Abstract
The purpose of this article is to analyze and understand the mechanism of action, effectiveness, cost and time benefits, advantages and disadvantages of the femtosecond laser (FSL) assisted cataract surgery. A PubMed search was done using the topic and the keywords. Research shows considerable improvements in corneal incisions, anterior capsulotomy, and phacofragmentation using FSL. We will also discuss and compare FSL with conventional cataract extraction techniques in terms of both short-term and long-term advantages and disadvantages. Limitations of the studies reviewed include small sample size and short-term follow-up. The major dilemma is still considered to be its heavy financial feasibility to date.
Keywords: Capsulotomy, cataract, clear corneal incision, femtosecond laser, LensAR, LenSx and Victus, OptiMedica, phacoemulsification, phacofragmentation
Introduction
Femtosecond laser (FSL) is being used in ocular surgery since 2001 for LASIK flaps, and recently in 2010 it got approved by Food and Drug Administration (FDA) for cataract surgery. The reason for its success in cataract surgery is that it has ultrafast pulses in the range of 10−15 which require less energy as compared to phacoemulsification for tissue destruction, thus increasing its safety margin.[1,2]
Since, its approval in 2010 following operating systems got FDA approval for their use in FSL assisted cataract surgery[3] (FLACS):
OptiMedica Catalys (Optimedica Corp, CA, USA)
LensAR (LensAR Inc., FL, USA)
Alcon LenSx (Alcon Laboratories, Ft Worth, TX, USA)
Technolas Victus (Technolas Perfect Vision GmbH, Germany; and Bausch + Lomb, NYSE, USA).
Materials and Methods
A PubMed search was conducted in July 2014 using the following keywords: FSL, cataract, phacoemulsification, phacofragmentation, capsulotomy, and clear corneal incisions (CCIs), OptiMedica, LensAR, LenSx, and Victus. The search revealed 336 articles from which 30 were shortlisted to include in our study. The references of articles selected were also utilized as a resource.
Femtosecond Laser: Mechanism of Action
FSL functions by means of its highly targeted near infrared scanning pulse focused to 3 um with an accuracy of 1 um.[1] Basic mechanism of action lies in the fact that this highly focused energy beam can raise the energy of matter to a level where plasma is created. This plasma of free electrons and ionized molecules rapidly expands, creating cavitation bubbles. The force of cavitation bubble creation separates the tissue.[3] This process of converting laser energy into mechanical energy is known as photodisruption. FSL uses near infrared waves that are not absorbed by cornea so it can also be used for targeting anterior chamber at various depths without the risk of the damaging cornea.[4]
Steps in Femtosecond Laser Assisted Cataract Surgery
The main steps required in this procedure include:
Pupillary dilatation
Topical anesthesia.
Docking
It requires applanation of cornea with a docking system to distribute the pressure evenly on cornea. This requires the patient to be flat on the table with minimal neck support. It may represent a contraindication for older patients with significant kyphosis and scoliosis. The head must be secured with a slight tilt, so the operated eye is in a higher plane to clear the nose.[1,4] Docking involves placing a suction ring on the eye that is separate from the patient interface [Figure 1]. A separate surgical microscope is positioned off to the side to visualize the eye for docking. FSL has both soft and hard docking platforms. During cataract surgery, soft docking is preferred to keep the intraocular pressure low. This is in contrast to docking done for corneal refractive surgery, where hard docking techniques were used. The eye is then docked using different interfaces that can be divided into contact (applanating) and noncontact (nonapplanating). Contact systems tend to have a smaller diameter and may fit a smaller orbit better. Noncontact devices, in addition to less increase in intraocular pressure, cause less subconjunctival hemorrhage and offer a wider field of view.[1,4] Following are the different docking techniques used by different companies:
Figure 1.
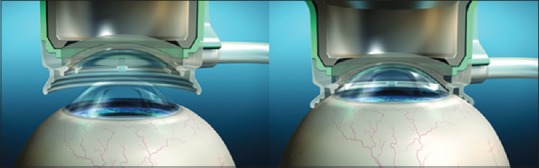
Docking: Platform for clear imaging and laser delivery
LenSx: Curved contact lens
LenAR: Liquid optics
OptiMedica: Liquid optics
Victus: Curved glass interface.
Rise in intraocular pressure following docking is considerably lower using liquid optics as compared to contact lens and curved glass interface.
Anterior segment imaging
It is done for better visualization of anatomical landmarks and for mapping out iris and posterior surface of the lens to avoid posterior capsular rent. It involves high-resolution, three-dimensional (3D), wide-field imaging of the anterior segment [Figure 2a and b]. Detailed visualization of the cornea, iris, iridocorneal angle and lens (including anterior and posterior capsule) is the key to success and safety with FLACS. Inaccuracy at this stage increases the risk of incomplete capsulotomy, imprecise corneal incisions, damage to the iris, and posterior capsular rupture.[5]
Figure 2.
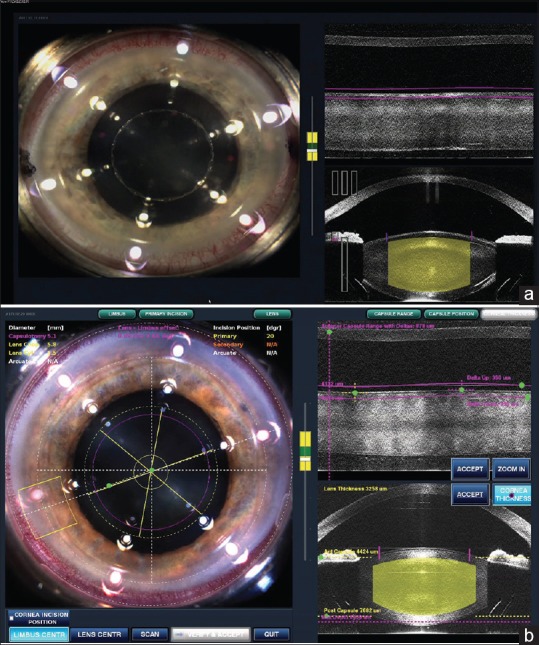
(a) Anterior segment imaging (b) anterior segment imaging using optical coherence tomography
There are two main techniques for this purpose namely optical coherence tomography (OCT) and Scheimpflug imaging technique. OCT delivers high-resolution because it is based on light, rather than sound or radio frequency. An optical beam is directed at the tissue, and a small portion of this light that reflects from sub-surface features is collected. Note that most light is not reflected but, rather, scatters off at large angles. In conventional imaging, this diffusely scattered light contributes background that obscures an image. However, in OCT, a technique called interferometry is used to record the optical path length of received photons allowing rejection of most photons that scatter multiple times before detection. Thus, OCT can build up clear 3D images of thick samples by rejecting background signal while collecting light directly reflected from surfaces of interest. Furthermore, it shows live sub-surface images and instant direct imaging of tissue morphology.
The Scheimpflug principle images the anterior eye with a camera at an angle to a slit-beam creating an optic section of the cornea and lens. More recent instrumentation has been designed to rotate around the visual axis capturing multiple images to create a 3D image of the anterior chamber. Scheimpflug measures of central corneal thickness and anterior chamber depth are accurate and have good repeatability.[6] However, owing to the refracting surfaces and the tilt of the camera, the unprocessed Scheimpflug image is distorted. The image is decreased in size perpendicular to the direction of the optical axis. Therefore, the curvatures of subsequent radii are reduced, such as the posterior corneal surface, and both the lens surfaces, and axial depths are increased, such as the corneal thickness and anterior chamber depth.[7] Following are imaging techniques used by different companies.
LenSx: OCT
LensAR: Scheimpflug
OptiMedica: OCT
Victus: OCT.
Clear corneal incisions (CCIs)
Preprogrammed partial thickness clear incisions are then made using FSL. FSL produces consistent and stable incisions.[8] This has been attributed to the controlled, reproducible generation of the incisions and the configuration of the corneal wound created[9] [Figure 3]. Studies have found that CCIs created with FSLs are square and significantly more resistant to deformation and leakage with increased stability compared with manually created incisions.[9,10] However, this will require a large amount of data to confirm and definitive results will not be present for some time.
Figure 3.
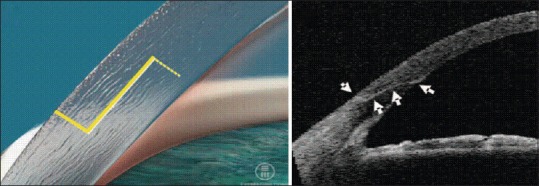
Precise, interlocking corneal incision using femtosecond laser
In addition to creating the main cataract surgical wound, FSLs are used to create limbal relaxing incisions (LRIs). When placed in specific areas of a person's cornea, LRIs can reduce the amount of astigmatism present, which can, and often does, improve postoperative uncorrected visual acuity.
Anterior capsulotomy
It is laser assisted, better sized, more circular and better centered.[11] The FSL is able to create a near perfect, round opening in the anterior capsule by dissecting it with a spiral laser pattern crossing the anterior capsule [Figure 4]. It can, however, cause the release of gas bubbles that can distort the anatomy and affect the capsulotomy. For this reason, the spiral pattern is applied first posterior to the capsule and advances anteriorly.[12]
Figure 4.
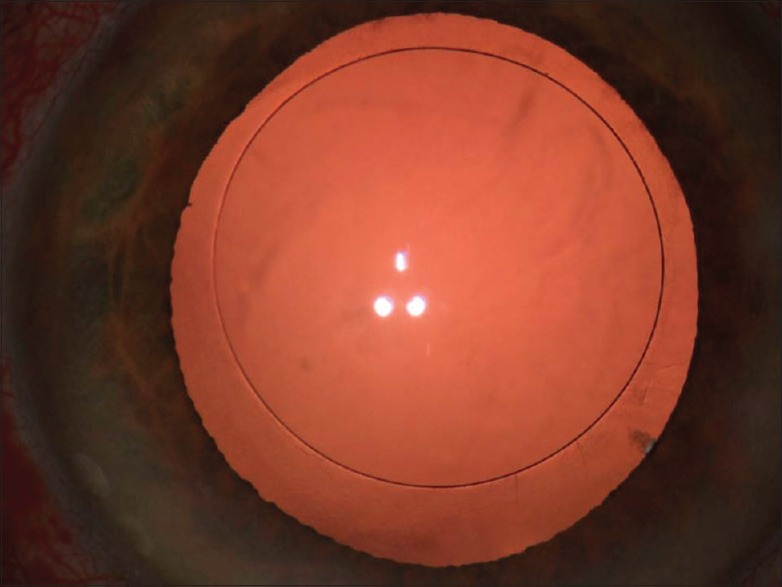
Anterior capsulotomy
Phacofragmentation
The FSL has the capability to assist the fragmentation (breaking up) of the cataract. The laser applies a number of pulses to the lens in a predesigned pattern [Figure 5] which then allows the surgeon to remove the lens matter using very low if at all required, phacoemulsification power. This has been shown to reduce the average time and energy required to break up and remove the lens by approximately 50%.[13] Inherently, this should make the overall procedure safer and less traumatic to the eye, which may further reduce the risk of postoperative swelling and lead to a faster visual recovery.
Figure 5.
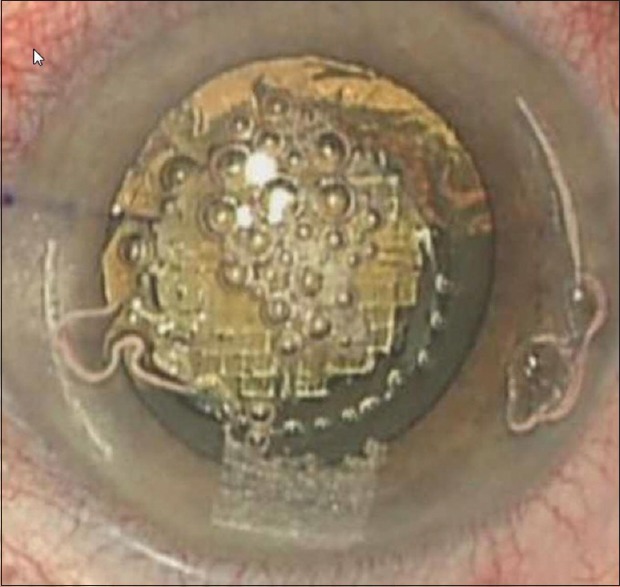
Phacofragmentation using femtosecond laser
Finally, the patient is taken to the operating room where the surgeon converts partial thickness incision to full thickness incision; removes lens fragments by phacoemulsification and implants an intraocular lens (IOL).
How can Femtosecond Laser Improve Cataract Surgery?
To answer the above question, we must first point out the shortcomings in previous cataract operating techniques and compare them with FSL. Following are the major side effects of previous procedures that have been proposed to be better controlled with the application of FSL:
Tears in capsulorrhexis
Decentration and tilt of lens
Risk of corneal injury
Postoperative endophthalmitis.[14]
Tears in capsulorrhexis
Manual capsulorrhexis is considered to be very tricky and most difficult part of cataract surgery leading to tears.[15] FSL appears to be a solution to this problem as its highly targeted lasers via OCT minimize the chances of human errors. FSL makes well targeted and highly precise capsulotomies.
Decentration and tilt
Unpredictable diameter seen in manual capsulorrhexis can cause decentration or tilt of IOL, which can affect the refractive power of the eye causing unpredictable anterior chamber depths and lead to increased rate of posterior capsular opacification.[16,17,18] According to Norrby IOL position was the largest contributor to postoperative refractive error in cataract patients. However, FSL delivers a precisely planned and circular capsulorrhexis, that leads to a better IOL overlap and minimizes the chances of decentration.[17] However whether the circular capsulorrhexis made by FSL has a significant effect on postoperative vision of the patients still needs to be explored.
Risk of corneal injury
Studies conducted by Murano et al. and Shin et al. show that ultrasound exposure causes cell stress and necrosis leading to corneal endothelial damage[19,20] Thus to avoid or reduce corneal injury the time duration of ultrasound exposure to eye should be reduced. Conrad-Hengerer et al. showed that FLACS has less effective phacoemulsification time compared to conventional techniques.[21] Furthermore, after application of FSL, less instrumentation is required further reducing the chances of tissue damage.
Postoperative endophthalmitis
According to a study conducted by Xia et al., Anterior segment OCT after cataract surgery shows internal corneal wounds and detachment of descemet membrane that serve as a risk factor for endophthalmitis.[22] However, FSL is capable of making cuts to precise depth and length with more square architecture, making it resistant to leakage and thus minimizing chances of endophthalmitis.[10] However, evidence lacks and more elaborate and long-term studies need to be conducted to establish this fact in favor of FLACS.
Current Femtosecond Laser Assisted Cataract Surgery Studies
There have been very few studies that demonstrate the use of FSL in all steps of cataract surgery. However, there are multiple studies describing the use of FSL in individual steps of cataract surgery. We shall now review each FSL assisted step individually.
Results
Femtosecond laser assisted corneal incision
A study conducted by Masket et al., using cadaver eyes found that corneal incisions created with FSL were stable and reproducible.[9] In addition, Grewal et al. observed CCIs created using the FSL had significant morphological differences compared with those using a metal keratome. There were lower rates of endothelial gape, endothelial misalignment, and descemet membrane detachment in the FSL group than in keratome group. Furthermore, CCIs created using FLACS were within 10% of the intended programmed measurements for incision length, depth, and angle.[23] However, there is no definitive data regarding wound strength in FSL generated corneal incisions.
Femtosecond laser assisted capsulotomy
Nagy et al., observed a higher degree of circularity, fewer patients with incomplete capsulorrhexis and better IOL centration with FSL.[24] Another study conducted by Friedman et al., showed that laser created capsulotomies were significantly more precise in size and shape than manually created capsulorrhexis.[25] Kránitz et al. showed that there was a statistically significant difference between circularity of capsulotomy at postoperative week one; favoring laser-assisted surgery. However after a year, there was no longer a statistical difference between two groups.[26]
Abell et al., observed that there was a significantly increased rate of anterior capsule tears in FLACS group (1.87%) when compared to phacoemulsification group (0.12%). In 7 FLACS cases, the anterior capsule tear extended to the posterior capsule. Irregularities at capsule margin as well as multiple apparent misplaced laser pits in normal parts of tissue were also observed. A learning curve may account for an increased complication rate with FLACS.[27]
Femtosecond laser assisted phacofragmentation
The ability of FSL to fragment the lens results in the need for less energy to be expended inside the eye. Nagy et al. showed that the FSL reduced phacoemulsification power by 43% and operative time by 51% in porcine eye study.[13] Similarly, Conrad-Hengerer et al. showed that in FLACS mean laser time was 54.9 s and effective phacoemulsification time was 0.16 ± 0.21 s compared to 4.07 ± 3.14 s in the standard group.[21] Palanker et al. observed a decrease in the perceived hardness of nuclear sclerotic cataract after FLACS, estimated by the surgeon to decrease from grade four to grade two.[12]
Complications associated with FLACS
It is postulated that FLACS will revolutionize the cataract surgery in the coming years but for now it presents a unique set of clinical and financial challenges to cataract surgeon. Though FLACS showed excellent results in cataract surgery but still there are many complications to be considered. A retrospective study of the first FLACS, conducted by Ophthalmology Department of Semmelweis University, Budapest, Hungary showed following complications:[28]
Suction break 2%
Conjunctival redness and hemorrhage 34%
Capsular tags and bridges 20%
Anterior tears 4%
miosis 32%
Endothelial damage due to cut within endothelial layer 3%.
However, a major reason for the above mentioned complications is considered to be learning curve associated with initial cases. These can be avoided by cautious surgical techniques and more skillful surgery. In fact, Bali et al. conducted a study and they stated that these complications were a part of learning curve.[29]
There is also additional operating room shifting time involved with the use of FSL, i.e. the patient needs to be shifted to the operating theater after application of laser treatment. Although the laser treatment usually takes only a few minutes, there is time involved in getting the patient positioned under the laser as well as associated OCT imaging and treating the eye. The phacoemulsification portion of the procedure is done under the operating microscope so the patient will have to move from either the laser bed or be wheeled out from under the laser to the operating microscope. These logistical issues as well as the other previously mentioned issues could result in increased time spent with each individual patient. This overall increased time could influence the volume of cataract patients that a surgeon, surgical center, or hospital may be able to perform during a given operating room/block if not evaluated carefully. All of these considerations must be taken into account when determining patient charges for this procedure.[30]
Limitations of FSL are not well recognized at this time but it is considered that patients with tremors or dementia will do poorly in initial docking system. Similarly patients with corneal opacities, anterior basement membrane dystrophies, ocular surface disease, and pannus with encroaching blood vessels are poor candidates. Furthermore, glaucoma patients need further assessment due to change in IOP during initial docking system. Since, FLACS relies on good anterior chamber imaging so patients with poor pupillary dilatation and posterior synechiae also become poor candidates.[31]
There is a great dilemma regarding financial feasibility of FLACS. A survey performed by Dalton, involved 1047 ophthalmologists. Of which 72% stated that financial issues were their most important concern about adopting this technology. Reduced workflow efficiency, patient dissatisfaction and increased patient expectations were also noted.[32] FLACS currently costs around $1500-$4000 per eye with a good foldable IOL in Pakistan.
Conclusion
In conclusion, FLACS appears to be a safe, efficient and reproducible procedure with promising results. It may eventually be proven superior to conventional cataract surgery and entirely change the face of cataract surgery. However at this point, more studies need to be done to establish this. The coming years of research will show how far FSL will grow in cataract surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sugar A. Ultrafast (femtosecond) laser refractive surgery. Curr Opin Ophthalmol. 2002;13:246–9. doi: 10.1097/00055735-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 2.He L, Sheehy K, Culbertson W. Femtosecond laser-assisted cataract surgery. Curr Opin Ophthalmol. 2011;22:43–52. doi: 10.1097/ICU.0b013e3283414f76. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson KE, Braga-Mele R, Cabot F, Davidson R, Dhaliwal DK, Hamilton R, et al. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39:1753–63. doi: 10.1016/j.jcrs.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Juhasz T, Loesel F, Kurtz R, Horvath C, Bille J, Mourou G. Corneal refractive surgery with femtosecond lasers. IEEE J Select Topics Quantum Electron. 1999;5:902–10. [Google Scholar]
- 5.Trikha S, Turnbull AM, Morris RJ, Anderson DF, Hossain P. The journey to femtosecond laser-assisted cataract surgery: New beginnings or a false dawn? Eye (Lond) 2013;27:461–73. doi: 10.1038/eye.2012.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koretz JE, Strenk SA, Strenk LM, Semmlow JL. Scheimpflug and high-resolution magnetic resonance imaging of the anterior segment: A comparative study. J Opt Soc Am A Opt Image Sci Vis. 2004;21:346–54. doi: 10.1364/josaa.21.000346. [DOI] [PubMed] [Google Scholar]
- 7.Fink W. Refractive correction method for digital charge-coupled device-recorded Scheimpflug photographs by means of ray tracing. J Biomed Opt. 2005;10:024003. doi: 10.1117/1.1899683. [DOI] [PubMed] [Google Scholar]
- 8.Steinert R. Presentation. Femtosecond Laser Refractive Cataract Surgery. 63rd Annual Proctor Lecture. 2011 [Google Scholar]
- 9.Masket S, Sarayba M, Ignacio T, Fram N. Femtosecond laser-assisted cataract incisions: Architectural stability and reproducibility. J Cataract Refract Surg. 2010;36:1048–9. doi: 10.1016/j.jcrs.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Ernest PH, Kiessling LA, Lavery KT. Relative strength of cataract incisions in cadaver eyes. J Cataract Refract Surg. 1991;17(Suppl):668–71. doi: 10.1016/s0886-3350(13)80681-5. [DOI] [PubMed] [Google Scholar]
- 11.Krasnov MM. Laser-phakopuncture in the treatment of soft cataracts. Br J Ophthalmol. 1975;59:96–8. doi: 10.1136/bjo.59.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palanker DV, Blumenkranz MS, Andersen D, Wiltberger M, Marcellino G, Gooding P, et al. Femtosecond laser-assisted cataract surgery with integrated optical coherence tomography. Sci Transl Med. 2010;2:58ra85. doi: 10.1126/scitranslmed.3001305. [DOI] [PubMed] [Google Scholar]
- 13.Nagy Z, Takacs A, Filkorn T, Sarayba M. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. J Refract Surg. 2009;25:1053–60. doi: 10.3928/1081597X-20091117-04. [DOI] [PubMed] [Google Scholar]
- 14.Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, Sweet PM, et al. Acute endophthalmitis following cataract surgery: A systematic review of the literature. Arch Ophthalmol. 2005;123:613–20. doi: 10.1001/archopht.123.5.613. [DOI] [PubMed] [Google Scholar]
- 15.Dooley IJ, O’Brien PD. Subjective difficulty of each stage of phacoemulsification cataract surgery performed by basic surgical trainees. J Cataract Refract Surg. 2006;32:604–8. doi: 10.1016/j.jcrs.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 16.Dick HB, Peña-Aceves A, Manns M, Krummenauer F. New technology for sizing the continuous curvilinear capsulorhexis: Prospective trial. J Cataract Refract Surg. 2008;34:1136–44. doi: 10.1016/j.jcrs.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34:368–76. doi: 10.1016/j.jcrs.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Hollick EJ, Spalton DJ, Meacock WR. The effect of capsulorhexis size on posterior capsular opacification: One-year results of a randomized prospective trial. Am J Ophthalmol. 1999;128:271–9. doi: 10.1016/s0002-9394(99)00157-9. [DOI] [PubMed] [Google Scholar]
- 19.Murano N, Ishizaki M, Sato S, Fukuda Y, Takahashi H. Corneal endothelial cell damage by free radicals associated with ultrasound oscillation. Arch Ophthalmol. 2008;126:816–21. doi: 10.1001/archopht.126.6.816. [DOI] [PubMed] [Google Scholar]
- 20.Shin YJ, Nishi Y, Engler C, Kang J, Hashmi S, Jun AS, et al. The effect of phacoemulsification energy on the redox state of cultured human corneal endothelial cells. Arch Ophthalmol. 2009;127:435–41. doi: 10.1001/archophthalmol.2009.39. [DOI] [PubMed] [Google Scholar]
- 21.Conrad-Hengerer I, Hengerer FH, Schultz T, Dick HB. Effect of femtosecond laser fragmentation on effective phacoemulsification time in cataract surgery. J Refract Surg. 2012;28:879–83. doi: 10.3928/1081597X-20121116-02. [DOI] [PubMed] [Google Scholar]
- 22.Xia Y, Liu X, Luo L, Zeng Y, Cai X, Zeng M, et al. Early changes in clear cornea incision after phacoemulsification: An anterior segment optical coherence tomography study. Acta Ophthalmol. 2009;87:764–8. doi: 10.1111/j.1755-3768.2008.01333.x. [DOI] [PubMed] [Google Scholar]
- 23.Grewal DS, Basti S. Comparison of morphologic features of clear corneal incisions created with a femtosecond laser or a keratome. J Cataract Refract Surg. 2014;40:521–30. doi: 10.1016/j.jcrs.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Nagy ZZ, Kránitz K, Takacs AI, Miháltz K, Kovács I, Knorz MC. Comparison of intraocular lens decentration parameters after femtosecond and manual capsulotomies. J Refract Surg. 2011;27:564–9. doi: 10.3928/1081597X-20110607-01. [DOI] [PubMed] [Google Scholar]
- 25.Friedman NJ, Palanker DV, Schuele G, Andersen D, Marcellino G, Seibel BS, et al. Femtosecond laser capsulotomy. J Cataract Refract Surg. 2011;37:1189–98. doi: 10.1016/j.jcrs.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Kránitz K, Takacs A, Miháltz K, Kovács I, Knorz MC, Nagy ZZ. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J Refract Surg. 2011;27:558–63. doi: 10.3928/1081597X-20110623-03. [DOI] [PubMed] [Google Scholar]
- 27.Abell RG, Davies PE, Phelan D, Goemann K, McPherson ZE, Vote BJ. Anterior capsulotomy integrity after femtosecond laser-assisted cataract surgery. Ophthalmology. 2014;121:17–24. doi: 10.1016/j.ophtha.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Nagy ZZ, Takacs AI, Filkorn T, Kránitz K, Gyenes A, Juhász É, et al. Complications of femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2014;40:20–8. doi: 10.1016/j.jcrs.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 29.Bali SJ, Hodge C, Lawless M, Roberts TV, Sutton G. Early experience with the femtosecond laser for cataract surgery. Ophthalmology. 2012;119:891–9. doi: 10.1016/j.ophtha.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Hatch KM, Talamo JH. Laser-assisted cataract surgery: Benefits and barriers. Curr Opin Ophthalmol. 2014;25:54–61. doi: 10.1097/ICU.0000000000000013. [DOI] [PubMed] [Google Scholar]
- 31.Moshirfar M, Churgin DS, Hsu M. Femtosecond laser-assisted cataract surgery: A current review. Middle East Afr J Ophthalmol. 2011;18:285–91. doi: 10.4103/0974-9233.90129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalton M. Laser-Assisted Cataract Surgery; Bringing New Technologies into the Fold. EyeWorld. 2011. [Last accessed on 2014 Oct 02]. pp. 30–1. Available at: http://www.eyeworld.org/article-bringing-new-technologies-into-the-fold .


