Abstract
Background: Telehealth has the potential to improve chronic disease management and outcomes, but data regarding direct benefit of telehealth in patients with heart failure (HF) have been mixed. The objective of this study was to determine whether the Health Buddy Program (HBP) (Bosch Healthcare, Palo Alto, CA), a content-driven telehealth system coupled with care management, is associated with improved outcomes in Medicare beneficiaries with HF. Materials and Methods: This was a retrospective cohort study of 623 Medicare beneficiaries with HF offered HBP enrollment compared with a propensity score-matched control group of Medicare beneficiaries with HF from the Medicare 5% sample. Associations between availability of the HBP and all-cause mortality, hospitalization, hospital days, and emergency department visits were evaluated. Results: Beneficiaries offered enrollment in the HBP had 24.9% lower risk-adjusted all-cause mortality over 3 years of follow-up (hazard ratio [HR]=0.75; 95% confidence interval [CI], 0.63–0.89; p=0.001). Patients who used the HBP at least once (36.9%) had 57.2% lower mortality compared with matched controls (HR=0.43; 95% CI, 0.31–0.60; p<0.001), whereas patients who did not use the HBP had no significant difference in survival (HR=0.96; 95% CI, 0.78–1.19; p=0.69). Patients offered the HBP also had fewer hospital admissions following enrollment (Δ=−0.05 admissions/quarter; p=0.011), which was primarily observed in patients who used the HBP at least once (Δ=−0.10 admissions/quarter; p<0.001). Conclusions: The HBP, a content-driven telehealth system coupled with care management, was associated with significantly better survival and reduced hospitalization in Medicare beneficiaries with HF. Prospective study is warranted to determine the mechanism of this association and opportunities for optimization.
Key words: : telemonitoring, survival, Medicare, heart failure, outcomes
Introduction
Heart failure (HF) is the leading hospital discharge diagnosis in patients >65 years old in the United States and costs approximately $30 billion annually.1 HF is particularly costly in Medicare patients, accounting for approximately 37% of all costs and 50% of inpatient costs in part because of the high prevalence of comorbid conditions.2 Despite advances in therapy, approximately 50% of HF patients will die within 5 years of diagnosis.1 Remote monitoring has been identified as a potential approach to managing HF patients and can range from phone contacts to complex systems that remotely acquire patient data.3,4 Telehealth is a type of remote monitoring that uses communication technology to transmit patient data to providers.5 Telehealth can be delivered by interactive systems to engage patients in management of their health and to detect signs of disease progression so providers may intervene prior to decompensation.6
Data regarding the impact of telemonitoring on HF outcomes have been mixed, and routine use of telemonitoring is not currently part of management guidelines.7 A Cochrane Review found that telemonitoring was associated with a reduction in all-cause mortality and all-cause hospitalizations,8 whereas the Telemonitoring to Improve Heart Failure Outcomes Trial (Tele-HF), the largest trial of telemonitoring to date, showed no improvement in outcomes in recently hospitalized HF patients compared with routine care.9
Many studies have focused on associations between patient outcomes and unaccompanied telemonitoring rather than telemonitoring integrated with care plans and/or patient education. For example, Tele-HF sites were encouraged to act on abnormal patient-reported values but were not provided prespecified responses. Indeed, the Cochrane Review of telemonitoring concluded “The aim for future use of structured telephone support and telemonitoring should be to use these interventions to tailor HF disease management programs to the population needs and resources, to the geography of the population and most importantly, to patient preferences.”8
The Health Buddy Program (HBP) (Bosch Healthcare, Palo Alto, CA) couples care management with the Health Buddy, an electronic device with a high-resolution screen and four large buttons located in patients' homes and connected to remote care managers. The Health Buddy asks patients daily for vital signs and other health-related information (e.g., “Are you more fatigued, tired, and/or unable to do routine activities like cooking, dressing, bathing over the past two weeks?”), providing feedback and education based on responses (e.g., “Increased fatigue or tiredness, and an inability to do your daily routine may be a sign of worsening heart failure”).6 After each session, the Health Buddy transmits patient data to the Health Buddy server, after which HBP categorizes patients as low, medium, or high risk based on daily responses using algorithms developed in accordance with evidence-based guidelines. Once a patient's risk status is determined, data are transmitted to the Health Buddy Desktop Application where providers could monitor patient status and triage follow-up. Depending on a patient's estimated risk, care managers may initiate interventions such as calling the patient, notifying the physician, or scheduling an office appointment for the patient. The Health Buddy Desktop Application also identifies patients who have not responded to their daily sessions, and action may be taken by care managers depending on duration and reason for nonresponse.10
A Centers for Medicare and Medicaid Services (CMS) demonstration study of Medicare beneficiaries with HF, chronic obstructive pulmonary disease, or diabetes mellitus suggested that patients offered the HBP had a risk-adjusted reduction in all-cause mortality over 2 years (20.3% versus 23.0%).11 Furthermore, patients offered enrollment in the HBP had an 18% reduction in all-cause hospitalizations compared with matched controls, and mortality benefits over 2 years were significant for HF patients in subgroup analysis.12 We present a retrospective analysis of HF patients who were offered enrollment in the HBP CMS demonstration study over 3 years. We hypothesized that Medicare beneficiaries with HF offered the HBP would have reductions in all-cause mortality and healthcare utilization compared with matched controls. We also hypothesized that HBP patients who actively used the system would have a greater benefit than those who did not.
Materials and Methods
The design of the HBP CMS demonstration project has been described previously.11 The study used a population-based “community intervention” design within a specific geographic area.10 The study did not randomize patients but rather compared health status and resource utilization of enrollees at baseline to the demonstration period. An initial cohort was identified in 2006, followed by a second cohort in 2007 to expand the study and account for attrition. Medicare Parts A and B claims data were obtained for all patients offered enrollment in the HBP, and data from control patients were obtained from the CMS 5% sample. Demographics and outcomes were derived using claims data, and disease conditions were identified using International Classification of Disease, Ninth Revision, Clinical Modification-9 (ICD-9 CM) terms. If a patient died, date of death was determined using enrollment files linked to Social Security Administration death records.
For the present analysis, patients were selected if at least one inpatient claim or two claims in any other setting with an ICD-9 CM code for HF (398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx) occurred within 12 months of the start of the program. Intervention patients were classified as “engaged” if they used the Health Buddy at least once during the study period, whereas patients were classified as “nonengaged” if they never used the Health Buddy. The details of identifying the overall sample of control patients have been described previously.11 In brief, control HF patients who resided in areas geographically similar to Bend, OR and Wenatchee, WA were selected from Medicare beneficiaries in the CMS 5% sample using the same ICD-9 CM criteria as for the intervention patients.
The primary outcome for this analysis was time to all-cause mortality over 3 years after being offered HBP enrollment. Secondary outcomes were changes in quarterly measures of healthcare resource utilization from baseline to the end of the study period including all-cause inpatient admissions, all-cause hospital days (conditional on hospitalization), and all-cause emergency department (ED) visits. As described previously, data from quarters immediately preceding and following HBP initiation were excluded to account for uncertainty regarding exact start date, and the quarter of a patient's death was excluded due to increased resource utilization immediately prior to death.11,12
The HBP demonstration project was designed to improve coordination of care and could potentially impact multiple types of outcomes. Accordingly, we matched on the propensity of being offered the HBP to enable evaluation of multiple outcomes including hospitalization, hospital days, and ED visits. Propensity matching helped identify control patients most similar to HBP patients prior to the intervention as described previously.11,12 The propensity of being offered the HBP was estimated using logistic regression. Baseline characteristics potentially associated with being offered HBP enrollment were considered, including demographics, comorbid conditions, overall health indicators (e.g., Medicare Hierarchical Condition Category score, Elixhauser comorbidity index, and high-risk comorbidities), total healthcare costs, all-cause resource utilization (e.g., hospitalizations, outpatient visits, ED visits, and healthcare provider visits), and HF-related healthcare utilization (e.g., hospitalizations and ED visits with a diagnosis of HF). A high-risk comorbidity was defined as any condition that could impede use of the Health Buddy device (see Supplementary Data; available online at www.liebertpub.com/tmj).
Baseline comorbidities were identified based on the presence of at least two claims (excluding laboratory and radiology claims) with related ICD-9 CM codes. Healthcare provider visits were defined as outpatient nonlaboratory services provided by physicians, physician assistants, or nurses. To optimize the match between intervention and control patients, separate algorithms were used for the 2006 and 2007 cohorts. Control and HBP patients were matched 1:1 with replacement based on closest propensity scores. A matched control with a propensity score within 0.01 was required for each intervention patient.
Mortality rates during the study period were compared descriptively using chi-squared tests. Unadjusted survival rates were compared between the intervention and control samples using Kaplan–Meier estimates. Cox proportional hazards models were used to compare the relative risk of all-cause mortality between intervention and control patients adjusting for year of enrollment and significant differences (p<0.10) in baseline characteristics after propensity matching for being offered the HBP. Changes in quarterly healthcare resource utilization in the baseline and study period were compared using t tests for continuous variables. Relative utilization changes were estimated using panel negative binomial models adjusting for year of enrollment and significant differences in baseline characteristics after matching. The significance of each prediction was estimated using bootstrapping. Analyses were repeated comparing subgroups of engaged and nonengaged intervention patients to their matched controls. A p value <0.05 was considered significant.
Results
In total, 644 intervention and 658 potential matched controls were analyzed. Of the 644 intervention patients, 21 were excluded because matched controls could not be identified based on propensity scores. Therefore, 623 patients and 623 matched controls were included in the analysis. Baseline characteristics were similar between the cohorts with the exception of age, depression, diabetes mellitus, ocular disorders, and high-risk comorbidities (Table 1). Among the intervention beneficiaries, 230/623 patients (36.9%) used the Health Buddy at least once. Those who engaged the Health Buddy entered information 389±310 days on average during the study period. Characteristics of engaged and nonengaged patients are shown in Table 2. The only significant differences between engaged and nonengaged beneficiaries were age (76.9±8.8 versus 79.8±9.1 years; p<0.001) and baseline outpatient visits in the previous year (16.0±12.2 versus 14.0±12.8; p=0.005).
Table 1.
Baseline Characteristics of Intervention Heart Failure Patients Versus Matched Controls
| CHARACTERISTIC | INTERVENTION | CONTROL | P VALUEa |
|---|---|---|---|
| Number of patients | 623 | 623 | |
| Demographics | |||
| Mean age (years) | 78.76±9.08 | 77.39±8.59 | 0.005 |
| Male | 353 (56.7%) | 326 (52.3%) | 0.125 |
| Under 65 years of age | 28 (4.5%) | 29 (4.7%) | 0.892 |
| Baseline comorbiditiesb | |||
| CHF-related comorbidities | |||
| Anemia | 113 (18.1%) | 113 (18.1%) | 1.000 |
| Anxiety, somatoform disorders, and personality disorders | 24 (3.9%) | 17 (2.7%) | 0.266 |
| Chronic atherosclerosis | 233 (37.4%) | 225 (36.1%) | 0.638 |
| Chronic liver disease | 3 (0.5%) | 9 (1.4%) | 0.082 |
| COPD | 208 (33.4%) | 187 (30.0%) | 0.201 |
| Dementia | 9 (1.4%) | 5 (0.8%) | 0.282 |
| Depression | 26 (4.2%) | 12 (1.9%) | 0.021 |
| Diabetes mellitus | 262 (42.1%) | 217 (34.8%) | 0.009 |
| Hypercholesterolemia | 52 (8.3%) | 63 (10.1%) | 0.282 |
| Hypertension | 309 (49.6%) | 314 (50.4%) | 0.777 |
| Malnutrition | 2 (0.3%) | 1 (0.2%) | 1.000 |
| Cancer | 152 (24.4%) | 140 (22.5%) | 0.422 |
| Myocardial infarction | 25 (4.0%) | 24 (3.9%) | 0.884 |
| Ocular disorder | 217 (34.8%) | 258 (41.4%) | 0.017 |
| Osteoporosis and osteoarthritis | 11 (1.8%) | 14 (2.2%) | 0.544 |
| Peripheral vascular disease | 55 (8.8%) | 48 (7.7%) | 0.471 |
| Renal failure | 79 (12.7%) | 92 (14.8%) | 0.285 |
| Respiratory failure | 20 (3.2%) | 16 (2.6%) | 0.499 |
| Cerebrovascular disease | 36 (5.8%) | 41 (6.6%) | 0.556 |
| Valvular heart disease | 60 (9.6%) | 72 (11.6%) | 0.269 |
| Obesity | 11 (1.8%) | 10 (1.6%) | 0.826 |
| Thyroid disorder | 37 (5.9%) | 34 (5.5%) | 0.714 |
| Sleep disorder | 9 (1.4%) | 3 (0.5%) | 0.082 |
| Pneumonia | 77 (12.4%) | 86 (13.8%) | 0.450 |
| Overall health indicators | |||
| Mean adjusted Elixhauser comorbidity index | 1.95±1.29 | 2.00±1.36 | 0.584 |
| Mean HCC score | 2.55±0.97 | 2.58±1.03 | 0.995 |
| End stage renal disease | 8 (1.3%) | 16 (2.6%) | 0.099 |
| Exclusionary comorbidity | 55 (8.8%) | 77 (12.4%) | 0.043 |
| Cost categories | |||
| Mean total healthcare costs | $19,838±$22,671 | $20,383±$24,422 | 0.685 |
| Resource utilization | |||
| All-cause resource utilization | |||
| Mean number of all-cause inpatient visits | 0.86±1.16 | 0.87±1.11 | 0.428 |
| Mean number of all-cause outpatient visits | 14.71±12.57 | 16.47±14.83 | 0.306 |
| Mean number of all-cause ED visitsc | 1.29±1.65 | 1.57±2.19 | 0.409 |
| Mean number of all-cause healthcare provider visitsd | 24.98±19.22 | 26.22±18.53 | 0.072 |
| CHF-related resource utilization | |||
| Mean number of CHF-related inpatient visits | 0.18±0.51 | 0.12±0.35 | 0.220 |
| Mean number of CHF-related ED visitsc | 0.18±0.55 | 0.20±0.59 | 0.684 |
Univariate comparisons of central tendencies used Wilcoxon tests for continuous variables and chi-squared tests for categorical variables.
Baseline comorbidities were diagnosed based on at least two claims with the related ICD-9 codes, excluding laboratory or radiology claims.
Emergency department (ED) visits were defined by claims in an institutional setting (inpatient and outpatient) with revenue center code “045.”
Healthcare provider visits were defined as services (excluding lab-related) provided by physicians, physician assistants, and nurses in any noninstitutional setting.
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HCC, hierarchical condition categories.
Table 2.
Baseline Characteristics of Engaged Heart Failure Patients Versus Nonengaged Intervention Heart Failure Patients
| CHARACTERISTIC | ENGAGED HEALTH BUDDYa | NONENGAGEDa | P VALUEb |
|---|---|---|---|
| Number of patients | 230 | 393 | |
| Demographics | |||
| Mean age (years) | 76.90±8.79 | 79.84±9.08 | <0.001 |
| Male | 141 (61.3%) | 212 (53.9%) | 0.074 |
| Under 65 years of age | 12 (5.2%) | 16 (4.1%) | 0.505 |
| Baseline comorbiditiesc | |||
| CHF-related comorbidities | |||
| Anemia | 42 (18.3%) | 71 (18.1%) | 0.952 |
| Anxiety, somatoform disorders, and personality disorders | 12 (5.2%) | 12 (3.1%) | 0.176 |
| Chronic atherosclerosis | 97 (42.2%) | 136 (34.6%) | 0.060 |
| Chronic liver disease | 1 (0.4%) | 2 (0.5%) | 1.000 |
| COPD | 81 (35.2%) | 127 (32.3%) | 0.459 |
| Dementia | 3 (1.3%) | 6 (1.5%) | 1.000 |
| Depression | 14 (6.1%) | 12 (3.1%) | 0.068 |
| Diabetes mellitus | 92 (40.0%) | 170 (43.3%) | 0.427 |
| Hypercholesterolemia | 21 (9.1%) | 31 (7.9%) | 0.589 |
| Hypertension | 115 (50.0%) | 194 (49.4%) | 0.878 |
| Malnutrition | (0.0%) | 2 (0.5%) | 0.534 |
| Cancer | 58 (25.2%) | 94 (23.9%) | 0.716 |
| Myocardial infarction | 12 (5.2%) | 13 (3.3%) | 0.241 |
| Ocular disorder | 81 (35.2%) | 136 (34.6%) | 0.877 |
| Osteoporosis and osteoarthritis | 5 (2.2%) | 6 (1.5%) | 0.545 |
| Peripheral vascular disease | 19 (8.3%) | 36 (9.2%) | 0.703 |
| Renal failure | 26 (11.3%) | 53 (13.5%) | 0.430 |
| Respiratory failure | 10 (4.3%) | 10 (2.5%) | 0.218 |
| Cerebrovascular disease | 12 (5.2%) | 24 (6.1%) | 0.646 |
| Valvular heart disease | 29 (12.6%) | 31 (7.9%) | 0.054 |
| Obesity | 1 (0.4%) | 10 (2.5%) | 0.062 |
| Thyroid disorder | 12 (5.2%) | 25 (6.4%) | 0.560 |
| Sleep disorder | 6 (2.6%) | 3 (0.8%) | 0.083 |
| Pneumonia | 29 (12.6%) | 48 (12.2%) | 0.885 |
| Overall health indicators | |||
| Mean adjusted Elixhauser comorbidity index | 1.99±1.40 | 1.92±1.23 | 0.867 |
| Mean HCC score | 2.63±1.03 | 2.51±0.93 | 0.236 |
| End stage renal disease | 2 (0.9%) | 6 (1.5%) | 0.717 |
| Exclusionary comorbidity | 18 (7.8%) | 37 (9.4%) | 0.500 |
| Cost categories | |||
| Mean total healthcare costs | $21,471±$23,492 | $18,882±$22,152 | 0.186 |
| Resource utilization | |||
| All-cause resource utilization | |||
| Mean number of all-cause inpatient visits | 0.87±1.10 | 0.85±1.19 | 0.519 |
| Mean number of all-cause outpatient visits | 15.98±12.16 | 13.97±12.75 | 0.005 |
| Mean number of all-cause ED visitsd | 1.18±1.44 | 1.35±1.76 | 0.453 |
| Mean number of all-cause healthcare provider visitse | 27.12±21.13 | 23.74±17.91 | 0.066 |
| CHF-related resource utilization | |||
| Mean number of CHF-related inpatient visits | 0.15±0.48 | 0.19±0.53 | 0.170 |
| Mean number of CHF-related ED visitsd | 0.17±0.52 | 0.19±0.57 | 0.533 |
Patients “engaged” the Health Buddy if they inputted information into the Health Buddy device at least once during the study period. Otherwise they were classified as “nonengaged.”
Univariate comparisons of central tendencies used Wilcoxon tests for continuous variables and chi-squared tests for categorical variables.
Baseline comorbidities were diagnosed based on at least two claims with the related ICD-9 codes, excluding laboratory or radiology claims.
Emergency department (ED) visits were defined by claims in an institutional setting (inpatient and outpatient) with revenue center code '“045.”
Healthcare provider visits were defined as services (excluding lab-related) provided by physicians, physician assistants, and nurses in any noninstitutional setting.
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HCC, hierarchical condition categories.
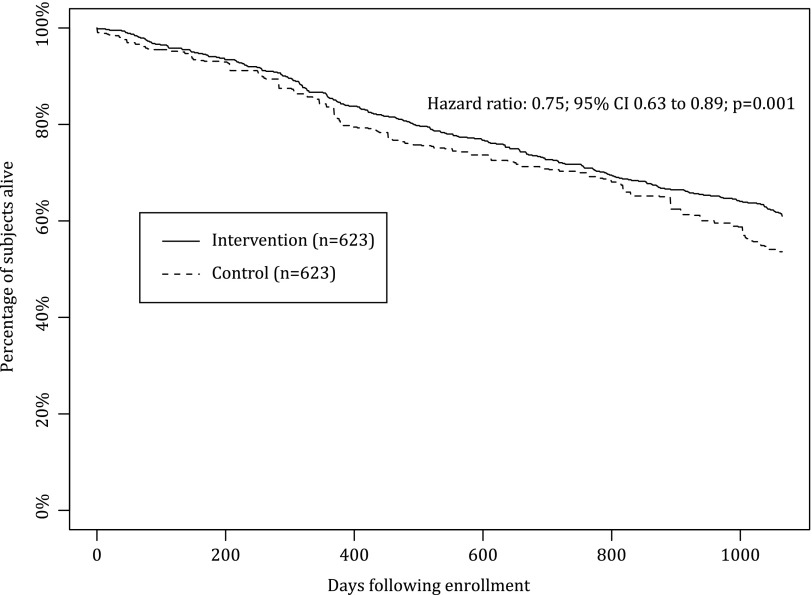
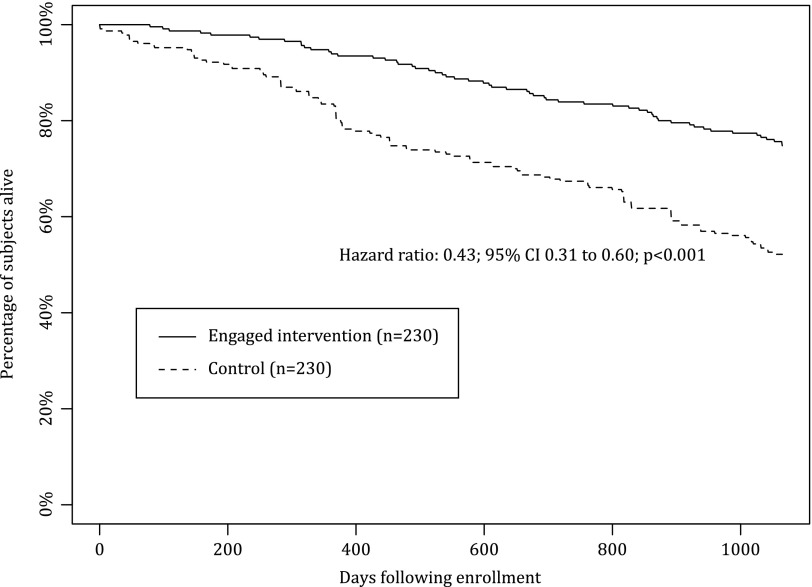
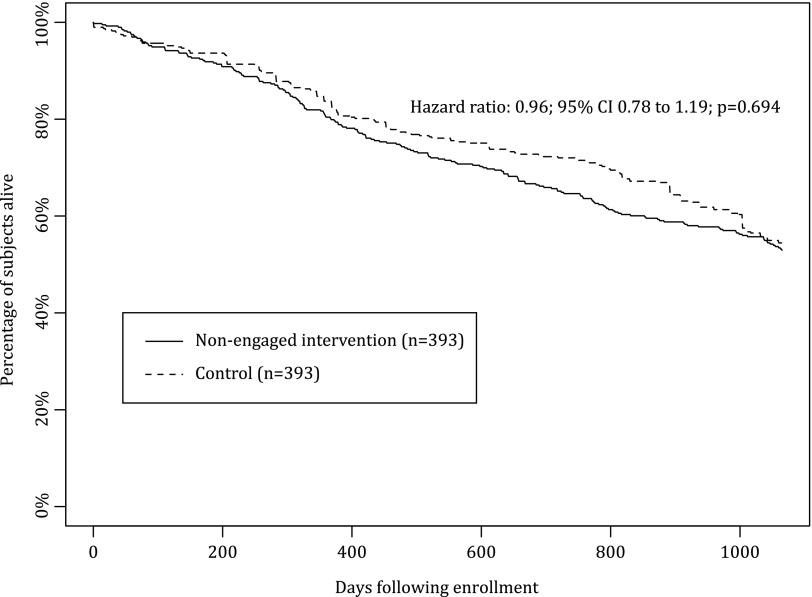
All-cause mortality during the study period was 39.0% in the intervention group and 46.4% among matched controls (p=0.008) (Table 3). Mortality risk in the intervention cohort was significantly lower than the control cohort (adjusted hazard ratio [HR]=0.75; 95% confidence interval [CI], 0.63–0.89; p<0.001) (Fig. 1) adjusting for age, liver disease, depression, diabetes mellitus, ocular disorders, sleep disorders, end-stage renal disease, and high-risk comorbidities. The reduction in mortality risk was greatest among engaged patients who had substantially lower overall mortality (25.2% versus 47.8%; p<0.001) (Table 3) and adjusted mortality risk compared with matched controls (adjusted HR=0.43; 95% CI, 0.31–0.60; p<0.001) (Fig. 2). Engaged intervention patients showed reduced mortality within the first study year (6.1% versus 15.2%; p<0.001) that persisted throughout the study period. There was no significant difference in either overall mortality (47.1% versus 45.5%; p=0.67) or adjusted mortality risk among nonengaged patients offered the HBP compared with matched controls (HR=0.96; 95% CI, 0.78–1.19; p=0.69) (Fig. 3).
Table 3.
Mortality Rates During the Study Period for Intervention Heart Failure Patients Versus Matched Controls
| SAMPLE | INTERVENTION | CONTROL | P VALUEa |
|---|---|---|---|
| Engaged Health Buddy (n=230/arm)b | 58 (25.2%) | 110 (47.8%) | <0.001 |
| Nonengaged (n=393/arm)b | 185 (47.1%) | 179 (45.5%) | 0.668 |
| Overall (n=623/arm) | 243 (39.0%) | 289 (46.4%) | 0.008 |
Patients were followed up for 3 years or until death in the study period.
p values were calculated using chi-squared tests.
Patients “engaged” the Health Buddy if they inputted information into the Health Buddy device at least once during the study period. Otherwise they were classified as “nonengaged.”
Fig. 1.
Overall survival for intervention heart failure patients versus matched controls. The primary outcome of mortality risk in the intervention cohort was significantly lower than that in the control cohort (adjusted hazard ratio=0.75; 95% confidence interval [CI], 0.63–0.89; p=0.001).
Fig. 2.
Overall survival for engaged intervention heart failure patients versus matched controls. The reduction in mortality risk was greatest among the engaged patients, who had substantially lower relative mortality risk compared with matched controls (adjusted hazard ratio=0.43; 95% confidence interval [CI], 0.31–0.60; p<0.001).
Fig. 3.
Overall survival for nonengaged intervention heart failure patients versus matched controls. There was no significant difference in mortality risk among nonengaged patients who were offered the Health Buddy Program compared with matched controls (adjusted hazard ratio=0.96; 95% confidence interval [CI], 0.78–1.19, p=0.69).
Changes in healthcare utilization are shown in Table 4. A reduction of 22.7% in quarterly hospitalizations was noted in intervention patients versus matched controls (Δ=−0.05 hospitalizations/quarter; 95% CI, −0.09 to −0.01; p=0.012). As with mortality, this reduction was limited to engaged patients who had a 43.5% reduction in quarterly hospitalizations (Δ=−0.10; 95% CI, −0.16 to −0.04; p=0.002), whereas the difference between nonengaged patients and matched controls was not significant (Δ=−0.02; 95% CI, −0.07 to 0.03; p=0.49). There were no significant differences between intervention and matched control cohorts in all-cause hospital days per quarter or all-cause ED visits. Results were similar after adjusting for significant differences in baseline characteristics between the two groups.
Table 4.
Healthcare Resource Use Comparison for Intervention Heart Failure Patients Versus Matched Controls
| INTERVENTION | CONTROL | |||||||
|---|---|---|---|---|---|---|---|---|
| BASELINE | STUDYa | BASELINE | STUDYa | UNADJUSTED RELATIVE CHANGE (95% CI)b | P VALUEc | ADJUSTED RELATIVE CHANGE (95% CI)d | P VALUEc | |
| Quarterly number of inpatient admissionse | ||||||||
| Engaged Health Buddy | 0.23 | 0.14 | 0.18 | 0.19 | −0.10 (−0.16, −0.04) | 0.002 | −0.12 (−0.18, −0.05) | <0.001 |
| Nonengaged | 0.21 | 0.19 | 0.21 | 0.19 | −0.02 (−0.07, 0.03) | 0.491 | −0.01 (−0.05, 0.05) | 0.956 |
| Overall | 0.22 | 0.17 | 0.20 | 0.19 | −0.05 (−0.09, −0.01) | 0.012 | −0.05 (−0.09, −0.01) | 0.011 |
| Hospital days per quarter, conditional on hospitalizatione | ||||||||
| Engaged Health Buddy | 5.04 | 6.11 | 5.72 | 5.96 | 0.83 (−1.33, 2.99) | 0.449 | 0.91 (−0.39, 3.13) | 0.127 |
| Nonengaged | 5.23 | 6.09 | 5.73 | 6.16 | 0.44 (−0.89, 1.76) | 0.518 | 0.68 (−0.54, 1.83) | 0.287 |
| Overall | 5.15 | 6.10 | 5.72 | 6.09 | 0.59 (−0.56, 1.73) | 0.316 | 0.60 (−0.21, 1.65) | 0.131 |
| Quarterly number of emergency room visitsf | ||||||||
| Engaged Health Buddy | 0.28 | 0.24 | 0.31 | 0.29 | −0.01 (−0.09, 0.07) | 0.776 | −0.05 (−0.13, 0.03) | 0.254 |
| Nonengaged | 0.34 | 0.33 | 0.37 | 0.32 | 0.05 (−0.03, 0.12) | 0.226 | 0.07 (0.01, 0.16) | 0.033 |
| Overall | 0.32 | 0.29 | 0.35 | 0.31 | 0.02 (−0.04, 0.07) | 0.492 | 0.02 (−0.03, 0.08) | 0.310 |
Patients were followed up for 3 years or until death in the study period.
Unadjusted relative change was calculated as (Intervention sample study period outcome – Intervention sample baseline period outcome) – (Control sample study period outcome – Control sample baseline period outcome).
Univariate comparisons of central tendencies used t tests for continuous variables.
Predictions were estimated with panel-negative binomial models. Control variables were those criteria with p values of < 0.1 after matching.
Quarterly number of inpatient admissions was calculated based on the average total number of inpatient claims in each quarter. Inpatient admissions that occurred during the last quarter of a patient's baseline year, the first quarter of a patient's study period, and the same quarter that a patient died were not included in the analysis.
Emergency room visits were defined by claims in an institutional setting (inpatient and outpatient) with revenue center code "045." Quarterly number of emergency room visits was calculated based on the average total number of emergency room visits in each quarter. Emergency room visits that occurred during the last quarter of a patient's baseline year, the first quarter of a patient's study period, and the same quarter that a patient died were not included in the analysis.
CI, confidence interval.
Discussion
This propensity score-matched cohort analysis of Medicare beneficiaries is one of the largest HF studies to date of telehealth coupled with care management and shows availability of the HBP was associated with a 24.9% reduction in all-cause mortality and a 22.7% reduction in quarterly hospitalizations relative to matched controls. These findings were driven by a 57.2% reduction in mortality and a 43.5% reduction in quarterly hospitalizations in the 36.9% of beneficiaries who used the HBP at least once during the study period. Although our findings need validation, they suggest that the success of a telehealth system in improving outcomes in HF may be related to two important factors beyond the remote clinical data collected by the system: (1) the degree of patient engagement with the system and (2) the patient population itself. The manner in which remote monitoring data are presented to and used by providers and the inclusion of interactive patient education may also be a factor but was not directly addressed in this analysis. Optimization of data management and presentation, integration into provider workflow, and better understanding of predictors of patient engagement with telehealth systems like the HBP might also improve effectiveness, and further study is warranted.
The association between the HBP and improved survival might be explained by at least three mechanisms: (a) a direct beneficial effect of the HBP on clinical care delivery, (b) improved patient self-management associated with the HBP leading to better outcomes, or (c) HBP engagement as a marker for patients predisposed to effective self-management and healthcare utilization. Prior studies have found that use of the HBP was associated with greater compliance with prescription medications,13 titration of medications,14 and compliance with nonpharmacologic recommendations,15 suggesting the HBP likely improved both care delivery and patient self-management, which may have contributed to reductions in mortality and hospitalization observed in the present study. In addition, secondary analyses of multiple randomized trials have shown improved outcomes in patients who demonstrate high pharmacologic adherence in the placebo arm comparable to those receiving the study drug, supporting the possibility that patient engagement may be a marker for other beneficial characteristics.16–18 The lack of patient-level data in this analysis, particularly data on adherence to non-HBP interventions and provider responses to evidence of patient deterioration, makes it difficult to determine the mechanism of potential benefit from the HBP. Prospective studies to elucidate the mechanism linking HBP engagement and improved survival in this trial may help define the role of the HBP and other telehealth technologies in HF management.
The Telemonitoring in Patients with Heart Failure (TEHAF) trial was a randomized study that investigated the association between the HBP and HF outcomes but found no reduction in HF hospitalizations or mortality during 1 year of follow-up.19 Due in part to a lower than expected hospitalization rate and shorter study period compared with the present analysis (1 versus 3 years), TEHAF was underpowered to evaluate its outcomes. The rate of adverse outcomes may also have been low because patients were recruited from HF clinics and had high degrees of adherence at baseline to recommended HF therapies such as beta-blockers (82%) and angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists (90%). Impact of patient engagement was not tested, but the authors reported an overall daily dialogue compliance of 90% with the HBP, a metric of compliance not available in our study. Limitations of the present study and TEHAF preclude firm conclusions that explain the contrasting results, but it is likely that patient populations and baseline management strategies were different. It is therefore possible that the HBP benefits some patient populations more than others.
Tele-HF randomized 1,653 patients recently hospitalized with HF to either telephone-based monitoring or guideline-based usual care and failed to detect a difference in the composite endpoint of re-admission or death over 18 months.9 In total, 85.6% of patients activated the system at least once, suggesting a higher level of engagement than in the present study. Unlike the HBP, the voice-response system in Tele-HF was not interactive and did not contain an education component. Patient contact was driven by clinician assessment of patient status rather than as an integral part of the system design, and the authors speculated that an interactive system may have been more effective.9
The Telemedical Interventional Monitoring in Heart Failure (TIM-HF) randomized stable ambulatory HF patients on optimal medical therapy from internal medicine practices to usual care or telemonitoring using a wireless system to collect electrocardiogram measurements, blood pressure, weight, and a patient self-assessment. As with Tele-HF, patient contact was initiated based on patient data. The system was not interactive and had no education component.20 No reduction in mortality was observed after 26 months. A high rate of patient engagement was observed, but outcomes in engaged and nonengaged patients were not compared.21 Based on these results, the authors concluded that remote telemonitoring does not improve survival in stable, optimally treated patients with HF. In addition to the intrinsic differences in the telemonitoring systems between TIM-HF and the present study, it was not possible to determine whether patients in the present study were receiving optimal HF treatment at baseline or how this may have changed following HBP initiation. It is therefore possible that baseline medical therapy and/or medical compliance after HBP enrollment may partially explain differences in outcomes between the studies.
This study has several limitations. It was a retrospective study, and all findings must be validated. Although the control cohort was constructed using rigorous propensity-score matching, unobserved differences between the intervention and control cohorts may have affected outcomes. Predictors of patient engagement that were unavailable may also lead to improved HF outcomes independent of HBP use, in which case the propensity match may have been incomplete. Because they were taken from the CMS 5% sample, control patients received care from different clinical sites than intervention patients, and some observed differences in outcomes could have been due to clinic-specific variations in care. Because the current study was restricted to HF patients in the Pacific Northwest, results may not be generalizable to other regions or patient populations. Temporal granularity in the Medicare data was limited to quarters, and associations between daily HBP data were not studied. Future analyses that associate patient-level data from the HBP with clinical events may help determine the role of the HBP in improving outcomes. In addition, this study was based solely on Medicare claims data but did not include Medicare Part D, so patient-level clinical and pharmacy data were not available to verify accuracy of diagnoses, determine quality of HF therapy, or assess medical compliance. Consequently, a prospective randomized controlled trial of the HBP including medication adherence data is necessary to validate our findings.
Conclusions
Availability of the HBP, a content-driven telehealth system coupled with care management, was associated with improved survival and reduced hospitalization for Medicare beneficiaries with HF driven by substantial benefits to patients who used the system. Further investigation into the mechanisms of this benefit and methods for optimizing patient engagement may enhance the benefit of the HBP and other telehealth platforms.
Supplementary Material
Acknowledgments
D.P.K. was supported by grants T32 HL007822-12 and L30 HL110124 from the National Institutes of Health and by Jacqueline's Research Fund funded by the Jacqueline Marie Leaffer Foundation and the University of Colorado Center for Women's Health Research. Analysis Group, Inc., the employer of D.M., H.G.B., J.L.J., and U.S.D., received unrestricted grants from Robert Bosch Healthcare for this study.
Disclosure Statement
J.L. has consulted for Medtronic, Boston Scientific, St. Jude, Abbott, and RESMED. D.P.K. has consulted for Gliimpse, Inc. D.M., H.G.B., J.L.J., and U.S.D. are employees of Analysis Group, Inc. R.L.P. II declares no competing financial interests exist.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013;127:e6–e245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Page RL, Lindenfeld J. The comorbidity conundrum: A focus on the role of noncardiovascular chronic conditions in the heart failure patient. Curr Cardiol Rep 2012;14:276–284 [DOI] [PubMed] [Google Scholar]
- 3.Clark RA, Inglis SC, McAlister FA, Cleland JG, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: Systematic review and meta-analysis. BMJ 2007;334:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palaniswamy C, Mishkin A, Aronow WS, Kalra A, Frishman WH. Remote patient monitoring in chronic heart failure. Cardiol Rev 2013;21:141–150 [DOI] [PubMed] [Google Scholar]
- 5.Cleland JG, Coletta AP, Castiello T, Clark AL. Clinical trials update from the European Society of Cardiology Heart Failure meeting 2011: TEHAF, WHICH, CARVIVA, and atrial fibrillation in GISSI-HF and EMPHASIS-HF. Eur J Heart Fail 2011;13:1147–1151 [DOI] [PubMed] [Google Scholar]
- 6.LaFramboise LM, Todero CM, Zimmerman L, Agrawal S. Comparison of Health Buddy with traditional approaches to heart failure management. Fam Community Health 2003;26:275–288 [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803–869 [DOI] [PubMed] [Google Scholar]
- 8.Inglis SC, Clark RA, McAlister FA, Ball J, Lewinter C, Cullington D, Stewart S, Cleland JG. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev 2010;(8):CD007228. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med 2010;363:2301–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCall N, Cromwell J, Smith K, Urato C. Evaluation of Medicare care management for high cost beneficiaries (CMHCB) demonstration: The Health Buddy Consortium (HBC). 2011. Available at www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/McCall_Eval_of_CMHCB_Demo_April_2011.pdf (last accessed September11, 2014)
- 11.Baker LC, Johnson SJ, Macaulay D, Birnbaum H. Integrated telehealth and care management program for Medicare beneficiaries with chronic disease linked to savings. Health Aff (Millwood) 2011;30:1689–1697 [DOI] [PubMed] [Google Scholar]
- 12.Baker LC, Macaulay DS, Sorg RA, Diener MD, Johnson SJ, Birnbaum HG. Effects of care management and telehealth: A longitudinal analysis using medicare data. J Am Geriatr Soc 2013;61:1560–1567 [DOI] [PubMed] [Google Scholar]
- 13.Kobb R, Hoffman N, Lodge R, Kline S. Enhancing elder chronic care through technology and care coordination: Report from a pilot. Telemed J E Health 2003;9:189–195 [DOI] [PubMed] [Google Scholar]
- 14.Schofield RS, Kline SE, Schmalfuss CM, Carver HM, Aranda JM, Pauly DF, Hill JA, Neugaard BI, Chumbler NR. Early outcomes of a care coordination-enhanced telehome care program for elderly veterans with chronic heart failure. Telemed J E Health 2005;11:20–27 [DOI] [PubMed] [Google Scholar]
- 15.Ramaekers BL, Janssen-Boyne JJ, Gorgels AP, Vrijhoef HJ. Adherence among telemonitored patients with heart failure to pharmacological and nonpharmacological recommendations. Telemed J E Health 2009;15:517–524 [DOI] [PubMed] [Google Scholar]
- 16.The Coronary Drug Project Research Group. Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med 1980;303:1038–1041 [DOI] [PubMed] [Google Scholar]
- 17.Horwitz RI, Viscoli CM, Berkman L, Donaldson RM, Horwitz SM, Murray CJ, Ransohoff DF, Sindelar J. Treatment adherence and risk of death after a myocardial infarction. Lancet 1990;336:542–545 [DOI] [PubMed] [Google Scholar]
- 18.Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, Yusuf S, Michelson EL, Pfeffer MA; CHARM Investigators. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: Double-blind, randomised, controlled clinical trial. Lancet 2005;366:2005–2011 [DOI] [PubMed] [Google Scholar]
- 19.Boyne JJ, Vrijhoef HJ, Crijns HJ, De Weerd G, Kragten J, Gorgels AP; TEHAF Investigators. Tailored telemonitoring in patients with heart failure: Results of a multicentre randomized controlled trial. Eur J Heart Fail 2012;14:791–801 [DOI] [PubMed] [Google Scholar]
- 20.Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, Boll H, Kim SS, Koehler K, Lücke S, Honold M, Heinze P, Schweizer T, Braecklein M, Kirwan BA, Gelbrich G, Anker SD; TIM-HF Investigators. Telemedical Interventional Monitoring in Heart Failure (TIM-HF), a randomized, controlled intervention trial investigating the impact of telemedicine on mortality in ambulatory patients with heart failure: Study design. Eur J Heart Fail 2010;12:1354–1362 [DOI] [PubMed] [Google Scholar]
- 21.Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD; Telemedical Interventional Monitoring in Heart Failure Investigators. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The Telemedical Interventional Monitoring in Heart Failure study. Circulation 2011;123:1873–1880 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.