thickness, although they did not analyze the prevalence of atheromatous plaque either. The atheromatous plaque is an easier parameter to measure than carotid intima-media thickness and is very useful when evaluating overall cardiovascular risk in our patients. The approach is noninvasive, reliable, reproducible, and inexpensive; therefore, it is the technique of choice for the detection of subclinical atherosclerosis and makes it possible to establish a classification that goes beyond common risk factors.
CONCLUSION
Young men with AGA should be made aware of an increased risk of IR and its consequences later in life for diseases such as dyslipidemia, hypertension, and diabetes. AGA patients with higher BMI or abdominal obesity (suggested by higher WC) should be advised regarding lifestyle modifications for weight reduction.
It is suggested that since patients with AGA have higher frequency of MetS and a tendency to develop carotid atheromatous plaques, so all the patients of early onset AGA should be screened for cardiovascular abnormalities by MetS criteria and carotid ultrasound studies for early detection of atheromatous plaques. It is further suggested that preventive treatment should be initiated before CVD become established.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Trüeb RM. Molecular mechanisms of androgenetic alopecia. Exp Gerontol. 2002;37:981–90. doi: 10.1016/s0531-5565(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair RD, Dawber RP. Androgenetic alopecia in men and women. Clin Dermatol. 2001;19:167–78. doi: 10.1016/s0738-081x(00)00128-0. [DOI] [PubMed] [Google Scholar]
- 3.Sasmaz S, Senol M, Ozcan A, Dogan G, Tuncer C, Akyol O, et al. The risk of coronary heart disease in men with androgenetic alopecia. J Eur Acad Dermatol Venereol. 1999;12:123–5. [PubMed] [Google Scholar]
- 4.Mansouri P, Mortazavi M, Eslami M, Mazinani M. Androgenetic alopecia and coronary artery disease in women. Dermatol Online J. 2005;11:2. [PubMed] [Google Scholar]
- 5.Matilainen V, Koskela P, Keinänen-Kiukaanniemi S. Early androgenetic alopecia as a marker of insulin resistance. Lancet. 2000;356:1165–6. doi: 10.1016/S0140-6736(00)02763-X. [DOI] [PubMed] [Google Scholar]
- 6.Ahouansou S, Le Toumelin P, Crickx B, Descamps V. Association of androgenetic alopecia and hypertension. Eur J Dermatol. 2007;17:220–2. doi: 10.1684/ejd.2007.0152. [DOI] [PubMed] [Google Scholar]
- 7.Sadighha A, Zahed GM. Evaluation of lipid levels in androgenetic alopecia in comparison with control group. J Eur Acad Dermatol Venereol. 2009;23:80–1. doi: 10.1111/j.1468-3083.2008.02704.x. [DOI] [PubMed] [Google Scholar]
- 8.Mosley JG, Gibbs AC. Premature grey hair and hair loss among smokers: A new opportunity for health education? BMJ. 1996;313:1616. doi: 10.1136/bmj.313.7072.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawk E, Breslow RA, Graubard BI. Male pattern baldness and clinical prostate cancer in the epidemiologic follow-up of the first National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 2000;9:523–7. [PubMed] [Google Scholar]
- 10.Oh BR, Kim SJ, Moon JD, Kim HN, Kwon DD, Won YH, et al. Association of benign prostatic hyperplasia with male pattern baldness. Urology. 1998;51:744–8. doi: 10.1016/s0090-4295(98)00108-3. [DOI] [PubMed] [Google Scholar]
- 11.Su LH, Chen TH. Association of androgenetic alopecia with smoking and its prevalence among Asian men: A community-based survey. Arch Dermatol. 2007;143:1401–6. doi: 10.1001/archderm.143.11.1401. [DOI] [PubMed] [Google Scholar]
- 12.Nabaie L, Kavand S, Robati RM, Sarrafi-Rad N, Kavand S, Shahgholi L, et al. Androgenic alopecia and insulin resistance: Are they really related? Clin Exp Dermatol. 2009;34:694–7. doi: 10.1111/j.1365-2230.2008.03118.x. [DOI] [PubMed] [Google Scholar]
- 13.Shahar E, Heiss G, Rosamond WD, Szklo M. Baldness and myocardial infarction in men: The atherosclerosis risk in communities study. Am J Epidemiol. 2008;167:676–83. doi: 10.1093/aje/kwm365. [DOI] [PubMed] [Google Scholar]
- 14.Abdel Fattah NS, Darwish YW. Androgenetic alopecia and insulin resistance: Are they truly associated? Int J Dermatol. 2011;50:417–22. doi: 10.1111/j.1365-4632.2010.04677.x. [DOI] [PubMed] [Google Scholar]
- 15.Matilainen V, Laakso M, Hirsso P, Koskela P, Rajala U, Keinänen-Kiukaanniemi S. Hair loss, insulin resistance, and heredity in middle-aged women. A population-based study. J Cardiovasc Risk. 2003;10:227–31. doi: 10.1097/01.hjr.0000070200.72977.c6. [DOI] [PubMed] [Google Scholar]
- 16.Severi G, Sinclair R, Hopper JL, English DR, McCredie MR, Boyle P, et al. Androgenetic alopecia in men aged 40-69 years: Prevalence and risk factors. Br J Dermatol. 2003;149:1207–13. doi: 10.1111/j.1365-2133.2003.05565.x. [DOI] [PubMed] [Google Scholar]
- 17.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 18.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: A 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–90. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 19.Su LH, Chen TH. Association of androgenetic alopecia with metabolic syndrome in men: A community-based survey. Br J Dermatol. 2010;163:371–7. doi: 10.1111/j.1365-2133.2010.09816.x. [DOI] [PubMed] [Google Scholar]
- 20.Schnohr P, Lange P, Nyboe J, Appleyard M, Jensen G. Gray hair, baldness, and wrinkles in relation to myocardial infarction: The Copenhagen City Heart Study. Am Heart J. 1995;130:1003–10. doi: 10.1016/0002-8703(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Freedman DS, Byers T. Baldness and ischemic heart disease in a national sample of men. Am J Epidemiol. 1996;143:651–7. [Google Scholar]
- 22.Lotufo PA, Chae CU, Ajani UA, Hennekens CH, Manson JE. Male pattern baldness and coronary heart disease: The Physicians' Health Study. Arch Intern Med. 2000;160:165–71. doi: 10.1001/archinte.160.2.165. [DOI] [PubMed] [Google Scholar]
- 23.Lesko SM, Rosenberg L, Shapiro S. A case-control study of baldness in relation to myocardial infarction in men. JAMA. 1993;269:998–1003. [PubMed] [Google Scholar]
- 24.Miric D, Fabijanic D, Giunio L, Eterovic D, Culic V, Bozic I, et al. Dermatological indicators of coronary risk: A case-control study. Int J Cardiol. 1998;67:251–5. doi: 10.1016/s0167-5273(98)00313-1. [DOI] [PubMed] [Google Scholar]
- 25.Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, Buendía-Eisman A, Naranjo-Sintes R. Androgenetic alopecia and cardiovascular risk factors in men and women: A comparative study. J Am Acad Dermatol. 2010;63:420–9. doi: 10.1016/j.jaad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Rebora A. Baldness and coronary artery disease: The dermatologic point of view of a controversial issue. Arch Dermatol. 2001;137:943–7. [PubMed] [Google Scholar]
- 27.Hamilton JB. Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. 1951;53:708–28. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 28.Norwood OT. Male pattern baldness: Classification and incidence. South Med J. 1975;68:1359–65. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;16(285):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.WHO⁄IASO⁄IOTF. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 31.Assmann G, Schulte H, Seedorf U. Cardiovascular risk assessment in the metabolic syndrome: Results from the prospective cardiovascular munster (PROCAM) study. Int J Obes (Lond) 2008;32(Suppl 2):S11–6. doi: 10.1038/ijo.2008.29. [DOI] [PubMed] [Google Scholar]
- 32.Hirsso P, Rajala U, Hiltunen L, Jokelainen J, Keinänen-Kiukaanniemi S, Näyhä S. Obesity and low-grade inflammation among young Finnish men with early-onset alopecia. Dermatology. 2007;214:125–9. doi: 10.1159/000098570. [DOI] [PubMed] [Google Scholar]
- 33.Mumcuoglu C, Ekmekci TR, Ucak S. The investigation of insulin resistance and metabolic syndrome in male patients with early-onset androgenetic alopecia. Eur J Dermatol. 2011;21:79–82. doi: 10.1684/ejd.2010.1193. [DOI] [PubMed] [Google Scholar]
- 34.Acibucu F, Kayatas M, Candan F. The association of insulin resistance and metabolic syndrome in early androgenetic alopecia. Singapore Med J. 2010;51:931–6. [PubMed] [Google Scholar]
- 35.Spinler SA. Challenges associated with metabolic syndrome. Pharmacotherapy. 2006;26(12 Pt 2):209S–17S. doi: 10.1592/phco.26.12part2.209S. [DOI] [PubMed] [Google Scholar]
- 36.Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43:513–8. doi: 10.1038/sj.sc.3101744. [DOI] [PubMed] [Google Scholar]
- 37.Canoy D. Distribution of body fat and risk of coronary heart disease in men and women. Curr Opin Cardiol. 2008;23:591–8. doi: 10.1097/HCO.0b013e328313133a. [DOI] [PubMed] [Google Scholar]
- 38.Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: A population-based prospective study. Circulation. 2007;116:2933–43. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 39.1st International Congress on “Prediabetes and the Metabolic Syndrome”; Berlin, Germany. 2005. [Google Scholar]
- 40.Matilainen VA, Mäkinen PK, Keinänen-Kiukaanniemi SM. Early onset of androgenetic alopecia associated with early severe coronary heart disease: A population-based, case-control study. J Cardiovasc Risk. 2001;8:147–51. doi: 10.1177/174182670100800305. [DOI] [PubMed] [Google Scholar]
- 41.Guzzo CA, Margolis DJ, Johnson J. Lipid profiles, alopecia, and coronary disease: Any relationship? Dermatol Surg. 1996;22:481. doi: 10.1111/j.1524-4725.1996.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 42.Shepherd J, Hunninghake DB, Barter P, McKenney JM, Hutchinson HG. Guidelines for lowering lipids to reduce coronary artery disease risk: A comparison of rosuvastatin with atorvastatin, pravastatin, and simvastatin for achieving lipid-lowering goals. Am J Cardiol. 2003;91:11C–7C. doi: 10.1016/s0002-9149(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 43.Sharrett AR, Sorlie PD, Chambless LE, Folsom AR, Hutchinson RG, Heiss G, et al. Relative importance of various risk factors for asymptomatic carotid atherosclerosis versus coronary heart disease incidence: The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;149:843–52. doi: 10.1093/oxfordjournals.aje.a009900. [DOI] [PubMed] [Google Scholar]
- 44.Hirsso P, Laakso M, Matilainen V, Hiltunen L, Rajala U, Jokelainen J, et al. Association of insulin resistance linked diseases and hair loss in elderly men. Finnish population-based study. Cent Eur J Public Health. 2006;14:78–81. doi: 10.21101/cejph.b0045. [DOI] [PubMed] [Google Scholar]
- 45.Sainte Marie Y, Toulon A, Paus R, Maubec E, Cherfa A, Grossin M, et al. Targeted skin overexpression of the mineralocorticoid receptor in mice causes epidermal atrophy, premature skin barrier formation, eye abnormalities, and alopecia. Am J Pathol. 2007;171:846–60. doi: 10.2353/ajpath.2007.060991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi KM, Kim SM, Kim YE, Choi DS, Baik SH, Lee J. International Diabetes Federation. Prevalence and cardiovascular disease risk of the metabolic syndrome using National Cholesterol Education Program and International Diabetes Federation definitions in the Korean population. Metabolism. 2007;56:552–8. doi: 10.1016/j.metabol.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 48.Salaroli LB, Barbosa GC, Mill JG, Molina MC. Prevalence of metabolic syndrome in population-based study, Vitória, ES-Brazil. Arq Bras Endocrinol Metabol. 2007;51:1143–52. doi: 10.1590/s0004-27302007000700018. [DOI] [PubMed] [Google Scholar]
- 49.Martínez Candela J, Franch Nadal J, Romero Ortiz J, Cánovas Domínguez C, Gallardo Martín A, López Yepes ML. Predictive capacity of the diagnostic criteria of metabolic syndrome on the insulin-resistance and the coronary risk. Med Clin (Barc) 2007;129:601–6. doi: 10.1157/13111806. [DOI] [PubMed] [Google Scholar]
- 50.Calbo Mayo JM, Terrancle de Juan I, Fernández Jiménez P, Rodríguez Martín MJ, Martínez Díaz V, Santisteban López Y, et al. Prevalence of metabolic syndrome in the province of Albacete (Spain) Rev Clin Esp. 2007;207:64–8. [PubMed] [Google Scholar]
- 51.Cipriani R, Ruzza G, Foresta C, Veller Fornasa C, Peserico A. Sex hormone-binding globulin and saliva testosterone levels in men with androgenetic alopecia. Br J Dermatol. 1983;109:249–52. doi: 10.1111/j.1365-2133.1983.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 52.Cotton SG, Nixon JM, Carpenter RG, Evans DW. Factors discriminating men with coronary heart disease from healthy controls. Br Heart J. 1972;34:458–64. doi: 10.1136/hrt.34.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dogramaci AC, Balci DD, Balci A, Karazincir S, Savas N, Topaloglu C, et al. Is androgenetic alopecia a risk for atherosclerosis? J Eur Acad Dermatol Venereol. 2009;23:673–7. doi: 10.1111/j.1468-3083.2009.03137.x. [DOI] [PubMed] [Google Scholar]


