Abstract
Electromyography is a commonly used method to determine relative effort and neuromuscular drive to skeletal muscle. A limitation of the interpretation of EMG within the literature is the many methods used to determine the intensity of muscle activation. In the current study, ten healthy young adults performed a level walking task while EMG was recorded from the tibialis anterior, medial gastrocnemius and fibularis longus. The EMG data were rectified and smoothed using the root mean squared (RMS). Peak RMS (pRMS), mean RMS (mRMS) and integrated EMG (iEMG) were normalized to the peak value within the subject and were used to determine EMG amplitude. A 3×3 repeated measures analysis of variance was used to determine significant differences between the methods of determining EMG amplitude. The findings of the current study show that pRMS produced significantly lower EMG amplitudes than mRMS or iEMG values. Furthermore, mRMS and iEMG produced nearly identical normalized EMG amplitudes. Based on the findings of this study and the components of each measurement of EMG amplitude, it is suggested to use mRMS to determine EMG amplitude.
Keywords: EMG, methods, muscle
INTRODUCTION
Many methods are used to investigate the different components of human movement in healthy and pathological conditions including motion capture (3, 4, 17, 18) and electromyography (1, 2, 8, 12–14). The control of human movement has been a research focus for many years and investigators have examined many aspects of the central and peripheral nervous systems as they pertain to vertebrate (6, 7, 15) and human movement (2, 6, 7, 11, 12). A popular tool for research and clinical assessment of peripheral nervous drive is electromyography (EMG). However, EMG has inherent weaknesses including cross talk and amplitude cancellation (9). Questions have been raised as to the validity of surface and fine wire EMG (10). Furthermore, the limitations of EMG have led to questions pertaining to muscular function during human movement (9, 10, 16) based on the aqueous nature of muscle.
In addition to the limitations of the EMG signals, many different methods exist to process and present EMG data. A few of these methods include the peak root mean squared (pRMS), mean root mean squared (mRMS) and integrated EMG (iEMG). A limiting factor in the interpretation of different EMG studies is the difference between the methods used to determine the amplitude of muscle activation. The pRMS uses a single value, the maximum of the RMS smoothed signal, to represent muscle activation; however, it is not robust against movement artifact and error inherent within the EMG signal. The mRMS is a robust measure that limits the effects of movement artifact, however is also less sensitive to changes in the EMG signal and may mask differences in muscle activation intensity between experimental conditions. The iEMG is more robust than pRMS to movement artifact, but is sensitive to temporal changes in onset and offset of muscle activation. Due to the temporal component of the iEMG signal, it may not provide an accurate measure of the amplitude of muscle activity in different groups or experimental conditions. Though each method properly used will provide useful information pertaining to the neural control of the movement in question, each has inherent limitations. The use of a single method of determining EMG amplitude provides internal validity, however it remains unclear as to whether these methods produce the same value for muscle activation intensity. Therefore the purpose of this study is to compare three methods of determining the amplitude of muscle activation using surface EMG. The null hypothesis was that the three different methods would not yield statistically different EMG amplitude values. The alternate hypothesis was that each of the three methods of determining EMG amplitude values would be statistically different.
METHOD
Participants
Twelve subjects (6 male; 6 females) between the ages of 18 years and 25 years (age: 22.9±1.4 yrs, height: 1.69±0.25 m, mass: 77.9±18.0 kg) participated in the current study. Subjects were healthy and free of lower extremity injury for the previous six months and had no history of major lower extremity injury or neurological disorder. All participants signed an informed consent statement approved by the University of Texas of the Permian Basin Institutional Review Board.
Protocol
Each participant performed seven level walking trials at a self-selected pace. Gait velocity was maintained within 10% (±5%) of the self-selected velocity determined during three practice trials during data collections. Surface electromyography (2000Hz, BTS Engineering, Bolgona, Italy) was collected from the medial head of the Gastrocnemius (MG), Tibialis Anterior (TA) and Fibularis Longus (FL). MG surface electrodes were placed parallel to the muscle fibers over the belly of the medial head of the gastrocnemius (5). Surface electrodes used to measure TA muscle activity were placed over the largest area of muscle mass parallel to and just lateral to the longitudinal axis of the anterior aspect of the tibia (5). FL muscle activation was assessed using surface electrodes placed over the largest muscle mass of the fibularis longus muscle in parallel with muscle fibers approximately one-fourth of the distance between the head of the fibula and the lateral malleolus (5). The skin beneath each electrode placement site was shaved, abraded and cleansed to minimize skin resistance.
Data Processing and Reduction
EMG signals from each trial were rectified and smoothed using the root mean squared with a 20ms smoothing window. Surface EMG signals were evaluated over the stance phase of the gait cycle. The magnitude of EMG activation was determined using three methods: peak RMS (pRMS), mean RMS (mRMS) and integrated EMG (iEMG). Peak RMS was calculated as the maximum value of the RMS signal during the stance phase of gait. Conversely, mean RMS was calculated as the mean of the RMS signal during the stance phase of gait. For the integrated EMG analysis, EMG signals were integrated across the stance phase of gait. All EMG values were normalized to the highest EMG value within that measurement type for each subject (i.e. pRMS/max pRMS).
Statistical Analysis
Subject means used in statistical analyses were calculated as the mean of the seven trials performed by each subject using the three candidate methods. A 3×3 (muscle × measurement) repeated measures analysis of variance (ANOVA) with a Tukey’s post-hoc was used to assess statistical differences between the three methodologies (SPSS 16.0, SPSS Inc., Chicago, IL, USA). Alpha level was set at p < 0.05.
RESULTS
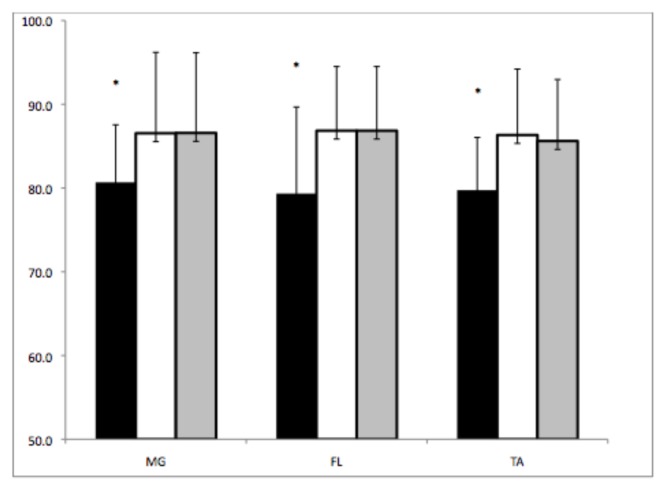
The three methods used produced visually similar results (Figure 1). The peak RMS measurement produced significantly lower normalized activation intensities than mean RMS (p = 0.001) and integrated EMG (p = 0.001; Table 1, Figure 1) measurements. Mean RMS and integrated EMG values were similar in each of the three tested muscles (p = 0.258; Table 1). There were no significant muscle (p = 0.974) or muscle by method interactions (p = 0.535).
Figure 1.
Activation intensities of the medial gastrocnemius (MG), fibularis longus (FL) and tibialis anterior (TA) measured by peak RMS (black), mean RMS (white) and integrated EMG (gray). * denotes pRMS is statistically different from mRMS and iEMG values.
Table 1.
Muscle activation intensities in the medial gastrocnemius (GM), fibularis longus (FL) and tibialis anterior (TA) as measured by peak RMS (pRMS), mean RMS (mRMS) and integrated EMG (iEMG). Presented mean (STD).
| Muscle | pRMS | mRMS | iEMG |
|---|---|---|---|
| MG | 80.7 (6.9) | 86.5 (9.7) a | 86.6 (9.6) a |
| FL | 79.3 (10.3) | 86.8 (7.7) a | 86.8 (7.7) a |
| TA | 79.7 (6.3) | 86.3 (7.9) a | 85.6 (7.3) a |
Note:
Significantly different than pRMS value.
DISCUSSION
In biomechanics and exercise science, many methods are used to measure the amplitude of muscle activation; however differences in methodology can often lead to difficulties in interpreting the findings of research studies. The purpose of the current study was to compare three methods of determining EMG amplitude in movement studies to determine their inter-relationships. The research question pertains to the similarity of muscle activation amplitude using each of the three methods.
The findings of the current study demonstrate that the measurement method used to determine EMG amplitude may affect the research results. The mean RMS and integrated EMG values were significantly higher than the peak RMS values. These differences in normalized EMG amplitude, though statistically significant, were not substantially large and would not inhibit interpretation of the research results. Given that these three methods were used on the same EMG signal, it is interesting to note that the peak RMS produced lower activation intensities. The limitation of using peak RMS to determine EMG signal amplitude is that it does not provide a robust measure regarding movement artifact and signal noise. Using the root mean square to smooth and process the EMG data limits the effect of outliers and noise; however, the normalization factor is also measured using peak RMS and may lead to erroneous normalized EMG values. Peak RMS may not be the best measure of EMG amplitude in the presence of noisy EMG data and a more robust measure may be preferred.
EMG amplitudes were similar when measured using mean RMS and integrated EMG. Both mean RMS and integrated EMG values are more robust measures than peak RMS regarding instantaneous noise and movement artifact. However, due to the temporal component of integrated EMG care must be taken in normalizing iEMG data. In the present study, iEMG data were analyzed over the stance phase, which was temporally maintained within 10% (±5%). If the experimental movement or population does not allow for consistency in the temporal component of the movement in question, integrated EMG may have limitations in the interpretation of the data. Furthermore, it is pertinent to note that if a repeated measures design is used to assess an intervention, the temporal component of the movement must be maintained for the accurate use of integrated EMG.
The mean RMS value is the most robust measure of EMG amplitude to movement artifact, signal noise and temporal changes in the movement. In the current study, the mean RMS produced similar EMG amplitudes to the integrated EMG measurement. However, in a repeated measures design which may alter the temporal component of the movement the mean RMS would not be affected by the changes in the time component. The amplitude of the EMG signal measured by iEMG, however, would be affected by the altered time component. Moreover, it is less affected by the presence of movement artifact or signal noise than peak RMS. These characteristics of the mRMS method suggest it is the best method tested for the determination of EMG signal amplitude.
A limitation of the current study is that data were only collected in a single movement condition and the differences in methodology could not be tested in multiple movement conditions which would alter the temporal component of the data. Additionally, the current data were collected in level walking and a more dynamic activity such as running, cutting or landing may have led to a lower quality of data including movement artifact. The addition of a more dynamic movement condition would have tested these three methods of determining EMG amplitude more thoroughly. The participants in the current study were healthy, young adults with no movement limitations. A patient population or an elderly population may have movement limitations leading to more erratic data which also would have improved the applicability of the current study.
The findings of the study demonstrate that the measure used to determine EMG amplitude is an important decision in study design. An important consideration in choosing the appropriate measure of EMG amplitude is the robustness of each measure and its inherent resistance to error. In the presence of artifact-free surface EMG data, each of these measures is sensitive to repeated measures and would provide internally valid statistical analyses. A further extension of these data would be to provide empirical evidence confirming the internal validity of these data in a repeated measures design.
REFERENCES
- 1.Babault N, Pousson M, Ballay Y, Van Hoecke J. Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J Appl Physiol. 2001;91(6):2628–2634. doi: 10.1152/jappl.2001.91.6.2628. [DOI] [PubMed] [Google Scholar]
- 2.Bastiaanse CM, Duysens J, Dietz V. Modulation of cutaneous reflexes by load receptor input during human walking. Exp Brain Res. 2000;135(2):189–198. doi: 10.1007/s002210000511. [DOI] [PubMed] [Google Scholar]
- 3.Bhambhani Y, Maikala R. Gender differences during treadmill walking with graded loads: biomechanical and physiological comparisons. Eur J Appl Physiol. 2000;81(1–2):75–83. doi: 10.1007/PL00013800. [DOI] [PubMed] [Google Scholar]
- 4.Byrne JE, Stergiou N, Blanke D, Houser JJ, Kurz MJ, Hageman PA. Comparison of gait patterns between young and elderly women: an examination of coordination. Percept Mot Skills. 2002;94(1):265–280. doi: 10.2466/pms.2002.94.1.265. [DOI] [PubMed] [Google Scholar]
- 5.Cram JR, Kasman GS. Introduction to Surface Electromyography. Gaithersburg, Maryland: Aspen Publishers; 1998. [Google Scholar]
- 6.Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture. 2000;11(2):102–110. doi: 10.1016/s0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 7.Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80(1):83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Earles DR, Koceja DM, Shively CW. Environmental changes in soleus H-reflex excitability in young and elderly subjects. Int J Neurosci. 2000;105(1–4):1–13. doi: 10.3109/00207450009003261. [DOI] [PubMed] [Google Scholar]
- 9.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96(4):1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 10.Farina D, Merletti R, Indino B, Graven-Nielsen T. Surface EMG crosstalk evaluated from experimental recordings and simulated signals. Reflections on crosstalk interpretation, quantification and reduction. Methods Inf Med. 2004;43(1):30–35. [PubMed] [Google Scholar]
- 11.Ferris DP, Aagaard P, Simonsen EB, Farley CT, Dyhre-Poulsen P. Soleus H-reflex gain in humans walking and running under simulated reduced gravity. J Physiol. 2001;530(1):167–180. doi: 10.1111/j.1469-7793.2001.0167m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouad K, Bastiaanse CM, Dietz V. Reflex adaptations during treadmill walking with increased body load. Exp Brain Res. 2001;137(2):133–140. doi: 10.1007/s002210000628. [DOI] [PubMed] [Google Scholar]
- 13.Haddad JM, van Emmerik RE, Whittlesey SN, Hamill J. Adaptations in interlimb and intralimb coordination to asymmetrical loading in human walking. Gait Posture. 2006;23(4):429–434. doi: 10.1016/j.gaitpost.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman M, Koceja DM. Hoffmann reflex profiles and strength ratios in postoperative anterior cruciate ligament reconstruction patients. Int J Neurosci. 2000;104(1–4):17–27. doi: 10.3109/00207450009035006. [DOI] [PubMed] [Google Scholar]
- 15.Hortobagyi T, Del Olmo MF, John CR. Age reduces cortical reciprocal inhibition in humans. Exp Brain Res. 2006;171(3):322–329. doi: 10.1007/s00221-005-0274-9. [DOI] [PubMed] [Google Scholar]
- 16.Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol. 2004;556(1):267–282. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerrigan DC, Lee LW, Collins JJ, Riley PO, Lipsitz LA. Reduced hip extension during walking: healthy elderly and fallers versus young adults. Arch Phys Med Rehabil. 2001;82(1):26–30. doi: 10.1053/apmr.2001.18584. [DOI] [PubMed] [Google Scholar]
- 18.Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehabil. 1998;79(3):317–322. doi: 10.1016/s0003-9993(98)90013-2. [DOI] [PubMed] [Google Scholar]