Abstract
This work reviews concepts regarding oxidative stress and the mechanisms by which endogenous and exogenous factors produce reactive oxygen species (ROS). It also surveys the relationships between oxidative stress, circadian rhythms, and retinal damage in humans, particularly those related to light and photodamage. In the first section, the production of ROS by different cell organelles and biomolecules and the antioxidant mechanisms that antagonize this damage are reviewed. The second section includes a brief review of circadian clocks and their relationship with the cellular redox state. In the third part of this work, the relationship between retinal damage and ROS is described. The last part of this work focuses on retinal degenerative pathology, age-related macular degeneration, and the relationships between this pathology, ROS, and light. Finally, the possible interactions between the retinal pigment epithelium (RPE), circadian rhythms, and this pathology are discussed.
1. Introduction
Over millions of years of evolution, organisms have developed diverse protective systems to control excess reactive oxygen species (ROS), which produce oxidative stress (OS). This term refers to elevated intracellular levels of ROS that cause damage to lipids, proteins, and DNA, a process that has been considered to be linked to a myriad of pathologies in humans.
The mechanisms of ROS production (e.g., via aerobic respiration or flavin-containing oxidases) and its rapid removal (e.g., via catalase) are present in almost all of the cell types found in organisms. OS effects depend on the intensity of damage induced by the ROS in the cell and the cellular response to this damage; if the cell is unable to overcome the damage and recover its function or if exogenous and endogenous antioxidant defenses (AOXs) cannot counter it, the cell can die. However, ROS have also been shown to function as second messengers by transducing extracellular signals to generate specific cellular responses. Proteins and other molecules that participate in signaling pathways can be modified by redox changes [1]. Numerous studies have substantially contributed to the development of the concepts of OS and the mechanisms involved in the production and regulation of ROS as well as their participation in cellular signaling processes (for a review, see [2]). The present work briefly reviews some of the mechanisms of ROS production and scavenging in addition to the participation of ROS in complex processes such as aging and biological rhythms. Due to the growing interest in the involvement of ROS in degenerative pathologies, the last part of this review is focused on the effects of OS on retinal degenerative processes, particularly on age-related macular degeneration (AMD). Due to the importance of circadian rhythms in the development of degenerative pathologies, a short survey of the possible relationships between circadian rhythms and AMD is included in the last part of this work.
2. Reactive Oxygen Species
ROS are formed either during metabolic processes that are linked to life-sustaining, enzyme-catalyzed reactions, such as aerobic respiration, or during responses to stress reactions when organisms, including humans, are exposed to biotic and abiotic stress factors, such as situations like hypoxia or anoxia. ROS encompass a variety of diverse chemical species including singlet oxygen, superoxide anion radical, hydroxyl radical (OH), hydrogen peroxide (H2O2), hydroxylperoxyl radical, alkoxyl radicals, and peroxyl radicals. Superoxide anion (O−) is converted to H2O2 by the enzyme superoxide dismutase (SOD), and the hydroxyl radical (OH) is a byproduct of the Fenton reaction. Nitric oxide (NO) and singlet oxygen are examples of reactive species. Some of these species, such as superoxide or hydroxyl radicals, are extremely unstable, whereas others, such as H2O2, are freely diffusible and relatively long-lived. These various radical species can be generated either exogenously by physical or chemical factors that induce stress reactions or through cell-dependent mechanisms via several different mechanisms, such as cytosolic enzyme systems or mitochondrial mechanisms. The cytosolic systems include, among others, the family of NADPH oxidases (NOX) [3], whereas the production of mitochondrial superoxide radicals occurs primarily at two discrete points in the electron transport chain (ETC), namely, complex I (NADH dehydrogenase) and complex III (ubiquinone-cytochrome c reductase) [4]. Mitochondrial production of ROS will be discussed in the next section. Some of the exogenous chemical sources of ROS include the xanthine/xanthine oxidase system, which produces O−; the Fenton reagent, which generates HO−; and photosensitizers such as rose bengal and benzoporphyrin derivatives, which produce 1O2 upon photosensitization [5, 6]. Physical abiotic factors such as UV radiation and visible light result in the formation of radical (O− and H•) and nonradical (1O2) ROS by Type I and Type II reactions, respectively (for a review, see [7]) (Figure 1). Various endogenous pigments in organisms, such as porphyrins, bilirubins, melanins, and pterins, are known to act as photosensitizers by absorbing radiation or visible light. This leads to the formation of the singlet photosensitizer state, which forms the triplet excited state. The excited photosensitizer undergoes either electron transport, forming O2 −, H2O2, and HO• or energy transfer, forming 1O2 (Type II reaction) [8, 9]. In Type I reaction, electron transport leads to the production of O2 − via the formation of photosensitizer anion radicals and substrate cation radicals or vice versa [10]. Spontaneous or enzymatically driven dismutation of O2 − leads to the formation of H2O2, which subsequently forms OH• via the Fenton reaction or other metal-catalyzed reactions. In a Type II reaction, triplet-singlet energy transfer from the excited photosensitizer to molecular oxygen forms 1O2 [11].
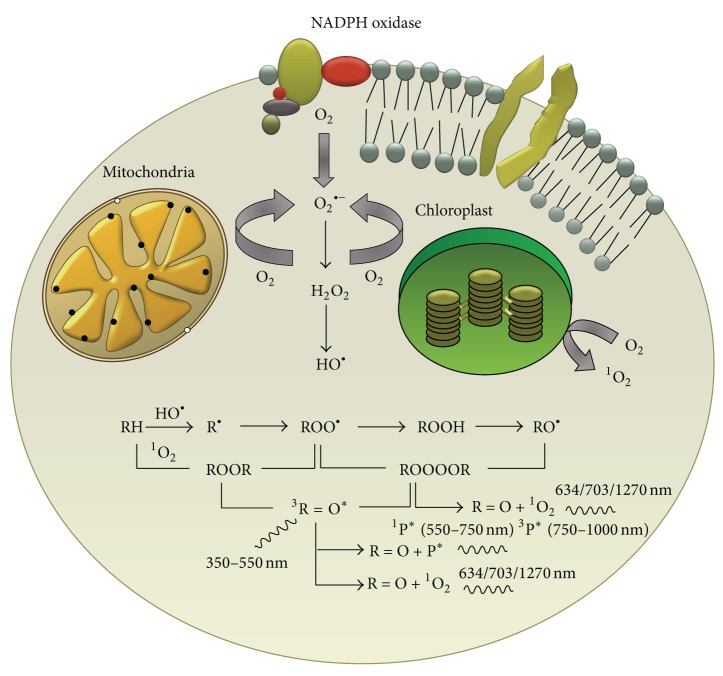
Figure 1.
A model showing the formation of reactive oxygen species (ROS) in different organelles of the cell. Superoxide anion radical (O2 −) is produced via the membrane-bound enzyme complex NADPH oxidase (nicotinamide adenine dinucleotide phosphate-oxidase) which is found embedded within the plasma membranes and membranes of various organelles such as mitochondria, chloroplasts, and phagosomes. The dismutation of O2 − is accompanied by the formation of hydrogen peroxide (H2O2) and then the hydroxyl radical (HO•) via the Fenton reaction. The highly reactive HO• has the capability to oxidize all types of biomolecules such as lipids, proteins, and nucleic acids. The oxidation of biomolecules is accompanied by the formation of high-energy intermediates such as dioxetane (ROOR) and tetroxide (ROOOOR), which upon further decomposition, generate electronically excited species such as triplet excited carbonyl, singlet and triplet excited pigments, and singlet oxygen (1O2). From Pospišíl et al. [7] with the permission of the authors and Elsevier.
Among all of the biomolecules that can be attacked by ROS, lipids are likely the most susceptible to oxidation. All cell membranes are rich in polyunsaturated fatty acids (PUFAs), which are easily injured by oxidizing agents. Peroxidation of cellular lipids modifies membrane properties and produces cytotoxic compounds, although some peroxidation products also play useful roles. Lipid peroxidation (autoxidation), a process that leads to the oxidation of PUFAs due to the presence of several double bonds in their structure, involves the production of peroxides and reactive organic free radicals (FRs). The latter can then react with other fatty acids, initiating a FR reaction cascade.
ROS can also react with nucleic acids by attacking nitrogenous bases and the sugar phosphate backbone. Furthermore, some of their primary effects include basic DNA damage and single- and double-strand DNA breaks. To a large extent, the oxidative radical damage that occurs in nucleic acids results from the reaction of DNA with radicals such as H• and •OH. Hydroxyl radicals are known to attack DNA, causing single- and/or double-strand breaks as well as pyrimidine and purine lesions, both of which can affect the integrity of the genome [12]. The inability of cells to repair the damage incurred in this process may lead to their death; alternatively, mutations may occur in the DNA, leading to carcinogenesis or the development of neurodegenerative diseases [13].
Mitochondrial DNA (mtDNA) is more susceptible to oxidative damage than its nuclear counterpart. Mutations in mtDNA can cause disturbances in the respiratory chain and loss of the control of ROS production. mtDNA is not protected by histones or other associated proteins, and because it has intronless regions and a high transcription rate, mtDNA is more susceptible to oxidative modifications in its coding regions. The reduced effectiveness of the repair system for mtDNA damage may be the cause of the accumulation of OS and its consequences. The formation of carbonyl derivatives is the most widely studied OS-induced protein modification [14]. Carbonyl formation can occur through a variety of mechanisms, including the direct oxidation of certain amino acid side chains and oxidation-induced peptide cleavage. Although all organs and proteins can potentially be modified by OS, certain tissues and protein targets may be especially susceptible [3]. Other protein modifications are NO-dependent. For example, NO reacts with O2 •− to generate ONOO−, which is capable of initiating further protein oxidation and nitration [15, 16].
The nitrogen dioxide radical, which is biologically formed from the reaction of N2 with oxygen or by the decomposition of ONOO−, reacts with tyrosine residues, resulting in the formation of 3-nitro-tyrosine. The addition of NO to the thiol groups of proteins via S-nitrosation (also referred to as S-nitrosylation) has also been reported to be associated with neurodegenerative diseases [16].
2.1. Mitochondria and ROS
Mitochondria are a major intracellular generator of ROS (mitoROS) [17]. Multiple producers of O− have been reported in mitochondria, including flavins in complexes I and II; ubiquinone binding sites in complexes I, II, and III; flavoprotein-Q oxidoreductase-mediated fatty acid beta-oxidation; and glycerol-3-phosphate, 2-oxoglutarate, and pyruvate dehydrogenases [18–21]. The major players in mitoROS production are complexes I and III of the mitochondrial ETC [18], although other ROS-producing mitochondrial sites include mitochondrial glycerol-3-phosphate dehydrogenase, the electron-transferring flavoprotein/ETF: ubiquinone oxidoreductase system of fatty acid oxidation, dihydroorotate dehydrogenase, the dihydrolipoamide dehydrogenase, and 2-oxoacid dehydrogenase complexes. In addition, the 2-oxoglutarate dehydrogenase complex, the branched-chain 2-oxoacid dehydrogenase complex, the pyruvate dehydrogenase complex, and proline dehydrogenase, among others, have been implicated in ROS production (for a review, see [22]). The mitochondrial ETC consists of four mitochondrial redox carriers that are known as complexes I, II, III, and IV. Electrons that are donated by NADH and FADH2 toward complexes I and II, respectively, are transferred to complex III and then eventually to complex IV to reduce oxygen molecules to water [23]. In complex I, O− production is driven by the presence of NADH, which donates electrons to a series of redox enzymes within the complex itself; this series includes flavin mononucleotide, Fe−S clusters, and coenzyme Q10 (ubiquinone) [24]. O− production has been linked to two sites within complex I: reduced flavin mononucleotides and quinone binding sites [24]. In isolated mitochondria, complex I can reduce O2 to O− under two different conditions: (1) when ATP is in low demand and the proton motive force (PMF) is maximal and (2) when the NADH/NAD+ ratio is high and ATP is continuously synthesized, resulting in a low PMF [25]. The first mechanism, which is also known as reverse electron transfer (RET), occurs when the PMF is maximal or when electrons donated from succinates in complex II are reverse transferred to complex I. This action causes electron binding to quinone binding sites, which allows electrons to leak out and reduce oxygen molecules [25]. The second mechanism, which is known as forward electron transfer, requires the donation of electrons from reduced form to oxygen molecules, producing O−. This process depends on a high NADH/NAD+ ratio or inhibition of the respiratory chain through mitochondrial damage, ischemia, the loss of cytochrome c, or mutations. It also causes the loading of electrons onto flavin and the subsequent leakage of electrons that reduce oxygen molecules.
In addition to the NADH-driven ROS production from complex I and, subsequently, complex III, complex II has also been demonstrated to be a source of ROS production [26, 27]. Complex II, or succinate dehydrogenase, is composed of four subunits: flavoprotein, the Fe–S cluster, and two transmembrane cytochrome b heme subunits [21, 28]. In complex II, O− production is driven by the presence of succinate, which sequentially donates two electrons to flavin, the Fe–S cluster and ubiquinone [27, 29, 30]. Complex IV, in contrast, has yet to be shown to directly produce ROS, although it affects the course of O− production by complexes I, II, and III and seems to be the terminal and rate-limiting complex that influences the flow of electrons across the ETC.
2.2. Autophagy and ROS
Autophagy is a lysosome-mediated degradation process for nonessential or damaged cellular components [31]. Physiologically, autophagy preserves the balance between organelle biogenesis and both the synthesis and clearance of proteins. This process is emerging as an important mediator of pathological responses because it involves cross talk between ROS and RNS (reactive nitrogen species) during both cell signaling and protein damage. Dysregulated redox signaling or mitochondrial dysfunction can also influence autophagic activities. OS is inseparably linked to mitochondrial dysfunction because mitochondria are both generators and targets of reactive species. Mitochondrial turnover depends on autophagy, which declines with age and is frequently dysfunctional in neurodegenerative diseases [32]. OS can also lead to nonspecific posttranslational modification of proteins, and it contributes to protein aggregation (for a review, see [33]).
2.3. Antioxidants
Organisms have natural AOXs that diminish the harmful effects of continuous ROS production. There are both endogenous and exogenous defense mechanisms against oxidative attack. OS and its biomarkers are the biochemical products of an imbalance between ROS production and the ability of biological AOXs to counteract the effects of ROS metabolites [34]. These defenses are located in the cytoplasm, the cellular membrane, and the extracellular space, and they include enzymatic defense systems, FR scavengers, and chelating agents for transitional metals. The enzymatic systems consist of intracellular molecules, such as SODs, catalase, glutathione peroxidase (GPx), and reductase [35]. FR scavengers slow down oxidation reactions, trapping FRs and transforming them into less aggressive compounds. They can be hydrosoluble and cytosolic (e.g., GSH and vitamin C) or liposoluble and membrane-bound (e.g., vitamin E and carotenoids). Vitamin C is considered to be the main hydrosoluble AOX system, and it may also regenerate tocopherol (vitamin E), which is one of the main liposoluble AOXs in cellular membranes, along with carotenoids (β-carotene, lutein, zeaxanthin, and lycopenes). GSH is considered the most abundant AOX system in the cytosol, nucleus, and mitochondria. The relevant chelating agents include molecules that bind to iron and copper as well as flavonoids. In this way, these chelating agents prevent these metals from participating in Fenton and Haber-Weiss reactions. Depending on their source, AOX agents can be either endogenous, as in GSH, SOD, and CAT, or exogenous (only obtained from nutrients), as in vitamins C and E, carotenoids, flavonoids, and oligo elements [36].
2.3.1. Melatonin as an Antioxidant
Melatonin (N-acetyl-5-methoxytryptamine) is an important AOX molecule. It is a ubiquitous substance that is secreted by the pineal gland of all mammals, including humans. In addition, its presence has been confirmed in many animals, plants, and unicellular organisms [37–39]. Melatonin participates in diverse functions in the body, including sleep, circadian rhythm regulation, and immunoregulation, and it may have anticarcinogenic effects [39]. As a chelating agent, melatonin is a potent FR scavenger and a regulator of redox-active enzymes [40]. This hormone is secreted during darkness and plays a key role in various physiological responses, including the regulation of circadian rhythms, sleep, homeostasis, retinal neuromodulation, and vasomotor responses. It scavenges hydroxyl, carbonate, and various organic radicals and a number of RNS. Melatonin also enhances the AOX potential of cells by stimulating the synthesis of AOX enzymes, including SOD, GPx, and glutathione reductase, and by augmenting glutathione levels [41]. This hormone preserves mitochondrial homeostasis, reduces FR generation, and protects mitochondrial ATP synthesis by stimulating complexes I and IV, thereby counteracting oxidative mtDNA damage and restoring the mitochondrial respiratory control system [41]. Because reduced complex I activity and is a sign of enhanced electron leakage, the resulting increase in OS is sufficient to induce apoptosis. The ability of melatonin to return complex I activity to normal levels suggests its significance in overall health via the prevention of age-associated degenerative changes [42].
2.4. ROS and Aging
Aging has been considered to involve the progressive accumulation of changes with time that are associated with or responsible for the ever-increasing susceptibility to disease and death that accompanies advancing age. In 1956, Harman [43] proposed that endogenously generated oxygen FRs induce the macromolecular oxidative damage that is responsible for senescence (for a review, see [44]) and is associated with a decline in physiological fitness during aging. This “free radical hypothesis” was modified and merged with the “oxidative stress hypothesis,” resulting in the term “oxidative stress” [45], which was defined as a disturbance in the pro-oxidant-antioxidant balance that results in a cellular state in which the AOXs are insufficient for complete eradication of various ROS [44]. Both propositions postulate that the progression of age-related deleterious alterations is a function of the imbalance between ROS fluxes and AOXs, and both predict that the narrowing of this gap should reduce the amount of structural damage and thereby prolong the life span. “Therefore, from this historical perspective, the postulated mechanism by which ROS are implicated in the aging process can be aptly characterized as the structural damage-based oxidative stress hypothesis” [44]. Over the past several decades, however, there has been a shift in ideology concerning the role of ROS in cell physiology. Some oxidants, particularly H2O2, have now been recognized as essential for cell survival due to their regulatory roles in a wide range of functions, including gene regulation, cell signaling, protein activation/deactivation, cellular differentiation, and apoptosis (reviewed in [46, 47]). Now, ROS have been recognized to act as signals [48, 49]. Although some of these signaling pathways promote cell death [50], others, such as oxygen-sensing and hypoxic responses [51] or the induction of autophagy [52], promote cell survival. Therefore, in theory, low (nontoxic) levels of ROS can promote lifespan extension by activating pathways that promote cellular resistance to various stresses.
Although there is little doubt that high levels of ROS are detrimental, mounting evidence indicates that mild to moderate ROS elevation extends lifespan. Dietary caloric restriction, inhibition of insulin-like growth factor-I signaling, and inhibition of the nutrient-sensing mechanistic target of rapamycin are robust longevity-promoting interventions [53] that all appear to elicit retrograde mitochondrial signaling processes that may even spread to other cells.
Other factors, such as biological rhythms, also seem to contribute to aging [54]. Age-associated changes in the day/night rhythm of melatonin production have been identified, with phase advances encountered more frequently in the elderly compared with young women [55]. Suprachiasmatic nucleus (SCN) function has also been shown to decline with age, particularly in patients with aging-associated neurodegenerative disorders, which are a major cause of dementia, and other poor health conditions that are common in elderly populations [56, 57]. A decline in melatonin production and altered melatonin rhythms can be major contributing factors to increased levels of OS and the associated degenerative changes that are observed in the elderly. Nevertheless, individuals of the same chronological age can exhibit dissimilar degrees of senescence-associated functional impairment, differences that may be attributable to well-documented interindividual variations in melatonin levels [58–61]. Variations in the degenerative changes that occur in cells and tissues have been attributed to variations in melatonin production; these changes are more often determined by an individual's physiological age rather than his chronological age [62]. Recently, genetic variation in the enzyme ASMT (HIOMT), which performs a metabolic step that determines the amount of melatonin produced [63], has been demonstrated.
3. Circadian Clocks and ROS
Circadian rhythmicity is a fundamental biological phenomenon that is universally important. This endogenous, innate oscillation with a period of approximately one day has been identified in all of the organisms that have been studied to date, from bacteria to eukaryotes. Temporal variations that are driven by a circadian oscillator are evident in many cellular functions including gene expression, metabolic flux rates, concentrations of signaling molecule, and cellular substructures. In multicellular organisms, circadian rhythms can be studied at different integration levels, from cell-to-cell interactions to organ physiology and from endocrine and neural communications to behavior. Although the control and coordination of circadian rhythms in metazoans are typically organized by specialized pacemaker structures, primary oscillations are generated at the cellular level. These rhythms are widely accepted to be genetically determined, and several genetic clocks have been identified in different taxa, including unicellular organisms [64].
The core of the circadian clock is based on an intracellular time-tracking system that enables organisms to anticipate environmental changes and thereby adapt their behavior and physiology to the appropriate time of day [65]. It is well known that in some animals, such as insects and mammals, a specific set of transcription factors constitutes the molecular architecture of the circadian clock. These factors are organized into positive and negative regulatory feedback loops that function in a cell-autonomous manner and that are rhythmically controlled by a master oscillatory system, which coordinates tissue-specific rhythms according to the input it receives from the rhythms of the outside world [66].
The one or more endogenous oscillators that function to generate a free-running period that is close to 24 h when the organism is maintained under constant environmental conditions are a central component of the circadian system. At the molecular level, these oscillators are based both on the products of “clock genes,” which are organized into transcriptional-translational feedback loops (TTLs), and on oscillations in posttranslational modifications of proteins, which contribute significantly to circadian oscillations [67]. Some of the clock genes encode transcriptional activators, whereas others encode negative feedback elements that inhibit their own expression by disrupting the activity of their activators. Meanwhile, kinases and phosphatases regulate the speed and precision of the clock [68]. Components of the oscillators receive environmental information through input pathways, allowing the oscillators to remain synchronized with the 24 h solar day. This time-of-day information from the oscillator(s) is then relayed through output pathways to regulate the expression of circadian clock-controlled genes and overt rhythmicity. One mechanism by which the output pathways are predicted to be rhythmically controlled is through transcription factors or signaling molecules that are themselves components of the oscillator. These factors, which are activated by the circadian clock, may in turn regulate the downstream clock control genes in a time-of-day-specific manner [69].
The internal clock would be useless if it was not able to synchronize with environmental time or if the cells within a tissue were not synchronized to each other. Therefore, input pathways to the circadian oscillator are vital to maintain the proper timing of the oscillator with respect to the environment. In a process called entrainment, input pathways reset the oscillator so that the period of the oscillator conforms to the 24 h period of the environment [69]. Input pathways detect environmental cues and utilize various mechanisms to increase or decrease the levels or activity of components of the molecular oscillator to set the clock to the correct time of day. One of the most ubiquitous time-giving cues is light, but nonphotic environmental cues, including nutrition, temperature, and social interactions, can also entrain the circadian clock [70–73]. In addition, the clock utilizes a strategy called gating to restrict responses to environmental cues at certain times of day. For example, diurnal mammals are typically insensitive to a light pulse during the day, but, during the night, a light pulse can advance or delay the clock to synchronize it with the environment [74], just as we would adjust our watches to match the local time. In organisms of various complexities, cells vary in their ability to support a molecular oscillator that can be entrained by environmental signals. In unicellular organisms, each cell has a fully entrainable oscillator that primarily responds to light [75]. However, in complex multicellular organisms, not all cell types have the necessary sensory capabilities, such as photoreception, to entrain the circadian oscillator. The cellular oscillators and overall rhythmicity of the organism are broken down into components that include a master pacemaker and peripheral oscillators [76]. To integrate multiple sensory inputs, organisms that possess a nervous system typically delegate the ability to sense environmental cues to a central oscillator or pacemaker rather than to individual cells. In mammals, sensory inputs to the clock are integrated in the brain, where signals from the master pacemaker entrain the oscillators in other tissues throughout the organism. Light is perceived by nonvisual retinal ganglion cells that transmit information via neural connections to the master pacemaker, which is located in a region of the hypothalamus, the SCN. The SCN pacemaker synchronizes oscillators in other tissues by a mechanism that utilizes circadian input pathways from the SCN to individual cells in the periphery. In addition to maintaining the entrainment of peripheral oscillators by the environment, this system ensures that cellular oscillations within tissues are properly in phase to provide resonance between individual cellular rhythms [77].
Melatonin acts as an important synchronizer in mammals and provides temporal feedback to oscillators within the SCN, which regulates the circadian phase and maintains rhythmic stability [78]. Various studies in birds and mammals, both in vitro and in vivo, have demonstrated that melatonin adjusts the circadian “clock” by acting directly on the molecular timing system [78, 79]. In humans, as in other mammals, melatonin is presumed to influence circadian rhythms by acting directly on receptors in the SCN [80].
For at least three decades, the cellular redox state in plants and animals has been known to change over circadian time [81], although many chronobiologists have long assumed that metabolic rhythms are a functional readout of the circadian clock and that redox oscillators simply provide feedback to the central TTL pacemaker [82].
Recent discoveries have, however, uncovered redox-based circadian oscillators that are conserved across both eukaryotic and prokaryotic species. Circadian rhythms in ROS generation and scavenging have been observed in a variety of species, including fungi [83], plants [84], and animals [85]. Further evidence of the involvement of the circadian clockwork in the regulation of redox systems is supported by studies of mutants, where the deletion or disruption of clock genes also results in the perturbation of redox systems. In mammals, 24 h oscillations in concentrations of oxidized NADPH and the reduced form of FAD have been observed in organotypic slices of the rodent SCN [86]. The rhythms of these coenzymes are believed to be dependent on the molecular clockwork because Bmal1−/− mice exhibit stochastic, but not circadian, FAD, and NADPH rhythms. These studies also revealed links between the redox state and the membrane excitability of SCN neurons, given that oxidizing and reducing agents can produce hyperpolarization and depolarization, respectively. Redox-dependent modulation of K+ channel conductance is believed to underlie these oscillations [86]. All of these findings support complex interdependence among the redox state, cellular energetics, and circadian clockwork in mammals.
The links between circadian physiology, prooxidizing changes in the redox state, as reflected by a decline in redox potential, and the process of aging seem to be coherent and well established [87]. Hence, in the present work, the OS damage generated in the human eye and its relationship with AMD is briefly discussed. Here, the impact of OS from the accumulation of ROS, which is probably due to circadian alterations, and its relevance to chronic pathologies, is briefly reviewed, with a particular emphasis on retinal neurodegenerative diseases such as AMD.
4. The Retina and Oxidative Stress
In humans, the retina is very prone to the generation of ROS compared with other tissues. This structure is a photosensitive tissue with high oxygen levels in the choroid and a high metabolic rate that is also exposed to light. Furthermore, the retina contains a higher concentration of PUFAs than other body tissues [88]. Lipids in the outer segment membranes of photoreceptors can be oxidized by radicals produced during photonic activation, and the endogenous oxygen species that are generated in the eyes through this process can induce ROS-related acute or chronic retinal damage. All of these factors, combined with the very high oxygen levels in the choroid, the high metabolic rate, and the exposure to light, make our retinas vulnerable to the effects of light, especially to light of shorter wavelengths [89]. “Each day, the retina of the average human absorbs approximately 1012 to 1015 photons, and this amount can be greatly increased by the workplace, sunlight exposure, or medical imaging of the retina during an eye examination. Such high levels of exposure to visible light can cause irreparable damage to the retina” [90]. Recently, Roehlecke et al. [91] demonstrated that ROS are generated and OS occurs directly in the outer segments of photoreceptors after blue light irradiation.
Among all of the retinal cell organelles, the mitochondria are particularly sensitive to OS due to their handling of electrons in the respiratory chain. In addition, after blue light exposure, more electrons deviate from the respiratory chain in the mitochondria, resulting in further damage. In fact, inhibiting the mitochondrial transport chain in retinal pigment epithelium (RPE) cells or adding mitochondria-specific AOXs blocks ROS formation and cell death [89]. Furthermore, chromophores in general and cytochromes in particular can be sources of ROS [36]. OS-induced inflammation initiates a functional decline in tear production, and dry eye is the first symptom of this type of damage to the eye [92].
In the human eye, OS in the RPE is widely accepted as a contributing factor for retinal disorders. The RPE, a monostratified cell layer, constitutes the outer blood-retinal barrier (BRB), controls fluid and metabolic exchange between the retina and the choriocapillaris, and participates in several functions linked to photoreceptor physiology, such as the phagocytosis of shed photoreceptor outer segments and transportation of molecules to and from the retina [93].
In addition, RPE cells release factors that control neuronal survival and angiogenesis (e.g., pigment epithelium-derived factor, PEDF) and that promote photoreceptor survival and have an antiangiogenic effect on the choriocapillaris [94, 95]. The RPE secretes PEDF on the apical side; in contrast, the angiogenic factor vascular endothelial growth factor (VEGF) is secreted on the basolateral side to maintain fenestration of the choriocapillaris. An imbalance in the expression of PEDF and VEGF appears to be involved in ocular pathologies. Because PEDF has antioxidative and antiangiogenic properties, this factor may protect against vision-threatening angiogenic mechanisms. In contrast, VEGF is generally accepted as the most potent inducer of endothelial activation and angiogenesis, a process in which new vessels develop from the preexisting vasculature (for a review, see [96]). Under ischemic or hypoxic conditions, which occur in many neovascular diseases, retinal expression and production of VEGF are dramatically increased [97, 98].
The oxidation of polyunsaturated fats in the retina leads to lipid peroxidation products such as carboxyethylpyrrole and 4-hydroxy-2-nonenal, which can form adducts with proteins and accumulate in the outer retina and in drusen [99]. The levels of AOX proteins such as catalase and SOD are increased in RPE homogenates derived from the eyes of late-stage AMD patients, indicating a response to increased OS in the eyes of these patients [100].
As indicated in previous sections, the NOX [101] family of enzymes has recently been recognized as a generator of ROS in rod or cone photoreceptors after they are damaged due to serum deprivation in a model of retinitis pigmentosa [102]. Roehlecke et al. [103] further demonstrated that blue light irradiation results in increased superoxide anion production.
Lipofuscin is the generic name given to a heterogeneous group of complex and autofluorescent bisretinoids, lipid peroxides, and proteins and to various fluorescent compounds that are formed from modified lipids or that are derived from vitamin A. The major substrate for lipofuscin in the RPE is a nondegradable end product that results from phagocytosis of the photoreceptor outer segments, which are rich in PUFAs and vitamin A. This substrate is located within the RPE, where it accumulates in lysosomes with age. Lipofuscin is a byproduct of the visual cycle [104, 105] that is produced when phagocytosed material is not entirely degraded within the RPE lysosomes, resulting in the accumulation of this complex over time. Lipofuscin is continually exposed to high oxygen tensions (70 mmHg) and to visible light (400–700 nm) during daylight hours, creating a prime environment for the generation of ROS that have the potential to damage cellular proteins and lipid membranes. Thus, lipofuscin is a photoinducible generator of ROS that increases the risk of oxidative injury [106]. A lipofuscin fluorophore, A2E, is now known to mediate the blue light-induced apoptosis of RPE cells, which inhibits the degradative capacity of lysosomes and disrupts membrane integrity [107, 108]. This complex phototoxic effect is wavelength-dependent; more superoxide anions are generated in granules exposed to blue light (400–520 nm) than in granules exposed to red light (660–730 nm) or full white light [109]. Lipofuscin induces the apoptosis of cultured RPE cells, leading to a decline in mitochondrial activity that is associated with the translocation of cytochrome c, an apoptosis-inducing factor, and two apoptosis-inducing proteins into the cytoplasm and nucleus [110].
However, whether A2E or lipofuscin may impair lysosomal function is still controversial. Recently, Saadat et al. [111] demonstrated that A2E and impaired autophagy mediate RPE cell damage. These authors proposed that decreased lysosomal capacity might also result in decreased autophagy in RPE cells, which would not affect autophagosome biogenesis or fusion with lysosomes but would instead impair the terminal stage of the degradation process. Thus, the accumulation of A2E in aged RPE cells, in which autophagy is already impaired, may result in RPE cell damage. The authors also considered the possibility that A2E inhibited lysosomal function, which then resulted in the accumulation of autophagosomes rather than an increase in autophagic flux through this pathway.
Some authors have maintained [112] that the RPE secretes apolipoprotein B particles into Bruch's membrane and that these particles accumulate with age and may in turn form a lipid wall, a precursor of the basal linear deposit. Then, certain constituents of the described aggregates may interact with ROS, resulting in proinflammatory, peroxidized lipids and leading to the upregulation of cytokines/chemokines, which then promotes neovascularization. However, recent publications indicate that, in the RPE, OS is also capable of inducing protective pathways, such as the phosphatidylinositide 3-kinase (PI3K)/Akt and nuclear factor erythroid-2-related factor 2 pathways. In addition, VEGF and neuroprotectin D1 signaling act to protect the retina [113, 114].
The phagocytosis of oxidized fatty acids from photoreceptor outer segments contributes to metabolic failure in the RPE [115]. Importantly, the FR byproducts of mitochondrial energy metabolism damage the RPE [116]. In addition, the mitochondrial ETC generates superoxide radicals through single-electron leaks at respiratory complexes I and III [18].
Flavin-dependent enzymes in the mitochondrial matrix may also be large contributors of ROS [22]. Superoxide can directly damage mitochondrial DNA, proteins, and lipids, and it can also be converted to H2O2 by manganese SOD in the mitochondria [22]. The potential for RPE light damage is influenced by a number of factors, including age, diurnal fluctuations, and pathology [117].
Otherwise, circadian photoreception decreases with age due to disruptions to circadian rhythms, age-related pupillary miosis and reduced crystalline lens transmission, particularly of blue light. Circadian studies have revealed loss of the control of pupil size and the crystalline lens in aging subjects [118].
5. ROS and Age-Related Macular Degeneration (AMD)
AMD, a major cause of visual impairment in the elderly, is linked to pathological changes involving the RPE. AMD is a progressive neurodegenerative disease of the central retinal area (macula lutea) and represents the most common cause of legal blindness in industrialized countries [119]. Epidemiologic studies from several countries have shown a dramatic increase in the prevalence and severity of AMD with age. Despite intensive basic and clinical research, its pathogenesis remains unclear, likely due to its multifactorial nature [120]. In addition to the strong age dependence of the disease, complex interactions between metabolic, functional, genetic, and environmental factors create a platform for the development of chronic changes in the ocular structures of the macular region (e.g., choriocapillaris, Bruch's membrane, RPE, and photoreceptors), and changes in each of these structures may contribute to varying degrees to the onset of AMD (Figure 2). Photoreceptors renew their light-sensitive outer segments continuously and shed their distal tips daily. The adjacent RPE efficiently consumes shed photoreceptor outer segment fragments (POSs) and recycles or digests their components via phagocytosis [121]. Outer segment renewal is crucial for the photoreceptor function and survival. Experimental studies have demonstrated that a lack of efficient POS phagocytosis by RPE cells in some rat strains causes rapid photoreceptor degeneration [122]. A delay in POS digestion can directly cause the accumulation of undigested POS materials, such as lipofuscin, in the RPE, which is detrimental to the RPE and the retina and may contribute to the development or progression of AMD [123, 124].
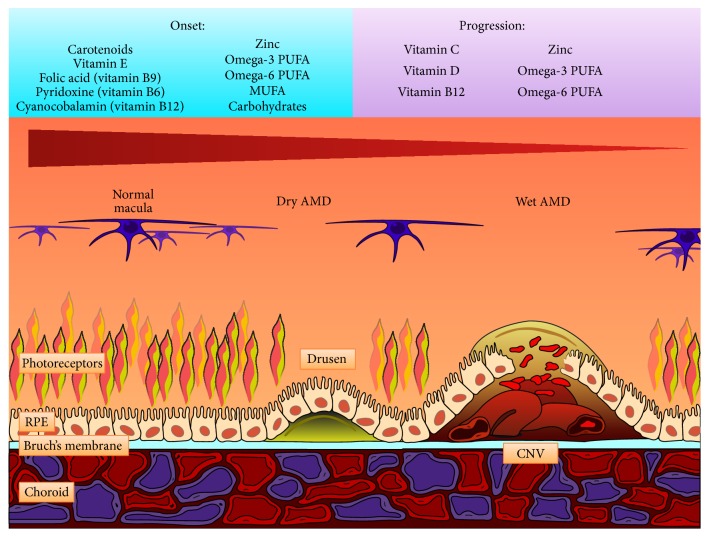
Figure 2.
Development of AMD. AMD is mainly due to photochemical damage and oxidative stress. In the figure, the progression of AMD is represented by the cellular structure of the normal macula (left side), the dry AMD (center), and the wet AMD (right side). In the left side of the image, the cells in the macula are normal. In the dry form of AMD (center), drusen impair the metabolic connection between the choroid and the upper layers of macula, leading to the degeneration of the RPE and photoreceptors. In the wet form of AMD (right side), the production of neovascular factors results in the formation of choroidal neovascularization (CNV) with subsequent fluid leakage and major degeneration of the RPE and photoreceptors. In the normal ageing process, lipids accumulate in Bruch's membrane, causing the membrane to thicken and improving the oxidative distress. Moreover, Bruch's membrane lacks an adequate intrinsic antioxidant system. Furthermore, the lipids can bind the macrophages, inducing the secretion of vascular endothelial growth factor (VEGF). The production of inflammatory factors improves the damage to the RPE and photoreceptors and induces the formation of functional microvascular networks (choroidal neovascularization). Antioxidant factors might prevent the disease and delay its progression. In the upper portion of the image, the pool of nutrients involved in the onset (left side) and progression (right side) of AMD are listed. From Zampatti et al. [131], with the permission of the authors and Elsevier. Rightlinks License number is 3679500345328.
Based on its clinical presentation, AMD is categorized into early, intermediate, and late stages [125]. Early and intermediate AMD are characterized by soft, yellowish deposits that vary in size from small to large (drusen) and by pigmentation changes in the macula, with little or no visual loss. In late AMD, visual loss appears in two forms, that is, neovascular AMD (also called “wet” or “exudative” AMD), in addition to drusen and atrophy. This form of AMD is characterized by the presence of edema and hemorrhage within or below the retina or RPE and geographic atrophy (also called “dry” AMD). Currently, there are no treatments for geographic atrophy, but neovascular AMD is treated with VEGF inhibitors, which, although not curative, are often effective in preventing severe visual loss [126]. The dry form, also known as age-related maculopathy, is characterized by the presence of drusen under the RPE that is accompanied by either the loss or focal accumulation of melanin pigment. This form of AMD is typically characterized by a progressive course that leads to degeneration of the RPE and photoreceptors. The exudative form is linked to choroidal neovascularization that is directed toward the subretinal macular region, with subsequent bleeding and/or fluid leakage that can result in a sudden loss of central vision. It is the most rapidly progressing form of AMD. Both the atrophic and exudative forms are associated with severe visual impairment [119]. The pathophysiology of AMD is complex, and, in addition to genetic predispositions, at least 4 processes contribute to the disease: lipofuscinogenesis, drusogenesis, local inflammation and neovascularization (in the case of the wet form), and immunological mechanisms [127].
The current pathophysiological conception of AMD assigns a primary role to age-related, cumulative oxidative damage to the RPE due to an imbalance between the generation and elimination of ROS [128]. In particular, lipofuscin has been hypothesized to be the primary source of the ROS responsible for both the cellular and extracellular matrix alterations found in AMD [129, 130].
The accumulation of lipofuscin and other lipid peroxides and potentially toxic substances may dramatically influence RPE physiology, as described above. This accumulation greatly reduces the phagocytic capacity, lysosomal enzyme activities, and AOX potential of the human RPE in vitro [133, 134]. The first clinical sign of early AMD is drusen, as mentioned above. Drusen are lipid-rich, sub-RPE deposits that contain a variety of proteins, including vitronectin, components of the terminal complement cascade, and b-amyloid [135, 136]. Microscopic analysis of eyes donated by patients with AMD revealed lipid deposits within Bruch's membrane and apoptosis of RPE cells as features of the disease that are distinct from normal aging. The advanced form of dry AMD, which leads to geographic atrophy, was characterized by breakdown of the RPE, the choriocapillaris, and photoreceptors in regions of the retina, often where large drusen were present [137]. Dysregulation of the phagocytosis of oxidized fatty acids from photoreceptor outer segments is a contributing factor to the metabolic failure of RPE cells [115, 123]. In addition to all of these factors, the damage caused by the FR byproducts of mitochondrial energy metabolism has been implicated in age-related damage to the RPE [116].
As reviewed above, the mitochondrial ETC generates superoxide radicals through single-electron leaks at respiratory complexes I and III [18], and flavin-dependent enzymes in the mitochondrial matrix may be large contributors of ROS [29].
Otherwise, starvation and hypoxia, which can result from poor perfusion, are generally associated with increased amounts of ROS, which promote autophagy via several complex signaling mechanisms [138] (Figure 3). In response to OS, autophagy is significantly increased in an attempt to remove oxidatively damaged organelles such as mitochondria. At this time, accumulating evidence linking the impairment of autophagy with a range of age-related neurodegenerative diseases, including AMD, has suggested that autophagy occurs in the RPE to maintain homeostasis because these cells are exposed to sustained OS. However, insufficient digestion due to impaired autophagy or lysosomal degradation in the RPE can lead to an accumulation of damaged organelles, toxic proteins (including lipofuscin), and extracellular drusen deposits, all of which can contribute to RPE dysfunction or RPE cell death, which have been associated with the pathogenesis of AMD [139]. In addition, the AOXs of the retina (e.g., via macular molecules such as lutein and zeaxanthin) are reduced in AMD [140].
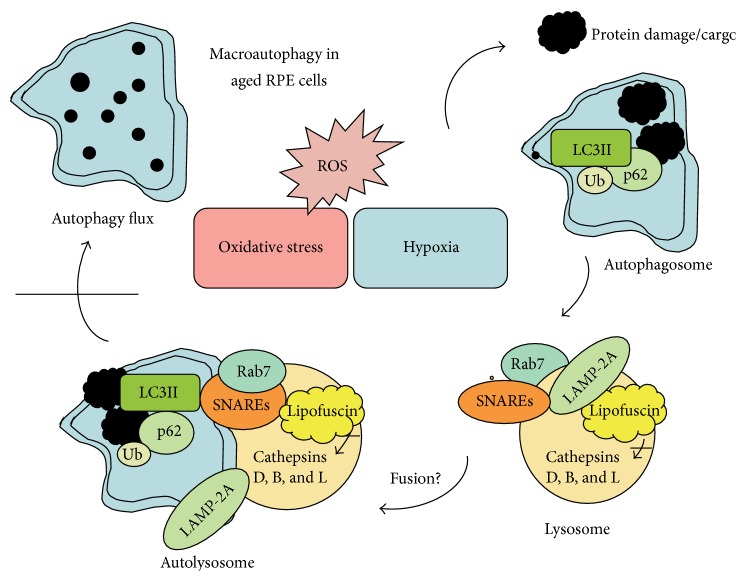
Figure 3.
Schematic presentation of the macroautophagy process in aged retinal pigment epithelial (RPE) cells. Oxidative stress, (ROS), and hypoxia lead to protein damage and aggregation, which induces autophagy. The substrate (cargo) for autophagy is degraded by lysosomal acid hydrolases, including cathepsins D, B, and L, after the fusion of lysosomes and autophagosomes to form autolysosomes. Rab7, LAMP-2A, and SNARE proteins are critical for the lysosome and autophagosome fusion process. Ubiquitin (Ub), LC3II, and p62 are complexed to the cargo and connect autophagy to the proteasomal clearance system. Macroautophagy is prevented in AMD because lysosomal lipofuscin disturbs cathepsin activity and autophagy flux. Fusion mechanisms in the RPE cells are under investigation. From Blasiak et al. [132], with the permission of the authors.
5.1. VEGF and AMD
VEGF is the most potent inducer of endothelial activation and angiogenesis. It is mainly expressed in retinal neurons and glial cells and is present in only scant amounts in blood vessels [141]. Under ischemic conditions, retinal expression and production of VEGF is increased [142]. This factor has also been implicated in the development of retinal neovascularization in ischemic retinopathies such as AMD [98]. Through a paracrine mechanism, VEGF binds to its cell-surface receptors, including VEGFR1/Flt-1, VEGFR2/Flk-1/KDR, and VEGFR3, and promotes endothelial cell survival, proliferation, migration, and tubular structure formation [142]. Among these receptors, VEGFR2 is the crucial receptor that mediates angiogenic and vascular permeability, whereas VEGFR3 mainly mediates lymphangiogenic functions. The activation of VEGFR1 plays a dual role and can either stimulate or inhibit angiogenesis, whereas the activation of VEGFR2 seems to only stimulate angiogenesis [143, 144].
Upon binding VEGF, VEGFR2 undergoes dimerization and autophosphorylation, resulting in the activation of its downstream kinases, including mitogen-activated protein kinase (MAPK), ERK1/2, p38, JNK, and PI3K/Akt, and of endothelial NO synthase (e-NOS), which may lead to further alterations in endothelial cell survival, proliferation, and migration. Recent reviews provide further information on this important factor [145, 146].
A constant oxygen supply is clearly essential for proper homeostasis and normal functioning in all retinal tissues. Cellular responses to reduced oxygen levels are mediated by the transcriptional regulator hypoxia-inducible factor-1 (HIF-1), a heterodimeric protein complex that consists of an oxygen-dependent subunit (HIF-1α) and a constitutively expressed nuclear subunit (HIF-1β). Under normoxic conditions, de novo synthesized cytoplasmic HIF-1α is degraded by the 26S proteasome. Under hypoxic conditions, HIF-1α is stabilized, binds to HIF-1β, and activates the transcription of various target genes. These genes play a key role in the regulation of angiogenesis in various visual pathologies, such as AMD [147].
5.2. AMD and the Complement Pathway
Growing evidence indicates that AMD is downstream of a chronic inflammatory condition in which activation of the immune system plays an important role. Metabolic products accumulate in the extracellular space between Bruch's membrane and the RPE and activate the complement system through a significant increase in OS, similar to the processes that occur in atherosclerosis or Alzheimer's disease [148]. These findings as well as those of many studies over the past decade have changed the understanding of the molecular mechanisms underlying AMD and led scientists to explore the targeting of specific molecular components of the complement pathway [149–153]. The complement system is a major component of innate immunity that plays a role in defense against invading microorganisms, the clearance of apoptotic cells, and the modulation of immune responses [154]. The complement cascade's four activation pathways converge upon a common terminal pathway that culminates in the formation of the cytolytic membrane attack complex (MAC). Binding of circulating C1q to antigen antibody complexes activates the classical pathway. The lectin pathway is activated by mannan-binding lectin following its recognition of, and binding to, molecular patterns on pathogen surfaces. The recently characterized intrinsic pathway is activated by proteases that cleave C3 and C5 directly. In contrast to the other three pathways, the alternative pathway is continuously active at a low level and is characterized by the spontaneous hydrolysis of C3 into the C3a and C3b fragments. C3b binds complement factor B (CFB), and once bound, CFB is cleaved by complement factor D (CFD) into Ba and Bb, thereby forming the active C3 convertase (C3bBb). C3bBb cleaves additional C3 molecules, which generates more C3a and C3b and thereby promotes further amplification of the cascade. In addition to the 30 or more complement components and fragments, numerous soluble and membrane-bound regulatory proteins modulate the complement system [153]. Complement factor H (CFH) is an important regulatory complement protein and is a major inhibitor of the alternative complement cascade that prevents excessive activation of the complement components. CFH regulates complement activity by inhibiting the activation of C3 to C3a and C3b and by inactivating existing C3b [154].
The discovery that drusen contain alternative complement pathway proteins led to the hypothesis that drusen could be involved in local complement-mediated inflammation [153]. The reports of an association between AMD and genetic variants in the cfh gene, a major inhibitor of the alternative pathway, support the inflammation model [154]. Other AMD risk variants have been found in genes underlying the alternative pathway, principally the formation of unstable C3 convertase, C3bBb, which cleaves C3 to generate the active segment C3b. Deposition of C3b on the target surface triggers the effector molecules C3a and C5a and the MAC, resulting in inflammation and cell lysis. In addition to CFH, several other AMD risk variants have been identified in genes underlying the alternative pathway. Variations in the factor B (BF) and complement component 2 (C2) genes are also associated with AMD [155].
A third line of evidence in support of complement involvement in AMD was provided by studies that showed that AMD patients have higher levels of complement activation products in their blood [156].
In 2001, data collected from the Age-Related Eye Disease Study (AREDS) revealed that patients who were treated with zinc, either alone or in combination with vitamins, displayed reduced progression to advanced AMD. AREDS results led to the recommendation that persons who are older than 55 years of age and who are at risk of developing advanced AMD should consider taking vitamin supplements plus zinc. A previous report published by the Blue Mountains Eye Study, a population-based study, confirmed the beneficial effect of zinc in AMD patients [157]. A recent study also provided evidence that daily administration of 50 mg of zinc sulfate can inhibit complement involvement in AMD patients who have increased complement activation [158].
5.3. AMD, RPE, and Daily and Circadian Rhythms
The retinal circadian system involves a unique structure. It contains a complete circadian system with multiple generation sites of numerous circadian rhythms, each of which deserves its own review. However, in the vertebrate retina, the intimate reciprocal relationship that exists between the neural retina and the underlying RPE is crucial for vision, while the diurnal and circadian rhythmicity of the RPE is critical for photoreceptor support and retinal function. Thus, in this section, we briefly review some of the rhythmic functions of the RPE that contribute to normal and pathological vision.
The association between AMD and biological rhythms has been poorly studied; however, there is a strong link between ocular physiology and circadian rhythms in both humans and animals. The renewal and elimination of aged photoreceptor outer segment tips by cells from the RPE is a daily rhythmic process that is crucial for long-term vision. Photoreceptors indefinitely renew their light-sensitive outer segments by disk shedding and the subsequent formation of new disks from the cilium of the inner segment. In higher vertebrates, outer segment renewal is synchronized by circadian rhythms [121, 159].
This shedding occurs once per day. In mice and rats, rod shedding is synchronized with light onset [160, 161]. To maintain the constant length of photoreceptors, the outer segment needs to be shed, and the formation of new outer segments must be coordinated.
The task of the adjacent RPE is to absorb shed POSs by phagocytosis and to recycle or digest their components. Outer segment renewal is crucial for photoreceptor function and survival, and a lack of efficient phagocytosis is sufficient to cause rapid photoreceptor degeneration by disk shedding [121, 162]. Photoreceptor disk shedding and subsequent phagocytosis by the RPE must be precisely regulated [163]. Alterations, such as delayed termination of shedding or defective digestion in the RPE, can cause the accumulation of lipofuscin [123]. Outer segment renewal and RPE phagocytosis are synchronized under circadian control and are triggered by the dark/light periods of the daily rhythm [163, 164].
Studies in higher vertebrates have revealed differences between cone- and rod-dominant species. Rod shedding mainly occurs in the morning, resulting in complementary RPE phagocytic activity by an increased number of phagosomes within the first 2 h after light onset, whereas cone shedding is more variable and mainly occurs either during the night or during the first 2 h after light onset [163, 164]. Any disruption in this process causes photoreceptor dysfunction and blindness in animal models and retinal disease in humans [93]. The synchronization of shedding with light seems to be crucial to photoreceptor physiology and survival because the accumulation of undigested material is detrimental to the RPE and retina and may contribute to the development or progression of AMD [123, 124]. These rhythms that synchronize with light are perceived by photosensitive retinal ganglion cells that contain the pigment melanopsin. Daily information is transmitted to the master circadian oscillator located in the SCN via the retinohypothalamic tract [66]. The action spectrum of light information for the circadian biological rhythm shows a peak at a shorter wavelength (464 nm) than that for visual information (approximately 555 nm).
Pivotal studies [161–164] have conclusively demonstrated that, in the rat retina, the diurnal rhythm of rod POS shedding and RPE phagocytosis is under circadian regulation. The mammalian retina displays persistent rhythmic activity even when it is isolated from the brain. In rats with transected nerves, POS shedding and RPE phagocytosis continue diurnally despite the loss of synaptic connections between the eye and the brain, suggesting that this rhythm is generated and controlled locally in the eye [165]. However, this rhythm cannot be reset by light unless the optic nerve remains intact [166]. Thus, the circadian renewal of POS involves both local control within the retina and central regulation by the brain.
The cellular and molecular mechanisms that underlie circadian regulation of shedding/phagocytosis are complex and are not the subject of this review. Numerous genes, proteins and signaling pathways play important roles in the engulfment of POS and the subsequent lysosomal degradation of spent photoreceptor disks within the RPE.
The lack of POS phagocytosis or digestion leads to photoreceptor dystrophy and blindness [122] due to the absence of the engulfment activity of the RPE. In rats that are deficient in MerTK, this absence causes dramatic and early onset retinal degeneration [122, 167]. Because the ingestion rate of POS by RPE cells exhibits a pronounced circadian rhythm that peaks around subjective dawn in both rat and mouse strains [159, 168], Prasad and colleagues [169] suggested that a feature of the TAM receptor system, such as ligand and/or receptor expression levels, might be regulated as a function of position in the circadian cycle.
Despite our vast knowledge of circadian biology and angiogenesis, the role of the circadian clock in the regulation of angiogenesis and vascular patterning remains poorly understood. Experimental animal models may help define the relationships between circadian rhythms and some retinal pathologies, such as AMD. Jensen et al. [170] showed that disruption of the circadian clock by both constant exposure to light and genetic manipulation of key genes in zebrafish led to impaired developmental angiogenesis. The disruption of crucial circadian regulatory genes, including Bmal1 and Period2, resulted in either marked impairment or enhancement of vascular development. At the molecular level, these authors showed that the circadian regulator Bmal1 directly targets the promoter region of the vegf gene in zebrafish, leading to elevated VEGF expression. Interestingly, deletion of these E-boxes in the promoter region of the zebrafish vegf gene resulted in inactivation of the promoter. These findings can be reasonably extended to developmental angiogenesis in mammals and even to pathological angiogenesis in humans [171]. Disruption of the circadian clock system not only affects the physiological activity of an organism but also often leads to the onset, development and progression of various diseases [172].
An important circadian hormone that is involved in vertebrate retinal circadian rhythms is melatonin, which is synthesized and produced by photoreceptors and shows a clear daily rhythm, with an acrophase at night [173]. This indolamine seems to have protective effects on other retinal cell types, including RPE cells and photoreceptors. Melatonin protects cultured RPE cells from OS and ischemia-induced cell death [174, 175]. Several studies have reported that melatonin is involved in the pathogenesis of AMD. In 2005, Yi et al. [176] reported that daily administration of melatonin (3 mg) may protect the retina and delay the progression of AMD. Rosen et al. [177] reported that the production of melatonin is decreased in AMD patients compared with age-matched controls, suggesting that a deficiency in melatonin may play a role in the occurrence of AMD. A further indication of the possible role of melatonin in age-related pathologies is the observation that retinal melatonin synthesis decreases during aging. In 2012, Tosini et al. [178] proposed that melatonin could affect the circadian clocks in photoreceptors and RPE cells and could thereby affect metabolism in these cells.
Melatonin and dopamine, two regulatory signals that play important roles in retinal physiology, have been proposed to be involved in the control of circadian POS shedding and RPE phagocytosis. In the retina, the production and release of melatonin and dopamine are under circadian control [179, 180]. Although there is insufficient clinical and experimental evidence to demonstrate a direct relationship between melatonin, circadian rhythms, and AMD, some reports have suggested that the melatonin rhythm is reversed in AMD patients [181].
6. Conclusion
This review highlights the role of OS as one of the main causes of AMD etiologies. In this retinal disease, as in other metabolic and degenerative pathologies, complex interactions among metabolic, functional, genetic, and environmental factors create a platform for the development of chronic changes in the ocular structures of the macular region in addition to a strong age dependence. In addition to genetic predispositions, at least four processes contribute to the disease: lipofuscinogenesis, drusogenesis, local inflammation and neovascularization (in the case of the wet form), and immunological mechanisms.
The current pathophysiological conception of AMD assigns a primary role to the age-related, cumulative oxidative damage to the RPE that occurs due to an imbalance between the generation and elimination of ROS. In particular, lipofuscin has been hypothesized to be the primary source of ROS and to be responsible for both the cellular and extracellular matrixes alterations in AMD. However, there may also be an association between the increasing levels of environmental sun radiation, especially short wavelengths in the violet and blue spectrum, due to both the ozone hole and climate change over the last decades, particularly given that AMD remains the leading cause of irreversible vision loss among the elderly in developed nations. In 2004, the overall global prevalence of AMD was approximately 8.7 percent, and the number of AMD patients was projected to rise to 196 million people worldwide by 2020 and to 288 million by 2040 [182]. Interestingly, the association between AMD and circadian rhythms, particularly different RPE rhythms, suggests a role for the circadian clock in AMD-related circadian abnormalities, which have generally been considered to be a consequence of neurodegeneration. However, recent evidence suggests that circadian disruption might actually contribute to the degenerative process and thus might be a modifiable cause of cell or neural injury.
Circadian clock genes have been shown to regulate VEGF signaling in tumorigenesis [183], and dopamine has been shown to modulate the effects of VEGF receptor activation on vascular endothelial cells [184, 185]. Recent results also indicate that Period genes may play a similar role in regulating vascularization signals in the retina in retinopathy disease models [186]. Regardlessly, retinal clock gene expression is disrupted in proliferative neovascularizing diseases [187].
Although circadian disturbances due to aging and neurodegenerative diseases have been duly noted, a key question is whether these disturbances influence the pathology of AMD. This question deserves further investigation.
List of Abbreviations
- AMD:
Age-related macular degeneration
- AOX:
Antioxidant defense
- BRB:
Blood-retinal barrier
- CFH:
Complement factor H
- ETC:
Electron transport chain
- FR:
Free radical
- GPx:
Glutathione peroxidase
- HIF-1:
Hypoxia-inducible factor-1
- Melatonin:
N-acetyl-5-methoxytryptamine
- MAC:
Membrane attack complex
- mitoROS:
Mitochondrially generated ROS
- mtDNA:
Mitochondrial DNA
- NOX:
NADPH oxidases
- OS:
Oxidative stress
- PEDF:
Pigment epithelium-derived factor
- PMF:
Proton motive force
- POSs:
Photoreceptor outer segment fragments
- PUFAs:
Polyunsaturated fatty acids
- RET:
Reverse electron transfer
- RNS:
Reactive nitrogen species
- ROS:
Reactive oxygen species
- RPE:
Retinal pigment epithelium
- SCN:
Suprachiasmatic nucleus
- SODs:
Superoxide dismutases
- TTL:
Transcriptional-translational feedback loop
- VEGF:
Vascular endothelial growth factor.
Disclosure
María Luisa Fanjul-Moles is a retired Professor.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Halliwell B. The antioxidant paradox: less paradoxical now? British Journal of Clinical Pharmacology. 2013;75(3):637–644. doi: 10.1111/j.1365-2125.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schieber M., Chandel N. S. ROS function in redox signaling and oxidative stress. Current Biology. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel T., Holbrook N. J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 4.Turrens J. F. Superoxide production by the mitochondrial respiratory chain. Bioscience Reports. 1997;17(1):3–8. doi: 10.1023/A:1027374931887. [DOI] [PubMed] [Google Scholar]
- 5.Kuppusamy P., Zweier J. L. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. Journal of Biological Chemistry. 1989;264(17):9880–9884. [PubMed] [Google Scholar]
- 6.DeRosa M. C., Crutchley R. J. Photosensitized singlet oxygen and its applications. Coordination Chemistry Reviews. 2002;233-234:351–371. doi: 10.1016/S0010-8545(02)00034-6. [DOI] [Google Scholar]
- 7.Pospíšil P., Prasad A., Rác M. Role of reactive oxygen species in ultra-weak photon emission in biological systems. Journal of Photochemistry and Photobiology B: Biology. 2014;139:11–23. doi: 10.1016/j.jphotobiol.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Kruft B. I., Greer A. Photosensitization reactions in vitro and in vivo. Photochemistry and Photobiology. 2011;87(6):1204–1213. doi: 10.1111/j.1751-1097.2011.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebel F., Kaur S., Ruvolo E., Kollias N., Southall M. D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. Journal of Investigative Dermatology. 2012;132(7):1901–1907. doi: 10.1038/jid.2011.476. [DOI] [PubMed] [Google Scholar]
- 10.Buettner G. R. Molecular targets of photosensitization—some biological chemistry of singlet oxygen. In: Smith K. C., editor. Photobiology Sciences on Line. American Society for Photobiology; 2011. http://www.photobiology.info/Buettner.html. [Google Scholar]
- 11.Kanofsky J. R. Measurement of singlet-oxygen in vivo: progress and pitfalls. Photochemistry and Photobiology. 2011;87(1):14–17. doi: 10.1111/j.1751-1097.2010.00855.x. [DOI] [PubMed] [Google Scholar]
- 12.Ziech D., Franco R., Pappa A., Panayiotidis M. I. Reactive oxygen species (ROS)—induced genetic and epigenetic alterations in human carcinogenesis. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2011;711(1-2):167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Evans M. D., Dizdaroglu M., Cooke M. S. Oxidative DNA damage and disease: induction, repair and significance. Mutation Research/Reviews in Mutation Research. 2004;567(1):1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Dalle-Donne I., Rossi R., Giustarini D., Milzani A., Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clinica Chimica Acta. 2003;329(1-2):23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 15.Pacher P., Beckman J. S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological Reviews. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill B. G., Dranka B. P., Bailey S. M., Lancaster J. R., Jr., Darley-Usmar V. M. What part of NO don't you understand? Some answers to the cardinal questions in nitric oxide biology. The Journal of Biological Chemistry. 2010;285(26):19699–19704. doi: 10.1074/jbc.r110.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turrens J. F. Mitochondrial formation of reactive oxygen species. The Journal of Physiology. 2003;552(2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brand M. D. The sites and topology of mitochondrial superoxide production. Experimental Gerontology. 2010;45(7-8):466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo J., Lemire B. D. The ubiquinone-binding site of the Saccharomyces cerevisiae succinate-ubiquinone oxidoreductase is a source of superoxide2. Journal of Biological Chemistry. 2003;278(48):47629–47635. doi: 10.1074/jbc.m306312200. [DOI] [PubMed] [Google Scholar]
- 20.Paranagama M. P., Sakamoto K., Amino H., Awano M., Miyoshi H., Kita K. Contribution of the FAD and quinone binding sites to the production of reactive oxygen species from Ascaris suum mitochondrial complex II. Mitochondrion. 2010;10(2):158–165. doi: 10.1016/j.mito.2009.12.145. [DOI] [PubMed] [Google Scholar]
- 21.Yankovskaya V., Horsefield R., Törnroth S., et al. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299(5607):700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- 22.Quinlan C. L., Goncalves R. L. S., Hey-Mogensen M., Yadava N., Bunik V. I., Brand M. D. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. Journal of Biological Chemistry. 2014;289(12):8312–8325. doi: 10.1074/jbc.m113.545301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Fiskum G., Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. Journal of Neurochemistry. 2002;80(5):780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirst J., Carroll J., Fearnley I. M., Shannon R. J., Walker J. E. The nuclear encoded subunits of complex I from bovine heart mitochondria. Biochimica et Biophysica Acta. 2003;1604(3):135–150. doi: 10.1016/s0005-2728(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 25.Murphy M. P. How mitochondria produce reactive oxygen species. Biochemical Journal. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gleason C., Huang S., Thatcher L. F., et al. Mitochondrial complex II has a key role in mitochondrial-derived reactive oxygen species influence on plant stress gene regulation and defense. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(26):10768–10773. doi: 10.1073/pnas.1016060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinlan C. L., Orr A. L., Perevoshchikova I. V., Treberg J. R., Ackrell B. A., Brand M. D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. Journal of Biological Chemistry. 2012;287(32):27255–27264. doi: 10.1074/jbc.m112.374629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hägerhäll C. Succinate: quinone oxidoreductases: variations on a conserved theme. Biochimica et Biophysica Acta—Bioenergetics. 1997;1320(2):107–141. doi: 10.1016/s0005-2728(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 29.Anderson R. F., Hille R., Shinde S. S., Cecchini G. Electron transfer within complex II. Succinate:ubiquinone oxidoreductase of Escherichia coli . Journal of Biological Chemistry. 2005;280(39):33331–33337. doi: 10.1074/jbc.m506002200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J., Frerman F. E., Kim J.-J. P. Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16212–16217. doi: 10.1073/pnas.0604567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J., Giordano S., Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochemical Journal. 2012;441(2):523–540. doi: 10.1042/bj20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malkus K. A., Tsika E., Ischiropoulos H. Oxidative modifications, mitochondrial dysfunction, and impaired protein degradation in Parkinson's disease: how neurons are lost in the Bermuda triangle. Molecular Neurodegeneration. 2009;4, article 24:24. doi: 10.1186/1750-1326-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong S. J., Low I. C. C., Pervaiz S. Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion. 2014;19:39–48. doi: 10.1016/j.mito.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Dröge W. Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Experimental Gerontology. 2002;37(12):1333–1345. doi: 10.1016/s0531-5565(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 35.Birben E., Sahiner U. M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organization Journal. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman K. Studies on free radicals, antioxidants and co-factors. Clinical Interventions in Aging. 2007;2(2):219–236. [PMC free article] [PubMed] [Google Scholar]
- 37.Balzer I., Hardeland R. Photoperiodism and effects of indoleamines in a unicellular alga, Gonyaulax polyedra . Science. 1991;253(5021):795–797. doi: 10.1126/science.1876838. [DOI] [PubMed] [Google Scholar]
- 38.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27(2):119–130. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 39.Pandi-Perumal S. R., Srinivasan V., Maestroni G. J. M., Cardinali D. P., Poeggeler B., Hardeland R. Melatonin: nature's most versatile biological signal? FEBS Journal. 2006;273(13):2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 40.Galano A., Tan D. X., Reiter R. J. Melatonin as a natural ally against oxidative stress: a physicochemical examination. Journal of Pineal Research. 2011;51(1):1–16. doi: 10.1111/j.1600-079x.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H.-M., Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. Journal of Pineal Research. 2014;57(2):131–146. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 42.Acuña-Castroviejo D., Coto-Montes A., Monti M. G., Ortiz G. G., Reiter R. J. Melatonin is protective against MPTP-induced striatal and hippocampal lesions. Life Sciences. 1996;60(2):PL23–PL29. doi: 10.1016/s0024-3205(96)00606-6. [DOI] [PubMed] [Google Scholar]
- 43.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 44.Sohal R. S., Orr W. C. The redox stress hypothesis of aging. Free Radical Biology & Medicine. 2012;52(3):539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sies H. Biochemistry of oxidative stress. Angewandte Chemie International Edition in English. 1986;25(12):1058–1071. doi: 10.1002/anie.198610581. [DOI] [Google Scholar]
- 46.Brandes N., Schmitt S., Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxidants & Redox Signaling. 2009;11(5):997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klomsiri C., Karplus P. A., Poole L. B. Cysteine-based redox switches in enzymes. Antioxidants & Redox Signaling. 2011;14(6):1065–1077. doi: 10.1089/ars.2010.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bae Y. S., Oh H., Rhee S. G., Yoo Y. D. Regulation of reactive oxygen species generation in cell signaling. Molecules and Cells. 2011;32(6):491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finkel T. Signal transduction by mitochondrial oxidants. Journal of Biological Chemistry. 2012;287(7):4434–4440. doi: 10.1074/jbc.r111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamata H., Honda S.-I., Maeda S., Chang L., Hirata H., Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120(5):649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 51.Chandel N. S., Maltepe E., Goldwasser E., Mathieu C. E., Simon M. C., Schumacker P. T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(20):11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherz-Shouval R., Shvets E., Fass E., Shorer H., Gil L., Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. The EMBO Journal. 2007;26(7):1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long Y. C., Tan T. M. C., Takao I., Tang B. L. The biochemistry and cell biology of aging: metabolic regulation through mitochondrial signaling. American Journal of Physiology—Endocrinology and Metabolism. 2014;306(6):E581–E591. doi: 10.1152/ajpendo.00665.2013. [DOI] [PubMed] [Google Scholar]
- 54.Banks G., Heise I., Starbuck B., et al. Genetic background influences age-related decline in visual and nonvisual retinal responses, circadian rhythms, and sleep. Neurobiology of Aging. 2015;36(1):380–393. doi: 10.1016/j.neurobiolaging.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skene D. J., Swaab D. F. Melatonin rhythmicity; effect of age and Alzheimer's disease. Experimental Gerontology. 2003;38(1-2):199–206. doi: 10.1016/s0531-5565(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 56.Pandi-Perumal S. R., Seils L. K., Kayumov L., et al. Senescence, sleep, and circadian rhythms. Ageing Research Reviews. 2002;1(3):559–604. doi: 10.1016/s1568-1637(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y.-H., Swaab D. F. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer's disease. Sleep Medicine. 2007;8(6):623–636. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 58.Ferrari E., Cravello L., Falvo F., et al. Neuroendocrine features in extreme longevity. Experimental Gerontology. 2008;43(2):88–94. doi: 10.1016/j.exger.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Travis R. C., Allen N. E., Peeters P. H. M., Van Noord P. A. H., Key T. J. Reproducibility over 5 years of measurements of 6-sulphatoxymelatonin in urine samples from postmenopausal women. Cancer Epidemiology Biomarkers & Prevention. 2003;12(8):806–808. [PubMed] [Google Scholar]
- 60.Grof E., Grof P., Brown G. M., Arato M., Lane J. Investigations of melatonin secretion in man. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1985;9(5-6):609–612. doi: 10.1016/0278-5846(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 61.Bergiannaki J. D., Soldatos C. R., Paparrigopoulos T. J., Syrengelas M., Stefanis C. N. Low and high melatonin excretors among healthy individuals. Journal of Pineal Research. 1995;18(3):159–164. doi: 10.1111/j.1600-079x.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 62.Barzilai N., Gabriely I. Genetic studies reveal the role of the endocrine and metabolic systems in aging. The Journal of Clinical Endocrinology & Metabolism. 2010;95(10):4493–4500. doi: 10.1210/jc.2010-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pagan C., Goubran-Botros H., Poirier K., et al. Mutation screening of ASMT, the last enzyme of the melatonin pathway, in a large sample of patients with intellectual disability. BMC Medical Genetics. 2011;12(1, article 17) doi: 10.1186/1471-2350-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mackey S. R., Golden S. S., Ditty J. L. The itty-bitty time machine: genetics of the cyanobacterial circadian clock. Advances in Genetics. 2011;74:13–53. doi: 10.1016/B978-0-12-387690-4.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dunlap J. C. Molecular bases for circadian clocks. Cell. 1999;96(2):271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 66.Reppert S. M., Weaver D. R. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 67.Hardin P. E. The circadian timekeeping system of Drosophila . Current Biology. 2005;15(17):R714–R722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 68.Vanselow J. T., Kramer A. Posttranslational regulation of circadian clocks. Protein Reviews. 2010;12:79–104. doi: 10.1007/978-1-4419-1262-6-3. [DOI] [Google Scholar]
- 69.Johnson C. H., Elliott J. A., Foster R. Entrainment of circadian programs. Chronobiology International. 2003;20(5):741–774. doi: 10.1081/CBI-120024211. [DOI] [PubMed] [Google Scholar]
- 70.Carneiro B. T. S., Araujo J. F. Food entrainment: major and recent findings. Frontiers in Behavioral Neuroscience. 2012;6, article 83 doi: 10.3389/fnbeh.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palma-Anzures I., Prieto-Sagredo J., Fanjul-Moles M. L. Temperature pulses synchronise the crayfish locomotor activity rhythm. Biological Rhythm Research. 2012;43(1):15–24. doi: 10.1080/09291016.2011.638116. [DOI] [Google Scholar]
- 72.Bloch G., Herzog E. D., Levine J. D., Schwartz W. J. Socially synchronized circadian oscillators. Proceedings of the Royal Society of London B: Biological Sciences. 2013;280(1765) doi: 10.1098/rspb.2013.0035.20130035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biological Reviews. 1996;71(3):343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 74.Pittendrigh C. Circadian systems: entrainment. In: Aschoff J., editor. Handbook of Behavioral Neurobiology, Vol 4 Biological Rhythms. New York, NY, USA: Plenum Press; 1981. pp. 95–124. [Google Scholar]
- 75.Bell-Pedersen D., Cassone V. M., Earnest D. J., et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nature Reviews Genetics. 2005;6(7):544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohawk J. A., Green C. B., Takahashi J. S. Central and peripheral circadian clocks in mammals. Annual Review of Neuroscience. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dibner C., Schibler U., Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annual Review of Physiology. 2009;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 78.Cassone V. M. Effects of melatonin on vertebrate circadian systems. Trends in Neurosciences. 1990;13(11):457–464. doi: 10.1016/0166-2236(90)90099-V. [DOI] [PubMed] [Google Scholar]
- 79.Wagner G. C., Johnston J. D., Tournier B. B., Ebling F. J. P., Hazlerigg D. G. Melatonin induces gene-specific effects on rhythmic mRNA expression in the pars tuberalis of the Siberian hamster (Phodopus sungorus) European Journal of Neuroscience. 2007;25(2):485–490. doi: 10.1111/j.1460-9568.2006.05291.x. [DOI] [PubMed] [Google Scholar]
- 80.Weaver D. R., Stehle J. H., Stopa E. G., Reppert S. M. Melatonin receptors in human hypothalamus and pituitary: implications for circadian and reproductive responses to melatonin. Journal of Clinical Endocrinology & Metabolism. 1993;76(2):295–301. doi: 10.1210/jc.76.2.295. [DOI] [PubMed] [Google Scholar]
- 81.Hardeland R., Coto-Montes A., Poeggeler B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiology International. 2003;20(6):921–962. doi: 10.1081/cbi-120025245. [DOI] [PubMed] [Google Scholar]
- 82.Merrow M., Roenneberg T. Circadian clocks: running on redox. Cell. 2001;106(2):141–143. doi: 10.1016/s0092-8674(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 83.Yoshida Y., Iigusa H., Wang N., Hasunuma K. Cross-talk between the cellular redox state and the circadian system in Neurospora . PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028227.e28227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lai A. G., Doherty C. J., Mueller-Roeber B., Kay S. A., Schippers J. H. M., Dijkwel P. P. Circadian clock-associated 1 regulates ROS homeostasis and oxidative stress responses. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(42):17129–17134. doi: 10.1073/pnas.1209148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fanjul-Moles M. L. ROS signaling pathways and biological rhythms: perspectives in crustaceans. Frontiers in Bioscience. 2013;18(2):665–675. doi: 10.2741/4129. [DOI] [PubMed] [Google Scholar]
- 86.Wang T. A., Yu Y. V., Govindaiah G., et al. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science. 2012;337(6096):839–842. doi: 10.1126/science.1222826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bednářová A., Kodrík D., Krishnan N. Nature's timepiece-molecular coordination of metabolism and its impact on aging. International Journal of Molecular Sciences. 2013;14(2):3026–3049. doi: 10.3390/ijms14023026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bazan N. G. Survival signaling in retinal pigment epithelial cells in response to oxidative stress: significance in retinal degenerations. Advances in Experimental Medicine and Biology. 2005;572:531–540. doi: 10.1007/0-387-32442-9_74. [DOI] [PubMed] [Google Scholar]
- 89.King A., Gottlieb E., Brooks D. G., Murphy M. P., Dunaief J. L. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochemistry and Photobiology. 2004;79(5):470–475. doi: 10.1562/le-03-17.1. [DOI] [PubMed] [Google Scholar]
- 90.Hunter J. J., Morgan J. I. W., Merigan W. H., Sliney D. H., Sparrow J. R., Williams D. R. The susceptibility of the retina to photochemical damage from visible light. Progress in Retinal and Eye Research. 2012;31(1):28–42. doi: 10.1016/j.preteyeres.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roehlecke C., Schumann U., Ader M., et al. Stress reaction in outer segments of photoreceptors after blue light irradiation. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0071570.e71570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uchino Y., Kawakita T., Miyazawa M., et al. Oxidative stress induced inflammation initiates functional decline of tear production. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0045805.e45805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strauss O. The retinal pigment epithelium in visual function. Physiological Reviews. 2005;85(3):845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 94.Dawson D. W., Volpert O. V., Gillis P., et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285(5425):245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 95.King G. L., Suzuma K. Pigment-epithelium-derived factor—a key coordinator of retinal neuronal and vascular functions. The New England Journal of Medicine. 2000;342(5):349–351. doi: 10.1056/NEJM200002033420511. [DOI] [PubMed] [Google Scholar]
- 96.Witmer A. N., Vrensen G. F. J. M., Van Noorden C. J. F., Schlingemann R. O. Vascular endothelial growth factors and angiogenesis in eye disease. Progress in Retinal and Eye Research. 2003;22(1):1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 97.Yoshida Y., Yamagishi S.-I., Matsui T., et al. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes/Metabolism Research and Reviews. 2009;25(7):678–686. doi: 10.1002/dmrr.1007. [DOI] [PubMed] [Google Scholar]
- 98.Do J. Y., Choi Y. K., Kook H., Suk K., Lee I. K., Park D. H. Retinal hypoxia induces vascular endothelial growth factor through induction of estrogen-related receptor γ . Biochemical and Biophysical Research Communications. 2015;460(2):457–463. doi: 10.1016/j.bbrc.2015.03.055. [DOI] [PubMed] [Google Scholar]
- 99.Hollyfield J. G., Bonilha V. L., Rayborn M. E., et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature Medicine. 2008;14(2):194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Decanini A., Nordgaard C. L., Feng X., Ferrington D. A., Olsen T. W. Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. American Journal of Ophthalmology. 2007;143(4):607–615. doi: 10.1016/j.ajo.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bedard K., Krause K.-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 102.Bhatt L., Groeger G., McDermott K., Cotter T. G. Rod and cone photoreceptor cells produce ROS in response to stress in a live retinal explant system. Molecular Vision. 2010;16:283–293. [PMC free article] [PubMed] [Google Scholar]
- 103.Roehlecke C., Schumann U., Ader M., Knels L., Funk R. H. W. Influence of blue light on photoreceptors in a live retinal explant system. Molecular Vision. 2011;17:876–884. [PMC free article] [PubMed] [Google Scholar]
- 104.Bazan H. E. P., Bazan N. G., Feeney-Burns L., Berman E. R. Lipids in human lipofuscin-enriched subcellular fractions of two age populations. Comparison with rod outer segments and neural retina. Investigative Ophthalmology & Visual Science. 1990;31(8):1433–1443. [PubMed] [Google Scholar]
- 105.Ng K.-P., Gugiu B., Renganathan K., et al. Retinal pigment epithelium lipofuscin proteomics. Molecular & Cellular Proteomics. 2008;7(7):1397–1405. doi: 10.1074/mcp.m700525-mcp200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wassell J., Davies S., Bardsley W., Boulton M. The photoreactivity of the retinal age pigment lipofuscin. The Journal of Biological Chemistry. 1999;274(34):23828–23832. doi: 10.1074/jbc.274.34.23828. [DOI] [PubMed] [Google Scholar]
- 107.Schütt F., Davies S., Kopitz J., Boulton M., Holz F. G. A retinoid constituent of lipofuscin, A2-E, is a photosensitizer in human retinal pigment epithelial cells. Der Ophthalmologe. 2000;97(10):682–687. doi: 10.1007/s003470070037. [DOI] [PubMed] [Google Scholar]
- 108.Sparrow J. R., Nakanishi K., Parish C. A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investigative Ophthalmology & Visual Science. 2000;41(7):1981–1989. [PubMed] [Google Scholar]
- 109.Boulton M., Dontsov A., Jarvis-Evans J., Ostrovsky M., Svistunenko D. Lipofuscin is a photoinducible free radical generator. Journal of Photochemistry and Photobiology B: Biology. 1993;19(3):201–204. doi: 10.1016/1011-1344(93)87085-2. [DOI] [PubMed] [Google Scholar]
- 110.Suter M., Remé C., Grimm C., Suter M., et al. Age-related macular degeneration. The lipofusion component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment epithelial cell. The Journal of Biological Chemistry. 2000;275(50):39625–39630. doi: 10.1074/jbc.m007049200. [DOI] [PubMed] [Google Scholar]
- 111.Saadat K. A. S. M., Murakami Y., Tan X., et al. Inhibition of autophagy induces retinal pigment epithelial cell damage by the lipofuscin fluorophore A2E. FEBS Open Bio. 2014;4:1007–1014. doi: 10.1016/j.fob.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Curcio C. A., Johnson M., Rudolf M., Huang J.-D. The oil spill in ageing Bruch membrane. British Journal of Ophthalmology. 2011;95(12):1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Faghiri Z., Bazan N. G. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Experimental Eye Research. 2010;90(6):718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Klettner A. Oxidative stress induced cellular signaling in RPE cells. Frontiers in Bioscience. 2012;4(2):392–411. doi: 10.2741/s275. [DOI] [PubMed] [Google Scholar]
- 115.Kaarniranta K., Sinha D., Blasiak J., et al. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9(7):973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jarrett S. G., Boulton M. E. Consequences of oxidative stress in age-related macular degeneration. Molecular Aspects of Medicine. 2012;33(4):399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McConnell V., Silvestri G. Age-related macular degeneration. Ulster Medical Journal. 2005;74(2):82–92. [PMC free article] [PubMed] [Google Scholar]
- 118.Turner P. L., Mainster M. A. Circadian photoreception: ageing and the eye's important role in systemic health. British Journal of Ophthalmology. 2008;92(11):1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Lookeren Campagne M., LeCouter J., Yaspan B. L., Ye W. Mechanisms of age-related macular degeneration and therapeutic opportunities. Journal of Pathology. 2014;232(2):151–164. doi: 10.1002/path.4266. [DOI] [PubMed] [Google Scholar]
- 120.Nowak J. Z. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacological Reports. 2006;58(3):353–363. [PubMed] [Google Scholar]
- 121.Young R. W., Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. The Journal of Cell Biology. 1969;42(2):392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mullen R. J., LaVail M. M. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science. 1976;192(4241):799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- 123.Finnemann S. C., Leung L. W., Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sparrow J. R., Boulton M. RPE lipofuscin and its role in retinal pathobiology. Experimental Eye Research. 2005;80(5):595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 125.Ferris F. L., III, Wilkinson C. P., Bird A., et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scott A. W., Bressler S. B. Long-term follow-up of vascular endothelial growth factor inhibitor therapy for neovascular age-related macular degeneration. Current Opinion in Ophthalmology. 2013;24(3):190–196. doi: 10.1097/ICU.0b013e32835fefee. [DOI] [PubMed] [Google Scholar]
- 127.Zhou J., Jang Y. P., Kim S. R., Sparrow J. R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wiktorowska-Owczarek A., Nowak J. Z. Oxidative damage in age-related macular degeneration (AMD) and antioxidant protection as a therapeutic strategy. Polish Journal of Environmental Studies. 2006;15:69–72. [Google Scholar]
- 129.Hollyfield J. G., Salomon R. G., Crabb J. W. Proteomic approaches to understanding age-related macular degeneration. Advances in Experimental Medicine and Biology. 2003;533:83–89. doi: 10.1007/978-1-4615-0067-4_11. [DOI] [PubMed] [Google Scholar]
- 130.Wolf G. Lipofuscin and macular degeneration. Nutrition Reviews. 2003;61(10):342–346. doi: 10.1301/nr.2003.oct.342-346. [DOI] [PubMed] [Google Scholar]
- 131.Zampatti S., Ricci F., Cusumano A., Marsella L. T., Novelli G., Giardina E. Review of nutrient actions on age-related macular degeneration. Nutrition Research. 2014;34(2):95–105. doi: 10.1016/j.nutres.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 132.Blasiak J., Petrovski G., Veréb Z., Facskó A., Kaarniranta K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. BioMed Research International. 2014;2014:7. doi: 10.1155/2014/768026.768026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shamsi F. A., Boulton M. Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Investigative Ophthalmology and Visual Science. 2001;42(12):3041–3046. [PubMed] [Google Scholar]
- 134.Sundelin S., Wihlmark U., Nilsson S. E. G., Brunk U. T. Lipofuscin accumulation in cultured retinal pigment epithelial cells reduces their phagocytic capacity. Current Eye Research. 1998;17(8):851–857. doi: 10.1076/ceyr.17.8.851.5146. [DOI] [PubMed] [Google Scholar]
- 135.Crabb J. W., Miyagi M., Gu X., et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang L., Clark M. E., Crossman D. K., et al. Abundant lipid and protein components of drusen. PLoS ONE. 2010;5(4) doi: 10.1371/journal.pone.0010329.e10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holz F. G., Strauss E. C., Schmitz-Valckenberg S., Van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079–1091. doi: 10.1016/j.ophtha.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 138.Galluzzi L., Pietrocola F., Levine B., Kroemer G. Metabolic control of autophagy. Cell. 2014;159(6):1263–1276. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mitter S. K., Rao H. V., Qi X., et al. Retinal Degenerative Diseases. Vol. 723. Berlin, Germany: Springer; 2012. Autophagy in the retina: a potential role in age-related macular degeneration; pp. 83–90. (Advances in Experimental Medicine and Biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu J., Seregard S., Algvere P. V. Photochemical damage of the retina. Survey of Ophthalmology. 2006;51(5):461–481. doi: 10.1016/j.survophthal.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 141.Famiglietti E., Stopa E. G., McGookin E. D., Song P., LeBlanc V., Streeten B. W. Immunocytochemical localization of vascular endothelial growth factor in neurons and glial cells of human retina. Brain Research. 2003;969(1-2):195–204. doi: 10.1016/S0006-8993(02)03766-6. [DOI] [PubMed] [Google Scholar]
- 142.Vinores S. A., Youssri A. I., Luna J. D., et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histology and Histopathology. 1997;12(1):99–109. [PubMed] [Google Scholar]
- 143.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Science Signaling. 2009;2(59, article re1) doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 144.Somanath P. R., Malinin N. L., Byzova T. V. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12(2):177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li J., Wang J. J., Zhang S. X. NADPH oxidase 4-derived H2O2 promotes aberrant retinal neovascularization via activation of VEGF receptor 2 pathway in oxygen-induced retinopathy. Journal of Diabetes Research. 2015;2015:13. doi: 10.1155/2015/963289.963289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Metzger C. S., Koutsimpelas D., Brieger J. Transcriptional regulation of the VEGF gene in dependence of individual genomic variations. Cytokine. 2015;76(2):519–526. doi: 10.1016/j.cyto.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 147.Vadlapatla R. K., Vadlapudi A. D., Mitra A. K. Hypoxia-inducible factor-1 (HIF-1): a potential target for intervention in ocular neovascular diseases. Current Drug Targets. 2013;14(8):919–935. doi: 10.2174/13894501113149990015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weinberger A. W. A., Eddahabi C., Carstesen D., Zipfel P. F., Walter P., Skerka C. Human complement factor H and factor H-like protein 1 are expressed in human retinal pigment epithelial cells. Ophthalmic Research. 2014;51(2):59–66. doi: 10.1159/000351624. [DOI] [PubMed] [Google Scholar]
- 149.Ansari M., Mckeigue P. M., Skerka C., et al. Genetic influences on plasma CFH and CFHR1 concentrations and their role in susceptibility to age-related macular degeneration. Human Molecular Genetics. 2013;22(23):4857–4869. doi: 10.1093/hmg/ddt336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zipfel P. F., Skerka C. Complement regulators and inhibitory proteins. Nature Reviews Immunology. 2009;9(10):729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 151.Zipfel P. F., Lauer N. Defective complement action and control defines disease pathology for retinal and renal disorders and provides a basis for new therapeutic approaches. Advances in Experimental Medicine and Biology. 2013;734:173–187. doi: 10.1007/978-1-4614-4118-2-11. [DOI] [PubMed] [Google Scholar]
- 152.Khan M. A., Assiri A. M., Broering D. C. Complement and macrophage crosstalk during process of angiogenesis in tumor progression. Journal of Biomedical Science. 2015;22(1):p. 58. doi: 10.1186/s12929-015-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Anderson D. H., Radeke M. J., Gallo N. B., et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Progress in Retinal and Eye Research. 2010;29(2):95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Klein R. J., Zeiss C., Chew E. Y., et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gold B., Merriam J. E., Zernant J., et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nature Genetics. 2006;38(4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reynolds R., Hartnett M. E., Atkinson J. P., Giclas P. C., Rosner B., Seddon J. M. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Investigative Ophthalmology & Visual Science. 2009;50(12):5818–5827. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Smith W., Mitchell P., Webb K., Leeder S. R. Dietary antioxidants and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 1999;106(4):761–767. doi: 10.1016/s0161-6420(99)90164-1. [DOI] [PubMed] [Google Scholar]
- 158.Smailhodzic D., Van Asten F., Blom A. M., et al. Zinc supplementation inhibits complement activation in age-related macular degeneration. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0112682.e112682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.LaVail M. M. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194(4269):1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- 160.Young R. W. The renewal of photoreceptor cell outer segments. Journal of Cell Biology. 1967;33(1):61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goldman A. I., Teirstein P. S., O'Brien P. J. The role of ambient lighting in circadian disc shedding in the rod outer segment of the rat retina. Investigative Ophthalmology & Visual Science. 1980;19(11):1257–1267. [PubMed] [Google Scholar]
- 162.Young R. W. The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Investigative Ophthalmology & Visual Science. 1978;17(2):105–116. [PubMed] [Google Scholar]
- 163.LaVail M. M. Circadian nature of rod outer segment disc shedding in the rat. Investigative Ophthalmology & Visual Science. 1980;19(4):407–411. [PubMed] [Google Scholar]
- 164.Bobu C., Craft C. M., Masson-Pevet M., Hicks D. Photoreceptor organization and rhythmic phagocytosis in the nile rat Arvicanthis ansorgei: a novel diurnal rodent model for the study of cone pathophysiology. Investigative Ophthalmology & Visual Science. 2006;47(7):3109–3118. doi: 10.1167/iovs.05-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Terman J. S., Remé C. E., Terman M. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Research. 1993;605(2):256–264. doi: 10.1016/0006-8993(93)91748-h. [DOI] [PubMed] [Google Scholar]
- 166.Teirstein P. S., Goldman A. I., O'Brien P. J. Evidence for both local and central regulation of rat rod outer segment disc shedding. Investigative Ophthalmology and Visual Science. 1980;19(11):1268–1273. [PubMed] [Google Scholar]
- 167.D'Cruz P. M., Yasumura D., Weir J., et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Human Molecular Genetics. 2000;9(4):645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 168.Gibbs D., Kitamoto J., Williams D. S. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(11):6481–6486. doi: 10.1073/pnas.1130432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Prasad D., Rothlin C. V., Burrola P., et al. TAM receptor function in the retinal pigment epithelium. Molecular and Cellular Neuroscience. 2006;33(1):96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 170.Jensen L. D., Cao Z., Nakamura M., et al. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Reports. 2012;2(2):231–241. doi: 10.1016/j.celrep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 171.Jensen L. D., Cao Y. Clock controls angiogenesis. Cell Cycle. 2013;12(3):405–408. doi: 10.4161/cc.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sahar S., Sassone-Corsi P. Circadian clock and breast cancer: a molecular link. Cell Cycle. 2007;6(11):1329–1331. doi: 10.4161/cc.6.11.4295. [DOI] [PubMed] [Google Scholar]
- 173.Tosini G., Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272(5260):419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 174.Liang F.-Q., Green L., Wang C., Alssadi R., Godley B. F. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Experimental Eye Research. 2004;78(6):1069–1075. doi: 10.1016/j.exer.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 175.Fu Y., Tang M., Fan Y., Zou H., Sun X., Xu X. Anti-apoptotic effects of melatonin in retinal pigment epithelial cells. Frontiers in Bioscience. 2012;17(4):1461–1468. doi: 10.2741/3997. [DOI] [PubMed] [Google Scholar]
- 176.Yi C., Pan X., Yan H., Guo M., Pierpaoli W. Effects of melatonin in age-related macular degeneration. Annals of the New York Academy of Sciences. 2005;1057(1):384–392. doi: 10.1196/annals.1356.029. [DOI] [PubMed] [Google Scholar]
- 177.Rosen R., Hu D.-N., Perez V., et al. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Molecular Vision. 2009;15:1673–1679. [PMC free article] [PubMed] [Google Scholar]
- 178.Tosini G., Baba K., Hwang C. K., Iuvone P. M. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Experimental Eye Research. 2012;103:82–89. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tosini G., Dirden J. C. Dopamine inhibits melatonin release in the mammalian retina: in vitro evidence. Neuroscience Letters. 2000;286(2):119–122. doi: 10.1016/s0304-3940(00)01117-4. [DOI] [PubMed] [Google Scholar]
- 180.Doyle S. E., Grace M. S., McIvor W., Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Visual Neuroscience. 2002;19(5):593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- 181.Schmid-Kubista K. E., Glittenberg C. G., Cezanne M., Holzmann K., Neumaier-Ammerer B., Binder S. Daytime levels of melatonin in patients with age-related macular degeneration. Acta Ophthalmologica. 2009;87(1):89–93. doi: 10.1111/j.1755-3768.2008.01173.x. [DOI] [PubMed] [Google Scholar]
- 182.Wong W. L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health. 2014;2(2):e106–e116. doi: 10.1016/s2214-109x(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 183.Koyanagi S., Kuramoto Y., Nakagawa H., et al. A molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Research. 2003;63(21):7277–7283. [PubMed] [Google Scholar]
- 184.Sarkar C., Chakroborty D., Mitra R. B., Banerjee S., Dasgupta P. S., Basu S. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. American Journal of Physiology—Heart and Circulatory Physiology. 2004;287(4):H1554–H1560. doi: 10.1152/ajpheart.00272.2004. [DOI] [PubMed] [Google Scholar]
- 185.Sinha S., Vohra P. K., Bhattacharya R., Dutta S., Sinha S., Mukhopadhyay D. Dopamine regulates phosphorylation of VEGF receptor 2 by engaging Src-homology-2-domain-containing protein tyrosine phosphatase 2. Journal of Cell Science. 2009;122(18):3385–3392. doi: 10.1242/jcs.053124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bhatwadekar A. D., Yan Y., Qi X., et al. Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow. Diabetes. 2013;62(1):273–282. doi: 10.2337/db12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Busik J. V., Tikhonenko M., Bhatwadekar A., et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. The Journal of Experimental Medicine. 2009;206(13):2897–2906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]