Abstract
Background
In this study, we aimed to identify a novel extracellular proteinase ADAMTS-18 that could be a potential tumor suppressor gene.
Results
We successfully constructed Adamts-18 knockout mice with BALB / c background. RT-PCR analysis showed syngeneic mammary tumor cell line 4 T1 per se has weakly endogenous ADAMTS-18 expression. Orthotopic inoculation of 4 T1 cells within the mammary fat pad of host mice, we found no significant difference in tumor growth and metastasis between Adamts-18 knockout mice and widetype control.
Conclusions
We did not confirm that ADAMTS-18 in the host tissues is relevant for breast tumor progress in a murine 4 T1 breast cancer model.
Keywords: ADAMTS-18, Breast cancer, Knockout mice
Background
Matrix metalloproteinases are traditionally linked to tumor dissemination through their ability to degrade most extracellular matrix (ECM) components, thus facilitating tumor cell invasion and metastasis [1]. However, recent studies suggest that some metalloproteinases contribute to inhibit tumorigenesis [2]. ADAMTS-18 (a disintegrin and metalloproteinase with thrombospondin motif 18), as yet no known function or substrate, is a member of ADAMTS family of secreted proteinases. It has been shown that ADAMTS-18 gene was inactivation in many carcinomas especially breast tumors and, therefore, is regarded as a novel functional tumor suppressor [3]. We previously reported that the C-terminal ADAMTS-18 fragment induces oxidative platelet fragmentation, dissolves platelet aggregates, and protects against carotid artery occlusion and cerebral stroke [4]. To further study the role of ADAMTS-18 in vivo, we generated Adamts-18 knockout mice (unpublished data). The aim of this study was to investigate the effect of ADAMTS-18 on tumorigenesis in a murine 4 T1 breast cancer model.
Methods
Animal studies
In our laboratory Adamts-18 heterozygote mice (Adamts-18+/-) with C57BL6/129SV were developed (unpublished data). They backcrossed to wild-type BALB/c mice for 7 generations to obtain BALB/c background Adamts-18+/- mice. Adamts-18+/- mice with BALB/c background are intercrossed to generate Adamts-18 knockout mice (Adamts-18-/-) and wildtype control (Adamts-18+/+) for further experiment. Syngeneic mammary tumor cell line 4 T1 cells were orthotopically implanted within the mammary fat pad of females aged 8–10 weeks. Cells implantation in the mammary fat pad was achieved using a (1/2)- inch 26 gauge needle and gentle pressure during delivery of 100 μl cells. Mice were watched daily to monitor the growth of the tumor and once a substantial size of tumor mass was found, the mice were sacrificed and the tumor mass were collected and weighted. The surface metastatic nodules on the lungs and other organs were counted, and then histologic analyses were performed. All procedures in animal experiments were approved by the Institutional Animal Care and Use Committee of East China Normal University (ECNU). All the mice used in this study are female and maintained in a specific pathogen-free facility at ECNU.
Western blotting
Proteins from the brain, kidney, liver or xenograft tumors of both mice were separated on a 12 % SDS–PAGE under reducing conditions and then transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked in blocking buffer (PBS, 0.5 % Tween-20, and 5 % non-fat dry milk powder) and then incubated with rabbit anti-ADAMTS18 IgG (sc-68416, Santa Cruz Biotechnology, Inc.) for 1 h at RT. After washing, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at RT. The immunoreactive bands were visualized with enhanced chemiluminescence (ECL) western blot kit.
Semi-quantitative RT-PCR analysis
The endogenous expression level of ADAMTS-18 gene in murine 4 T1 tumor cells was detected by semi-quantitative RT-PCR. Total RNA was isolated from cultured cells using the TRIzol reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Semi-quantitative RT-PCR was performed with cDNA reverse-transcribed from 1 μg of total RNA using AMV reverse transcriptase (Takara, Otsu, Japan). The cycle threshold values were normalized to the expression of the housekeeping gene β-actin. Band density was scanned and calculated. The primers are list as following: β-actin forward: 5′-ACGGCCAGGTCATCACTATTG-3′, β-actin reverse: 5′-CCTGCTTGCTGATCCACATCT; ADAMTS-18 forward (mouse specific): 5′-TGGAAAGTCACAAAATGGTCTCA-3′; ADAMTS-18 reverse (mouse specific): 5′- AACCACAATGTTTATGTCGCTTC-3′.
Histological analysis
Tumor tissues were fixed in 10 % formaldehyde in phosphate-buffered saline (PBS), pH 7.4, dehydrated and embedded in paraffin and sectioned with a microtome. Tumor malignant degree and mitotic counts in sections were evaluated by hematoxylin and eosin (H.E) staining. Intratumoral vascular density was assessed by staining with goat anti-mouse CD31 antibody (Santa Cruz Biotechnology Inc., CA). Microvessel density (MVD) was determined by light microscopy in areas of invasive tumor containing the highest numbers of capillaries and microvessels per area. MVD was expressed as the number of microvessel per high-power field.
Statistical analysis
P values were determined through the two-tailed Student’s t test. Data are presented as mean ± SD. Differences were considered statistically significant when P < 0.05.
Results and discussion
The potential role of ADAMTS-18 in tumor genesis is first suggested by the genetic analysis [5]. It has been shown that the loss of 16q23 region is strongly associated with a variety of cancers. Since ADAMTS-18 is one of these genes located around 16q23 region, it has been studied as a candidate oncogene [3, 5–10]. Because ADAMTS-18 is often inactivated in breast tumors, we therefore develop Adamts-18 knockout mouse with BALB/c background, and inoculate syngeneic mammary tumor cell line 4 T1 cells to evaluate the role of ADAMTS-18 in breast cancer.
First, Adamts-18 heterozygote mice (Adamts-18+/-) with C57BL6/129SV background were backcrossed to wild-type BALB/C mice for 7 generations to obtain Adamts-18+/- mice with BALB/ c background. They are intercrossed to get Adamts-18 knockout mice (Adamts-18-/-) and wildtype control (Adamts-18+/+) for further experiment (Fig. 1a-b). Immunoblot analysis for ADAMTS-18 expression in different tissues from both mice further confirmed the knockout effectiveness (Fig. 1c). The results of orthotopic inoculation of syngeneic mammary tumor cell line 4 T1 cells (106/mouse) within the mammary fat pad showed that the percentage of tumor incidence reached 100 % after six and seven generations (Fig. 1d). In the seventh generation of mice, 10 of 10 mice bear tumor when the inoculation doses of 4 T1 cells were 106 per mice (Fig. 1e). Representative photograph of breast tumor (Fig. 1f) and hematoxylin-eosin (HE) staining are shown (Fig. 1g).
Fig. 1.
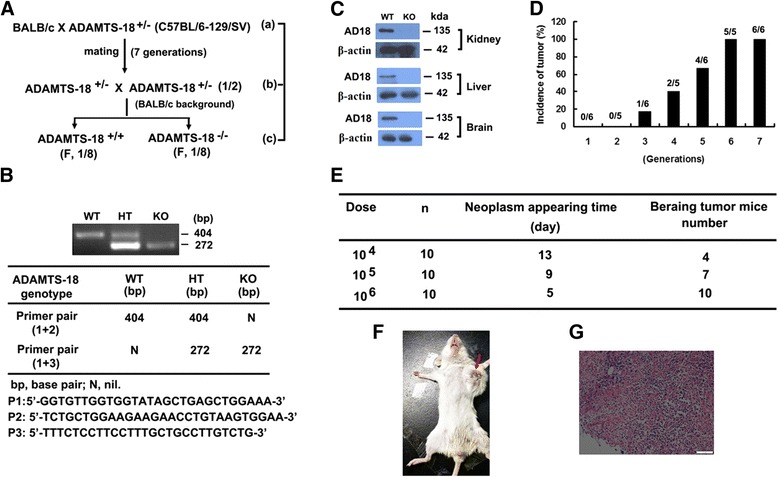
Generation and characterization of Adamts-18 knockout mice with BALB/c background. a The mating strategy for producing BALB/c inbred Adamts-18 knockout mice; (b) PCR genotyping of wildtype (WT), heterozygote (HT), and knockout (KO) mice; (c) Western blot analysis of Adamts-18 in kidney, liver, and brain from different mice, β-actin was used as a loading control. WT, wild-type mouse; KO, knockout mouse. d Incidence rate of breast cancer in different generation of mice with BALB/C background; (e) Bearing-tumor mice number in the seventh generation of BALB/c inbred mice after orthotopically implanted within the mammary fat pad of mice with different dose of 4 T1 cells. f Representative photograph of breast tumor. Arrow refers to breast tumor; (g) Representative HE staining, bar = 50 μm
The effect of tumor growth in some extent depends on tumor cell line per se. It has been shown that the expression of ADAMTS-18 was dramatically reduced or totally silenced in multiple human cancer cell lines, including cell lines of human breast cancer (BT549, MB231, MCF-7, and YCC-B1) due to methylation of promoter CpG Ilands (CGI) [5]. To test the expression level of ADAMTS-18 in murine 4T1 cells, RT-PCR was performed and the result showed 4T1 per se has weakly endogenous ADAMTS-18 expression (Fig. 2a, upper panel). In this regard, 4T1-knockout ADAMTS-18 cell line is needed in future studies. We then injected 4T1 cells to both Adamts-18 knockout mice (Adamts-18-/-) and wildtype control (Adamts-18+/+) mice. The result of Western blotting showed that the Adamts-18 expression is ~3.6 fold higher in xenograft tumors of WT mice than in KO mice (n = 3, **P < 0.01) (Fig. 2a, lower panel). However, we found no significant differences in tumor growth, survival rate of mice, tumor weight between Adamts-18-/- and Adamts-18+/+ mice (Fig. 2b-d). There was no significant difference in tumor malignant degree and mitotic counts between Adamts-18+/+ and Adamts-18-/- mice [mitotic counts, WT vs. KO, 10.75 ± 2.1 vs. 11 ± 1.4 per high power field (HFP), n = 8/group, P = 0.826). Moreover, microvessel density (MVD) had no significant difference between Adamts-18+/+ and Adamts-18-/- mice (MVD, WT vs. KO, 64 ± 6 vs. 67 ± 11 per high power field (HFP), n = 5/group, P = 0.947) (Fig. 2e). There is no significant difference in systemic metastasis (Fig. 3a), especially lung metastasis (Fig. 3b-d) between Adamts-18+/+ and Adamts-18-/- mice. These results indicated that ADAMTS-18 in host tissues (e.g. stroma components, endothelium, tumor-associated fibroblasts, and immune cells etc) likely exerts little effect on breast tumor progress. However, further studies are needed to assess the effect of ADAMTS-18 on other tumor types (e.g. melanoma, spontaneous cancers, etc) or its synergy effect with other tumor-related genes.
Fig. 2.
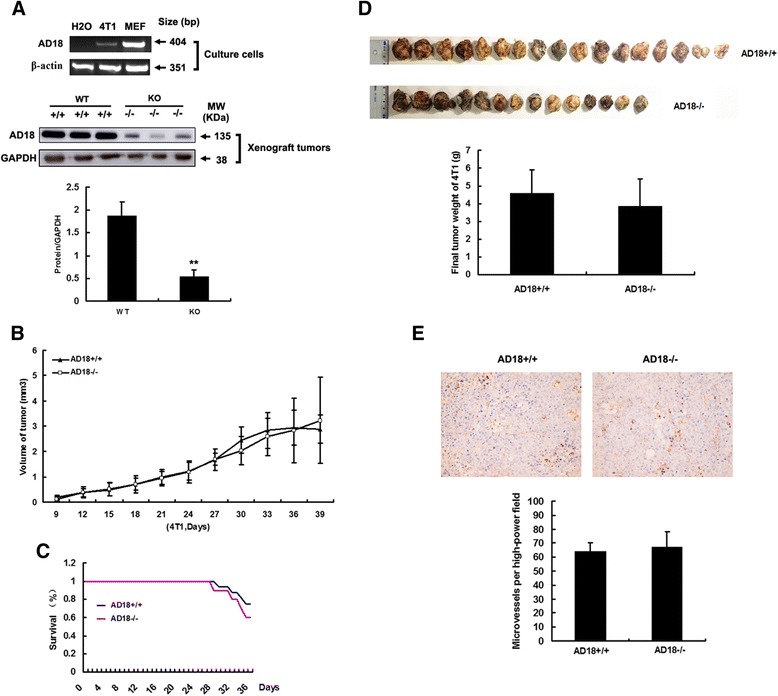
Syngeneic mammary tumor cell line 4 T1 subcutaneously transplanted growth. a The endogenous ADAMTS-18 expression in cultured 4 T1 cells (upper panel) or in xenograft 4 T1 tumors (lower panel). MEF, mouse embryonic fibroblast; bp, base pair; MW, molecular weight; **indicates P < 0.01. b Mice were challenged with 106 4 T1 cells, and tumor volumes were monitored for six weeks. c Survival rate of mice; (d) Final tumor weight in different genotype of mice. Adamts-18 +/+, wildtype mice; Adamts-18 -/-, knockout mice. e CD31 (40 × magnification) positive microvessels in each group (n = 5/group). Data are expressed as mean ± SD
Fig. 3.
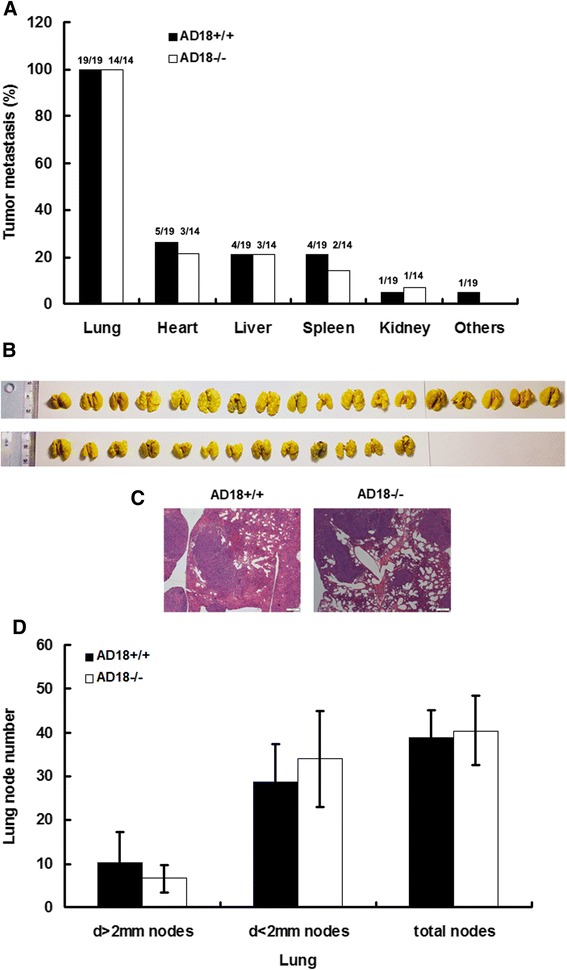
Tumor metastasis in different genotype of mice. a Systemic tumor metastasis; (b) Representative photograph of lung metastasis; (c) Representative HE staining of lung metastasis; (d) Bar graph showing statistic analysis of lung tumor nodes
Many ADAMTSs have proved to be anti-angiogenic through their metalloproteinase-dependent and –independent activities, which contribute to inhibit cancer development and progression. ADAMTS-1 displays both types of mechanism [11]. It induces the generation of anti-angiogenic fragments by cleaving matrix-bound thrombospondins-1 and -2 [12], and also sequesters angiogenic factors such as the basic fibroblast growth factor (bFGF) and vascular endothelial growth 1(VEGF165) [13, 14]. ADAMTS-9 also demonstrates metalloproteinase-dependent and –independent activities, but the mechanism remains unclear [15]. Other ADAMTSs, including ADAMTS-2, -4, -5, and -12, displays metalloproteinase-independent inhibition of neovascularization, which is closely related to their thrombospondin type 1 sequence repeat (TSR) domain [16–19]. The peptides (termed Adamtsostatins) corresponding to the TSRs of some family members have anti-angiogenic properties, indicating that this may be a general activity of ADAMTSs. In this study, we didn’t find difference in microvessel densities of tumors between Adamts-18+/+ and Adamts-18-/- mice. Nevertheless, more sophisticated assays for angiogenesis such as wound healing, tube formation, and ischemia assays are needed in future studies.
Conclusions
The current data suggest that the murine 4 T1 breast tumor cell per se has weakly endogenous ADAMTS-18 expression, and ADAMTS-18 in the host mouse exerts little effect on breast tumor progression in murine 4 T1 breast cancer model.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81570389, 81170481, 81200352).
Abbreviations
- ADAMTS-18
a disintegrin and metalloproteinase with thrombospondin motif 18
- ECM
extracellular matrix
- HE
hematoxylin-eosin
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WZ and SYD conceived the idea, designed the research, and wrote the manuscript. ML, TTL, and JF performed the research. WZ and SYD analyzed the data. WZ and SYD contributed reagents, materials and analytical tools. All authors read and approved the final manuscript.
Contributor Information
Min Liu, Email: minliu920120@163.com.
Tiantian Lu, Email: tianmissing@sina.com.
Fang Jing, Email: fangjing@126.com.
Suying Dang, Email: suyindang@shsmu.edu.cn.
Wei Zhang, Phone: +86 21 32530498, Email: wzhang@sat.ecnu.edu.cn.
References
- 1.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 2.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordgard SH, Johansen FE, Alnaes GIG, Bucher E, Syvanen AC, Naume B, et al. Genome-wide analysis identifies 16q deletion associated with survival, molecular subtypes, mRNA expression, and germline haplotypes in breast cancer patients. Genes Chromosomes Cancer. 2008;47:680–96. doi: 10.1002/gcc.20569. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Nardi MA, Li YS, Zhang W, Pan RM, Dang SY, et al. C-terminal ADAMTS-18 fragment induces oxidative platelet fragmentation, dissolves platelet aggregates, and protects against carotid artery occlusion and cerebral stroke. Blood. 2009;113:6051–60. doi: 10.1182/blood-2008-07-170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin H, Wang X, Ying J, Wong AHY, Li H, Lee KY, et al. Epigenetic identification of ADAMTS18 as a novel 16q23.1 tumor suppressor frequently silenced in esophageal, nasopharyngeal and multiple other carcinomas. Oncogene. 2007;26:7490–8. doi: 10.1038/sj.onc.1210559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Zhang W, Shao Y, Zhang C, Wu Q, Yang H, et al. High-resolution melting analysis of ADAMTS18 methylation levels in gastric, colorectal and pancreatic cancers. Med Oncol. 2010;27:998–1004. doi: 10.1007/s12032-009-9323-8. [DOI] [PubMed] [Google Scholar]
- 7.Wei XM, Prickett TD, Viloria CG, Molinolo A, Lin JC, Cardenas-Navia I, et al. Mutational and functional analysis reveals ADAMTS18 metalloproteinase as a novel driver in melanoma. Mol Cancer Res. 2010;8:1513–25. doi: 10.1158/1541-7786.MCR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Otin C, Matrisian LM. Tumour micro environment - opinion - emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–8. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 9.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 10.Wagstaff L, Kelwick R, Decock J, Edwards DR. The roles of ADAMTS metalloproteinases in tumorigenesis and metastasis. Front Biosci Landmark. 2011;16:1861–72. doi: 10.2741/3827. [DOI] [PubMed] [Google Scholar]
- 11.Tan Ide A, Ricciardelli C, Russell DL. The metalloproteinase ADAMTS1: a comprehensive review of its role in tumorigenic and metastatic pathways. Int J Cancer. 2013;133:2263–76. doi: 10.1002/ijc.28127. [DOI] [PubMed] [Google Scholar]
- 12.Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, et al. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 2006;25:5270–83. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krampert M, Kuenzle S, Thai SN, Lee N, Iruela-Arispe ML, Werner S. ADAMTS1 proteinase is up-regulated in wounded skin and regulates migration of fibroblasts and endothelial cells. J Biol Chem. 2005;280:23844–52. doi: 10.1074/jbc.M412212200. [DOI] [PubMed] [Google Scholar]
- 14.Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem. 2003;278:23656–65. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- 15.Koo BH, Coe DM, Dixon LJ, Somerville RP, Nelson CM, Wang LW, et al. ADAMTS9 is a cell-autonomously acting, anti-angiogenic metalloprotease expressed by microvascular endothelial cells. Am J Pathol. 2010;176:1494–504. doi: 10.2353/ajpath.2010.090655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubail J, Kesteloot F, Deroanne C, Motte P, Lambert V, Rakic JM, et al. ADAMTS-2 functions as anti-angiogenic and anti-tumoral molecule independently of its catalytic activity. Cell Mol Life Sci. 2010;67:4213–32. doi: 10.1007/s00018-010-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu YP, Staton CA, Cross N, Buttle DJ. Anti-angiogenic properties of ADAMTS-4 in vitro. Int J Exp Pathol. 2012;93:70–7. doi: 10.1111/j.1365-2613.2011.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Sharghi-Namini S, Rao N, Ge R. ADAMTS5 functions as an anti-angiogenic and anti-tumorigenic protein independent of its proteoglycanase activity. Am J Pathol. 2012;181:1056–68. doi: 10.1016/j.ajpath.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 19.El Hour M, Moncada-Pazos A, Blacher S, Masset A, Cal S, Berndt S, et al. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene. 2010;29:3025–32. doi: 10.1038/onc.2010.49. [DOI] [PubMed] [Google Scholar]


