Abstract
Introduction
Gastric bypass is today the most frequently performed bariatric procedure,but, despite of it, several complications can occur with varied morbimortality. Probably all bariatric surgeons know these complications, but, as bariatric surgery continues to spread, general surgeon must be familiarized to it and its management. Gastric bypass complications can be divided into two groups: early and late complications, taking into account the two weeks period after the surgery. This paper will focus the early ones.
Method
Literature review was carried out using Medline/PubMed, Cochrane Library, SciELO, and additional information on institutional sites of interest crossing the headings: gastric bypass AND complications; follow-up studies AND complications; postoperative complications AND anastomosis, Roux-en-Y; obesity AND postoperative complications. Search language was English.
Results
There were selected 26 studies that matched the headings. Early complications included: anastomotic or staple line leaks, gastrointestinal bleeding, intestinal obstruction and incorrect Roux limb reconstruction.
Conclusions
Knowledge on strategies on how to reduce the risk and incidence of complications must be acquired, and every surgeon must be familiar with these complications in order to achieve an earlier recognition and perform the best intervention.
Keywords: Postoperative complications; Follow-up studies; Gastric bypass; Anastomosis, Roux-en-Y; Obesity
Abstract
Introdução
O bypass gástrico é hoje o procedimento bariátrico mais realizado, mas, apesar disso, várias complicações podem ocorrer com variada morbimortalidade. Provavelmente todos os cirurgiões bariátricos conhecem essas complicações, mas como a cirurgia bariátrica continua a se espalhar, o cirurgião geral deve estar familiarizado com essas complicações e seu manuseio. As complicações do bypass gástrico podem ser divididas em dois grupos: as precoces e tardias, tendo em conta o período de duas semanas após a operação. Este artigo irá focar as precoces.
Método
Foi realizada revisão da literatura utilizando as bases Medline/PubMed, Cochrane Library, SciELO, e informações adicionais sobre sites institucionais de interesse cruzando os descritores: bypass gástrico AND complicações; seguimento AND complicações; complicações pós-operatórias AND anastomose, Roux-en-Y; obesidade AND complicações pós-operatórias. A língua usada para a busca foi o inglês.
Resultados
Foram selecionados 26 artigos que combinavam com os descritores. As complicações imediatas foram: fístula na linha de grampeamento, sangramento gastrointestinal, obstrução intestinal e reconstrução incorreta da alça em Roux.
Conclusão
O conhecimento sobre as estratégias de como reduzir o risco e incidência das complicações deve ser adquirido ao longo do tempo, e cada cirurgião deve estar familiarizado com essas complicações, a fim de reconhecê-las precocemente e realizar a melhor intervenção.
INTRODUCTION
Among all the bariatric procedures, the Roux-en-Y gastric bypass (RYGB) is the most frequently performed13. It belongs to the group of combined procedures because it generates restriction and malabsorption.
The restriction is generated by cutting the proximal stomach, thereby reducing its volume and creating a pouch of approximately 10 to 25 ml, leaving the rest of the stomach excluded.
In the other hand, the malabsorption is generated by dividing the small intestine into an alimentary limb (Roux limb) and a biliopancreatic limb. The alimentary limb of Roux-en-Y is created by dividing the jejunum 50 cm below the duodenojejunal ligament. Then the alimentary limb is measured and a side-to-side stapled jejunojejunostomy is created, typically 150 cm below the gastrojejunal anastomosis.
Despite of it well documented safety1,7,11,28,30, several complications can occur with varying degrees of morbidity and mortality risk. These complications includes: anastomotic or staple line leaks, gastrointestinal bleeding, intestinal obstruction, anastomotic strictures, marginal ulceration and gastro-gastric fistula and less common, incorrect Roux limb reconstruction.
METHOD
Literature review was carried out using Medline/PubMed, Cochrane Library, SciELO, and additional information on institutional sites of interest crossing the headings: gastric bypass AND complications; follow-up studies AND complications; postoperative complications AND anastomosis, Roux-en-Y; obesity AND postoperative complications. Search language was English. There were selected 26 studies that matched the headings. Early complications included: anastomotic or staple line leaks, gastrointestinal bleeding, intestinal obstruction and less common, incorrect Roux limb reconstruction.
Anastomotic or staple line leaks
This complication can be defined as inadequate tissue healing allowing for exit of gastrointestinal material through the staple or suture line. It remains as one of the most common causes of death after RYGB, leak-associated mortality can be up to 37.5-50%8,9,31 representing the second cause of death and together with pulmonary embolism represent more than 50% of the causes of death in patients undergoing bariatric surgery.
The incidence of this complication ranges from 0 to 5.6% in large series and does not differ significantly between laparoscopic and open RYGB8.
There are five potential sites of leaking after RYGB: gastrojejunostomy, gastric pouch staple line, roux limb staple line, jejunojejunostomy and gastric remnant staple line. The frequency of these locations is shown in Table 1.
Table 1.
Frequency and leak locations
| Location | Incidence |
|---|---|
| Gastrojejunostomy | 67.8% |
| Gastric pouch | 10.2% |
| Gxcluded stomach | 3.4% |
| Jejunojejunal anastomosis | 5% |
| Gastrojejunostomy plus pouch | 3.4% |
| Pouch plus excluded stomach | 3.4% |
| Undetermined sites | 6.8% |
Different risk factors for developing a leak have been studied and it has been shown that patients at higher risk are primarily those who are older super-obese, men, and those with multiple co-morbidities and previous or revisional bariatric operations8,14,27,28,31.
In the other hand, operative technique can also be related with the leak rate: appropriate staple firing and its size, staple line reinforcement with biologic buttress material11, use of fibrin sealant5,17, intraoperative leak testing, anastomosis under tension, and ischemia can affect the incidence of anastomotic leaks after laparoscopic RYGB17.
Although most anastomotic leaks occur five to seven days after surgery and are thought to be related to ischemia, 95% of anastomotic leaks that occur within two days of surgery probably result from technical error20. Regarding to the staple firing, one possible error can occur when the staples do not engage or do not completely close when the endoscopic stapler is fired. This may occur when the wrong-sized staple cartridge is selected. As a result, the staples appear to have fired and seated properly, but some or all of the staples may become dislodged and a leak occurs.
Another error can occur when a loose staple is retained at the apex of the previously fired staple line. Firing the device across the loose staple can damage subsequent staples as they are deployed or the loose staple may damage the stapler firing mechanism leading to wedge-band bypass failure17. Wedge-band bypass failure occurs when the cutting blade of the stapler is pushed off its track as a free staple is dragged by the blade. As a result, one side staples and seals while the other side cuts and opens. Because of these potential problems, every staple firing should be closely inspected on both sides for the quality and integrity of the staple line and all free staples located in the apex of the staple line should be removed prior to the next firing2.
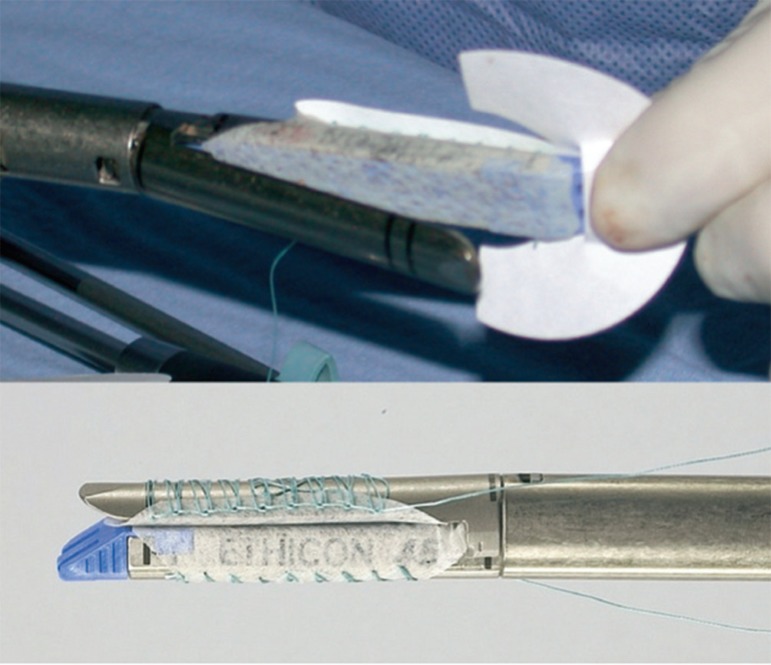
Biologic buttress material like polyglycolide acid and trimethylene carbonate or bovine pericardial strips have been proposed as a reinforcement of the stapler line in order to reduce the leak rate (Figure 2). Results in terms of reducing the leak rate are not as conclusive as the decrease of the bleeding risk at the staple line3,6,17. The polyglycolide acid and trimethylene carbonate staple reinforcement material (polyglycolide 67%, trimethylene carbonate 33%) called Seamguard - WL Gore & Associates) has an advantage over bovine pericardium which is that the latter is nonabsorbable while the Seamguard is completely absorbed within six months and is therefore less likely to cause fistula or erode.
FIGURE 2.
Staple line reinforcement sleeves supplied in pairs: one sleeve fits on cartridge jaw of stapler and other fits on anvil jaw of stapler23
Fibrin sealants have been used with increasing frequency in a variety of surgical fields for their unique hemostatic and adhesive abilities. Synthesized from pooled human fresh frozen plasma, they contain fibrinogen, factor XIII, thrombin, ionized calcium, and fibronectin. In the presence of calcium, thrombin facilitates the activated factor XIII to polymerize fibrin monomer to form insoluble fibrin clot. The process mimics the last step of the coagulation cascade. Fibrinogen gives the system both its adhesive and its hemostatic capabilities. Fibrin glue is solidified into a firm, white, rubberlike mass with strong adhesive properties within a few seconds of being mixed. The application of this fibrin glue in the suture lines would reduce the incidence of leakage as some studies have shown16.
Like was said before, most anastomotic leaks occur at the gastrojejunostomy (Table 1); therefore, surgeons must use some method to intraoperatively test the integrity of this anastomosis, either via instillation of methylene blue through an orogastric tube or air insufflation through an orogastric tube or flexible gastroscope with the anastomosis submerged28. Anastomotic tension has been proposed as a risk factor for leaks after gastric bypass surgery because it may result in stress that exceeds the disruptive pressures of a stapled or sutured anastomosis. The main technical factor that has been studied and reported is the role of Roux limb orientation in the development of anastomotic leaks after RYGB.
Theoretically, compared with the antecolic route, the retrocolic Roux limb has a more direct path to the gastric pouch and may be associated with lower gastrojejunal anastomotic tension. The studies presented until now have reported conflicting results. Edwards et al.12 reported that leaks may occur more commonly after antecolic (3%) versus retrocolic (0.5%) laparoscopic RYGB. However, Bertucci et al.5 reported no anastomotic leaks after 141 retrocolic and 200 antecolic procedures, and Carrasquilla et al.10 reported an anastomotic leak rate of 0.1% after 1000 antecolic procedures versus 1.85% after 108 retrocolic procedures. Therefore a prospective randomized study is still needed to prove this asseveration.
The diagnosis of leaks relies on clinical grounds, with or without the help of radiographic19. A patient who does not progress favorably after the first postoperative day and experiences increasing abdominal pain, persistent tachycardia, fever, tachypnea, purulent drain output, oliguria or any combination of these symptoms requires investigation19,26,28. Some studies have shown that sustained tachycardia with a heart rate in excess of 120 beats per minute was a good indicator of a leak19.
Some groups have questioned the necessity of routine upper gastrointestinal contrast studies27; however, such routine testing within the first 24-36 hours postoperatively is a standard practice among bariatric surgeons17,19.
Some other methods that can be used to detect the leaks besides the upper gastrointestinal contrast, are computed tomography scan or oral administration of methylene blue and observation to see if it comes out through the drains28.
If is decided to perform contrast studies, findings such as fluid collections adjacent to the pouch, diffuse abdominal fluid, free intraperitoneal air, and trace amount of oral contrast in the drain tract can confirm the diagnosis.
Early recognition and management is the mainstay of treatment of leaks following RYGB. Depending on the patient's clinical condition and the magnitude of the leak, different treatments can be offered, from a minimal invasive treatment to reoperation.
Conservative management can be effective in non-septic, hemodynamically stable patients with contained leaks. The mainstay of this treatment are intravenous antibiotics, monitoring of secretions through drains, nasoenteral nutrition or total parenteral nutrition (depending on the case and the location of the leak), and if the leak is contained and accessible, a percutaneous treatment can be performed28. This approach has been shown to be successful and lacks the morbidity associated with a reoperation28.
But if the patient is hemodynamically unstable, has a complicated leak, or signs of sepsis, an operative treatment is mandatory. The operative goals are: to confirm and repair the leak, remove gastrointestinal contents from the abdominal cavity and place closed suction drains.
The repair of the leak would be the ideal situation, but often suturing the place of the leak can be challenging, as the acutely inflamed tissues might not be amenable to suture placement. In such cases, the removal of gastrointestinal contents and placement of drainage tubes may be the safest option. Depending on the surgical team skills the approach could be laparoscopic or open.
Other options that have been described to control the leak are the omental reinforcement of the area of the leak24 and the endoscopic injection of fibrin sealant at the site of the leak18.
Maintain the nutrition is mandatory to allow healing the tissues in the place of the leak. In order to achieve this, the placement of a feeding gastrostomy into the gastric remnant or a feeding jejunostomy should be considered. This would allow for continued enteral nutrition while bowel rest is maintained at the site of the leak.
Anastomotic or staple line leaks are the main concern for bariatric surgeons when performing a RYGB, although its incidence is low its complications can be devastating. Caution should be taken when firing the stapler, according the guidelines previously mentioned. If the leaks occurs, an early recognition is essential to avoid further complication and to reduce the morbidity and mortality. The line of treatment will vary according to the clinical status of the patient.
Gastrointestinal bleeding
Among the early complications, bleeding is one the most feared by surgeons. The literature reports an incidence between 1.9% and 4.4%19,22and its incidence can be higher in patients with previous abdominal surgery due to adhesions requiring adhesiolysis intraoperatively.
Interestingly, a systematic review comparing open versus laparoscopic RYGB have noted that the frequency of gastrointestinal tract bleeding was significantly higher in the laparoscopic RYGB (LRYGB) series (1.9% vs 0.6%). Some hypothesis to explain this increased incidence of bleeding in the LRYGB in the minimally invasive surgery era are the overuse of DVT chemoprophylaxis and the decreased of the practice of oversewing the staple lines.
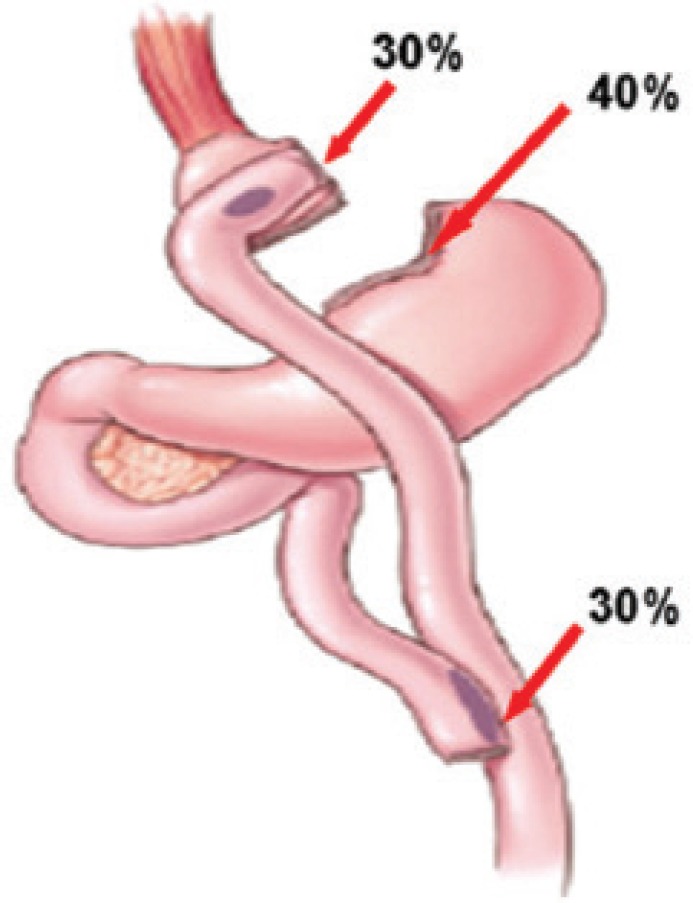
The bleeding after LRYGB can originate at one of five potential staple lines: the gastric pouch, excluded stomach, Roux limb staple line, gastrojejunostomy, and jejunojejunostomy. Staple-line bleeding occurs at the transected tissue edges or at the sites of staple penetration of the tissue. In order of frequency, the sites of staple lines bleeding are: 40% were from the gastric remnant staple line, 30% from the gastrojejunal staple line, and 30% from the jejunojejunal staple line (Figure 3). Additional sites of bleeding include the liver, spleen, and trocar sites.
FIGURE 3.
Sites of staple lines bleeding15
There are two types of postoperative hemorrhage noted to occur following LRYGB: intraperitoneal or intraluminal. The first is bleeding into the abdominal cavity, possibly from staple lines at the gastrojejunostomy, the gastric pouch, the jejunojejunostomy or the excluded stomach22. The second, occurs into the lumen of the digestive tract at the aforementioned sites. The latter usually occurs as a late bleeding while the intraperitoneal bleeding occurs as an early bleeding.
Like any bleeding associated with surgery, early recognition is essential. The clinical signs and symptoms are crucial in determining the most appropriate steps for managing this life-threatening complication. Some surgeons advocate the use of drains for the early recognition of bleeding. However, as in other areas of gastrointestinal surgery, drains are not always a reliable indicator, particularly in the case of intraluminal bleeding. Therefore, once again, a heavy reliance on clinical parameters and laboratory work-up become most important. The presence of pallor, dizziness, confusion, tachycardia, hypotension, hematemesis, bright red blood per rectum, drop in the hemoglobin level, large quantity of bloody fluid from the abdominal drains and low urine output should alert the surgeon to ongoing postoperative bleeding19.
The treatment depends on the timing of onset and the clinical presentation. In cases of late presentation (>48 hours) of gastrointestinal bleeding after surgery, it can be managed conservatively in most cases, especially when associated with no acute clinical symptoms, and melena, which might indicate the passage of old blood and inactive bleeding. In these cases discontinuation of DVT chemoprophylaxis and watchful waiting with supportive therapy can be successful.
In the other hand, early postoperative bleeding, occurring within a few hours after the surgery, manifested by hematemesis or bright red blood per rectum in the presence of clinical signs of bleeding is a clear indication for urgent surgical intervention. Abdominal re-exploration using either a laparoscopic or open approach must be performed. If the patient is hemodynamically unstable, laparoscopy is relatively contraindicated because the increased intra-abdominal pressure during pneumoperitoneum can result in worsening of the hemodynamics.
The goals are to evacuate the majority of the clots, attempt to identify and control the site of hemorrhage if it is readily apparent15 or to oversew all staple lines if the patient is hemodynamically unstable and does not have an obvious bleeding site22. Finding a dilated excluded stomach can it be due to be filled with clots, and in these cases it is necessary to evacuate the clots and place a gastric tube for continuous decompression. Not infrequently, no obvious source of bleeding can be determined during re-explorations, but the patient can still benefit from the evacuation of intraperitoneal hematoma, which might speed the recovery process through shortening the duration of postoperative ileus.
An important amount of blood can be lost with an acute postoperative gastrointestinal hemorrhage before overt clinical abdominal signs develop. If is suspected intra-abdominal bleeding based on clinical signs - such as hypotension, tachycardia, or a falling hematocrit -, in the absence of any obvious gastrointestinal source, re-exploration should not be delayed.
Although hematemesis suggests a gastrojejunostomy origin, brisk, bright red blood per rectum might originate from the gastric remnant or jejunojejunostomy anastomosis.
If is suspected that the bleeding source is proximal intraluminal the best treatment option is an endoscopic intervention, which is invaluable in controlling bleeding from the gastric pouch or gastrojejunostomy. Thermal coagulation, injection of vasoconstrictors, and clipping are all effective ways of controlling bleeding from these sites4.
Endoscopy has limited application for management of bleeding at the jejunojejunostomy because of the long length of the Roux limb, particularly in patients with a 150-cm Roux limb, and the large amount of intraluminal clots prohibiting good visualization. Although, successful endoscopic management of bleeding at the jejunojejunostomy has been described4, there is no role for endoscopic management of staple-line bleeding arising from the bypassed stomach which is inaccessible to the endoscope.
There are some potential methods for prevention of staple-line bleeding. One method is to use a linear stapler with a shorter staple height. For example, using a white linear stapler load (2.5 mm) instead of a blue stapler load (3.5 mm) for the creation of the jejunojejunostomy or a blue stapler load instead of a green stapler load (4.8 mm) for the creation of the gastric pouch. The shorter staple height provides more compression of the tissues and hence results in better hemostasis. However, shorter staple height does not completely prevent staple line bleeding and it can increase the risk of leaking due to inadequate tissue approximation.
Another method for prevention of staple-line bleeding is the use of a staple-line reinforcement product. Peri-Strips Dry® (Synovis, Saint Paul, MN) are composed of two strips of biological tissue derived from bovine pericardium that are applied to the linear stapler and act as a buttressing material at the staple-lines6. Seamguard® (W. L. Gore & Associates, Flagstaff, AZ) staple-line reinforcement works in a similar way using ePTFE instead of biologic tissue, but these products are nonabsorbable. The presence of a foreign body next to the gastro intestinal tract could lead to infection of the foreign body and possible erosion. So, a bioabsorbable Seamguard® composed of absorbable Maxon® suture material which is degraded within six weeks after surgery could be a better option.
Another potential method for prevention of gastrointestinal bleeding performed by many surgeons is to routinely oversewing of all staple-lines at the primary operation. However, this is a time-consuming task.
Bleeding is a potential complication after gastric bypass. Its incidence appears to be higher in LRYGB than in open RYGB. Timing of intervention should be based on the patient's clinical status, including vital signs, hematocrit, and other indications of ongoing hemorrhage. Endoscopic management of bleeding from the gastric pouch may be successful. Laparoscopic exploration will be mandatory in case of intraperitoneal bleeding and oversewing of all staple-lines should be performed. In some patients, a gastric tube with clot evacuation will be necessary. Preventive measures include the use of staples with shorter staple height, routine oversewing of staple-lines, and/or the use of staple-line reinforcement products.
Intestinal obstruction
The most common causes of small bowel obstruction following LRYGB are related to internal hernias which is a feared and well-recognized complication after RYGB. An internal hernia can be defined as a protrusion of intestine through a defect within the abdominal cavity. Most internal hernias present later in the postoperative period rather than early.
Compared with the open approach, the incidence of internal hernia is greater after LRYGB, estimated between 3-4.5%25. Some hypotheses postulate that the laparoscopic approach reduce the bowel manipulation and peritoneal irritation so it generates fewer postoperative adhesions, and therefore less fixation of small bowel to adjacent structures. In addition, rapid weight loss after LRYGB results in reduced intraperitoneal fat and larger mesenteric defects7.
Bowel obstruction secondary to internal hernias usually presents in the later postoperative period while early small bowel obstructions (in less than one month) usually result from technical problems with the Roux limb. Causes include complete blockage or partial narrowing of the gastrojejunostomy or jejunojejunostomy, acute angulation of the Roux limb, and narrowing of the Roux limb at the level of the transverse mesocolon. The latter obstruction also is seen as a late complication due to scarring at the transverse mesocolon defect.
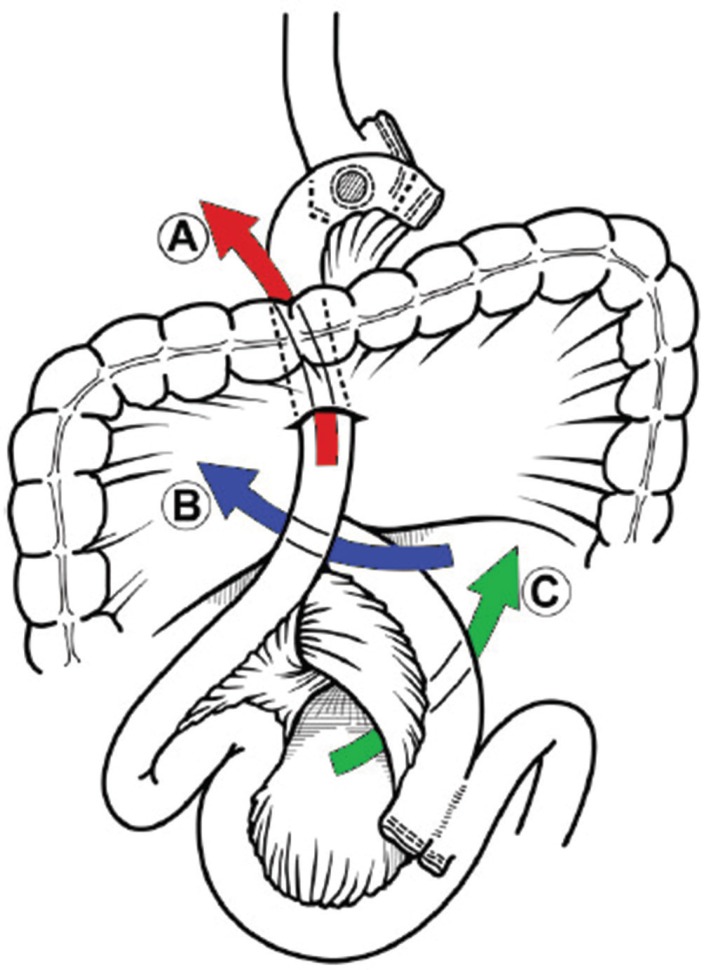
RYGB can be accomplished using either an antecolic or retrocolic approach. Depending on the chosen approach a number of potential mesenteric defects are created (Figure 4). The retrocolic approach creates three defects: one in the transverse mesocolon, one at the site of the jejunojejunostomy and a Petersen defect (a space created between the Roux limb and the transverse mesocolon). While the antecolic approach creates only two mesenteric defects: one at the jejunojejunostomy and another in the Petersen defect.
FIGURE 4.
Mesenteric defects: A) transverse mesocolic; B) Petersen's space; and C) jejunojejunostomy mesentery32
The most common location for internal hernias and its relation to Roux limb configuration has been a subject of debate. Understandably, mesocolic defect hernias are unique to a retrocolic approach and are not seen with an antecolic approach. In some reports, mesocolic defects were the most common among all internal hernias33. Some authors reported that transverse mesocolic hernias were the most common, followed by jejunojejunostomy and Petersen's space hernias25. In an antecolic approach, however, both Petersen's and jejunojejunostomy mesenteric defect hernias are reported, with hernias at the jejunojejunostomy defect being more common in some other series. Other investigators report a higher incidence of Petersen's and jejunojejunostomy hernias with a retrocolic approach. A significant decrease in small bowel obstruction have been reported by some authors after switching from a retrocolic to an antecolic technique.
Dull abdominal pain with or without intestinal obstruction is the most common presentation of an internal hernia. Usually the presentation is delayed, occurring several months to years after the operation, but it can occur in the immediate postoperative period (being more common in these cases the technical problems with the Roux limb).
Some patients report previous episodes of undefined gastrointestinal upset and frequent mild symptoms of intermittent obstruction before their main presentation. The small bowel may intermittently become trapped and then reduced at the site of the internal hernia, causing this subtle presentation and atypical bowel obstruction features. Nausea, emesis, and postprandial abdominal pain (usually in the left upper quadrant) are common complaints and because of change in the gastrointestinal anatomy, patients may not present with typical signs and symptoms of bowel obstruction.
A diagnosis of small bowel obstruction can be made by performing an upper gastrointestinal series. However, the specific cause may not be evident. Findings that favor a diagnosis of internal hernia include a cluster of dilated bowel segments in the left upper or middle abdomen, which remain relatively fixed in this high position on views obtained with the patient in an erect position.
CT scans can be helpful in depicting signs of an internal hernia. In trans mesenteric internal hernia, when the Roux limb is herniated, CT scanning shows a cluster of dilated bowel segments in the expected position of the Roux-en-Y loop. Other CT findings suggestive of an internal hernia include small bowel mesentery traversing the transverse colon mesentery and location of the jejunojejunostomy superior to the transverse colon. In addition, crowding, stretching, and engorgement of the main mesenteric trunk to the right and signs of small bowel obstruction may be seen. A swirled appearance of mesenteric fat or vessels was found to be the best single predictor of hernia, with a sensitivity of approximately 80% and a specificity of 90%.
However, CT scanning is not always diagnostic and the percentage of negative CT scanning in patients with internal hernias can be up to for 20%. Therefore any patient with unexplained abdominal pain that does not correlate with physical findings should be considered to have an internal hernia. A high index of suspicion is crucial for early intervention and avoidance of an abdominal catastrophe, such as long segment of small bowel ischemia.
To prevent bowel obstruction after gastric bypass, specific measures should be taken. Routine closure of the mesenteric defect at the jejunojejunostomy, transverse mesocolon mesenteric defect, and the Petersen defect is recommended. Some authors advocate the placement of an ''anti-obstruction suture'' at the jejunojejunostomy to prevent bowel obstruction at the afferent limb29. Other authors recommend placement of a suture on the proximal Roux limb that fixes it to the remnant stomach to prevent angulation of the Roux limb's proximal part in case of an antecolic, antegastric technique33.
Whether to use absorbable or non-absorbable, running or interrupted suture have been also a matter of debate. Some authors who have modified their technique from absorbable to non-absorbable sutures and from an interrupted to a running technique have reported a reduction in the incidence of internal hernias.
Leaving aside the internal hernias, the second most common cause of small bowel obstruction after LRYGB is obstruction at the jejunojejunostomy, occurring in approximately 1.8% of antecolic LRYGB procedures. It can also be a complication of the retrocolic approach. Early obstructions at the jejunojejunostomy can be caused by technical problems, such as bowel kinking, narrowing, or acute angulation of the anastomosis. Other causes include postsurgical anastomotic edema, stenosis, ischemia, and staple-line bleeding with intraluminal hematoma formation. Early obstructions at other locations usually result from edema or technical problems with the Roux limb position, such as an extrinsic compression of the Roux limb as it traverses the transverse mesocolic defect from thickened cicatrix formation in this area.
Other less-common causes of small bowel obstruction after LRYGB include trocar site incisional hernias (port sites larger than 10 mm should be closed routinely to prevent port-site herniation), adhesive bands, bezoars, anastomotic strictures, and jejunojejunostomy intussusception. Rarely, superior mesenteric artery syndrome might complicate the course of LRYGB secondary to rapid weight loss and cause gastric outlet obstruction symptoms.
Surgical exploration of patients with suspected internal hernia should be performed without delay. A doubtful operative decision can result in the development of a closed loop obstruction, a potentially devastating problem. Despite normal complementary studies, a diagnostic laparoscopy is recommended if the clinical symptoms suggest an internal hernia. The entire small bowel and all the potential hernia defects should be carefully evaluated. If hernias are found, the repair involves reducing the hernia and closing defects. Remaining defects should be closed if they have not already been closed.
Lysis of adhesions should be performed if a strangulated band causes obstruction. In presence of a dilated gastric remnant decompression using a long needle or placement of a gastrostomy tube it's recommended.
Narrowing of the jejunojejunostomy due to incorrect stapling of the jejunojejunostomy usually requires creation of a new enteroenterostomy proximal to the obstruction site. Angulation of the Roux limb at the jejunojejunostomy requires repositioning of the Roux limb and placement of an antiobstruction suture.
The possibility of performing a laparoscopic approach to manage a bowel obstruction will depend on the extent of bowel dilation and the site of bowel obstruction. In case of distal obstruction with concomitant severe bowel dilation often complicates a safe laparoscopic entry and may require laparotomy.
Roux limb obstruction due to edema of the jejunojejunostomy or gastrojejunostomy usually requires conservative treatment consisting of suspending the oral feeding and the administration of intravenous fluids. Total parenteral nutrition is rarely needed because this problem usually resolves within a few days. Obstruction at the transverse mesocolon is also typically managed conservatively.
Bowel obstruction is a relatively frequent complication after LRYGP. Closure of all mesenteric defects is highly recommended to prevent internal hernias. The antegastric, antecolic approach could reduce the incidence of internal hernias at the transmesocolon defect. Early diagnosis and surgical exploration in suspected cases is essential to a successful outcome.
Incorrect Roux limb reconstruction
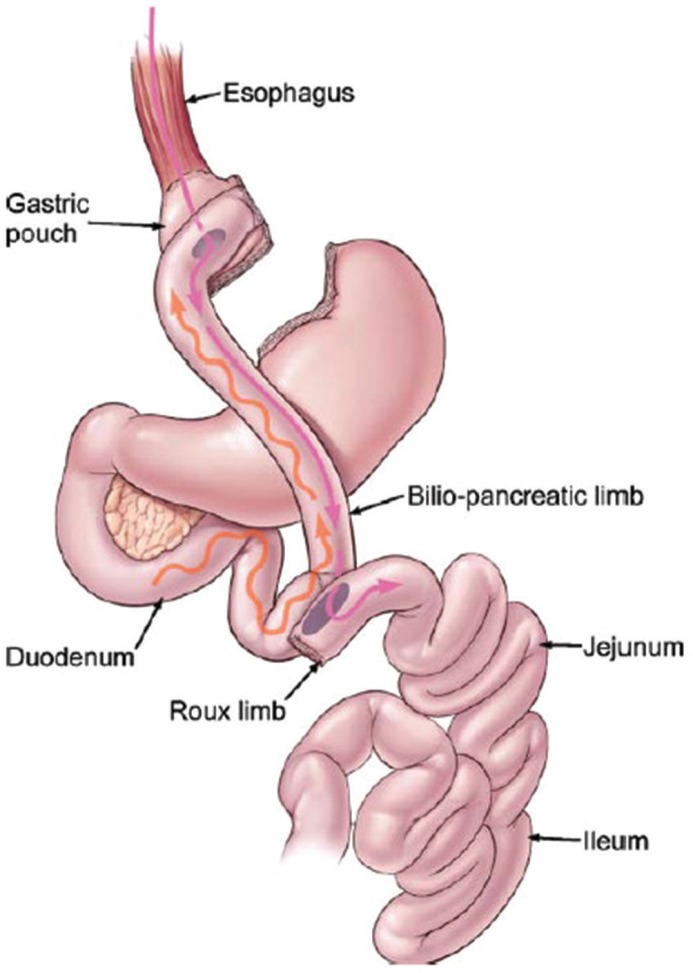
This complication, although being rare, can be potentially devastating. Involves the inadvertent anastomosis of the proximal biliopancreatic limb of the jejunum to the gastric pouch in conjunction with a misplaced jejunojejunostomy. This so called Roux-en-O construction gives rise to a blind loop (Figure 5). Although this seems to be an atypical complication infrequently reported in the literature, must be present in the surgeons mind because it can be easily avoided, and if it does occur, it poses unique diagnostic challenges and profoundly increases patient morbidity3.
FIGURE 5.
The Roux-en-O configuration: the bilio-pancreatic limb is inadvertently anastomosed to the gastric pouch; the wavy line represents peristalsis and flow of bile; the solid line represents movement of a food bolus32
Patients with the Roux-en-O configuration typically present with abdominal pain, biliary emesis, esophagitis and severe dehydration. This occurs promptly in the postoperative period. Usually there is an important delay between the patient's initial symptoms presentation and the time at which the diagnosis is determined. During this period, physician usually request numerous contrast radiologic studies and endoscopies, which commonly fail to highlight any important pathology. Ultimately, it seems as though only hepatobiliary iminodiacetic acid scanning is able to facilitate the diagnosis of the complication accurately by revealing prompt reflux of radioactive tracer from the duodenum to the esophagus. In the reviewed published case reports about this complication, patients had undergone repeated operative interventions, numerous complications, protracted hospital admissions and severe delay in the commencement of oral intake3.
The best management strategy for this problem is to avoid creating the Roux-en-O anastomosis at the initial surgery. The lack of surgical experience with bariatric techniques may be the most important predisposing factor to this complication. Some technical tips to avoid this complication are to make the biliopancreatic limb no longer than 50 cm, thus precluding its easier anastomosis to the gastric pouch. Furthermore, the Roux limb should be marked with a suture, short segment Penrose drain or Weck clip promptly after the jejunum is divided to facilitate easy differentiation between itself and the biliopancreatic limb. Finally, before fashioning the jejunojejunostomy, the biliopancreatic limb should be traced back to the duodenojejunal ligament so that proper orientation is assured.
If intraoperative detection of a Roux-en-O was missed and a patient presents postoperatively with suspicious symptoms and little radiographic evidence of pathology, a hepatobiliary iminodiacetic acid scanning should be obtained before surgical intervention to help with diagnosis, as the aberrant construction is sometimes hard to detect intraoperatively in a hostile abdomen.
CONCLUSION
Knowledge on strategies on how to reduce the risk and incidence of complications must be acquired, and every surgeon must be familiar with these complications in order to achieve an earlier recognition and perform the best intervention.
FIGURE 1.
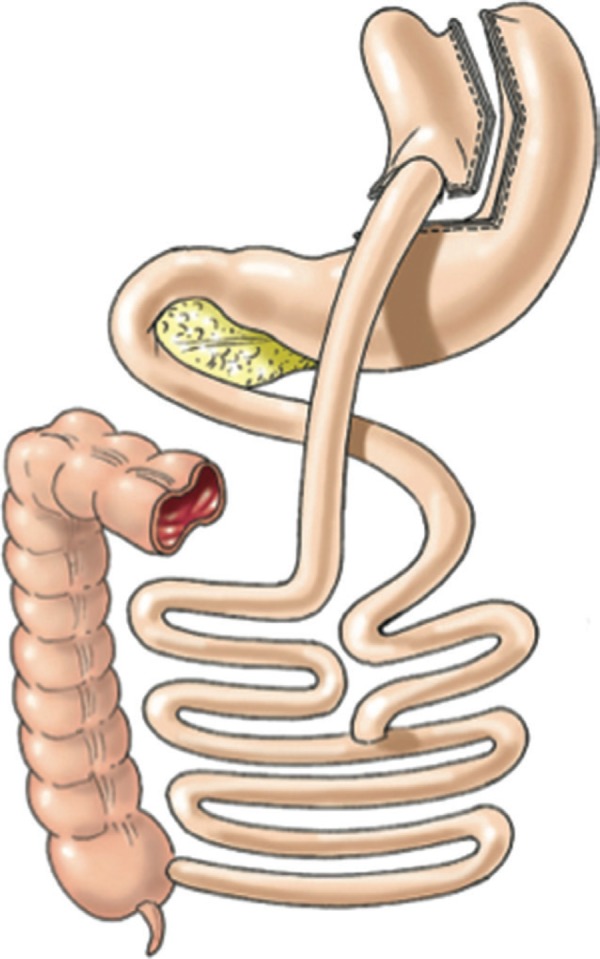
Roux-en-Y gastric by pass (Laparoscopic Gastrointestinal Surgery. Palermo, Gimenez, Gagner. Cadiere and Dapri chapter) AMOLCA 2014
Footnotes
Financial source: none
REFERENCES
- 1.Ahmed AR, Rickards G, Husain S, et al. Trends in internal hernia incidence after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17(12):1563–1566. doi: 10.1007/s11695-007-9260-6. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed AR, Rickards G, Messing S, et al. Roux limb obstruction secondary to constriction at transverse mesocolon rent after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2009;5(2):194–198. doi: 10.1016/j.soard.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Baker MT, Lara MD, Kothari SN. Superior mesenteric artery syndrome after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2(6):667–667. doi: 10.1016/j.soard.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Behrns KE, Smith CD, Sarr MG. Prospective evaluation of gastric acid secretion and cobalamin absorption following gastric bypass for clinically severe obesity. Dig Dis Sci. 1994;39(2):315–320. doi: 10.1007/BF02090203. [DOI] [PubMed] [Google Scholar]
- 5.Bertucci W, Yadegar J, Takahashi A, Alzahrani A, Frickel D, Tobin K, Kapur K, Namdari B, Dutson E, Gracia C, Mehran A. Antecolic laparoscopic Roux-en-Y gastric bypass is not associated with higher complication rates. Am Surg. 2005;71(9):735–737. [PubMed] [Google Scholar]
- 6.Blachar A, Federle MP. Gastrointestinal complications of laparoscopic Roux-en-Y gastric bypass surgery in patients who are morbidly obese: findings on radiography and CT. AJR Am J Roentgenol. 179:1437–1442. doi: 10.2214/ajr.179.6.1791437. [DOI] [PubMed] [Google Scholar]
- 7.Braley SC, Nguyen NT, Wolfe BM. Late gastrointestinal hemorrhage after gastric bypass. Obes Surg. 2002;12(3):404–407. doi: 10.1381/096089202321088255. [DOI] [PubMed] [Google Scholar]
- 8.Brolin RE. (1995) The antiobstruction stitch in stapled Roux-en-Y enteroenterostomy. Am J Surg. 169:355–357. doi: 10.1016/S0002-9610(99)80175-5. [DOI] [PubMed] [Google Scholar]
- 9.Capella RF, Iannace VA, Capella JF. Bowel obstruction after open and laparoscopic gastric bypass surgery for morbid obesity. J Am Coll Surg. 2006;203(3):328–335. doi: 10.1016/j.jamcollsurg.2006.05.301. [DOI] [PubMed] [Google Scholar]
- 10.Carrasquilla C, English WJ, Esposito P, et al. Total stapled, total intra-abdominal (TSTI) laparoscopic Roux-en-Y gastric bypass: one leak in 1000 cases. Obes Surg. 2004;14(5):613–617. doi: 10.1381/096089204323093372. [DOI] [PubMed] [Google Scholar]
- 11.Champion JK, Williams M. Small bowel obstruction and internal hernias after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13(4):596–600. doi: 10.1381/096089203322190808. [DOI] [PubMed] [Google Scholar]
- 12.Edwards MA, Jones DB, Ellsmere J, Grinbaum R, Schneider BE. Anastomotic leak following antecolic versus retrocolic laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2007;17(3):292–297. doi: 10.1007/s11695-007-9048-8. [DOI] [PubMed] [Google Scholar]
- 13.Garza E, Jr, Kuhn J, Arnold D, et al. Internal hernias after laparoscopic Roux-en-Y gastric bypass. Am J Surg. 2004;188(6):796–800. doi: 10.1016/j.amjsurg.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez R, Lin E, Venkatesh KR, et al. Gastrojejunostomy during laparoscopic gastric bypass: analysis of 3 techniques. Arch Surg. 2003;138(2):181–184. doi: 10.1001/archsurg.138.2.181. [DOI] [PubMed] [Google Scholar]
- 15.Heneghan HM, Meron-Eldar S, Yenumula P, Rogula T, Brethauer SA, Schauer PR. Incidence and management of bleeding complications after gastric bypass surgery in the morbidly obese. Surg Obes Relat Dis. 2012 Nov-Dec;8(6):729–735. doi: 10.1016/j.soard.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Higa KD, Boone KB, Ho T. (2000) Complications of the laparoscopic Roux-en-Y gastric bypass: 1,040 patients-what have we learned? Obes Surg. 10:509–513. doi: 10.1381/096089200321593706. [DOI] [PubMed] [Google Scholar]
- 17.Higa KD, Ho T, Boone KB. Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg. 2003;13(3):350–354. doi: 10.1381/096089203765887642. [DOI] [PubMed] [Google Scholar]
- 18.Huang CS, Forse RA, Jacobson BC, et al. Endoscopic findings and their clinical correlations in patients with symptoms after gastric bypass surgery. Gastrointest Endosc. 2003;58(6):859–866. doi: 10.1016/s0016-5107(03)02310-1. [DOI] [PubMed] [Google Scholar]
- 19.Kravetz AJ, Reddy S, Murtaza G, et al. A comparative study of handsewn versus stapled gastrojejunal anastomosis in laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2011;25:1287–1292. doi: 10.1007/s00464-010-1362-x. [DOI] [PubMed] [Google Scholar]
- 20.Lewis CE, Jensen C, Tejirian T, et al. Early jejunojejunostomy obstruction after laparoscopic gastric bypass: case series and treatment algorithm. Surg Obes Relat Dis. 2009;5(2):203–207. doi: 10.1016/j.soard.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Lockhart M, Tessler FN, Canon CL, Smith J Kevin, Larrison MC, Fineberg NS, Roy BP, Clements RH. (2007) Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls. AJR Am J Roentgenol. 188:745– 750. doi: 10.2214/AJR.06.0541. [DOI] [PubMed] [Google Scholar]
- 22.Mason EE, Munns JR, Kealey GP, et al. Effect of gastric bypass on gastric secretion. 1977. Surg Obes Relat Dis. 2005;1(2):155–60. doi: 10.1016/j.soard.2005.02.014. [discussion: 161-2] [DOI] [PubMed] [Google Scholar]
- 23.Miller KA, Pump A. Use of bioabsorbable staple reinforcement material in gastric ?bypass: a prospective randomized clinical trial. Surg Obes Relat Dis. 2007;3(4):?417–21. doi: 10.1016/j.soard.2007.03.244. [discussion: 422] [DOI] [PubMed] [Google Scholar]
- 24.Papasavas PK, Caushaj PF, McCormick JT, Quinlin RF, Hayetian FD, Maurer J, Kelly JJ, Gagne DJ. (2003) Laparoscopic management of complications following laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 17:610– 614. doi: 10.1007/s00464-002-8826-6. [DOI] [PubMed] [Google Scholar]
- 25.Papasavas PK, Gagne´ DJ, Donnelly PE, et al. Prevalence of Helicobacter pylori infection and value of preoperative testing and treatment in patients undergoing laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4(3):383–388. doi: 10.1016/j.soard.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Raman R, Raman B, Raman P, et al. Abnormal findings on routine upper GI series following laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17(3):311–316. doi: 10.1007/s11695-007-9057-7. [DOI] [PubMed] [Google Scholar]
- 27.Ramos AC, Silva AC, Ramos MG, Canseco EG, Galvão-Neto Mdos P, Menezes Mde A, Galvão TD, Bastos EL. Simplified gastric bypass: 13 years of experience and 12,000 patients operated. Arq Bras Cir Dig. 2014;27(Suppl 1):2–8. doi: 10.1590/S0102-6720201400S100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogula T, Yenumula PR, Schauer PR. A complication of Roux-en-Y gastric bypass: intestinal obstruction. Surg Endosc. 2007;21(11):1914–1918. doi: 10.1007/s00464-007-9535-y. [DOI] [PubMed] [Google Scholar]
- 29.Schirmer B, Erenoglu C, Miller A. Flexible endoscopy in the management of patients undergoing Roux-en-Y gastric bypass. Obes Surg. 2002;12:634–638. doi: 10.1381/096089202321019594. [DOI] [PubMed] [Google Scholar]
- 30.Schneider C, Cobb W, Scott J, Carbonell A, Myers K, Bour E. Rapid excess weight loss following laparoscopic gastric bypass leads to increased risk of internal hernia. Surg Endosc. 2011 May;25(5):1594–1598. doi: 10.1007/s00464-010-1444-9. [DOI] [PubMed] [Google Scholar]
- 31.Schweitzer MA, DeMaria EJ, Broderick TJ, Sugerman HJ. (2000) Laparoscopic closure of mesenteric defects after Roux-en-Y gastric bypass. J Laparoendosc Adv Surg Tech. 10:173–175. doi: 10.1089/lap.2000.10.173. [DOI] [PubMed] [Google Scholar]
- 32.Sherman V, Dan AG, Lord JM, Chand B, Schauer PR. Complications of gastric bypass: avoiding the Roux-en-O configuration. Obes Surg. 2009 Aug;19(8):1190–1194. doi: 10.1007/s11695-009-9875-x. [DOI] [PubMed] [Google Scholar]
- 33.Sugerman HJ. Gastric bypass surgery for severe obesity. Semin Laparosc Surg. 2002;9(2):79–85. [PubMed] [Google Scholar]