Abstract
Background
Cerebrospinal fluid (CSF) neurofilament light chain protein (NFL) is a sensitive marker of neuronal injury in a variety of neurodegenerative conditions, including the CNS dysfunction injury that is common in untreated HIV infection. However, an important limitation is the requirement for lumbar puncture. For this reason, a sensitive and reliable blood biomarker of CNS injury would represent a welcome advance in both clinical and research settings.
Methods
To explore whether plasma concentrations of NFL might be used to detect CNS injury in HIV infection, an ultrasensitive Single molecule array (Simoa) immunoassay was developed. Using a cross-sectional design, we measured NFL in paired CSF and plasma samples from 121 HIV-infected subjects divided into groups according to stage of their systemic disease, presence of overt HIV-associated dementia (HAD), and after antiretroviral treatment (ART)-induced viral suppression. HIV-negative controls were also examined.
Findings
Plasma and CSF NFL concentrations were very highly correlated (r = 0.89, P < 0.0001). While NFL was more than 50-fold lower plasma than CSF it was within the quantifiable range of the new plasma assay in all subjects, including the HIV negatives and the HIV positives with normal CSF NFL concentrations. The pattern of NFL changes were almost identical in plasma and CSF, both exhibiting similar age-related increases in concentrations along with highest values in HAD and substantial elevations in ART-naïve neuroasymptomatic subjects with low blood CD4+ T cells.
Interpretation
These results show that plasma NFL may prove a valuable tool to evaluate ongoing CNS injury in HIV infection that may be applied in the clinic and in research settings to assess the presence if active CNS injury. Because CSF NFL is also elevated in a variety of other CNS disorders, sensitive measures of plasma NFL may similarly prove useful in other settings.
Keywords: HIV, Cerebrospinal Fluid, CSF, Plasma, Neurofilament Light Chain, NFL, Biomarker, Central Nervous System, CNS, HIV Associated Dementia, HAD, Antiretroviral Treatment
Highlights
-
•
Plasma NFL is a sensitive marker of neuronal injury
-
•
Plasma and CSF NFL concentrations were highly correlated
-
•
Plasma NFL is useful to detect active axonal injury in treated and untreated HIV
A sensitive blood biomarker of neuronal injury has long been sought for. Here we describe an ultrasensitive quantification measurement of the axonal neurofilament light chain protein (NFL) in blood. We test this in the setting of HIV brain injury in which cerebrospinal fluid (CSF) NFL has been well characterized and noted to be a reliable biomarker of neuronal injury.
Blood and CSF NFL were highly correlated and measurement of plasma NFL was able to detect both severe and subclinical neuronal injury in HIV.
CSF NFL is elevated in a wide range of additional neurodegenerative settings, so the reported findings likely have broader implications, though this requires additional direct study.
1. Introduction
Infection of the central nervous system (CNS) is a nearly universal feature of systemic human immunodeficiency virus-1 (HIV) infection. It develops early in systemic HIV infection (Valcour et al., 2012) and continues throughout its untreated course (Gisslen et al., 1999). While often seemingly innocent, this infection can evolve to a form that is associated with CNS injury, most severely manifesting as HIV-associated dementia (HAD) with high morbidity and mortality (Price et al., 1988). However, infected individuals can also manifest less severe CNS injury that elides detection (Antinori, A, et al., 2007, Heaton, RK, et al., 2011). Individuals with HIV infection can suffer CNS dysfunction from a variety of other conditions that can confuse diagnosis. Current research diagnostic classification depends on performance on a neuropsychological test battery which may be difficult to implement and also can be confounded by other conditions and co-morbidities (Antinori et al., 2007). Moreover, in patients with neurocognitive impairment it may be difficult to distinguish ongoing CNS injury related to HIV infection from prior but now static CNS injury, particularly without clear longitudinal observation. In order to more objectively define ongoing CNS injury in individual patients and in research settings evaluating the prevalence or incidence of CNS disease or CNS treatment effects, sensitive and reliable objective biomarkers can prove to be of great value.
To date, measurement of neurofilament light chain (NFL) concentrations in the cerebrospinal fluid (CSF) appears the most sensitive and useful biomarker of active CNS injury in HIV infection — showing elevations in not only overt HAD but also in patients with less severe, inapparent or clinically confounded impairment (Jessen Krut, J, et al., 2014, Peterson, J, et al., 2014). However, the need to sample CSF has limited the application of this measurement, particularly in the clinical contexts of screening patients, assessing those who refuse lumbar puncture, or evaluating those who suffer other conditions that obscure evaluation. An assay that could be applied more broadly to cohort studies and clinical trials that do not include lumbar puncture would also be valuable in assessing the prevalence of CNS injury and its response to treatment. Hence, a biomarker requiring only blood sampling rather than lumbar puncture would clearly represent a useful advance in clinical management and research settings.
To address this need we have developed an ultra-sensitive immunoassay for plasma NFL using Single molecule array (Simoa) technology (Rissin et al., 2010). In this study we compare plasma with CSF NFL concentrations of NFL in a cross-sectional study of HIV-infected individuals and HIV-negative controls and show that the plasma analysis using this sensitive method yields results that are concordant with CSF analysis despite the differences of NFL concentrations in the two fluids.
2. Methods
2.1. Study Design and Patients
This was a cross-sectional study using archived blood and CSF samples originated from two cohort studies: one in Gothenburg, Sweden and the second in San Francisco, California. The samples and related background data were all obtained between 1992 and 2014 within the context of research protocols approved by the institutional review boards of the two study sites. All blood and CSF samples were obtained after informed consent of subjects under these IRB-approved protocols.
Blood and CSF were included from 7 defined HIV-infected subject groups (n = 121) as outlined in previous studies (Jessen Krut, J, et al., 2014, Peterson, J, et al., 2014): early or ‘primary’ HIV infection (PHI, defined as within the first twelve months after initial HIV-1 infection) (Spudich et al., 2011); four groups of chronically HIV-infected subject volunteers without a diagnosis of HAD and who were not being evaluated or treated for overt neurological symptoms or signs when recruited, designated as neuroasymptomatic (NA) and divided by blood CD4+ T cell counts into those with > 350, 200–349, 50–199, and CD4 < 50 cells/μL; and a group presenting with clinically overt HAD, most commonly of subacute onset. All of these subjects were either naïve to treatment or off treatment for at least 6 months at the time of sampling. Also included was a group of treated HIV-infected subjects with > 1 year of plasma virus suppression to below 50 copies/mL of HIV RNA (ART). A group of uninfected (HIV-neg) volunteer subjects (n = 19), confirmed by serological testing at study visit, were recruited from the San Francisco community for research assessments to provide comparison data for the HIV-infected subjects. Background clinical, laboratory and demographic data for each subject group are summarized in Table 1.
Table 1.
Background Subject Characteristics.
| Groups |
N |
Age |
Plasma HIV-RNA |
CSF HIV-RNA |
Blood CD4+ T cells |
|---|---|---|---|---|---|
| Median years (IQR) | Median Log10 (IQR) | Median Log10 (IQR) | Median cells/mL (IQR) | ||
| HIV negative (HIV-neg) | 19 | 34 (29–48) | NA | NA | 763 (686–993) |
| Primary HIV Infection (PHI) | 13 | 36 (30–46) | 4.70 (4.11–5.64) | 2.73 (1.96–3.94) | 539 (340–761) |
| Neuroasymtpomatic HIV (NA) | |||||
| CD4 > 350 | 19 | 41 (35–43) | 4.08 (3.30–4.44) | 3.23 (2.45–3.68) | 490 (402–582) |
| CD4 200–349 | 17 | 48 (36–56) | 4.91 (4.30–5.31) | 4.28 (3.44–4.68) | 240 (215–305) |
| CD4 50–199 | 19 | 44 (37–56) | 4.97 (4.44–5.25) | 3.96 (3.53–4.56) | 125 (100–170) |
| CD4 < 50 | 20 | 41 (36–49) | 5.40 (4.54–5.88) | 3.10 (2.30–3.81) | 22 (10–39) |
| HIV-associated dementia (HAD) | 11 | 43 (38–56) | 5.49 (4.83–5.83) | 4.34 (2.59–5.31) | 60 (30–144) |
| HIV, treated-suppressed (ART) | 22 | 44 (38–52) | 1.30 (1.30–1.30) | 1.30 (1.30–1.30) | 544 (275–725) |
2.2. Blood and CSF Sampling
Blood and CSF were obtained according to standard protocols as previously described (Price, RW, et al., 2001, Gisslen, M, et al., 2007). CSF was immediately subjected to low-speed centrifugation to remove cells, aliquoted and stored within 1 h of collection at ≤− 70 °C until the time of the neuronal biomarker assays. Blood was collected in EDTA tubes and plasma was aliquoted and stored in parallel with CSF for later batch assays.
2.3. Background Laboratory Methods
We explored correlations with background HIV clinical biomarkers including: blood CD4+ T cell counts: CSF and blood HIV RNA concentrations; CSF white blood cell (WBC) counts; CSF:serum albumin ratio as an indicator of blood–brain barrier permeability; and blood and CSF neopterin, a marker of macrophage and microglial activation (Hagberg et al., 2010).
HIV RNA levels were measured in cell-free CSF and plasma at each site using the Cobas TaqMan RealTime HIV-1 (version 1 or 2; Hoffmann-La Roche, Basel, Switzerland), or the Abbott RealTime HIV-1 assay (Abbot Laboratories, Abbot Park, IL, USA). All recorded viral loads that were below the lower limit of quantitation (40 copies/mL) were standardized to a defined ‘floor’ value of 20 copies/mL for descriptive purposes. Blood and CSF neopterin were analyzed using a commercially available immunoassay (BRAHMS, Hennigsdorf, Germany), with an upper normal reference value of 8.8 nmol/L in blood and 5.8 nmol/L in CSF (Hagberg et al., 2010). Each study visit included assessments by local clinical laboratories using routine methods to measure CSF white blood cell (WBC) count, CSF and blood albumin, and blood CD4+ and CD8+ T lymphocyte counts by flow cytometry.
2.4. Clinical Evaluations
All HIV-infected subjects and controls underwent routine clinical bedside screening for symptoms or signs of CNS opportunistic infections or other conditions that could impact CSF or blood biomarker concentrations. Diagnosis of HAD was based on clinicians' assessment at presentation which was characteristically subacute, and met American Academy of Neurology criteria (Anon., 1991). Many of these subjects were studied before publication of the more formal Frascati criteria (Antinori et al., 2007) and were diagnosed with AIDS dementia complex (ADC) stages 1–4 (Price and Brew, 1988) but met the functional criteria for the Frascati diagnosis of HAD without the requisite extensive formal neuropsychological assessment.
2.5. NFL Measurements
CSF NFL concentrations were measured using a sensitive sandwich method (NF-light® ELISA kit, UmanDiagnostics AB, Umeå, Sweden) as previously described (Jessen Krut, J, et al., 2014, Norgren, N, et al., 2003). The coefficient of variations (CVs) for the repeatability and intermediate precision for this assay is CV < 6% and CV < 7%, respectively, and the lower limit of quantification (LLOQ) is 50 pg/mL determined by repeated measurements (n = 15) of a CSF sample with low (52 pg/mL) concentration and still acceptable variability (CV = 12%). Plasma NFL levels were determined using the same monoclonal antibodies and calibrator as in the NF-light assay, transferred onto the Simoa platform using a homebrew kit (Quanterix, Lexington, MA, USA). The limit of detection (mean blank signal + 3 SD) for the Simoa NFL assay was 0.3 pg/mL and the lower limit of quantification (mean blank signal + 10 SD) was 2.7 pg/mL when compensated for a four-fold sample dilution. Samples were analyzed in duplicate, and the CV for the samples was below 10%.
All plasma samples were analyzed in a single run in the Laboratory of Neurochemistry at the University of Gothenburg by board-certified laboratory technicians blind to clinical data using a single batch of reagents for each assay; intra-assay coefficients of variation were below 10% for all analyses. Previously described laboratory cutoffs for CSF NFL were used for descriptive analysis (Jessen Krut et al., 2014). Based on age-related reference values, normal cutoff values in CSF for NFL were defined as < 380 ng/L (< 30 years), < 560 ng/L (30–39 years), < 890 ng/L (40–59 years), and < 1850 ng/L (> 59 years). Laboratory cutoffs for blood NFL have not yet been defined. Some of the CSF NFL results and results of the PHI subjects have been previously reported in a different context (Peterson, J, et al., 2014, Peluso, MJ, et al., 2013), including within a larger data set characterizing CSF in relation to age (Jessen Krut et al., 2014).
2.6. Statistical Methods
Descriptive statistics were performed using Prism (version 6, Graphpad Software Inc., La Jolla, CA) or SPSS (IBM SPSS version 21) software. Continuous variables were log10 transformed where appropriate to reduce skewness. Comparison of biomarker concentrations was performed with One-way ANOVA and Tukey's multiple comparison test for evaluation of multiple groups. Biomarker associations were analyzed with Pearson correlation analysis. The relationship between age and log10 plasma and CSF NFL levels, was analyzed with linear regression.
3. Results
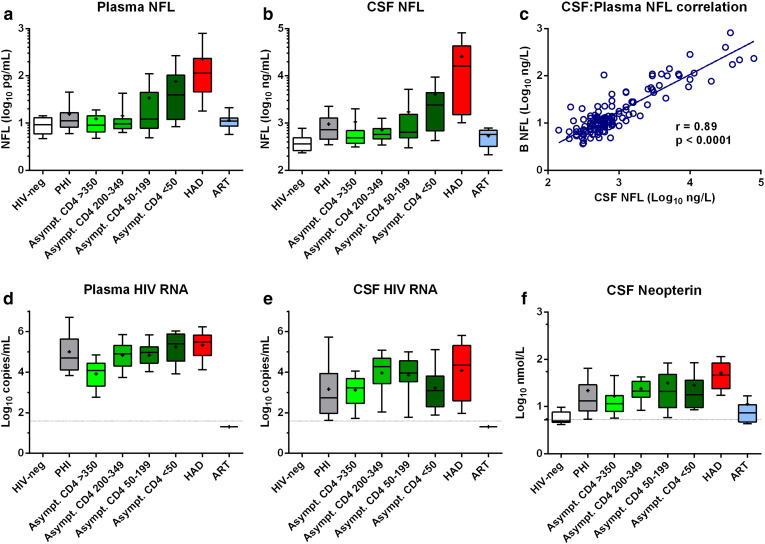
Plasma NFL concentrations were within the linear range of the assay in all samples, Fig. 1a. It was thus possible to quantify plasma NFL in all subjects analyzed, despite more than 50-fold lower concentrations than in CSF. This included the HIV-negative controls and other subjects with low normal CSF NFL levels, Table 2. CSF concentrations were likewise within the quantitative range of the standard assay, Fig. 1b. Notably, plasma NFL concentrations were highly correlated with those of CSF (r = 0.89, p < 0.0001), Fig. 1c. Fig. 1 provides general context for the NFL results, showing plasma and CSF HIV RNA (d and e) and CSF neopterin (f) concentrations are also shown in Fig. 1.
Fig. 1.
Association between CSF and plasma NFL and concentrations of biomarkers in 8 subject groups. Panels a–b and d–f, plot concentrations of different markers for the 8 subject groups, HIV-negative controls (HIV-neg); primary HIV infection (PHI); untreated neuroasymptomatic subjects in different CD4 cell strata (Asympt); HIV-associated dementia (HAD); and subjects on suppressive antiretroviral treatment (ART). Boxes depict median and IQR, whiskers show 10–90 percentiles and ‘+’ designates the means. Dotted horizontal lines in panels d–e show the limit of detection for HIV RNA (40 copies/mL), and in panel f the upper limit of normal for CSF neopterin (5.8 nmol/L). Panel c plots the correlation between log CSF and log plasma NFL.
Table 2.
Median (IQR) Plasma NFL (Left Columns) and CSF NFL (Right Columns) Concentrations in Different Groups.
| Groups |
N |
Plasma NFL |
CSF NFL |
|---|---|---|---|
| Median nmol/L (IQR) | Median nmol/L (IQR) | ||
| HIV negative (HIV-neg) | 19 | 9.3 (5.9–13.1) | 363 (264–487) |
| Primary HIV Infection (PHI) | 13 | 11.2 (8.2–16.6) | 732 (456–1288) |
| Neuroasymtpomatic HIV (NA) | |||
| CD4 > 350 | 19 | 9.0 (6.5–14.3) | 488 (374–694) |
| CD4 200–349 | 17 | 9.7 (7.6–12.2) | 573 (464–764) |
| CD4 50–199 | 19 | 12.1 (7.8–44.7) | 640 (490–1530) |
| CD4 < 50 | 20 | 39.6 (12.0–103) | 2415 (690–4420) |
| HIV-associated dementia (HAD) | 11 | 114 (46.0–235) | 16,185 (1513–43,010) |
| HIV, treated-suppressed (ART) | 22 | 11.1 (8.6–12.8) | 582 (322–706) |
The patterns of plasma and CSF NFL concentration changes across the HIV-infected groups were very similar. In agreement with previous studies, the CSF NFL elevations were greatest in the HAD groups but there was also a high prevalence of abnormal levels in untreated neuroasymptomatic subjects with low CD4+ T-cell counts (Jessen Krut, J, et al., 2014, Peterson, J, et al., 2014). Using the laboratory age-related cut-offs for CSF NFL, all of the HAD patients had elevated CSF NFL concentrations, and 75% of untreated neuroasymptomatic subjects with CD4+ T-cell count below 50 cells/μL and 58% with 50–199 CD4+ cells/μL had abnormal CSF NFL concentrations; additionally 24% of subjects with 200–349 CD4 cells and 16% of those with CD4 cell counts above 350 cells/μL had increased levels—indicating a high level of clinically unappreciated ongoing CNS injury that varied with degree of systemic disease progression. By comparison, in the treated group, and in HIV-negative subjects, the frequency was much lower, 4 and 5%, respectively. There also were moderate elevated CSF NFL levels in a large proportion of subjects with PHI, 54%, in accordance with previous reports (Spudich et al., 2011).
Unlike CSF, age-related reference values have not yet been established for plasma NFL, but the pattern of changes in the subject groups was very similar to that of CSF NFL. Again the HAD had highest plasma NFL concentrations, with all values exceeding the 90th percentile of the HIV negative control levels. Like CSF, there were also elevations of plasma NFL in a substantial portion of the neuroasymptomatic subjects with low CD4+ T cell counts.
The reason for the similarity in these patterns was the strong correlation between plasma and CSF NFL (Fig. 1c) and thus the plasma levels appear to reflect concentrations in CSF and, hence, the severity of CNS injury.
Statistical comparisons between groups using One-way ANOVA with Tukey's multiple comparison tests are listed in Table 3. The plasma and CSF NFL concentrations in the HAD group were significantly elevated compared to all other subgroups. The neuroasymptomatic CD4 < 50 group were also elevated above the other groups, except compared to neuroasymptomatic CD4 50–199, and, for CSF only, also to the PHI group.
Table 3.
Comparisons of Blood and CSF Neurofilament Light Protein (NFL) Concentrations Among Subject Groups.
| Blood NFL (log) | CSF NFL (log) | |
|---|---|---|
| Overall ANOVA p | ||
| Group Comparisons | < 0.0001 | < 0.0001 |
| Tukey's Multiple Comparison | ||
| HAD vs. HIV- | < 0.0001 | < 0.0001 |
| HAD vs. PHI | < 0.0001 | < 0.0001 |
| HAD vs. NA CD4 > 350 | < 0.0001 | < 0.0001 |
| HAD vs. NA CD4 200–349 | < 0.0001 | < 0.0001 |
| HAD vs. NA CD4 50–199 | < 0.0001 | < 0.0001 |
| HAD vs. NA CD4 < 50 | 0.008 | < 0.0001 |
| HAD vs. ART | < 0.0001 | < 0.0001 |
| NA CD4 < 50 vs. HIV- | < 0.0001 | < 0.0001 |
| NA CD4 < 50 vs. PHI | 0.005 | 0.063 |
| NA CD4 < 50 vs. NA CD4 > 350 | < 0.0001 | 0.001 |
| NA CD4 < 50 vs. NA CD4 200–349 | < 0.0001 | 0.001 |
| NA CD4 < 50 vs. NA CD4 50–199 | 0.056 | 0.129 |
| NA CD4 < 50 vs. ART | < 0.0001 | < 0.0001 |
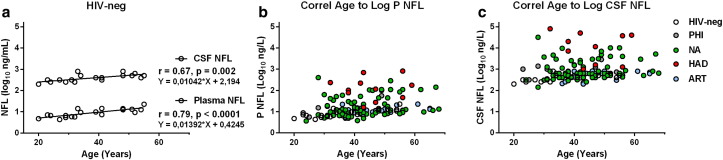
CSF NFL increases with age in the absence of HIV infection (Jessen Krut et al., 2014) and, as anticipated, there was a significant correlation between age and CSF NFL in the group of 19 HIV-negative controls (r = 0.67, p = 0.002). Similarly, plasma NFL was strongly associated with age in HIV-negative controls (r = 0.79, p < 0.0001). In Fig. 2, plasma (b) and CSF (c) NFL are plotted against age in the different groups studied. The slopes of the plasma and CSF NFL values against age in HIV-negative controls were similar to what has been reported previously in CSF (Jessen Krut et al., 2014), Fig. 2a.
Fig. 2.
Association between NFL and age. Panel a displays the regression lines for age to log plasma and log CSF NFL in HIV-negative controls. Age was closely correlated with plasma NFL (r = 0.79, p < 0.0001).
Plasma and CSF NFL were also significantly correlated with CD4+ T cell count, plasma and CSF HIV RNA levels, blood and CSF neopterin, and the albumin ratio. Correlation coefficients were similar for CSF and plasma NFL (Table 4).
Table 4.
Plasma NFL (Left Columns) and CSF NFL (Right Columns) Correlation With Background HIV Clinical Biomarkers.
| Log Plasma NFL |
Log CSF NFL |
|||
|---|---|---|---|---|
| r | p | r | p | |
| Log CD4 | − 0.43 | < 0.001 | − 0.36 | < 0.001 |
| Log P HIV RNA | 0.45 | < 0.001 | 0.41 | < 0.001 |
| Log CSF HIV RNA | 0.25 | 0.020 | 0.29 | 0.008 |
| Log S Neopterin | 0.48 | < 0.001 | 0.44 | < 0.001 |
| Log CSF Neopterin | 0.42 | < 0.001 | 0.30 | 0.002 |
| Log CSF/P albumin ratio | 0.31 | 0.004 | 0.30 | 0.002 |
4. Discussion
A sensitive and reliable blood biomarker of neural injury in CNS neurodegeneration has long been sought. The results of this study show that plasma levels of NFL provide a good indicator of active CNS injury in HIV infection that agrees well with the results of CSF NFL measurement. This thus promises to be a useful advance in the study of CNS injury in HIV infection. Additionally, because of the sensitivity of CSF NFL to CNS injury in other settings, these findings suggest that this same assay may be useful also in other neurodegenerative conditions.
Using an ultrasensitive immunoassay, NFL was detectable and quantifiable in blood in all subjects analyzed despite more than 50-fold lower concentrations than in CSF. This included detection in subjects with low normal CSF NFL levels, including HIV-negative controls and HIV-infected on suppressive ART. Thus, the assay could be used to analyze the full range of subjects included in this study and could be similarly deployed in other small and large clinical and research settings to assess active CNS injury.
We found elevated levels of NFL, both in CSF and plasma, in patients with HAD and in some neuroasymptomatic HIV-infected patients, mainly in subjects with low CD4+ cell count. This is in agreement with previous studies on CSF NFL, reflecting an ongoing subclinical axonal injury in these subjects (Jessen Krut et al., 2014). CSF NFL can predict subsequent development of HAD and we have previously shown that CSF NFL was increased 1–2 years before overt symptoms of dementia in patients developing HAD in the pre-ART era (Gisslen et al., 2007). The incidence of HAD has decreased substantially during recent years in the developed world when the majority of HIV-infected patients are on effective ART (Lescure et al., 2011). Nowadays, HAD develops almost exclusively in so called late presenters with low CD4+ T cell counts at time of diagnosis. ART effectively prevents HAD, and severe neurocognitive disease rarely develops in patients on effective ART. Exceptions are rare cases with ‘neurosymptomatic CSF escape’ characterized by new progressive CNS disease in patients on ART with undetectable or low HIV RNA in plasma but disproportionately increased viral loads in CSF (Canestri, A, et al., 2010, Peluso, MJ, et al., 2012). Although CSF NFL can be used to detect active neuronal injury in these settings, it is limited by the need for CSF sampling that sometimes could be an obstacle. While more severe CNS disease is uncommon in treated HIV, mild neurocognitive impairment is frequently found also in patients on ART (Antinori, A, et al., 2007, Heaton, RK, et al., 2010). In these treatment settings, plasma NFL may be a useful marker to help discriminate active ongoing neuronal injury from neurological or cognitive symptoms related to sequelae of prior CNS damage that occurred before treatment initiation (Munoz-Moreno et al., 2008), so called inactive disease, though this important issue needs further direct study. This distinction between active and static injury may have important implications for treatment, the first more amenable to intervention either with additional antiviral or adjuvant, anti-inflammatory, interventions. Previous efforts at mitigating neurocognitive impairment of treated-suppressed individuals may have been underpowered because of failure to distinguish between active and static neural injury. Importantly, a plasma assay of injury might prove valuable to assess neurological impact of treatment trials that have not included neurological assessments or lumbar puncture, opening a large experience with different treatment strategies and drug regimens to neurological outcome analysis.
Increased concentrations of CSF NFL are not specific to HIV-related brain damage; CSF NFL levels have been shown to be a sensitive indicator of CNS axonal injury in several neurological diseases (Norgren, N, et al., 2003, Constantinescu, R, et al., 2010, Gunnarsson, M, et al., 2011) including various infections (Studahl, M, et al., 2000, Grahn, A, et al., 2013), and, by extrapolation, plasma NFL has the potential to become a valuable marker in a wide range of neurological disease settings.
Plasma NFL increased with age in our controls in a pattern similar to that found in the CSF. This age-dependent increase in CSF NFL has been clearly defined in earlier, larger studies (Jessen Krut et al., 2014). The mechanisms underlying the age-dependent increase in CSF NFL have not been established, but these age effects need to be considered when analyzing NFL concentrations, including the effects of treatment in individuals with HIV followed over longer periods of time.
NFL is important for maintenance of the axonal caliber and morphological integrity and is expressed predominantly in large-caliber myelinated axons (Hoffman et al., 1987), but is also present in neurites (axons and dendrites) of CNS neurons in the cerebral and cerebellar cortex, hypothalamus and spinal cord (Trojanowski et al., 1986). Its CSF levels reflect leakage from injured or degenerating neurons, correlate with white-matter lesions and other injuries to subcortical brain regions (Jonsson et al., 2010), and predict severity and survival in several neurodegenerative diseases (Skillback et al., 2014). The robust correlation between plasma and CSF levels of NFL strongly suggests that plasma levels indeed reflect ongoing CNS injury, which is in agreement with a recent study on amyotrophic lateral sclerosis, however using a less sensitive method that does not allow proper quantification in all samples (Lu et al., 2015). However, an increase in plasma NFL without adjacent increase in CSF could theoretically also be a result of peripheral neuronal injury given that NFL is also found in peripheral nervous system neurons (Trojanowski et al., 1986). The magnitude of this effect needs to be evaluated in future studies of patients with the common HIV distal sensory and other neuropathies.
We have observed a close correlation between plasma and CSF NFL, including a parallel increase in both, in the presence of ongoing neuronal injury in HIV-infected patients. Therefore, we believe that plasma NFL has the potential of being a useful biomarker of CNS injury in HIV infection, and most likely in other neurodegenerative settings as well.
Contributors
M.G. initiated the study and wrote the paper. M.G., L.H., S.S., and R.W.P. recruited the subjects. H.Z., K.B., U.A., and N.N. developed the NFL assays and performed the NFL analyses. D.F. performed neopterin measurements. M.G., R.W.P., and S.N. analyzed the data and performed the statistics. All authors edited the manuscript, provided comments, and approved the final version..
Declaration of Interest
N.N. is employed by UmanDiagnostics AB, Umeå, Sweden. UmanDiagnostics did not play any role in the study design and was not involved in the data analysis or interpretation of the results. H.Z. and K.B. are co-founders of Brain Biomarkers Solutions in Gothenburg AB, a GU Holding-based platform company at the University of Gothenburg, Sweden. All other authors declare no competing financial interest.
Acknowledgments
This work was supported by the Swedish Research Council (K2011-58P-20931-01-4, K2010-63P-21562-01-4, K2011-61X-20401-05-6, 2013-2546, and K2013-61X-14002-13-5), the Sahlgrenska University Hospital (ALFGBG-430271, ALFGBG-441051, and ALFGBG-139671), the Knut and Alice Wallenberg Foundation, VINNOVA (# 2014-03496), the Torsten Söderberg Foundation at the Royal Swedish Academy of Sciences, and the United States National Institutes of Health (R21MH096619, P01MH094177, R01MH62701, UL1 RR024131, and P30 AI027763). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Anon. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41(6):778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- Antinori A., Arendt G., Becker J.T. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canestri A., Lescure F.X., Jaureguiberry S. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin. Infect. Dis. 2010;50(5):773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- Constantinescu R., Rosengren L., Johnels B., Zetterberg H., Holmberg B. Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson's disease and atypical Parkinsonian disorders. Parkinsonism Relat. Disord. 2010;16(2):142–145. doi: 10.1016/j.parkreldis.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Gisslen M., Fuchs D., Svennerholm B., Hagberg L. Cerebrospinal fluid viral load, intrathecal immunoactivation, and cerebrospinal fluid monocytic cell count in HIV-1 infection. J. Acquir. Immune Defic. Syndr. 1999;21(4):271–276. doi: 10.1097/00126334-199908010-00003. [DOI] [PubMed] [Google Scholar]
- Gisslen M., Hagberg L., Brew B.J., Cinque P., Price R.W., Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J. Infect. Dis. 2007;195(12):1774–1778. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- Grahn A., Hagberg L., Nilsson S., Blennow K., Zetterberg H., Studahl M. Cerebrospinal fluid biomarkers in patients with varicella-zoster virus CNS infections. J. Neurol. 2013;260(7):1813–1821. doi: 10.1007/s00415-013-6883-5. [DOI] [PubMed] [Google Scholar]
- Gunnarsson M., Malmestrom C., Axelsson M. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 2011;69(1):83–89. doi: 10.1002/ana.22247. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Cinque P., Gisslen M. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Clifford D.B., Franklin D.R., Jr. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Franklin D.R., Ellis R.J. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P.N., Cleveland D.W., Griffin J.W., Landes P.W., Cowan N.J., Price D.L. Neurofilament gene expression: a major determinant of axonal caliber. Proc. Natl. Acad. Sci. U. S. A. 1987;84(10):3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen Krut J., Mellberg T., Price R.W. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One. 2014;9(2):e88591. doi: 10.1371/journal.pone.0088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M., Zetterberg H., van Straaten E. Cerebrospinal fluid biomarkers of white matter lesions - cross-sectional results from the LADIS study. Eur. J. Neurol. 2010;17(3):377–382. doi: 10.1111/j.1468-1331.2009.02808.x. [DOI] [PubMed] [Google Scholar]
- Lescure F.X., Omland L.H., Engsig F.N. Incidence and impact on mortality of severe neurocognitive disorders in persons with and without HIV infection: a Danish nationwide cohort study. Clin. Infect. Dis. 2011;52(2):235–243. doi: 10.1093/cid/ciq041. [DOI] [PubMed] [Google Scholar]
- Lu C.H., Macdonald-Wallis C., Gray E. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84(22):2247–2257. doi: 10.1212/WNL.0000000000001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Moreno J.A., Fumaz C.R., Ferrer M.J. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res. Hum. Retrovir. 2008;24(10):1301–1307. doi: 10.1089/aid.2007.0310. [DOI] [PubMed] [Google Scholar]
- Norgren N., Rosengren L., Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003;987(1):25–31. doi: 10.1016/s0006-8993(03)03219-0. [DOI] [PubMed] [Google Scholar]
- Peluso M.J., Ferretti F., Peterson J. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26(14):1765–1774. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso M.J., Meyerhoff D.J., Price R.W. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J. Infect. Dis. 2013;207(11):1703–1712. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J., Gisslen M., Zetterberg H. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One. 2014;9(12):e116081. doi: 10.1371/journal.pone.0116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.W., Brew B.J. The AIDS dementia complex. J. Infect. Dis. 1988;158(5):1079–1083. doi: 10.1093/infdis/158.5.1079. [DOI] [PubMed] [Google Scholar]
- Price R.W., Brew B., Sidtis J., Rosenblum M., Scheck A.C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Price R.W., Paxinos E.E., Grant R.M. Cerebrospinal fluid response to structured treatment interruption after virological failure. AIDS. 2001;15(10):1251–1259. doi: 10.1097/00002030-200107060-00006. [DOI] [PubMed] [Google Scholar]
- Rissin D.M., Kan C.W., Campbell T.G. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 2010;28(6):595–599. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skillback T., Farahmand B., Bartlett J.W. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology. 2014;83(21):1945–1953. doi: 10.1212/WNL.0000000000001015. [DOI] [PubMed] [Google Scholar]
- Spudich S., Gisslen M., Hagberg L. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J. Infect. Dis. 2011;204(5):753–760. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studahl M., Rosengren L., Gunther G., Hagberg L. Difference in pathogenesis between herpes simplex virus type 1 encephalitis and tick-borne encephalitis demonstrated by means of cerebrospinal fluid markers of glial and neuronal destruction. J. Neurol. 2000;247(8):636–642. doi: 10.1007/s004150070134. [DOI] [PubMed] [Google Scholar]
- Trojanowski J.Q., Walkenstein N., Lee V.M. Expression of neurofilament subunits in neurons of the central and peripheral nervous system: an immunohistochemical study with monoclonal antibodies. J. Neurosci. 1986;6(3):650–660. doi: 10.1523/JNEUROSCI.06-03-00650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V., Chalermchai T., Sailasuta N. Central nervous system viral invasion and inflammation during acute HIV infection. J. Infect. Dis. 2012;206(2):275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]