Abstract
Hypertrichosis is a common side effect of topical minoxidil and has been reported to occur mainly close to the areas of application. In this paper, we present a case of a 26-year-old woman who developed generalized hypertrichosis 8 weeks after treatment with 5% topical minoxidil solution for alopecia areata. Generalized hypertrichosis is a rare side effect and has been described mainly in children and adolescents. Even though minoxidil is commonly prescribed for alopecia areata, there is insufficient evidence to support its systematic use and the occurrence of adverse effects should prompt drug interruption. Nonetheless, topical minoxidil has been shown to be a safe medication for adult patients, and we believe that the present case was an isolated one, possibly resulting from the misuse of the drug.
Keywords: Alopecia areata, hypertrichosis, minoxidil
INTRODUCTION
Minoxidil-induced hypertrichosis is not an unusual event and has been reported essentially in the vicinity of application areas.[1] Generalized hypertrichosis, however, is rare and has been described mainly in children and adolescents.[1,2,3,4] We present a case of minoxidil-induced generalized hypertrichosis in an adult woman with alopecia areata.
CASE REPORT
A 26-year-old woman presented to our hair clinic with multiple patches of alopecia areata on the scalp. A treatment plan consisting of topical clobetasol gel, intralesional triamcinolone, and 5% topical minoxidil solution was prescribed. After 8 weeks of treatment, the patient noticed the onset of facial hypertrichosis that progressively spread to the upper limbs and eventually to the entire body surface [Figures 1 and 2]. There were no systemic symptoms and no abnormalities in adrenal, gonadal, or thyroidal hormones were detected. The patient admitted overuse of topical minoxidil, having applied around 100 ml/week on the scalp. A few months after drug interruption, there was a complete remission of hypertrichosis.
Figure 1.
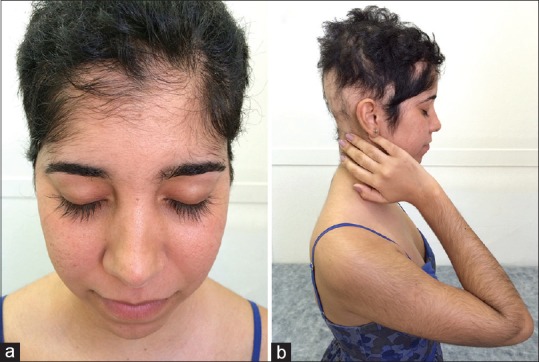
Patient presenting (a) Hypertrichosis on the face. Note trichomegaly of the eyelashes; (b) involvement of the upper limbs
Figure 2.
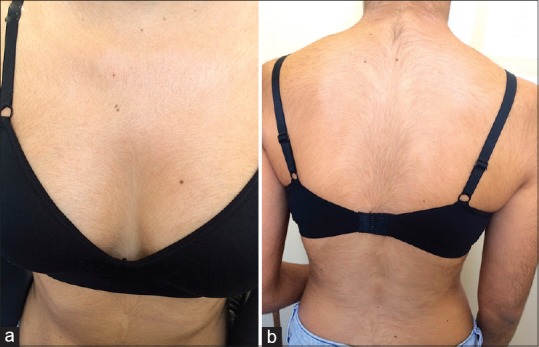
Generalized hypertrichosis involving the (a) Anterior and (b) posterior trunk
DISCUSSION
The mechanism of action through which oral and topical minoxidil induce hair growth remain incompletely understood.[5] Topical minoxidil is widely used for androgenetic alopecia and has been indicated as an off-label medication in the treatment of alopecia areata. One of its possible side effects is hypertrichosis; an excessive number of terminal hairs in nonandrogen-dependent areas, as observed in our patient. It must be distinguished from hirsutism wherein this excessive growth follows the male pattern of hair distribution.[6] In most patients, hypertrichosis is restricted to the face and upper limbs possibly by inadvertent application or transferring of the product through fomites.[7,8] In cases of generalized hypertrichosis, however, it is unlikely that the product reaches the entire body surface. Systemic absorption of topically applied minoxidil is minimal. However, it has been proposed that, in such patients, a higher sensitivity of the follicular apparatus might be responsible for the widespread growth of hair observed.[1,3] We believe that the clinical picture presented herein results mainly from the overuse of the drug leading to higher systemic absorption, but we cannot exclude that an increased susceptibility of the hair follicle to minoxidil might have played a role in this case.
CONCLUSION
We present a rare case of generalized hypertrichosis in an adult patient following exposure to topical minoxidil. Even though minoxidil is commonly prescribed for alopecia areata, there is insufficient evidence to support its systematic use and the occurrence of adverse effects should prompt drug interruption.[9] Nonetheless, topical minoxidil has been shown to be a safe medication for adult patients, and we believe that the present case was an isolated one, possibly resulting from the misuse of the drug.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.González M, Landa N, Gardeazabal J, Calderón MJ, Bilbao I, Díaz Pérez JL. Generalized hypertrichosis after treatment with topical minoxidil. Clin Exp Dermatol. 1994;19:157–8. doi: 10.1111/j.1365-2230.1994.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 2.Guerouaz N, Mohamed AO. Minoxidil induced hypertrichosis in children. Pan Afr Med J. 2014;18:8. doi: 10.11604/pamj.2014.18.8.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herskovitz I, Freedman J, Tosti A. Minoxidil induced hypertrichosis in a 2 year-old child. F1000Res. 2013;2:226. doi: 10.12688/f1000research.2-226.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farsani TT, Kane MJ, Kane KS. Piggyback-acquired hypertrichosis. Pediatr Dermatol. 2014;31:520–2. doi: 10.1111/pde.12329. [DOI] [PubMed] [Google Scholar]
- 5.Duque-Estrada B, Raso P, Gavazzoni MF, Abraham LS Coutinho A, Sodré CT. Hair disorders. In: Azulay RD, Azulay DR, Azulay-Abulafia L, editors. Dermatology. 6th ed. Rio de Janeiro Brasil: Guanabara Koogan; 2013. pp. 729–52. [Google Scholar]
- 6.Paschoal L, Droste D, Prearo C, China C, Peris E, Mendes M. Hair distribution in young women. An Bras Dermatol. 1990;65:20S–2S. [Google Scholar]
- 7.Dawber RP, Rundegren J. Hypertrichosis in females applying minoxidil topical solution and in normal controls. J Eur Acad Dermatol Venereol. 2003;17:271–5. doi: 10.1046/j.1468-3083.2003.00621.x. [DOI] [PubMed] [Google Scholar]
- 8.Peluso AM, Misciali C, Vincenzi C, Tosti A. Diffuse hypertrichosis during treatment with 5% topical minoxidil. Br J Dermatol. 1997;136:118–20. [PubMed] [Google Scholar]
- 9.Hordinsky M, Donati A. Alopecia areata: An evidence-based treatment update. Am J Clin Dermatol. 2014;15:231–46. doi: 10.1007/s40257-014-0086-4. [DOI] [PubMed] [Google Scholar]


