Sir,
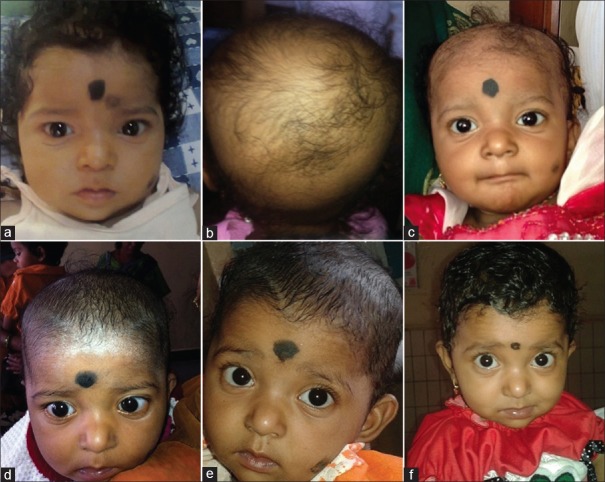
A 10-month-old, 7.4 kg, newly diagnosed hypothyroid infant (thyroid profile showed T3 0.25 ng/mL, T4 1.2 μg/dL, TSH 150 μIU/dL] [Figure 1a] was started on levothyroxine 100 μg/day (14 μg/kg/day). Sonography of the neck showed hypoplasia of thyroid gland. Antiperoxidase and antithyroglobulin antibodies were negative. Ten days after starting levothyroxine, the baby presented with sudden onset, diffuse of loss of scalp hair. The remaining hairs were thin and easily pluckable. On examination, infant had non-scarring alopecia involving 90% of the scalp area without the exclamation mark appearance [Figure 1b and c]. Thyroxine overdose was suspected as the cause of anagen effluvium. At this stage the serum T4 level was very high (Serum T4 = 18.2 μg/dL, TSH = 0.72 μIU/dL), suggesting hyperthyroidism. Levothyroxine dose was reduced by 50% to 50 μg/day. A follow up after 15 days showed dramatic regrowth of hairs [Figure 1d]. Six weeks later, T4 level was still slightly high and therefore the dose was decreased to 37.5 μg/day (5 μg/kg/day). In the follow ups (T4 = 9 μg/dL) scalp hair growth was normal without further hair loss [Figure 1e and f].
Figure 1.
Sequence of events and action. (a) At the time of diagnosis as hypothyroidism, before starting thyroxine. (b) After 20 days of 100 μg/day levothyroxine, on thyroxine 100 μg/day. (c) After 20 days of 100 μg/day levothyroxine, on thyroxine 100 μg/day. (d) After 15 days of decreased levothyroxine dose to 50 μg/day. (e) After 50 days of levothyroxine 50 μg/day. (f) After 50 days of levothyroxine 37.5 μg/day
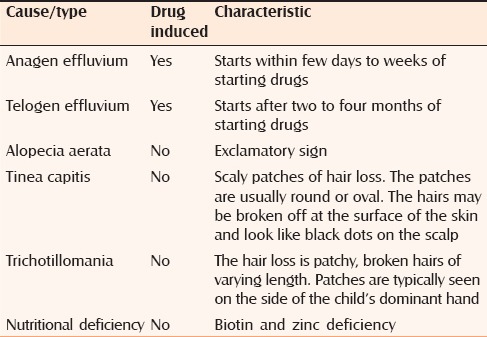
Normally, human hair growth and maintenance depends on three phases of their growth cycle: Anagen, catagen, and telogen. Most hair follicles would be in anagen at any given time, which is the mitotically active and growing stage.[1] Common causes of diffuse scalp hair loss are shown in Table 1. Medications can cause two types of hair loss, telogen effluvium and anagen effluvium. Telogen effluvium is the most common form and usually appears within two to four months after medication. Anagen effluvium occurs within a few days to weeks after medication, as in our patient. The severity of medication-induced hair loss depends on the type and dosage. When we diagnosed hypothyroidism in our child, scalp hair was absolutely normal in texture and distribution [Figure 1a]. Our case developed diffuse hair loss, which was secondary to overdose of levothyroxine; hair loss occurred within a few days of starting treatment and also reversed after correcting the dose [Figure 1b–d]. Diffuse alopecia areata is another cause of rapid, diffuse hair loss without any clinical inflammatory signs. Well-circumscribed round to oval patches of hairless skin interspersed with normal hair will be seen. Exclamation point hairs is characteristic of alopecia areata, which was not present in our case.[2] Clinically both hyper- and hypothyroidism are associated with hair loss.[3] In hypothyroidism, hair becomes dry, coarse, brittle, increasingly thinner, and slow growing. The relative proportions of telogen compared with anagen hairs in the scalp increase in patients with hair loss.[4] Confusingly, hyperthyroidism can also lead to hair loss where the scalp hair is often fine, soft, greasy, reduced hair shafts tensile strength together with thinned hair shaft diameter despite an apparently increased hair matrix proliferation.[4,5] Ahsan et al. demonstrated thyroid receptors in human hair follicles and through these receptors, thyroid hormone can exert a direct effect on hair follicles.[4] It is very difficult to explain why both hypo- and hyperthyroid states are associated with substantial hair loss.[3] T3 at both lower and higher levels is not suitable to promote the proliferation and/or metabolism of hair follicles. Probably the magnitude of the effects of thyroid hormone on hair growth is variable, and its expression may be influenced by local factors and/or other hormones.[4] Our child developed diffuse hair loss within two weeks of starting levothyroxine, when the estimated T4 and T3 levels were high. After decreasing the levothyroxine dose, there was a dramatic improvement in the scalp hair growth, suggesting anagen effluvium. Scalp hair growth was absolutely normal in texture and distribution while on thyoxine maintenance dose. To conclude, hair follicles need adequate and correct levels of thyroxine to have appropriate hair growth and all children on levothyoxine should be monitored for serum levels of T3 and T4.
Table 1.
Common causes of diffuse scalp hair loss
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kanwar AJ, Narang T. Anagen effluvium. Indian J Dermatol Venereol Leprol. 2013;79:604–12. doi: 10.4103/0378-6323.116728. [DOI] [PubMed] [Google Scholar]
- 2.Seetharam KA. Alopecia areata: An update. Indian J Dermatol Venerol Leprol. 2013;79:563–75. doi: 10.4103/0378-6323.116725. [DOI] [PubMed] [Google Scholar]
- 3.Paus R. Exploring the “thyroid-skin connection”: Concepts, questions, and clinical relevance. J Invest Dermatol. 2010;130:7–10. doi: 10.1038/jid.2009.359. [DOI] [PubMed] [Google Scholar]
- 4.Ahsan MK, Urano Y, Kato S, Oura H, Arase S. Immunohistochemical localization of thyroid hormone nuclear receptors in human hair follicles and in vitro effect of L-triiodothyronine on cultured cells of hair follicles and skin. J Med Invest. 1998;44:179–84. [PubMed] [Google Scholar]
- 5.van Beek N, Bodó E, Kromminga A, Gáspár E, Meyer K, Zmijewski MA, et al. Thyroid hormones directly alter human hair follicle functions: Anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381–8. doi: 10.1210/jc.2008-0283. [DOI] [PubMed] [Google Scholar]