Abstract
Background
Poor adherence to medications is a major cause of morbidity and inadequate drug effectiveness. Efforts to improve adherence have typically been either ineffective or too complex to implement in clinical practice. Lottery-based incentive interventions could be a scalable approach to improving adherence.
Methods
This was a randomized, controlled clinical trial of a daily lottery-based incentive in patients on warfarin stratified by baseline international normalized ratio (INR). The trial randomized 100 patients to either a lottery-based incentive or no lottery intervention. Main outcome was out-of-range INRs.
Results
Over 6 months, the overall percentage of out-of-range INRs did not differ between the 2 arms (mean 23.0% in lottery arm and 25.9% in control arm, adjusted odds ratio [OR] 0.93, 95% CI 0.62–1.41). However, among the a priori subgroup with a baseline INR below therapeutic range, there was a significant reduction in out-of-range INR in the lottery arm versus the control arm (adjusted OR 0.39, 95% CI 0.25–0.62), whereas there was no such effect among those with therapeutic INRs at baseline (adjusted OR 1.26, 95% CI, 0.76–2.09, P value for interaction = .0016). Among those with low INR at baseline, there was a nonsignificant 49% reduction in the odds of nonadherence with the intervention (OR 0.51, 95% CI 0.23–1.14).
Conclusions
Although a lottery-based intervention was not associated with a significant improvement in anticoagulation control among all study participants, it improved control among an a priori group of patients at higher risk for poor adherence.
Adherence to medications is difficult to maintain, particularly for chronic asymptomatic conditions. Poor adherence has tremendous impact on health outcomes and health care costs.1 It is of greatest concern for medications with a narrow therapeutic range because missed doses can rapidly reduce their effectiveness, whereas extra doses increase their risk. Unfortunately, attempts to enhance adherence to long-term medications are often ineffective or require significant resources to be effective.2 Novel and scalable methods are needed to improve medication adherence.
Warfarin is an ideal drug for studying the effectiveness of new methods of enhancing adherence. Partly due to its narrow therapeutic range and partly due to the lack of symptoms associated with the conditions it treats, adherence to warfarin therapy and anticoagulation control is generally poor.3,4 Low rates of adherence not only have direct effects in terms of reduced effectiveness and risks associated with poor anticoagulation control but also dissuade many physicians from prescribing warfarin to patients who could potentially benefit from it.3,5
Preliminary research supports the potential effectiveness in increasing warfarin adherence of lottery-based incentives,6 which have also been used successfully to promote weight loss.7 The purpose of the WIN trial was to test the effect of a daily lottery-based incentive on anticoagulation control among warfarin-treated patients in a controlled, randomized trial.
Methods
Study population
Patients ≥21 years undergoing care at the outpatient hospital of the University of Pennsylvania Anticoagulation Management Center were eligible to participate if they had a target international normalized ratio (INR) range of anywhere between 2 and 3.5 (eg, 2–3 or 2.5–3.5) and had, at any time in the past, achieved stable warfarin anticoagulation, defined as 2 INRs within their target range over 2 consecutive clinic visits. Exclusion criteria were no access to a telephone line (which was required to use the Med-eMonitor (Informedix, Rockville, MD), as discussed below), unwillingness to participate or to sign a consent form, dementia or any other impairment affecting ability to provide informed consent and/or use the Med-eMonitor, illness with anticipated life expectancy of 6 months or less, INR over the upper limit for the individual’s range at the time of enrollment (to avoid possibly exacerbating this over-anticoagulation if a patient’s adherence improved during the study), and antiphospholipid antibody syndrome or abnormal INR before starting warfarin.
Study protocol
The protocol was approved by the institutional review board of the University of Pennsylvania, and all participants provided written, informed consent before randomization. The study was registered at clinicaltrials.gov as Testing Strategies to Improving Warfarin Adherence, ID number NCT00622102. An independent data safety monitoring board monitored the trial.
All patients were provided with an Informedix Med-eMonitor System, which has a display screen and separate medication compartments in which to place their warfarin. The monitor connects to an analog telephone line. Participants were randomized to 1 of 2 study arms: (1) enrollment in a daily lottery, administered via the Med-eMonitor, with an expected daily value of $3, or (2) no lottery intervention. In the lottery arm, participants had a 1 in 5 chance of a $10 reward and 1 in 100 chance of a $100 reward each day if they opened the monitor’s pill compartment and confirmed that they took their warfarin as prescribed that day. If patients were told to not take warfarin on a particular day, they would only be eligible for the lottery if they did not take a pill that day. The device was programmed to communicate by telephone with a central database at 3 AM each night so that the prior day’s adherence could be ascertained and the winning lottery number could be matched to the monitor’s preassigned number to determine any winnings for that day. Notification of any lottery winnings and the amount of those winnings were sent via the telephone connection to the Med-eMonitor overnight so that patients could see their winnings on the Med-eMonitor screen the next morning. Patients who did not take their warfarin as directed on a given day were notified if they would have won (if their lottery number was drawn) and how much they would have been paid had they taken their medication correctly. The system was automated so that there were no personnel required to run the lotteries. All other reminder systems and feedback from the Med-eMonitor were disabled for both groups.
Participants were seen by their anticoagulation clinic practitioner as per usual practice and by the study staff at baseline, 2 weeks, 3 months, and 6 months (corresponding to times when patients were returning for regular clinic visits). The purpose of these study visits was to collect follow-up data, but there were no other interventions during these visits. The study coordinators who conducted these visits were unaware of the adherence data throughout the trial.
Randomization procedures
Randomization was carried out using a random-number generator and via permuted block randomization with variable block sizes of 2, 4, and 6. Because of the a priori hypothesis that patients within therapeutic INR range at the time of randomization might benefit less from the intervention than those with an INR below the target range, randomization was stratified by INR below range and INR within range at enrollment. Neither field staff nor study participants could be blinded to study arm because of the nature of the intervention; study investigators and analysts, however, remained blinded to intervention assignments until all follow-ups and data cleaning were completed.
Study outcomes
The primary outcome variable was anticoagulation control, measured as INR out of range as a repeated-measures variable. We used all INR values measured over the 6 months of the study regardless of whether they occurred during a study visit. Limiting the analysis to only study-specific INRs did not alter any of the results. Secondary outcomes were adherence measured via the Med-eMonitor, bleeding events, and thromboembolism. We chose anticoagulation control rather than adherence as the primary outcome because it is more clinically relevant and because, in contrast to adherence, it is not subject to potential participant manipulation. Although it is unlikely, participants could produce erroneous adherence results by, either intentionally or unintentionally, opening and closing their pill compartments without taking the drug.
Statistical analysis
All analyses were by intent to treat using a repeated-measures analysis for the primary outcome and the adherence outcomes and using generalized estimating equation (GEE) logistic regression to account for the lack of independence among an individual’s INR and adherence values over time. This analysis models the likelihood of being out of range, adjusting for time on study, and compares the chance of being in range across the treatment groups. The odds ratio (OR) from this analysis compares the odds of poor anticoagulation control in the treatment group with that of patients in the control group; values <1 indicate a greater likelihood of anticoagulation control in subjects on treatment. To test for possible confounding, we estimated the effect of the intervention after adjusting for each potential covariate. Any covariate that changed the main effect of the intervention by at least 10% was retained in the final analysis model. We a priori examined the effect of the intervention on the 2 groups in whom we stratified the randomization (below INR target range and within range at enrollment) and tested for interaction by stratum using the appropriate product term in the GEE model. The adherence outcome was similarly analyzed as days incorrect using GEE models. Bleeding and thromboembolism outcomes were compared using Fisher exact test. All tests were 2 sided and used a significance level of .05.
In post hoc analyses, we examined whether subgroups of patients were more likely to respond to the lottery intervention. This included subgroups by income, insurance, other predictors of poor adherence,8 and level of anticoagulation control before study enrollment.
The trial was designed to have 80% power to detect a 50% reduction in the primary end point of out-of-range INRs at an α of .05 based on an estimate from our prior work of approximately 40% out-of-range INRs in the control group, 7 INR checks over 6 months, and an intraclass coefficient among INRs of 0.05. Based on prior studies,3 an improvement in adherence from around 20% incorrect pills taken to around 8% would correspond to a 50% relative improvement in anticoagulation control. The sample size estimate was 50 patients per arm.
The sponsor of the study, the Aetna Foundation, had no role in the design of the study, execution of the study, or analysis, interpretation, and writing of the manuscript. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Results
Study population
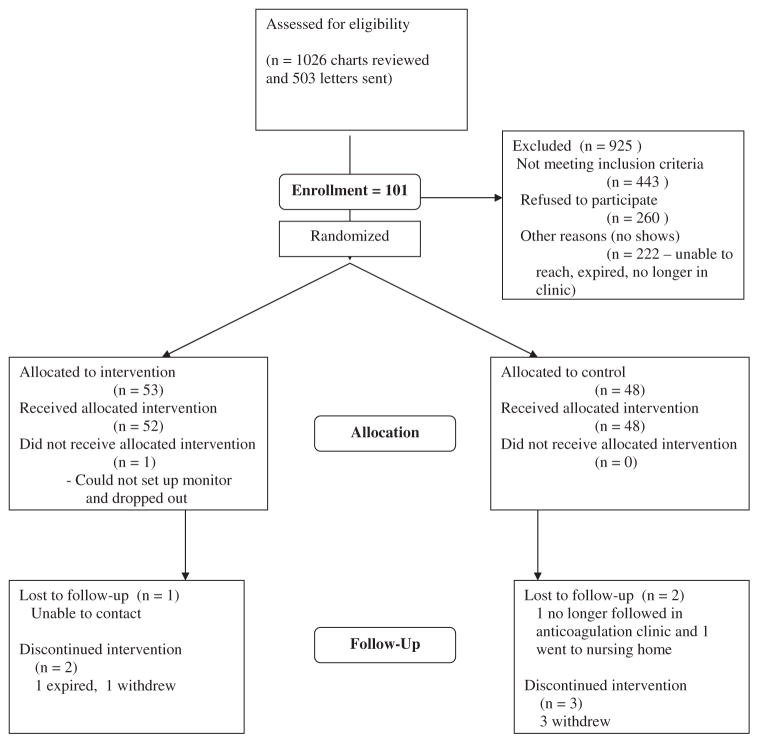
Figure 1 shows the flow of patients through the study. The lottery arm included 53 patients, and the control arm included 48 patients. One patient in the lottery arm could not successfully set up the Med-eMonitor and thus dropped out of the study, having never received the intervention. Although 2 patients in the intervention arm and 3 patients in the control arm stopped using the monitor before the 6-month follow-up point, they continued to be followed up and are included in the study and INR analyses. The mean number of follow-up INRs measured was 8.2 (SD 3.4) in the intervention arm and 8.0 (SD 2.6) in the control arm (P = .70).
Figure 1.
Patient flowchart.
Table I presents baseline characteristics of the study population. About 17% to 18% of the population had an INR below target range at enrollment. The lottery arm had somewhat better anticoagulation control in the preenrollment period than the control arm, although the difference was not statistically significant.
Table I.
Baseline characteristics
| Variable | Group | Lottery arm (n = 52) | Control arm (n = 48) | P |
|---|---|---|---|---|
| Age (y) | Median (Q1–Q3) | 64.0 (54.5–70.0) | 59.5 (48.5–66.0) | .1705 |
| Gender | Male | 34 (65.4%) | 22 (45.8%) | .0491 |
| Female | 18 (34.6%) | 26 (54.2%) | ||
| Education level | High school (9–12 y) | 22 (42.3%) | 20 (41.7%) | .8496 |
| College/trade school (13–16 y) | 16 (30.8%) | 17 (35.4%) | ||
| More than college (+17 y) | 14 (26.9%) | 11 (22.9%) | ||
| Employment status | Working | 20 (38.5%) | 15 (31.3%) | .3608 |
| Unemployed | 3 (5.8%) | 5 (10.4%) | ||
| Retired | 17 (32.7%) | 11 (22.9%) | ||
| Disabled | 12 (23.1%) | 17 (35.4%) | ||
| Self-reported insurance status | 1 = Any Medicare | 26 (50.0%) | 25 (52.1%) | .4575 |
| 2 = Medicaid | 4 (7.7%) | 7 (14.6%) | ||
| 3 = Private | 18 (34.6%) | 10 (20.8%) | ||
| 4 = Other | 2 (3.8%) | 4 (8.3%) | ||
| 5 = None | 2 (3.8%) | 2 (4.2%) | ||
| Federal poverty line | 1: <100% FPL | 12 (24.0%) | 11 (22.9%) | .9128 |
| 2: 100%–200% FPL | 8 (16.0%) | 8 (16.7%) | ||
| 3: 200%–300% FPL | 5 (10.0%) | 7 (14.6%) | ||
| 4: >300% FPL | 25 (50.0%) | 22 (45.8%) | ||
| Marital status | Married | 21 (40.4%) | 15 (31.3%) | .7501 |
| Separated/divorced | 12 (23.1%) | 15 (31.3%) | ||
| Widowed | 5 (9.6%) | 5 (10.4%) | ||
| Never married | 14 (26.9%) | 13 (27.1%) | ||
| Race: any African American | No | 23 (44.2%) | 17 (35.4%) | .3687 |
| Yes | 29 (55.8%) | 31 (64.6%) | ||
| Indication for warfarin | 1 = Atrial fibrillation/flutter | 21 (40.4%) | 17 (35.4%) | .7878 |
| 2 = Post DVT/PE | 11 (21.2%) | 13 (27.1%) | ||
| 3 = Mechanical heart valve | 10 (19.2%) | 6 (12.5%) | ||
| 4 = Dilated CMP | 4 (7.7%) | 4 (8.3%) | ||
| 5 = Other | 6 (11.5%) | 8 (16.7%) | ||
| Do not know | 1 (1.9%) | 0 (0.0%) | ||
| History prior warfarin use | No | 41 (82.0%) | 39 (81.3%) | .9236 |
| Yes | 9 (18.0%) | 9 (18.8%) | ||
| General health status | Excellent | 3 (5.8%) | 4 (8.3%) | .5769 |
| Very good | 12 (23.1%) | 9 (18.8%) | ||
| Good | 27 (51.9%) | 20 (41.7%) | ||
| Fair | 9 (17.3%) | 12 (25.0%) | ||
| Poor | 1 (1.9%) | 3 (6.3%) | ||
| Alcohol consumption frequency | Never | 21 (40.4%) | 17 (35.4%) | .2162 |
| Monthly or less | 11 (21.2%) | 13 (27.1%) | ||
| 2–4 times a m | 11 (21.2%) | 9 (18.8%) | ||
| 2–3 times a wk | 0 (0.0%) | 4 (8.3%) | ||
| ≥4 times a wk | 9 (17.3%) | 5 (10.4%) | ||
| Smoking status | 1 = Current | 9 (17.6%) | 10 (20.8%) | .5745 |
| 2 = Past | 22 (43.1%) | 24 (50.0%) | ||
| 3 = Never | 20 (39.2%) | 14 (29.2%) | ||
| Yes | 8 (15.4%) | 11 (22.9%) | ||
| INR below range at baseline visit | No | 43 (82.7%) | 39 (81.3%) | .8512 |
| Yes | 9 (17.3%) | 9 (18.8%) | ||
| Percentage of INR time out of range during preenrollment | Median (Q1–Q3) | 19.0 (5.3–37.6) | 29.2 (12.9–44.5) | .3545 |
Q, Quartile; FPL, federal poverty line; DVT/PE, deep vein thrombosis/pulmonary embolus; CMP, cardiomyopathy.
Primary and secondary outcomes
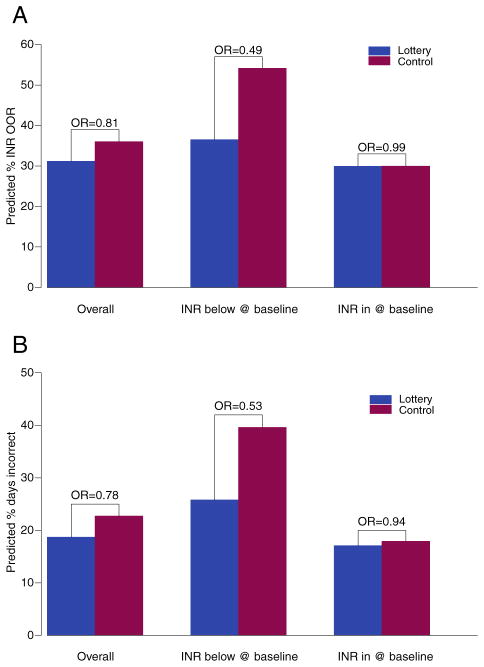
Out-of-range INRs did not differ between the 2 arms of the trial (Figure 2A and Table II). The intervention had minimal effects on the occurrence of low INRs (INR below range: mean 10.9% (SD 12.9%) vs 13.6% (18.0%) for lottery vs control arms, respectively) or high INRs (12.1% [SD 16.5%] vs 12.3% [14.6%]). We found that employment status was a confounder based on our a priori criteria and therefore included it in the adjusted GEE analyses (Table II). We assessed whether the intervention effect differed over time by including interaction terms between study arm and time since enrollment and did not find any statistically significant effects (all P > .5).
Figure 2.
A, Out-of-range INRs by study arm and a priori subgroup. Predictive probability of INR out of range. Primary outcome of out-of-range INRs by study arm and a priori subgroups of those with INR below range and INR within range at baseline. Results are presented as predicted probabilities from GEE logistic regression models. B, Nonadherence by study arm and a priori subgroup. Predictive probability of nonadherence. Secondary outcome of adherence by study arm and a priori subgroups of those with INR below range and INR within range at baseline. Results are presented as predicted probabilities from GEE logistic regression models.
Table II.
Effect of intervention on primary and secondary outcomes
| A. Effect of study arm (lottery vs control) on primary outcome: INR out of range
| ||||
|---|---|---|---|---|
| Unadjusted
|
Adjusted for employment status
|
|||
| OR (95% CI) | P | OR (95% CI) | P | |
| Overall | 0.81 (0.53–1.22) | .3051 | 0.93 (0.62–1.41) | .7390 |
| INR below at baseline (n = 18) | 0.49 (0.25–0.95) | .0338 | 0.39 (0.25–0.62) | <.0001 |
| INR in range at baseline (n = 82) | 0.99 (0.63–1.58) | .9972 | 1.26 (0.76–2.09) | .3744 |
| B. Effect of study arm (lottery vs control) on secondary outcome: nonadherence
| ||||
|---|---|---|---|---|
| Unadjusted
|
Adjusted for employment status
|
|||
| OR (95% CI) | P | OR (95% CI) | P | |
| Overall | 0.78 (0.49–1.25) | .3038 | 0.84 (0.55–1.28) | 4138 |
| INR below at baseline (n = 18) | 0.53 (0.24–1.17) | .1172 | 0.51 (0.23–1.14) | .1016 |
| INR in range at baseline (n = 82) | 0.94 (0.57–1.56) | .8216 | 0.99 (0.63–1.59) | .9959 |
Although the intervention did not result in statistically significant differences in INR control overall, there was a significant difference based on the a priori stratification by baseline INR. Among those with an INR below target range at baseline, there was a statistically significant effect of the lottery intervention on the primary outcome (adjusted OR 0.39, 95% CI 0.25–0.62, P < .001), whereas there was no statistically significant effect among those with an INR within target range at baseline (adjusted OR 1.26, 95% CI 0.76–2.09, P = .37). The difference between the effect of the intervention on these subgroups was statistically significant (interaction P value = .0016).
The effects on the secondary outcome of adherence (Table II and Figure 2B) mirror the results above. Adherence, overall, was better in the lottery arm than the control arm, but this difference was not statistically significant. The presence of an INR below therapeutic range at baseline was strongly associated with worse adherence during the trial, independent of the intervention. The OR for incorrect pill taking among those with a below-range INR compared with those with in-range INR at baseline was 2.28 (95% CI 1.38–3.77, P = .0013). Among the subgroup with INR below target range at baseline, the odds of incorrect dose taking was improved by almost 50% in the lottery versus control arm (OR 0.51, 95% CI 0.23–1.14), whereas there was no such effect among those with an INR within target range at baseline (OR 0.99, 95% CI, 0.63–1.59) (Table II and Figure 2B). The P value for this interaction was .16.
No patients in the study had a thromboembolism, and there were no bleeding events requiring hospitalization. There were 3 bleeding events requiring emergency department visits (1 for a nose bleed and 2 after patients cut themselves) in the lottery arm and none in the control arm (P = .25). There were 17 minor bleeding events (not requiring hospitalization) in the lottery arm and 10 in the control arm (P = .36).
Discussion
In this first randomized, clinical trial of a daily lottery-based incentive for medication adherence, there was no effect overall on anticoagulation control or adherence. However, among study participants with INRs below target range at baseline, significant improvements in anticoagulation control were observed. This was mirrored by an improvement in adherence, which did not meet statistical significance. Based on prior study, the approximately 50% improvement in adherence in this subgroup would be predicted to improve out-of-range INRs by about 40%.3 The subgroup results, within the level of precision of this study, are consistent with such an effect.
The lack of an effect of the intervention among the entire cohort could have several causes. First, the degree of anticoagulation control in the cohort was better than anticipated, both limiting the study’s power and the opportunity for the intervention to improve adherence (ie, if patients are already adherent, an adherence-based intervention will not further improve anticoagulation control). It is possible that simply being in the study improved adherence in all participants, including the control group. However, our prestudy estimate of poor adherence was based on a study in a similar cohort in which patients were monitored for adherence with electronic pill caps. In the subgroup with a subtherapeutic INR at baseline, the proportion of out-of-range INRs was similar to that predicted for the whole cohort (46%). This subgroup was more likely to be poorly adherent during the study (and thus likely to have been more nonadherent before the study) and appeared to respond to the intervention. Second, it is possible that the expected value of the lottery ($3 per day) was not sufficient to motivate behavioral changes. However, prior work suggests that higher expected value payments may be no more beneficial.6 Third, it is possible that the positive subgroup result, despite being prespecified and demonstrating a significant interaction by study arm, occurred by chance. Other limitations of the study include its inability to discern if patients took the correct number of pills from the Med-eMonitor compartment, use of a single anticoagulation clinic, and the potential for gaming of the system by opening pill doors but not taking the drug (which, although unlikely that patients would not go ahead and take the pill at that point, is why our primary outcome was INR control, not adherence).
The results of this trial are less striking than the findings of a pilot study in which anticoagulation control improved substantially in a noncontrolled study during a period of administering the lottery.6 That study was limited by not having a control group and demonstrates the importance of randomized trials in evaluating any health care intervention. In addition, the lottery system was not automated at the time the pilot study was performed, and participants had to be called on a regular basis to inform them of their lottery winnings. This personal contact may have enhanced the effects of the intervention on adherence.
The use of financial incentives for health care providers has been the focus of much research and debate.9–11 There is widespread agreement that incentive approaches that reward improved patient health instead of increased volume need to be developed and rigorously tested.12 Although many of the efforts to date have focused on pay-for-performance systems with provider incentives, there is growing interest in the use of patient incentives as an approach that will be increasingly used by large employers and, potentially, health systems to manage population health. However, the use of financial incentives for patients has received little attention.13 To our knowledge, this is the first test of whether a daily lottery-based intervention improves medication adherence. Such an approach has several appealing theoretical and practical characteristics: it can provide rapid and daily positive reinforcement14–17; it can motivate people based on their experience of past rewards and the prospect of future rewards18; it can provide incentives to avoid regret, a potent force in decision making19; and it is easily scalable with the use of modern technologies and could be cost-effective. With the work done in this study using the Med-eMonitor system, automation of a lottery-based incentive has proven to be feasible. Whether such an intervention ultimately proves to be cost-effective requires further study.
Given the public health consequences of poor medication adherence and the difficulty in improving adherence in a scalable manner, an automated lottery-based intervention could be a useful way to improve adherence for warfarin as well as other chronic medications. This study suggests that a lottery-based intervention could improve anticoagulation control in a subgroup of patients who are likely to have poor adherence. Further study in larger trials is warranted.
Acknowledgments
Stephen E. Kimmel, MD, MSCE, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The study was funded by the Aetna Foundation who had no role in the design of the study; execution of the study; analysis, interpretation, and writing of the manuscript; or decision to submit the manuscript for publication. Aetna also did not have access to the data. All authors had access to the data.
Footnotes
Trial registry name: Clinical Trials.gov; registration number NCT00904982 http://www.clinicaltrials.gov.
References
- 1.Peterson AM, Takiya L, Finley R, et al. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60:657–65. doi: 10.1093/ajhp/60.7.657. [DOI] [PubMed] [Google Scholar]
- 2.Haynes RB. Improving patient adherence: state of the art, with a special focus on medication taking for cardiovascular disorders. In: Burke LE, Ockene IS, editors. Compliance in Healthcare and Research. Armonk, NY: Futura Publishing Company, Inc; 2001. pp. 3–21. [Google Scholar]
- 3.Kimmel SE, Chen Z, Price M, et al. The influence of patient adherence on anticoagulation control with warfarin. Arch Intern Med. 2007;167:229–35. doi: 10.1001/archinte.167.3.229. [DOI] [PubMed] [Google Scholar]
- 4.Parker CS, Chen Z, Price M, et al. Adherence to warfarin assessed by electronic pill caps, clinician assessment, and patient reports: results from the IN-RANGE study. J Gen Intern Med. 2007;22:1254–9. doi: 10.1007/s11606-007-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the Anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–34. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 6.Volpp KG, Loewenstein G, Troxel AB, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8:272–7. doi: 10.1186/1472-6963-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpp KG, John LK, Troxel AB, et al. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300:2631–7. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platt AB, Localio AR, Brensinger CM, et al. Risk factors for nonadherence to warfarin: results from the IN-RANGE Study. Pharmacoepidemiol Drug Saf. 2008:853–60. doi: 10.1002/pds.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenthal MB, Frank RG, Li Z, et al. Early experience with pay-for-performance: from concept to practice. JAMA. 2005;294:1788–93. doi: 10.1001/jama.294.14.1788. [DOI] [PubMed] [Google Scholar]
- 10.Petersen LA, Woodard LD, Urech T, et al. Does pay-for-performance improve the quality of health care? Ann Intern Med. 2006;145:265–72. doi: 10.7326/0003-4819-145-4-200608150-00006. [DOI] [PubMed] [Google Scholar]
- 11.Glickman SW, Ou FS, DeLong ER, et al. Pay for performance, quality of care, and outcomes in acute myocardial infarction. JAMA. 2007;297:2373–80. doi: 10.1001/jama.297.21.2373. [DOI] [PubMed] [Google Scholar]
- 12.Davis K. Paying for care episodes and care coordination. NEJM. 2007;356:1166–8. doi: 10.1056/NEJMe078007. [DOI] [PubMed] [Google Scholar]
- 13.Volpp KG, Pauly MV, Loewenstein G, et al. P4P4P: an agenda for research on pay-for- performance for patients. Health Aff (Millwood) 2009;28:206–14. doi: 10.1377/hlthaff.28.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–96. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 15.Thaler RH. Some empirical evidence on time inconsistency. Rev Econ Stud. 1981;23:165–80. [Google Scholar]
- 16.Loewenstein G, Prelec D. Anomalies in intertemporal choice: evidence and an interpretation. Q J Econ. 1992;107:573–97. [Google Scholar]
- 17.Kirby K. Bidding on the future: evidence against normative discounting of delayed rewards. J Exp Psychol. 2009;126:54–70. [Google Scholar]
- 18.Camerer D, Ho T-H. Experience-weighted attraction learning in normal form games. Econometrica. 1999;67:837–74. [Google Scholar]
- 19.Connolly T, Butler DU. Regret in economic and psychological theories of choice. J Behav Decis Mak. 2006;19:148–58. [Google Scholar]