Abstract
Despite the emergence of JAK inhibitors, there is a need for disease-modifying treatments for Philadelphia-negative myeloproliferative neoplasms (MPNs). JAK inhibitors ameliorate symptoms and address splenomegaly, but because of the heterogeneous contributors to the disease process, JAK inhibitor monotherapy incompletely addresses the burden of disease. The ever-growing understanding of MPN pathogenesis has provided the rationale for testing novel and targeted therapeutic agents, as monotherapies or in combination, in preclinical and clinical settings. A number of intriguing options have emerged, and it is hoped that further progress will lead to significant changes in the natural history of MPNs.
Keywords: Myeloproliferative neoplasm, Myelofibrosis, Therapy, Targeted, Novel, Combination
Highlights
-
•
Targeting aberrant kinase activity other than that of JAK2 is of significant clinical interest.
-
•
Recognition of the genetic basis of MPNs has facilitated the way for drug development.
1. Introduction
The Philadelphia chromosome-negative (Ph −) MPNs include clonal disorders of myeloid progenitor cells, such as polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF). The latter can be sub-categorized as either primary (PMF) or as transformed from PV or ET (post-PV/ET MF) (Tefferi et al., 2009a). The incidence of classic Ph − MPNs in Europe is 1.8 cases per 100,000 person-years (Visser et al., 2012). In the US, between 2008 and 2010 (as assessed by a review of two large health plans), the prevalences of PV, ET, and MF were 44–57, 38–57, and 4–6 per 100,000, respectively (Mehta et al., 2012). In general, the MPNs may be associated with an increased risk of morbidity and mortality and may lead to a significant decrement in quality of life (QOL). Generally, MF differs from PV and ET in that it typically carries a worse prognosis and high symptom burden related to elevated cytokine levels, cytopenias, splenomegaly, and extramedullary hematopoiesis, all of which can result in fatigue, early satiety, abdominal discomfort, inactivity, night sweats, pruritus, bone pain and weight loss (Emanuel et al., 2012). Transformation to acute myelogenous leukemia (AML) has been the most feared complication of MPNs, particularly of MF.
Prognosis of MPNs varies greatly based on subtype. ET is associated with a 10-year and 15-year survival of 89 and 80%, leukemic transformation rate of 0.7% and 2.1%, and rate of progression to MF of 0.8% and 9.3%, respectively (Barbui et al., 2011). Among patients with PV, median survival has been shown to be 14.1 years, which is worse than that of the age- and sex-matched control population of the US (Tefferi et al., 2013). Evolution to MDS and leukemia was the main cause of death in a phase 3 study comparing the use of hydroxyurea (HU) to pibobroman among treatment-naïve PV patients under the age of 65 years (Kiladjian et al., 2011) In a large European epidemiological study of PV, 41% of deaths (1.5 deaths per 100 persons per year) were attributable to cardiovascular events (Marchioli et al., 2005). The median survival for patients with MF was shown to be 6.5 years, between years 1996 and 2007, in a European population (Cervantes et al., 2012).
A better understanding of the molecular pathogenesis of Ph − MPNs has been greatly facilitated by the 2005 discovery of the point mutation JAK2 V617F, which is present in almost 95% of PV cases and 50–60% of ET and PMF cases (Kralovics et al., 2005, Rampal and Levine, 2014). The mutation leads to constitutive activation of Janus kinase 2 (JAK2), a member of the Janus family of kinases, normally phosphorylated/activated by various cytokine receptors to drive signal transducer and activator of transcription (STAT) pathways in hematopoiesis (Fig. 1). The thrombopoietin receptor mutation MPL W515L, identified shortly after the discovery of JAK2 V617F, is another driver mutation that leads to activation of the JAK–STAT pathway and is present in a minority JAK2 V617F-negative cases of MF and ET (Pikman et al., 2006). In 2013, mutations of CALR, the gene that encodes the endoplasmic reticulum chaperone calreticulin, were identified among 67–88% of patients with JAK2/MPL negative ET and MF (Klampfl et al., 2013, Nangalia et al., 2013). The presence of these mutations may have prognostic implications for patients. For example, MF patients with CALR mutations have an improved overall survival as compared to patients with JAK2 mutations (Klampfl et al., 2013, Nangalia et al., 2013), and ET patients with CALR mutations have a decreased incidence of thrombosis (Rumi et al., 2014a). The absence of mutations of JAK2, CALR, and MPL (“triple negative” PMF) appears to ultimately promote leukemic transformation, as compared with CALR-mutated or JAK2-mutated patients (Rumi et al., 2014b). These findings suggest that mutational status may be able to serve as a prognosticator independently from established prognostic models that are based on clinical features. Since the discovery of JAK2 V617F, there has been the identification of numerous other somatic mutations in MPNs (e.g., JAK2 exon 12, IKZF1, DNMT3A, TP53, TET2, SRSF2, SF3B1, ASXL1, IDH1/2, and EZH2). Many of these genes play roles in epigenetic processes, leading to activation or suppression of gene expression, suggesting a rationale for epigenetic therapies. Among MF patients, mutations in ASXL1, SRSF2, IDH, or EZH2 have been associated with a poor survival or an increased risk for leukemic transformation (Vannucchi et al., 2013). Many of the other mutations seen in MPNs involve mRNA splicing and genes controlling cellular metabolism, and some may facilitate clonal selection and promote dominance of JAK2 V617F subclones (Nangalia and Green, 2014, Vainchenker et al., 2011). The role of systematic mutational profiling to predict for benefit to therapy is the subject of current investigations, which will likely be challenging endeavors given the clonal complexity of MPNs (Lundberg et al., 2014).
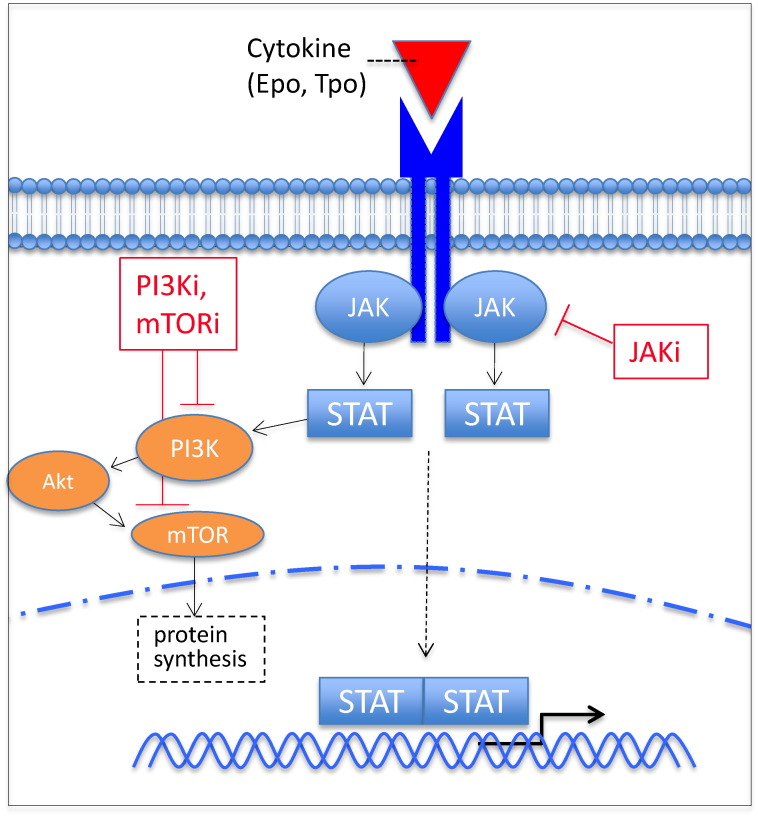
Fig. 1.
JAK-STAT signaling contributes to the pathogenesis of MPNs. Unregulated JAK-STAT signaling, leading to STAT-mediated hematopoiesis and activation of the PI3K/Akt/mTOR pathway, may result from a number of aberrations including point mutations JAK2 V617F, leading to constitutive activation of JAK2 kinase, and MPL W515L, an activating mutation of the thrombopoietin receptor. Number small molecule inhibitors of these pathways, including JAK, PI3K, and mTOR inhibitors, are in clinical development. Epo, erythropoietin; JAK, Janus kinase; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide-3 kinase; STAT, signal transducer and activator of transcription; Tpo, thrombopoietin; -i, inhibitor.
2. Current Treatment of Classic Ph − MPNs
Treatment decisions are generally based on MPN subtype, risk category, age, and disease manifestations. For PV and ET, thrombosis risk category often guides the decision to use cytoreductive and anti-platelet therapies (and phlebotomy for PV). Historically, treatment of MF has been guided by the patient's symptom profile and burden of disease, and has involved managing cytopenias (androgens, erythropoiesis stimulating agents, immunomodulatory agents) and splenomegaly (HU, busulfan, cladribine, splenectomy, splenic radiation, and immunomodulatory agents) and providing measures to prevent infections, control symptoms, and improve QOL (e.g., corticosteroids and stimulants) (Gowin et al., 2013, Tefferi et al., 2009b). Treatment has also been based in part on the risk category (as an aid for selecting transplant candidates), assessed by the International Prognostic Scoring System (IPSS) or Dynamic IPSS/plus. Allogeneic hematopoietic stem cell transplant, typically reserved for fit patients with intermediate-2 or high-risk disease, is the only curative treatment option and may resolve bone marrow fibrosis, lead to molecular remission, restore normal hematopoiesis, and lead to cure in 40–70% of patients (Kröger et al., 2009, Ballen et al., 2010). However, its association with significant morbidity and mortality limits its utility.
The oral selective JAK1/2 inhibitor ruxolitinib was assessed in a phase 1/2 study of 153 MF patients requiring therapy, identifying 25 mg orally twice daily or 100 mg once daily as the maximum tolerated doses, along with evidence of dose-dependent suppression of phosphorylated STAT3 (Verstovsek et al., 2010). In the phase 2 portion, based on responses (at least 50% reduction of splenomegaly), toxicity, and need for dose reductions, 15 mg twice-daily was established as the optimal starting dose, and most patients experienced rapid improvement in debilitating MF-related symptoms. Ruxolitinib was FDA- and EMA-approved for the treatment of MF in 2011 and 2012, respectively, based on the results of two phase 3 studies in which ruxolitinib was compared to either placebo (COMFORT-1) or to best available therapy (COMFORT-2) (Verstovsek et al., 2012, Harrison et al., 2012). Among the ruxolitinib-treated patients, a 35% or higher reduction in spleen size was achieved and maintained in 41.9% (versus 0.7% of placebo-treated patients) at week 24 in COMFORT-1 and in 32% and 28% (versus 0% of patients who received best available therapy) at weeks 24 and 48, respectively, in COMFORT-2. Moreover, in COMFORT-1, 45.9% of ruxolitinib-treated patients (versus 5.3%) achieved an improvement of at least 50% in the total symptom score (Verstovsek et al., 2012). In COMFORT-2, pre-specified exploratory analyses showed that ruxolitinib-treated patients experienced improvements in quality of life, role functioning, and physical condition and reductions in MF-associated symptoms as compared to the patients who received best available therapy (Harrison et al., 2012). The efficacy of ruxolitinib appeared to be dose-dependent and was not influenced by the presence or absence of the JAK2 V617F mutation, as patients with mutant and wild-type JAK2 responded similarly. Extended follow-up of these two studies demonstrated a survival advantage with the use of ruxolitinib among intermediate- and high-risk MF patients, while reduction in spleen size seemed to correlate with longer survival (Cervantes et al., 2013, Vannucchi et al., 2015a). In the UK open-label, phase 2 ROBUST trial, involving 48 MF patients, 50% of all patients and 57% of intermediate-1 risk patients achieved the primary composite endpoint of treatment success, which was defined as ≥ 50% reduction in palpable spleen length and/or a ≥ 50% decrease in the Myelofibrosis Symptom Assessment Form Total Symptom Score at 48 weeks (Mead et al., 2015). An exploratory analysis of COMFORT-1 showed a reduction in MF-related hepatomegaly among the ruxolitinib-treated patients, providing rationale for using ruxolitinib in MF patients who undergo splenectomy (Verstovsek et al., 2015). There are also reports of resolution of marrow fibrosis, reduction in JAK2 mutant allele burden, and even achievement of complete molecular remission with its long-term use (Wilkins et al., 2013, Deininger et al., 2015).
Ruxolitinib's FDA and EMA approvals were expanded in 2014 and 2015, respectively, to the treatment of PV patients who are intolerant of or resistant to HU, based on results from the RESPONSE trial, a phase 3 trial in which ruxolitinib was shown to be superior in regard to hematocrit control and spleen volume reduction as well as in suppressing disease-related symptoms (Vannucchi et al., 2015b). The most common adverse events, albeit usually low-grade, associated with ruxolitinib are anemia and thrombocytopenia, and other reported adverse events include headache, fatigue, diarrhea, nausea, and infections (Verstovsek et al., 2012, Harrison et al., 2012, Vannucchi et al., 2015b). Progressive multifocal leukoencephalopathy has been reported to occur in an elderly patient receiving the drug (Wathes et al., 2013). The infectious complications of ruxolitinib may be at least partially explained by its impairment of dendritic cell development and function, including impairment of the dendritic cell's activation, migration, and ability to induce T-cell responses (Heine et al., 2013b). These observations have prompted some investigators to recommend an infectious risk assessment and prophylaxis strategy (Heine et al., 2013a). Three-year follow-up of the COMFORT studies showed that the rates of many of the adverse events generally decreased with longer exposure to ruxolitinib treatment, with the highest rates occurring within the first 6 months (Cervantes et al., 2013). It is also important to keep in mind that sudden withdrawal of ruxolitinib has been associated with a shock-like syndrome from the reemergence of suppressed inflammatory cytokines (Tefferi and Pardanani, 2011). However, such a severe withdrawal syndrome was not reported in the COMFORT and RESPONSE studies, but return of baseline MF-related symptoms typically occurs within approximately a week upon discontinuation of ruxolitinib (Verstovsek et al., 2012, Harrison et al., 2012, Vannucchi et al., 2015b).
In assessing ruxolitinib's position in the grand scheme of MPN treatment, two critical appraisals of the data from both the COMFORT trials suggested that the evidence to support ruxolitinib for the treatment of MF was limited, for various reasons, including trial design bias, limited sample size, comparator choice, and outcome indirectness (Barosi et al., 2015, Marti-Carvajal et al., 2015). Alternatively, an argument could be made that the data to support ruxolitinib's role in the treatment of MPNs are derived from randomized controlled trials with a relatively large sample size for an orphan disease, particularly one in which there is a paucity of other effective therapies.
To further define ruxolitinib's role, cost effectiveness studies have been conducted. The National Institute for Health and Care Excellence (NICE) obtained clinical and cost-effectiveness data from the drug manufacturer (derived from COMFORT-1 and COMFORT-2), and with this data, an independent study group concluded that there was significant uncertainty of the manufacturer's cost-effectiveness model due to its limitations. Despite conceding that ruxolitinib was clinically effective, NICE decided that ruxolitinib was not a cost effective use of National Health Service resources for treatment MF-related splenomegaly or disease symptoms in adults (Wade et al., 2013). A Finnish study assessed the cost effectiveness of ruxolitinib for the treatment of MF by creating a survival-based decision model based on data from COMFORT-2 and found that ruxolitinib produced 2.43 incremental quality-adjusted life years when compared to best available therapy, concluding that these gains came at reasonable costs, given the improvements in overall survival (Hahl et al., 2015).
3. Other JAK Inhibitors
Beyond ruxolitinib, there are other JAK inhibitors under clinical investigation. The most advanced in development include momelotinib and pacritinib. As with ruxolitinib, momelotinib is a dual JAK1/2 inhibitor that has been shown to decrease splenomegaly and MPN-related symptoms in patients with intermediate or high-risk MF (Pardanani et al., 2013). Based on the International Working Group (IWG) criteria, 59% of evaluable patients in that phase I–II study experienced a response to anemia, and 70% of patients who had received red cell transfusions in the month prior to study entry achieved a minimum 12-week period of transfusion-independence. Grade 3/4 adverse reactions included thrombocytopenia in nearly third of the patients, while treatment-related grade 1 sensory peripheral neuropathy was seen in about a fifth of the patients. Currently ongoing are randomized studies of ruxolitinib versus momelotinib in MF patients (NCT01969838) and momelotinib versus best available therapy among MF patients who have already been treated with ruxolitinib (NCT02101268).
The other advanced phase JAK inhibitor, pacritinib, is a dual JAK2/FLT3 kinase inhibitor that has been shown to reduce splenomegaly and MF-related symptoms (Komrokji et al., 2015). A unique aspect of this inhibitor is the potential lack of significant anemia and thrombocytopenia noted with other JAK inhibitors. In a phase 2 study, in which 35 intermediate/high-risk MF patients were enrolled, 40% had hemoglobin < 10 g/dL and 43% had platelets < 100,000 × 109/L at entry (Komrokji et al., 2015). The percentage change in hemoglobin measurements at each study visit relative to the baseline visit stayed within a median of 6%. Platelet count measurements decreased by a median of 12% at week 12 and 17.6% at week 24, but these levels remained stable through week 60. Grade 1/2 diarrhea and nausea were the most common adverse events (AEs). Two ongoing phases 3 trials are comparing pacritinib to best available therapy (NCT01773187 and NCT02055781).
4. Novel Treatment Strategies: Beyond JAK Inhibitor Monotherapy
Given the limitations of JAK inhibition as monotherapy, such as its inability to lead to CR in most patients and the prompt return of symptoms upon treatment discontinuation, there is a significant effort to develop new classes of therapeutic agents for MPNs, many of which are being studied as combination therapy with ruxolitinib. Although promising, there are a number of potential challenges with developing drug combinations for MPN treatment, taking into account the burgeoning number of putative drug targets and experimental agents in development, the inability to reliably predict synergist activity in the preclinical setting, relatively low disease incidence, and the increased complexity of assessing disease response and clinical benefit, as compared to that of many other diseases.
5. Interferon
Interferon (IFN) alpha, a cytokine with antiviral, immunomodulatory, and growth inhibitory properties, has been of interest for the treatment of MPNs for decades (Kiladjian et al., 2008a, Kiladjian et al., 2008b). Standard IFN alpha has been associated with hematologic response rates close to 80% for PV and ET, but its use is limited by toxicities, such as cytopenias, flu-like symptoms, and fatigue, leading to treatment discontinuation in up to 20% of patients. In a multicenter phase 2 study of pegylated (peg)-IFN alpha-2a involving 40 PV patients, there was a 95% complete hematologic response rate among the evaluable patients at 12 months and a 90% molecular response, as assessed by JAK2 V617F allele percentage, which declined from 45% at baseline to 3% after 36 months (Kiladjian et al., 2008a, Kiladjian et al., 2008b). In another phase 2 study of peg-IFN, complete hematologic responses were achieved in 76% and 77% of patients with PV and ET, respectively, along with complete molecular response in 18% and 17% (Quintás-Cardama et al., 2013). However, twenty percent of the patients in this study discontinued the treatment because of drug-related toxicity. In addition to reductions in JAK2 V617F allele burden, CALR mutant molecular responses have also been noted among ET patients treated with peg-IFN (Verger et al., 2015). The next-generation, mono-pegylated IFN alpha-2b isoform, ropeginterferon alpha-2b, administered every 2 weeks, was assessed in a phase 1/2 study involving 51 PV patients, yielding an overall response rate of 90% and a complete response rate of 47% (Gisslinger et al., 2015). The complete and partial molecular response rates were 47% and 43%, respectively. Overall, interferon treatment has major efficacy in the treatment of PV and ET, while its efficacy in MF is more limited and its role in MF management not well-defined (Ianotto et al., 2013).
6. Epigenetic Therapies
As mentioned, a number of recurrent somatic mutations observed in MPN are involved with epigenetic processes and include the following: TET2, involved with methylcytosine residue hydroxylation (Delhommeau et al., 2009); DNMT3A, a cytosine methyltransferase (Abdel-Wahab et al., 2011b); IDH1/2, oxidoreductases leading to 2-hydroxyglutarate production that inhibits alpha-ketoglutarate-dependent enzymes such as TET2 (Tefferi et al., 2012); ASXL1, involved with HOX gene regulation via Polycomb repressive complex 2 (PRC2)-mediated histone methylation (Abdel-Wahab et al., 2012); and EZH2, a histone methyltransferase component of PRC2 (Abdel-Wahab et al., 2011a). Of note, JAK2 functions as an epigenetic modifier by affecting histone posttranslational modifications. Genome-wide methylation studies have revealed hyper- and hypomethylation in promoter regions and in non-CpG island loci among MPN samples, as compared with healthy controls, with differences also noted between PMF and PV/ET samples (Nischal et al., 2013). Particular methylomic signatures were associated with the presence of ASXL1 and TET2 mutations. Also supporting the role of epigenetic aberration in the pathogenesis of MPNs, histone deacetylase (HDAC) activity has been observed to be elevated in PMF patients as compared with other MPN patients and healthy volunteers, with HDAC levels correlating to degree of splenomegaly (Wang et al., 2008). Global gene expression profiling of blood from patients with MPNs has revealed abnormalities in the expression of various HDAC genes (Skov et al., 2012).
6.1. HDAC Inhibitors
Given the abundance of evidence that epigenetic deregulation is involved in MPN pathogenesis, targeting epigenetic processes is of great therapeutic interest. As a class, the HDAC inhibitors (HDACis), four of which are currently FDA-approved for use in T-cell lymphomas or multiple myeloma, along with the DNA methyltransferase inhibitors, have led the way in epigenetic therapy for the treatment of malignancies. Histone modification patterns, regulated by histone acetyl transferases and HDACs, guide the recruitment of various transcription factors to maintain and perform normal cellular functions (Mascarenhas et al., 2011). Dysregulation of this epigenetic process can result in suppression of transcription of tumor suppressor and cell differentiation genes, contributing to MPN pathogenesis (Fig. 2). There have been numerous preclinical and clinical studies in recent years that have provided proof of principle that HDAC inhibition confers some degree of anti-neoplastic activity among MPNs. The HDAC inhibitor givinostat was found to induce apoptosis in JAK2 V617F MPN cells to a greater degree than in JAK2 wild type cell lines, and global gene expression analysis revealed that it modulated expression of multiple genes that are implicated in cell cycle regulation and hematopoiesis (Amaru Calzada et al., 2012). Among MPN cell lines and CD34 + cells from MPN patients, givinostat inhibited proliferation and erythroid differentiation and increased histone H3 acetylation at the promoter of NFE2, a gene involved with hematopoiesis. Furthermore, it independently inhibited JAK–STAT signaling. In combination with hydroxyurea, it synergistically potentiated the induction of pro-apoptotic effects in the JAK2 V617F MPN cell lines (Amaru Calzada et al., 2013).
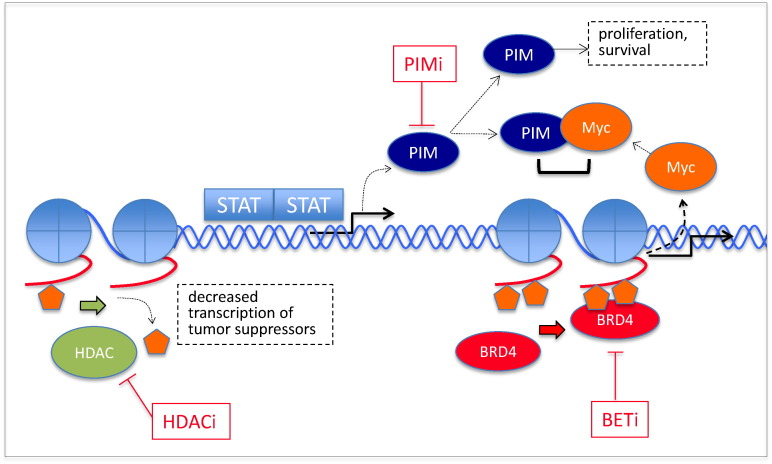
Fig. 2.
Examples of other aberrantly regulated molecular signaling pathways and targets in MPNs. HDAC-mediated deacetylation of the lysine residues of histone tails lead to chromatin condensation and transcriptional silencing of tumor suppressor genes (Wang et al., 2008). PIM kinase expression, induced by JAK-STAT signaling, is involved in a number of prosurvival functions, one of which is phosphorylation and stabilization of Myc. The BET family of BRD proteins includes BRD4, which has a pocket for acetylated lysine residues of the histone tail. BET, bromodomain and extraterminal family of bromodomain-containing proteins; BRD4, bromodomain-containing protein 4; HDAC, histone deacetylase; PIM; proviral integration of Moloney virus; STAT, signal transducer and activator of transcription, -i, inhibitor.
Two other HDAC inhibitors, trichostatin A and vorinostat, were able to restore expression of miR-375, which is a negative regulator of JAK–STAT signaling (Yin et al., 2015). Forced expression of miR-375 has been previously shown to inhibit constitutive and inducible JAK2/STAT signaling, suppresses cell proliferation, and decreases colony formation in hematopoietic progenitors from MPN patients. In a phase 2 study in which givinostat was administered to JAK2 V617F-mutated PV, ET, and MF patients (n = 29) starting at 50 mg orally twice daily and given for a median of 20 weeks, 3 major responses were observed among 16 MF patients (Rambaldi et al., 2010). Among 13 PV/ET patients, 1 complete and 6 partial responses were noted, while pruritus and splenomegaly reduction was observed in 75% of PV/ET and 38% of MF patients. Dose reduction and/or temporary interruption was required in 10 and 15 of the patients, respectively. In another phase 2 study, using two different doses of givinostat in combination with hydroxycarbamide (HC) among PV patients unresponsive to the maximum tolerated dose (MTD) of HC, complete and partial responses were observed in 55% and 50% of patients receiving 50 and 100 mg of givinostat, respectively (Finazzi et al., 2013). Pruritus reduction was observed in most of the patients. Among 22 intermediate/high-risk MF patients treated with the HDAC1 inhibitor pracinostat in a phase 2 study, 36% experienced clinical benefit, but 91%, 13%, and 21% experienced fatigue, grade 3/4 neutropenia, and grade 3/4 thrombocytopenia, respectively. Almost all of the patients discontinued therapy, primarily due to lack of efficacy (Quintás-Cardama et al., 2012). A phase 2 study involving 63 ET/PV patients demonstrated a 35% response rate along with pruritus and splenomegaly reduction and a reduction in JAK2 V617F allelic reduction, but more than half of the patients discontinued treatment mainly due to significant toxicity, including diarrhea, fatigue, and renal impairment (Andersen et al., 2013). Similarly, in a phase 2 study of pan-deacetylase inhibitor panobinostat in MF patients, some evidence of efficacy was noted, but toxicity led to significant treatment discontinuation (Deangelo et al., 2013). Given the seemingly low therapeutic index of HDACis for the treatment of MPN, studying HDACis at lower doses, in combination with other agents may be warranted. As supported by preclinical studies (Wang et al., 2009, Evrot et al., 2013), two clinical trials of panobinostat in combination with ruxolitinib for the treatment of MF are ongoing (NCT01693601 and NCT01433445).
6.2. Hypomethylating Agents
There is a potential role for the other major class of epigenetic modifying agents, DNA methyltransferase inhibitors, also known as hypomethylating agents (HMA), in treating MPNs, as evidenced by results of preclinical and clinical studies. Treatment of PMF CD34 + and normal CD34 + cells with the HMA decitabine, followed by HDAC inhibitor suberoylanilide hydroxamic acid or trichostatin A resulted in apoptosis in the former but not in the latter (Wang et al., 2010). With transplantation of HMA/HDAC inhibitor-treated JAK2 V617F + PMF CD34 + cells into non-obese diabetic/severe combined immunodeficient mice, the percentage of JAK2 V617F + cells progressively declined from 2 to 6 months, in comparison with that of mice transplanted with cells treated with cytokines (Wang et al., 2010). A retrospective study of 45 patients with MPN transformed to AML, MPN in accelerated phase, or high-risk PMF treated with decitabine revealed response rates that ranged from 29% to 82%, response durations of 6.5 to 9 months, and an improvement in overall survival among responders as compared with non-responders (Badar et al., 2015). There are clinical trials evaluating the activity of decitabine for patients with MF and high-risk MPNs, as a single agent and in combination with JAK inhibitors (NCT00095784, NCT02564536, and NCT02076191). Limited clinical activity of 5-azacitidine was observed in a trial involving 34 MF patients, with a 24% response rate after a median of 5 months of treatment (Quintás-Cardama et al., 2008). An assay for global DNA methylation showed a decrease from 53% methylation pre-therapy to 44% on day 14 and a return to 50% at the end of the first 28-day cycle. Trials of azacitidine in combination with ruxolitinib and with a hedgehog inhibitor are underway (NCT01787487 and NCT02129101).
6.3. Bromodomain Inhibition
Bromodomains are a highly conserved protein motif able to recognize acetylated lysine residues of histone and nonhistone proteins and serve as epigenetic readers and regulators that participate in DNA replication, chromatin remodeling, DNA damage, and transcriptional regulation (Fig. 2) (Dawson et al., 2012). They exist in a tandem structure at the N-terminal of more than 40 different human proteins and belong to one of nine major families, one of which is the bromodomain and extraterminal (BET) family, comprising bromodomain-containing proteins 2, 3, 4, and T. Bromodomain 4, for example, is involved with transcriptional initiation and elongation by associating with mediator complex and with positive transcription elongation factor b, respectively (Dawson et al., 2012). Abnormalities involving BET proteins may contribute to the pathogenesis of a number of malignancies, including multiple myeloma, Burkitt's lymphoma, AML with MLL translocations, NUT midline carcinomas, and MPNs (Dawson et al., 2012, Wyspianska et al., 2014). These observations have prompted ongoing efforts to develop BET inhibitors, which inhibit the ability of BET proteins to interact with acetylated lysine residues on histones. The BET inhibitor I-BET151 was shown to inhibit growth and, along with a JAK2 inhibitor, induce apoptosis among JAK2 inhibitor-resistant human erythroleukemic (HEL) cell lines via downregulation of LMO2, an oncogenic regulator that is dependent upon JAK2 kinase activity in HEL cells (Wyspianska et al., 2014). I-BET151 inhibited erythroid colony formation among JAK2 mutant but not wild-type JAK2 associated erythroid colonies taken from PV patients. Also of particular interest is BET's role in regulating MYC and that inhibition of BET bromodomain–promoter interactions results in a reduction of MYC transcript and protein levels resulting in G1 arrest and extensive apoptosis in various leukemia and lymphoma cell lines (Chan et al., 2015). Currently, two phase 1 trials assessing BET inhibitors are enrolling patients with myeloproliferative neoplasms (NCT02158858, NCT02431260).
7. PI3K/Akt/mTOR Pathway Inhibition
A number of agents that target various nodes of the phosphoinositide-3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway, including PI3K and mTOR inhibitors, are currently in development for the treatment of MPNs. This pathway is a crucial regulator of cell growth, survival, and metabolism conditions (Saleiro and Platanias, 2014, Mohindra et al., 2014). It mediates ribosomal translation of mRNA, via downstream effectors 4EBP1 and S6K, into proteins required for cell growth, cycle progression, and metabolism. The overactivation of this pathway, which can be result from constitutive activity of upstream JAK2 (Fig. 1), has been observed in MPNs, and its various roles in the pathogenesis of MPNs has been reviewed in detail elsewhere (Bartalucci et al., 2013a, Pandey and Kapur, 2015, Mclornan and Harrison, 2013). In brief, the PI3K/Akt/mTOR pathway may mediate disease progression as well as cellular drug resistance. The rapalog mTOR inhibitor everolimus, currently approved for the treatment of various advanced solid tumors, was shown to have clinical activity in MF in a phase 1/2 clinical trial (Guglielmelli et al., 2011). Of 30 evaluable high/intermediate-risk MF patients who had received prior therapy, splenomegaly reduction of > 50% and > 30% occurred in 20% and 44%, respectively, and 69% and 80% experienced complete resolution of constitutional symptoms, respectively. The responses were not associated with a reduced JAK2 V617F allele burden or with cytokine levels. Grade 1/2 stomatitis was the most common toxicity, occurring in the majority of subjects. Of great clinical interest, based on the results of multiple preclinical studies, is the combination of PI3K/Akt/mTOR inhibitors with JAK inhibitors (Choong et al., 2013, Bartalucci et al., 2013b, Szymańska et al., 2015). In a cellular screening assay, synergistic anti-proliferative activity was observed in JAK2-mutated MPN mouse Ba/F3 cells when subjected to combinations of various pan-class I PI3K inhibitors and JAK inhibitors (Choong et al., 2013). Moreover, in murine MPN models, the PI3K/JAK inhibitor doublets synergistically improved survival and delayed onset of splenomegaly (Choong et al., 2013). The use of BEZ235, a dual PI3K/mTOR inhibitor, alone or in combination with ruxolitinib, in in vitro and in vivo preclinical models of JAK2 V617F-mutated MPN, also led to synergism (Bartalucci et al., 2013b). Other classes of agents of interest include the catalytic mTORC1/2 inhibitors and AKT inhibitors. Currently, a number of clinical trials involving the use of PI3K/AKT/mTOR inhibitors alone or in combination are in progress (NCT01730248 and NCT02493530).
8. Telomerase Inhibition
Telomeres, which are nucleoproteins located at the ends of chromosomes, maintain genome stability by protecting the ends of chromosomes from degradation (Ouellette et al., 2011). Many neoplastic cells are able to obviate this replicative senescence by activating telomerase, a reverse transcriptase that maintains telomere length by adding nucleotides to the telomere during cell division. Telomerase inhibition has been demonstrated to induce this process among neoplastic cells (Ouellette et al., 2011). Imetelstat, an intravenously administered, 13-mer lipid-conjugated antisense oligonucleotide that targets the RNA template component of telomerase, conferred an 89% overall response rate in a phase 2 study of 18 ET patients for whom first-line therapy had failed (Baerlocher et al., 2015). Ten of the patients received therapy for a median of 17 months, and among the 8 patients with JAK2 V617F mutations, molecular responses were noted in 7. In a pilot study involving 33 MF patients, half of whom had received prior JAK inhibitor therapy, 21% attained a complete (n = 4) or partial remission (n = 7; CR, PR) of a median duration of 18 and 10 months, respectively (Tefferi et al., 2015). Response rates were higher among those with wild-type ASXL1, as compared to mutated ASXL1 (32% versus 0%, P = 0.07), and among those with a mutation in SF3B1 or U2AF1, as compared with their wild-type counterparts (38% versus 4%, P = 0.04). Among the CR patients, bone marrow fibrosis reversal and molecular responses were noted in 100% and 75%, respectively. Baseline telomere length was not predictive of response. In both of these studies, grade 3/4 cytopenias occurred in approximately 10–30% of patients, and many of the patients had low-grade liver function test abnormalities. In addition to antisense oligonucleotide strategies, the use of telomerase-targeting small molecules and immunotherapy represent potential strategies (Mocellin et al., 2013).
9. Hedgehog Pathway Inhibition
Hedgehog (Hh) is a highly conserved signaling pathway, components of which include cell surface receptor Patched-1 (PTCH1), transmembrane receptor Smoothened (SMO), and three glioma associated family transcription factors (GLI) (Khan et al., 2015). It is involved with multiple essential processes, including stem cell maintenance, cell cycle regulation, apoptosis, and cellular proliferation, and interacts with many other pro-survival pathways such as RAS–ERK–MAPK and PI3K/Akt/mTOR. There is evidence of Hh pathway dysregulation in a number of malignancies including MPNs, in which a 20–100 fold increase in expression of Hh target genes has been noted in granulocytes isolated from MPN patients and among preclinical models of PMF (Bhagwat et al., 2013). In a murine bone marrow transplant model of ET/MF, the use of the selective SMO inhibitor sonidegib (LDE-225) in combination with ruxolitinib significantly reduced blood counts, mutant allele burden, and bone marrow fibrosis as compared to that observed with ruxolitinib as monotherapy (Bhagwat et al., 2013). Among 23 intermediate/high-risk MF patients treated with the combination of these same two agents in the phase 1b portion of a phase 1b/2 study, 65% achieved a ≥ 50% reduction in palpable spleen length from baseline (Gupta et al., 2014). In a phase 1a study of SMO inhibitor PF-04449913 for patients with hematological malignancies (n = 32), five of the six MF patients attained stable disease, and the sixth stayed on study for over a year while achieving durable clinical improvement with a > 50% reduction in extramedullary disease (Jamieson et al., 2011). For both of these studies, the results from the phase 2 portions are awaited (NCT01787552 and NCT02226172). In a phase 2 study, oral SMO inhibitor IPI-926 was administered to 14 MF patients, all of whom discontinued treatment by 7.5 months primarily due to lack of effectiveness (Sasaki et al., 2015). There was no significant reduction in symptoms and splenomegaly and insignificant reductions in JAK2 V617F allele burden and marrow fibrosis. AEs consisted mainly of low-grade gastrointestinal and hepatic toxicities. The lackluster results did not support the continuation of the development of this particular agent. Another SMO inhibitor, erismodegib, is being assessed in combination with azacitidine in a phase 1 study for various myeloid malignancies, including MPNs (NCT02129101).
10. PIM Kinase Inhibition
Proviral Integration of Moloney virus (PIM) is a family of pro-survival serine/threonine kinases, comprising PIM1, PIM2, and PIM3, involved with regulating the cell cycle, cellular metabolism, proliferation, survival, and cross-talking with Akt kinase (Warfel and Kraft, 2015). PIM1 expression is induced by JAK2/STAT5 signaling (Fig. 2), suggesting some involvement of PIM activity in MPN pathogenesis (Lambert et al., 2014). PIM kinase inhibitors SGI-1776 and AZD1208 inhibited growth and viability of JAK2 V617F-dependent MPN model and MPN patient cells in a dose-dependent manner, and activity was enhanced when used in combination with ruxolitinib. Both of these PIM kinase inhibitors, as single agents, inhibited erythropoietin-independent erythroid colony formation of primary cells from MPN patients but not from normal controls. AZD1208 administered in combination with ruxolitinib synergistically inhibited colony formation of primary cells from MPN patients (Lambert et al., 2014). In another set of studies, PIM inhibitor LGH447 in combination with ruxolitinib led to significant modulation of PIM1/2 and pSTAT3/5, respectively, among JAK2 V617F-mutated MPN cell lines, and both agents inhibited pERK and pS6 in one of the cell lines (Saci et al., 2013). This drug combination also resulted in a greater reduction of overall disease burden and spleen weight than either agent alone in a mouse MPN model. The observation of the suppression of MYC protein levels among MPN cell lines after treatment with various PIM kinase inhibitors provides further support of PIM's role in MPN pathogenesis (Huang et al., 2014). Using a pooled small hairpin RNA library screen among an MPN cell line, MYC was identified as a top therapeutic target in the setting of JAK2 inhibition. Considering that PIM kinases directly phosphorylate MYC, resulting in stabilization of MYC, various pan-PIM inhibitors were tested, in combination with JAK2 inhibitor SAR302503, against MPN cell lines, demonstrating synergistic MPN cell growth inhibition, apoptosis, and eradication of JAK2 inhibitor-resistant MPN clones. There is currently a phase 1/2 trial evaluating pan-PIM inhibitor INCB053914 among patients with MF (NCT02587598) and a phase 1 trial evaluating another pan-PIM inhibitor PIM447 (formerly LGH447), ruxolitinib, and a CDK 4/6 inhibitor in doublet and triplet combinations for patients with MF (NCT02370706).
11. Glutaminase Inhibition
Among JAK2 V617F-mutant cells in vitro, as compared with their wild-type counterparts, there has been the observation of increased oxygen consumption, increased extracellular acidification, increased glutamine metabolism, and upregulated glutaminase (Zhan et al., 2015). Glutaminase expression was increased in JAK2 V617F-mutant peripheral blood CD34 + cells as compared to JAK2 wild-type progenitor cells from the same patients MPN patients, and its expression increased further with disease progression. The glutaminase inhibitor, BPTES, along with ruxolitinib, using various laboratory assays, led to an increased anti-proliferative effect among these JAK2 V617F-mutant cell lines and MPN patient peripheral blood CD34 + cells from MPN patients than that of ruxolitinib alone (Zhan et al., 2015). These observations, along with the knowledge that neoplastic cells generally rely upon aerobic glycolysis more than normal cells do (i.e., Warburg effect), provide the rationale for the clinical investigation of the role of glutaminase inhibitor therapy, with or without JAK inhibition, among MPN patients requiring therapy.
12. Other Therapeutic Strategies
There are multiple other classes of agents, some of which are in clinical development, with potential therapeutic benefit for MPN patients. These include agents that target heat shock protein-90, isocitrate dehydrogenase, and mitogen/extracellular signal-regulated kinase (MEK) (Weigert et al., 2012, Tefferi et al., 2012, Kong et al., 2014). PRM-151, a recombinant human pentraxin-2 with anti-fibrosing properties, is currently being evaluated in a phase 2 clinical trial for patients with MF (NCT01981850). Aurora A kinase inhibitors, which have been shown to induce megakaryocytic polyploidization, induce growth arrest and apoptosis of a megakaryoblastic cell line, and decrease disease burden in a murine model, are also of great clinical interest (Verstovsek et al., 2014, Goldenson et al., 2013). A multicenter pilot study involving the Aurora A kinase inhibitor alisertib for the treatment of MF patients has recently been launched (NCT02530619).
13. Conclusion
The need for optimal treatment options for many patients with MPN persists, despite the advent of ruxolitinib. In general, JAK inhibitor monotherapy has considerably improved patient well-being, ameliorating MPN symptoms and splenomegaly, but disease remission is not an expectation. Given the ability to relieve symptoms, JAK inhibitor therapy may become an essential cornerstone of combination therapy with other novel agents, which may address other aspects of disease pathogenesis.
14. Outstanding Questions
Efforts continue to identify relevant, targetable pathogenetic pathways in MPNs, with the goal of improving efficacy and decreasing off-target toxicity of therapy. Further therapeutic efforts may be facilitated by addressing the following questions or issues: a better understanding of the mechanisms of action of novel agents; accurate identification of predictive biomarkers of response to therapy; the optimal time to initiate a particular therapy for any given individual, given the clinical heterogeneity of MPNs and that many patients will fare well with currently established general treatment options, such as phlebotomy, aspirin, and use of cytoreductive agents; how clinical trials may be judiciously designed and conducted, given the relatively low incidence of MPNs along with the limitless potential drug combinations; with new, effective therapies, how allogeneic hematopoietic stem cell transplantation should be incorporated into the treatment paradigm.
15. Search Strategy and Selection Criteria
Data for this Review were identified by searches of PubMed, the abstract libraries of the American Society of Hematology and the American Society of Clinical Oncology, and www.clinicaltrials.gov, using the search terms “myeloproliferative neoplasm” “novel” “targeted therapy” “pathobiology” and the names of the classes of agents discussed in the Review.
Author Contribution
All authors contributed to literature search and writing.
Conflicts of Interest Disclosures
BLS serves as a consultant for Incyte, Corp. The other authors have no relevant disclosures to report.
Funding/Acknowledgements
The research work of LCP is supported by NIH grants CA155566 and CA77816 and a Merit Review grant from the Department of Veterans affairs.
References
- Abdel-Wahab O., Pardanani A., Patel J. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia. 2011;25(7):1200–1202. doi: 10.1038/leu.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Wahab O., Pardanani A., Rampal R. DNMT3A mutational analysis in primary myelofibrosis, chronic myelomonocytic leukemia and advanced phases of myeloproliferative neoplasms. Leukemia. 2011;25(7):1219–1220. doi: 10.1038/leu.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Wahab O. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22(2):180–193. doi: 10.1016/j.ccr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaru Calzada A. The HDAC inhibitor givinostat modulates the hematopoietic transcription factors NFE2 and C-MYB in JAK2 V617F Myeloproliferative Neoplasm Cells. Exp. Hematol. 2012;40(8):634–645. doi: 10.1016/j.exphem.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Amaru Calzada A. Givinostat and hydroxyurea synergize in vitro to induce apoptosis of cells from JAK2V617F myeloproliferative neoplasm patients. Exp. Hematol. 2013;41(3):253–260. doi: 10.1016/j.exphem.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Andersen C.L. A phase II study of vorinostat (MK-0683) in patients with polycythaemia vera and essential thrombocythaemia. Br. J. Haematol. 2013;162(4):498–508. doi: 10.1111/bjh.12416. [DOI] [PubMed] [Google Scholar]
- Badar T. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk. Res. 2015;39(9):950–956. doi: 10.1016/j.leukres.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerlocher G.M. Telomerase inhibitor imetelstat in patients with essential thrombocythemia. N. Engl. J. Med. 2015;373(10):920–928. doi: 10.1056/NEJMoa1503479. [DOI] [PubMed] [Google Scholar]
- Ballen K.K. Outcome of transplantation for myelofibrosis. Biol. Blood Marrow Transplant. 2010;16(3):358–367. doi: 10.1016/j.bbmt.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui T. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: a international study. J. Clin. Oncol. 2011;29(23):3179–3184. doi: 10.1200/JCO.2010.34.5298. [DOI] [PubMed] [Google Scholar]
- Barosi G., Rosti V., Gale R.P. Critical appraisal of the role of ruxolitinib in myeloproliferative neoplasm-associated myelofibrosis. OncoTargets Ther. 2015:1091–1102. doi: 10.2147/OTT.S31916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartalucci N., Guglielmelli P., Vannucchi A.M. Rationale for targeting the PI3K/Akt/mTOR pathway in myeloproliferative neoplasms. Clin. Lymphoma Myeloma Leuk. 2013;13(Suppl. 2):S307–S309. doi: 10.1016/j.clml.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Bartalucci N., Tozzi L. Co-targeting the PI3K/mTOR and JAK2 signalling pathways produces synergistic activity against myeloproliferative neoplasms. J. Cell. Mol. Med. 2013;17(11):1385–1396. doi: 10.1111/jcmm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat N. Improved efficacy of combination Of JAK2 and hedgehog inhibitors in myelofibrosis N. Bhagwat, ed. Blood. 2013;122(21):666. [Google Scholar]
- Cervantes F. Improving survival trends in primary myelofibrosis: an international study. J. Clin. Oncol. 2012;30(24):2981–2987. doi: 10.1200/JCO.2012.42.0240. [DOI] [PubMed] [Google Scholar]
- Cervantes F. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;120(25):4047–4054. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
- Chan C.H. BET bromodomain inhibition suppresses transcriptional responses to cytokine-Jak–STAT signaling in a gene-specific manner in human monocytes. Eur. J. Immunol. 2015;45:287–297. doi: 10.1002/eji.201444862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong M.L. Combination treatment for myeloproliferative neoplasms using JAK and pan-class I PI3K inhibitors. J. Cell. Mol. Med. 2013;17(11):1397–1409. doi: 10.1111/jcmm.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M.a., Kouzarides T., Huntly B.J.P. Targeting epigenetic readers in cancer. N. Engl. J. Med. 2012;367(7):647–657. doi: 10.1056/NEJMra1112635. [DOI] [PubMed] [Google Scholar]
- Deangelo D.J. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br. J. Haematol. 2013;162(3):326–335. doi: 10.1111/bjh.12384. [DOI] [PubMed] [Google Scholar]
- Deininger M. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood. 2015 doi: 10.1182/blood-2015-03-635235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhommeau F. Mutation in TET2 in Myeloid Cancers. N. Engl. J. Med. 2009;360(22):2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- Emanuel R.M. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J. Clin. Oncol. 2012;30(33):4098–4103. doi: 10.1200/JCO.2012.42.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrot E. JAK1/2 and pan-deacetylase inhibitor combination therapy yields improved efficacy in preclinical mouse models of JAK2V617F-driven disease. Clin. Cancer Res. 2013;19(22):6230–6241. doi: 10.1158/1078-0432.CCR-13-0905. [DOI] [PubMed] [Google Scholar]
- Finazzi G. A phase II study of Givinostat in combination with hydroxycarbamide in patients with polycythaemia vera unresponsive to hydroxycarbamide monotherapy. Br. J. Haematol. 2013;161(5):688–694. doi: 10.1111/bjh.12332. [DOI] [PubMed] [Google Scholar]
- Gisslinger H. Ropeginterferon alfa-2b, a novel IFN a-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015;126(15):1762–1770. doi: 10.1182/blood-2015-04-637280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenson B. Aurora a kinase is a novel therapeutic target in the myeloproliferative neoplasms Q. J. Wen, ed. Blood. 2013;122(21):109. [Google Scholar]
- Gowin K. The new landscape of therapy for myelofibrosis. Curr. Hematol. Malig. Rep. 2013;8(4):325–332. doi: 10.1007/s11899-013-0178-x. [DOI] [PubMed] [Google Scholar]
- Guglielmelli P. Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood. 2011;118(8):2069–2076. doi: 10.1182/blood-2011-01-330563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V. Phase 1b dose-escalation study of sonidegib (LDE225) in combination with ruxolitinib (INC424) in patients with myelofibrosis. Blood. 2014;124(21):712. [Google Scholar]
- Hahl J. Cost-effectiveness of ruxolitinib for the treatment of myelofibrosis in Finland. Economic evaluation based on finnish auria biobank data on health care resource utilization. Value Health. 2015;18(7):A669. [Google Scholar]
- Harrison C. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 2012;366(9):609–619. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- Heine A., Brossart P., Wolf D. Ruxolitinib is a potent immunosuppressive compound: is it time for anti-infective prophylaxis? Blood. 2013;122(23):3843–3844. doi: 10.1182/blood-2013-10-531103. [DOI] [PubMed] [Google Scholar]
- Heine A., Held S.A.E. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122(7):1192–1202. doi: 10.1182/blood-2013-03-484642. [DOI] [PubMed] [Google Scholar]
- Huang S.A. Combination of PIM and JAK2 inhibitors synergistically suppresses cell proliferation and overcomes drug resistance of myeloproliferative neoplasms. Oncotarget. 2014;5(10) doi: 10.18632/oncotarget.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianotto J.-C. Efficacy and safety of pegylated-interferon α-2a in myelofibrosis: a study by the FIM and GEM French cooperative groups. Br. J. Haematol. 2013;162(6):783–791. doi: 10.1111/bjh.12459. [DOI] [PubMed] [Google Scholar]
- Jamieson C. Phase 1 dose-escalation study of PF-04449913, an oral hedgehog (Hh) inhibitor, in patients with select hematologic malignancies. ASH Ann. Meet. Abstr. 2011;118(21):424. [Google Scholar]
- Khan A.A., Harrison C.N., McLornan D.P. Targeting of the Hedgehog pathway in myeloid malignancies: still a worthy chase? Br. J. Haematol. 2015;170(3):323–335. doi: 10.1111/bjh.13426. [DOI] [PubMed] [Google Scholar]
- Kiladjian J.-J., Chomienne C., Fenaux P. Interferon-alpha therapy in bcr-abl-negative myeloproliferative neoplasms. Leuk. Off. J. Leuk. Soc. Am. Leuk. Res. Fund UK. 2008;22(11):1990–1998. doi: 10.1038/leu.2008.280. [DOI] [PubMed] [Google Scholar]
- Kiladjian J.-J. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–3072. doi: 10.1182/blood-2008-03-143537. [DOI] [PubMed] [Google Scholar]
- Kiladjian J.J. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J. Clin. Oncol. 2011;29(29):3907–3913. doi: 10.1200/JCO.2011.36.0792. [DOI] [PubMed] [Google Scholar]
- Klampfl T. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 2013;369(25):2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- Komrokji R.S. Results of a phase 2 study of pacritinib (SB1518 ), a JAK2/JAK2 (V617F) inhibitor, in patients with myelo fi brosis. Blood. 2015;125(17):2649–2656. doi: 10.1182/blood-2013-02-484832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G. Combined MEK and JAK inhibition abrogates murine myeloproliferative neoplasm. J. Clin. Investig. 2014;124(6):2762–2773. doi: 10.1172/JCI74182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralovics R. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Kröger N. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis : a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114(26):5264–5270. doi: 10.1182/blood-2009-07-234880. [DOI] [PubMed] [Google Scholar]
- Lambert Q.T., Pradhan A., Reuther G.W. Pims: potential therapeutic targets for myeloproliferative neoplasms. Blood. 2014;124(21):4575. [Google Scholar]
- Lundberg P. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220–2228. doi: 10.1182/blood-2013-11-537167. [DOI] [PubMed] [Google Scholar]
- Marchioli R. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J. Clin. Oncol. 2005;23(10):2224–2232. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Marti-Carvajal A.J., Anand V., Sola I. Janus kinase-1 and Janus kinase-2 inhibitors for treating myelofibrosis. Cochrane Database Syst. Rev. 2015;4(4):CD010298. doi: 10.1002/14651858.CD010298.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas J. Epigenetic abnormalities in myeloproliferative neoplasms: a target for novel therapeutic strategies. Clin. Epigenetics. 2011;2(2):197–212. doi: 10.1007/s13148-011-0050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclornan D., Harrison C. Combination therapies in myeloproliferative neoplasms: Why do we need them and how to identify potential winners? J. Cell. Mol. Med. 2013;17(11):1410–1414. doi: 10.1111/jcmm.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead A.J. Response to ruxolitinib in patients with intermediate-1-, intermediate-2-, and high-risk myelofibrosis: results of the UK ROBUST Trial. Br. J. Haematol. 2015;2 doi: 10.1111/bjh.13379. ((March), p.n/a–n/a) [DOI] [PubMed] [Google Scholar]
- Mehta J. Need for patient reported outcomes (PRO) in adult acute lymphoblastic leukemia (ALL) ASH Ann. Meet. Abstr. 2012;120(21):4704. [Google Scholar]
- Mocellin S., Pooley K.a., Nitti D. Telomerase and the search for the end of cancer. Trends Mol. Med. 2013;19(2):125–133. doi: 10.1016/j.molmed.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Mohindra N.A., Giles F.J., Platanias L.C. Use of mTOR inhibitors in the treatment of malignancies. Expert. Opin. Pharmacother. 2014;15(7):979–990. doi: 10.1517/14656566.2014.899582. [DOI] [PubMed] [Google Scholar]
- Nangalia J., Green T.R. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2014. The evolving genomic landscape of myeloproliferative neoplasms; pp. 287–296. [DOI] [PubMed] [Google Scholar]
- Nangalia J. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013;369(25):2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nischal S. Methylome profiling reveals distinct alterations in phenotypic and mutational subgroups of myeloproliferative neoplasms. Cancer Res. 2013;73(3):1076–1085. doi: 10.1158/0008-5472.CAN-12-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M.M., Wright W.E., Shay J.W. Targeting telomerase-expressing cancer cells. J. Cell. Mol. Med. 2011;15(7):1433–1442. doi: 10.1111/j.1582-4934.2011.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey R., Kapur R. Targeting phosphatidylinositol-3-kinase pathway for the treatment of Philadelphia-negative myeloproliferative neoplasms. Mol. Cancer. 2015;14(1):118. doi: 10.1186/s12943-015-0388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanani A. Safety and efficacy of CYT387, a JAK1 and JAK2 Inhibitor, in myelofibrosis. Leukemia. 2013;27:1322–1327. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikman Y. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):1140–1151. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintás-Cardama A. A phase II study of 5-azacitidine for patients with primary and post-essential thrombocythemia/polycythemia vera myelofibrosis. Leukemia. 2008;22(5):965–970. doi: 10.1038/leu.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintás-Cardama A. Therapy with the histone deacetylase inhibitor pracinostat for patients with myelofibrosis. Leuk. Res. 2012;36(9):1124–1127. doi: 10.1016/j.leukres.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintás-Cardama A. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon α-2a. Blood. 2013;122(6):893–901. doi: 10.1182/blood-2012-07-442012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaldi A. A pilot study of the histone-deacetylase inhibitor givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br. J. Haematol. 2010;150(4):446–455. doi: 10.1111/j.1365-2141.2010.08266.x. [DOI] [PubMed] [Google Scholar]
- Rampal R., Levine R.L. A primer on genomic and epigenomic alterations in the myeloproliferative neoplasms. Best Pract. Res. Clin. Haematol. 2014;27(2):83–93. doi: 10.1016/j.beha.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Rumi E., Pietra D., Ferretti V. Thrombocythemia with substantially different clinical course and outcomes JAK2 or CALR mutation status de fi nes subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123(10):1544–1551. doi: 10.1182/blood-2013-11-539098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumi E., Pietra D., Pascutto C. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood. 2014;124(7):1062–1069. doi: 10.1182/blood-2014-05-578435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saci A. The combination of JAK inhibitor, ruxolitinib, and PIM inhibitor, LGH447, in preclinical models of myeloproliferative neoplasia Z. Alexander Cao, ed. Blood. 2013;122(21):4100. [Google Scholar]
- Saleiro D., Platanias L.C. Intersection of mTOR and STAT signaling in immunity. Trends Immunol. 2014;36(1):21–29. doi: 10.1016/j.it.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K. Phase II evaluation of IPI-926, an oral hedgehog inhibitor, in patients with myelofibrosis. Leuk. Lymphoma. 2015;56(7):2092–2097. doi: 10.3109/10428194.2014.984703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov V. Increased gene expression of histone deacetylases in patients with Philadelphia-negative chronic myeloproliferative neoplasms. Leuk. Lymphoma. 2012;53(1):123–129. doi: 10.3109/10428194.2011.597905. [DOI] [PubMed] [Google Scholar]
- Szymańska J. Pro-apoptotic activity of ruxolitinib alone and in combination with hydroxyurea, busulphan, and PI3K/mTOR inhibitors in JAK2-positive human cell lines. Adv. Clin. Exp. Med. 2015;24(503):195–202. doi: 10.17219/acem/32934. [DOI] [PubMed] [Google Scholar]
- Tefferi A., Pardanani A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin. Proc. 2011;86(12):1188–1191. doi: 10.4065/mcp.2011.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A., Thiele J., Vardiman J.W. The 2008 World Health Organization classification system for myeloproliferative neoplasms: order out of chaos. Cancer. 2009;115(17):3842–3847. doi: 10.1002/cncr.24440. [DOI] [PubMed] [Google Scholar]
- Tefferi A., Verstovsek S. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J. Clin. Oncol. 2009;27(27):4563–4569. doi: 10.1200/JCO.2008.21.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A. IDH mutations in primary myelofibrosis predict leukemic transformation and shortened survival: clinical evidence for leukemogenic collaboration with JAK2V617F. Leukemia. 2012;26(3):475–480. doi: 10.1038/leu.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874–1881. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N. Engl. J. Med. 2015;373(10):908–919. doi: 10.1056/NEJMoa1310523. [DOI] [PubMed] [Google Scholar]
- Vainchenker W. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;118(7):1–3. doi: 10.1182/blood-2011-02-292102. [DOI] [PubMed] [Google Scholar]
- Vannucchi A.M. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27(9):1861–1869. doi: 10.1038/leu.2013.119. [DOI] [PubMed] [Google Scholar]
- Vannucchi A.M., Kantarjian H.M. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase 3 trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015 doi: 10.3324/haematol.2014.119545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucchi A.M., Kiladjian J.-J. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N. Engl. J. Med. 2015;372(5):426–435. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger E. Clinical and molecular response to interferon — a therapy in essential thrombocythemia patients with CALR mutations. Blood. 2015;126(24):2585–2592. doi: 10.1182/blood-2015-07-659060. [DOI] [PubMed] [Google Scholar]
- Verstovsek S. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N. Engl. J. Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S. Phase 2 trial of PRM-151, an antifibrotic agent, in patients with myelofibrosis: Stage 1 results. ASCO Meet. Abstr. 2014;32(15_suppl):7114. [Google Scholar]
- Verstovsek S. Efficacy of ruxolitinib on hepatomegaly in patients with myelofibrosis. Leukemia. 2015;24(November):1–3. doi: 10.1038/leu.2015.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser O. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur. J. Cancer. 2012;48(17):3257–3266. doi: 10.1016/j.ejca.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Wade R. Ruxolitinib for the treatment of myelofibrosis: a NICE single technology appraisal. PharmacoEconomics. 2013;31(10):841–852. doi: 10.1007/s40273-013-0083-0. [DOI] [PubMed] [Google Scholar]
- Wang J.C. Enhanced histone deacetylase enzyme activity in primary myelofibrosis. Leuk. Lymphoma. 2008;49(12):2321–2327. doi: 10.1080/10428190802527699. [DOI] [PubMed] [Google Scholar]
- Wang Y. Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood. 2009;114(24):5024–5033. doi: 10.1182/blood-2009-05-222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Sequential treatment of CD34 + cells from patients with primary myelofibrosis with chromatin-modifying agents eliminate JAK2V617F-positive NOD/SCID marrow repopulating cells. Blood. 2010;116(26):5972–5982. doi: 10.1182/blood-2010-02-269696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel N.A., Kraft A.S. PIM kinase (and Akt) biology and signaling in tumors. Pharmacol. Ther. 2015;151:41–49. doi: 10.1016/j.pharmthera.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathes R., Moule S., Milojkovic D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N. Engl. J. Med. 2013;369(2):197–198. doi: 10.1056/NEJMc1302135. [DOI] [PubMed] [Google Scholar]
- Weigert O. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J. Exp. Med. 2012;209(2):259–273. doi: 10.1084/jem.20111694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B.S. Resolution of Bone marrow fibrosis in a patient receiving JAK1/JAK2 inhibitor treatment with ruxolitinib. Haematologica. 2013;98(12):1872–1876. doi: 10.3324/haematol.2013.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyspianska B. BET protein inhibition shows efficacy against JAK2V617F-driven neoplasms. Leukemia. 2014:88–97. doi: 10.1038/leu.2013.234. (2013 August ) [DOI] [PubMed] [Google Scholar]
- Yin L. Epigenetic deregulated miR-375 contributes to the constitutive activation of JAK2/STAT signaling in myeloproliferative neoplasm. Leuk. Res. 2015;39(4):471–478. doi: 10.1016/j.leukres.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Zhan H. Targeting glutamine metabolism in myeloproliferative neoplasms. Blood Cell Mol. Dis. 2015;55(3):241–247. doi: 10.1016/j.bcmd.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]