Abstract
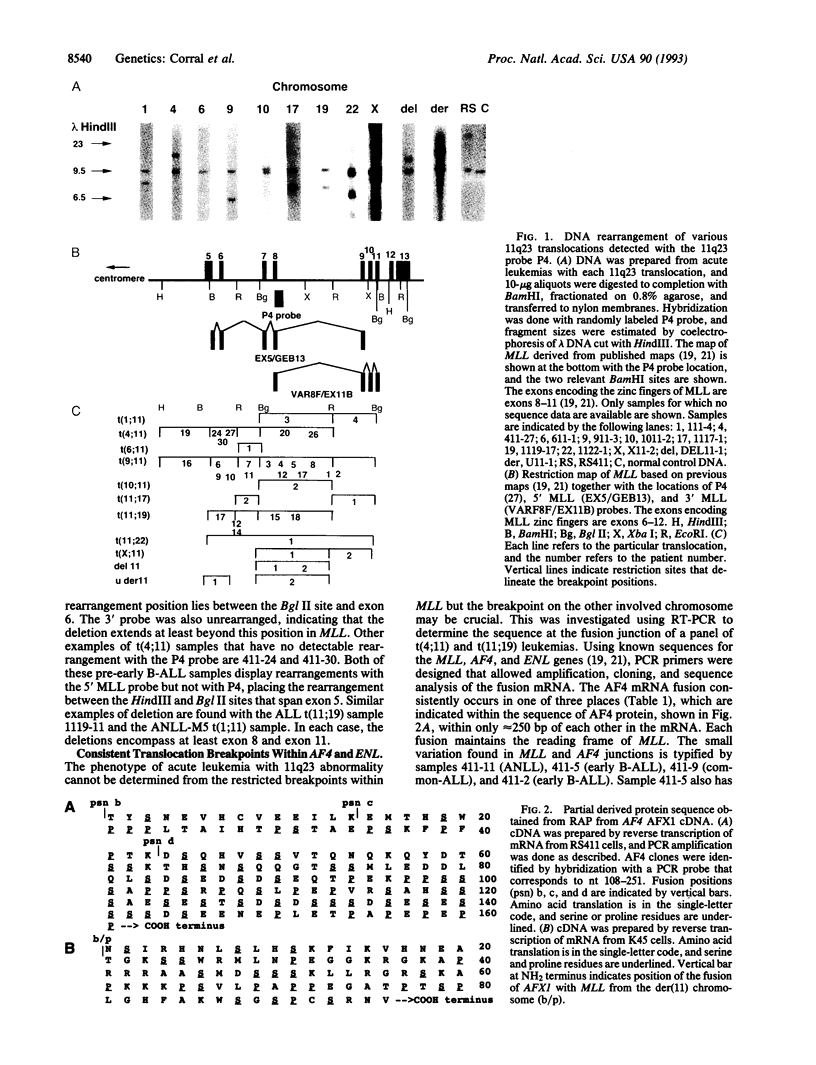
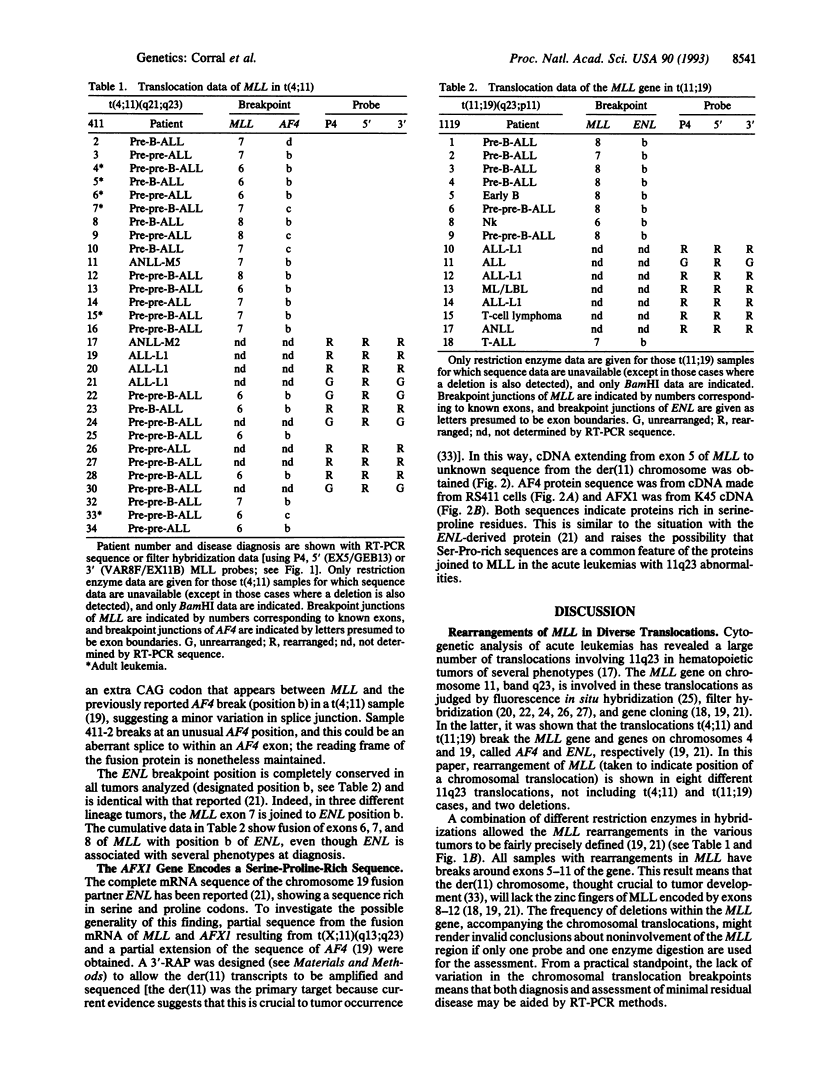
The MLL gene, on human chromosome 11q23, undergoes chromosomal translocation in acute leukemias, resulting in gene fusion with AF4 (chromosome 4) and ENL (chromosome 19). We report here translocation of MLL with nine different chromosomes and two paracentric chromosome 11 deletions in early B cell, B- or T-cell lineage, or nonlymphocytic acute leukemias. The mRNA translocation junction from 22 t(4;11) patients, including six adult leukemias, and nine t(11;19) tumors reveals a remarkable conservation of breakpoints within MLL, AF4, or ENL genes, irrespective of tumor phenotype. Typically, the breakpoints are upstream of the zinc-finger region of MLL, and deletion of this region can accompany translocation, supporting the der(11) chromosome as the important component in leukemogenesis. Partial sequence of a fusion between MLL and the AFX1 gene from chromosome X shows the latter to be rich in Ser/Pro codons, like the ENL mRNA. These data suggest that the heterogeneous 11q23 abnormalities might cause attachment of Ser/Pro-rich segments to the NH2 terminus of MLL, lacking the zinc-finger region, and that translocations occur in early hematopoietic cells, before commitment to distinct lineages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Harris A. W., Pinkert C. A., Corcoran L. M., Alexander W. S., Cory S., Palmiter R. D., Brinster R. L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985 Dec 12;318(6046):533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Akao Y., Seto M., Takahashi T., Saito M., Utsumi K. R., Nakazawa S., Ueda R. Rearrangements on chromosome 11q23 in hematopoietic tumor-associated t(11;14) and t(11;19) translocations. Cancer Res. 1991 Dec 15;51(24):6708–6711. [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Denning S. M., Haynes B. F., Waldmann T. A., Kirsch I. R. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Guglielmi P., Jonveaux P., Cherif D., Gisselbrecht S., Mauchauffe M., Berger R., Larsen C. J., Mathieu-Mahul D. Two distinct mechanisms for the SCL gene activation in the t(1;14) translocation of T-cell leukemias. Genes Chromosomes Cancer. 1990 Jan;1(3):194–208. doi: 10.1002/gcc.2870010303. [DOI] [PubMed] [Google Scholar]
- Boehm T., Buluwela L., Williams D., White L., Rabbitts T. H. A cluster of chromosome 11p13 translocations found via distinct D-D and D-D-J rearrangements of the human T cell receptor delta chain gene. EMBO J. 1988 Jul;7(7):2011–2017. doi: 10.1002/j.1460-2075.1988.tb03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Foroni L., Kaneko Y., Perutz M. F., Rabbitts T. H. The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4367–4371. doi: 10.1073/pnas.88.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Cheng J. T., Tasi L. H., Schneider N., Buchanan G., Carroll A., Crist W., Ozanne B., Siciliano M. J., Baer R. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J. 1990 Feb;9(2):415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. T., Yang C. Y., Hernandez J., Embrey J., Baer R. The chromosome translocation (11;14)(p13;q11) associated with T cell acute leukemia. Asymmetric diversification of the translocational junctions. J Exp Med. 1990 Feb 1;171(2):489–501. doi: 10.1084/jem.171.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djabali M., Selleri L., Parry P., Bower M., Young B. D., Evans G. A. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat Genet. 1992 Oct;2(2):113–118. doi: 10.1038/ng1092-113. [DOI] [PubMed] [Google Scholar]
- Don R. H., Cox P. T., Wainwright B. J., Baker K., Mattick J. S. 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991 Jul 25;19(14):4008–4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch P., Boehm T., Lavenir I., Larson T., Arno J., Forster A., Rabbitts T. H. T-cell acute lymphoblastic lymphoma induced in transgenic mice by the RBTN1 and RBTN2 LIM-domain genes. Oncogene. 1992 Dec;7(12):2389–2397. [PubMed] [Google Scholar]
- Gibbons B., Katz F. E., Ganly P., Chessells J. M. Infant acute lymphoblastic leukaemia with t(11; 19). Br J Haematol. 1990 Mar;74(3):264–269. doi: 10.1111/j.1365-2141.1990.tb02581.x. [DOI] [PubMed] [Google Scholar]
- Gu Y., Nakamura T., Alder H., Prasad R., Canaani O., Cimino G., Croce C. M., Canaani E. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992 Nov 13;71(4):701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- Harris A. W., Pinkert C. A., Crawford M., Langdon W. Y., Brinster R. L., Adams J. M. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med. 1988 Feb 1;167(2):353–371. doi: 10.1084/jem.167.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. C., Martinerie C., Sun L. H., Williams D., Showe L. C., Marteneire C. Translocations and rearrangements in T-cell acute leukemias with the t(11;14) (p13;q11) chromosomal translocations. Oncogene. 1989 Mar;4(3):341–349. [PubMed] [Google Scholar]
- Kamps M. P., Baltimore D. E2A-Pbx1, the t(1;19) translocation protein of human pre-B-cell acute lymphocytic leukemia, causes acute myeloid leukemia in mice. Mol Cell Biol. 1993 Jan;13(1):351–357. doi: 10.1128/mcb.13.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney L., Bower M., Gibbons B., Das S., Chaplin T., Nacheva E., Chessells J. M., Reeves B., Riley J. H., Lister T. A. Chromosome 11q23 translocations in both infant and adult acute leukemias are detected by in situ hybridization with a yeast artificial chromosome. Blood. 1992 Oct 1;80(7):1659–1665. [PubMed] [Google Scholar]
- Leder A., Pattengale P. K., Kuo A., Stewart T. A., Leder P. Consequences of widespread deregulation of the c-myc gene in transgenic mice: multiple neoplasms and normal development. Cell. 1986 May 23;45(4):485–495. doi: 10.1016/0092-8674(86)90280-1. [DOI] [PubMed] [Google Scholar]
- McCabe N. R., Burnett R. C., Gill H. J., Thirman M. J., Mbangkollo D., Kipiniak M., van Melle E., Ziemin-van der Poel S., Rowley J. D., Diaz M. O. Cloning of cDNAs of the MLL gene that detect DNA rearrangements and altered RNA transcripts in human leukemic cells with 11q23 translocations. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11794–11798. doi: 10.1073/pnas.89.24.11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire E. A., Rintoul C. E., Sclar G. M., Korsmeyer S. J. Thymic overexpression of Ttg-1 in transgenic mice results in T-cell acute lymphoblastic leukemia/lymphoma. Mol Cell Biol. 1992 Sep;12(9):4186–4196. doi: 10.1128/mcb.12.9.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G. J., Cotter F., Katz F. E., Ridge S. A., Domer P., Korsmeyer S., Wiedemann L. M. Breakpoints at 11q23 in infant leukemias with the t(11;19)(q23;p13) are clustered. Blood. 1992 Nov 1;80(9):2172–2175. [PubMed] [Google Scholar]
- Morrissey J., Tkachuk D. C., Milatovich A., Francke U., Link M., Cleary M. L. A serine/proline-rich protein is fused to HRX in t(4;11) acute leukemias. Blood. 1993 Mar 1;81(5):1124–1131. [PubMed] [Google Scholar]
- Nakamura T., Alder H., Gu Y., Prasad R., Canaani O., Kamada N., Gale R. P., Lange B., Crist W. M., Nowell P. C. Genes on chromosomes 4, 9, and 19 involved in 11q23 abnormalities in acute leukemia share sequence homology and/or common motifs. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4631–4635. doi: 10.1073/pnas.90.10.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Boehm T. Structural and functional chimerism results from chromosomal translocation in lymphoid tumors. Adv Immunol. 1991;50:119–146. doi: 10.1016/s0065-2776(08)60824-x. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A., Larson R., Nathan P. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat Genet. 1993 Jun;4(2):175–180. doi: 10.1038/ng0693-175. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H. Translocations, master genes, and differences between the origins of acute and chronic leukemias. Cell. 1991 Nov 15;67(4):641–644. doi: 10.1016/0092-8674(91)90057-6. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. The der(11) chromosome contains the critical breakpoint junction in the 4;11, 9;11, and 11;19 translocations in acute leukemia. Genes Chromosomes Cancer. 1992 Oct;5(3):264–266. doi: 10.1002/gcc.2870050316. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Loos U., Ludwig W. D. TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is overexpressed in acute T-cell leukaemia with the t(11;14)(p13;q11). Oncogene. 1991 Oct;6(10):1887–1893. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tkachuk D. C., Kohler S., Cleary M. L. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992 Nov 13;71(4):691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- Yoffe G., Schneider N., Van Dyk L., Yang C. Y., Siciliano M., Buchanan G., Capra J. D., Baer R. The chromosome translocation (11;14)(p13;q11) associated with T-cell acute lymphocytic leukemia: an 11p13 breakpoint cluster region. Blood. 1989 Jul;74(1):374–379. [PubMed] [Google Scholar]
- Ziemin-van der Poel S., McCabe N. R., Gill H. J., Espinosa R., 3rd, Patel Y., Harden A., Rubinelli P., Smith S. D., LeBeau M. M., Rowley J. D. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10735–10739. doi: 10.1073/pnas.88.23.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]