Abstract
Protein-based vaccines offer a safer alternative to live-attenuated or inactivated vaccines but have limited immunogenicity. The identification of adjuvants that augment immunogenicity, specifically in a manner that is durable and antigen-specific, is therefore critical for advanced development. In this study, we use the filovirus virus-like particle (VLP) as a model protein-based vaccine in order to evaluate the impact of four candidate vaccine adjuvants on enhancing long term protection from Ebola virus challenge. Adjuvants tested include poly-ICLC (Hiltonol), MPLA, CpG 2395, and alhydrogel. We compared and contrasted antibody responses, neutralizing antibody responses, effector T cell responses, and T follicular helper (Tfh) cell frequencies with each adjuvant's impact on durable protection. We demonstrate that in this system, the most effective adjuvant elicits a Th1-skewed antibody response and strong CD4 T cell responses, including an increase in Tfh frequency. Using immune-deficient animals and adoptive transfer of serum and cells from vaccinated animals into naïve animals, we further demonstrate that serum and CD4 T cells play a critical role in conferring protection within effective vaccination regimens. These studies inform on the requirements of long term immune protection, which can potentially be used to guide screening of clinical-grade adjuvants for vaccine clinical development.
Abbreviations: BME, beta mercaptoethanol; CD, cluster of differentiation; DSCF, Dwass, Steel, Critchlow-Fligner; ELISA, Enzyme linked immunosorbent assay; ELISPOT, enzyme-linked immunospot assay; FACS, fluorescence activated cell sorting; FBS, fetal bovine serum; GP, glycoprotein; IACUC, Institutional Animal Care and Use Committee; IM, intramuscular; IP, intraperitoneal; IQR, interquartile range; LN, lymph node; ma-EBOV, mouse-adapted Ebola virus; MPLA, monophosphoryl lipid A; NAb, neutralizing antibody; Ns, not significant; PBS, phosphate buffered saline; Pfu, plaque forming unit; PRR, pattern recognition receptor; PsVNA, pseudovirion neutralization assay; TLR, Toll-like receptor; USAMRIID, United States Army Medical Research Institute of Infectious Diseases; VLP, virus-like particle
Keywords: Vaccine, Adjuvant, Durable protection, Immune correlates, Ebola virus
Highlights
-
•
Adjuvants can prolong the protection afforded by protein-based vaccines and impact adaptive immune responses
-
•
Enhanced CD4 T cell responses, helper and effector, correlate with duration of protection
-
•
Durable protection from ma-EBOV is associated with Tfh frequency, Th1 antibody titers, and effector CD4 T cells
Protein-based vaccines are extremely safe, but they sometimes require the addition of adjuvants to enhance immunogenicity. In this study, we compared the impact of multiple adjuvants on immunogenicity, focusing on the duration of vaccine-mediated protection in mice. We then looked at how each adjuvant impacted the immune response in order to identify correlates of that long lasting immunity. The most effective adjuvant/vaccine combinations elicited multifunctional CD4 T cell responses and a Th1-skewed antibody response. By transferring antigen-experienced CD4 T cells and serum into naïve animals, we demonstrated that both CD4 T cells and serum were critical for durable vaccine-mediated protection.
1. Introduction
Traditional vaccine development has focused on highly immunogenic live-attenuated or inactivated pathogen platforms. However, safety concerns associated with these platforms, as well as advances in vaccine manufacturing and antigen characterization, have turned interest toward protein-based vaccines. Protein-based vaccines are designed to elicit an immune response against a specific antigen with known protective capabilities. While they are a safer alternative to traditional vaccines, they are also less immunogenic and confer less durable immune responses. Among protein-based vaccine platforms are virus-like particles (VLPs), which are essentially empty viral particles incapable of replicating in the host. VLPs have been heralded as one of the most promising future vaccine platforms, and some examples of VLPs are already in the clinic, including Cervarix, a Human Papilloma Virus (HPV) vaccine (Pitoiset et al., 2015, Einstein et al., 2014a, Einstein et al., 2014b).
Adjuvants are often included in protein-based vaccines in order to regulate antigen dispersal and to enhance immunogenicity. Recent advances in adjuvant discovery have highlighted the potential importance of pattern recognition receptor (PRR) ligands as vaccine adjuvants (Coffman et al., 2010, Steinhagen et al., 2011, Brunner et al., 2010, O'Hagan and Fox, 2015). Traditional alum-based vaccine adjuvants may not work for vaccines requiring a cytotoxic, Th1-skewed immune response, whereas PRR agonists can be used to direct the type of immune response elicited against the vaccine antigen. AS04, an aluminum salt adjuvant that includes the Toll-like receptor (TLR) 4 ligand monophosphoryl lipid A, has been approved in Fendrix and Cervarix, Hepatitis B and HPV vaccines, respectively, and several other PRR agonist-based adjuvants are currently in clinical trials (Surquin et al., 2011, Beran, 2008, Einstein et al., 2014a, Einstein et al., 2014b).
In this study, four adjuvants were tested in combination with the Ebola virus VLP vaccine to determine their impact on durable protection. Alhydrogel is a well-characterized aluminum hydroxide adjuvant, which is currently in several FDA-approved vaccines. Alhydrogel provides a depot effect whereby antigen is released more slowly in vivo, resulting in prolonged antigen exposure, which may or may not contribute to adjuvantcy (Hutchison et al., 2012). Additionally, alhydrogel has been shown to activate the inflammasome, which may contribute to the immunogenicity of alhydrogel-based vaccines (Guven et al., 2013, Gupta, 1998, Hogenesch, 2002, Marrack et al., 2009). PolyICLC is a double-strand RNA stabilized by poly-L-lysine in carboxymethylcellulose (Levy et al., 1975). It signals through TLR3 and potentially MDA5 receptors, eliciting a strong type I IFN response, and it skews the immune response toward a Th1 profile response (Wang et al., 2010, Alexopoulou et al., 2001, Nemes et al., 1969). PolyICLC has been in multiple clinical trials for both therapeutic and vaccine purposes (Martins et al., 2015b). There are three different classes of CpG molecules, which target different cell subsets and receptors and have different recognition in mouse and human cells (Verthelyi et al., 2001, Hartmann et al., 2003, Marshall et al., 2003). The CpG examined here is a class C CpG (2395), meaning that it signals through both pDC and B cells, impacting type I IFN production, antigen-presenting cell (APC) maturation, and NK cell activation (Marshall et al., 2003). CpG molecules (specifically 7909) have been in multiple clinical trials as vaccine adjuvants; the specific CpG tested in this work has not been in clinical trials but was selected as it targets both human and murine TLR9 and it has both Class A and B activation characteristics. Finally, MPLA is a TLR4 agonist, which is comparable to MPL, the active component of the GSK adjuvant AS04 (Einstein et al., 2014a, Einstein et al., 2014b). MPLA has been shown to be highly effective as an adjuvant, particularly in combination with an aluminum-based adjuvant like alhydrogel or a nanoparticle formulation (Bohannon et al., 2013).
Considerable work has gone into evaluating the impact of putative adjuvants on innate immune activation and on adaptive immune responses to model antigens and potential vaccines (Longhi et al., 2009, Kastenmuller et al., 2012, Trumpfheller et al., 2008, Stahl-Hennig et al., 2009, Perret et al., 2013, Caproni et al., 2012). It has been demonstrated that co-administration of adjuvant and antigen can be critical for optimizing the immune response, and formulation of adjuvants and antigen in nanoparticles for co-administration is currently being explored as a means of targeting the adjuvant effects (Quinn et al., 2013b, Hanson et al., 2015, Moon et al., 2012, Jain et al., 2011). However, adjuvants' impact on long lasting protective immunity is poorly understood, particularly in the context of a relevant challenge model (Seder et al., 2015).
The filovirus VLP vaccine has demonstrated efficacy in the murine, guinea pig, and nonhuman primate models of filovirus infection (Swenson et al., 2005, Swenson et al., 2008a, Swenson et al., 2008b, Warfield et al., 2003, Warfield et al., 2004, Warfield et al., 2007, Martins et al., 2014, Martins et al., 2015a). To evaluate durable protection, we developed a rigorous murine model for Ebola virus challenge. We then tested the ability of the aforementioned adjuvants to augment protection under this model. Correlates of durable protection were identified by comparing and contrasting immune parameters associated with different levels of protection. These data inform broadly on the impact of classic TLR agonists to enhance the durable protection of a protein-based vaccine, and they demonstrate that adjuvant selection can determine the quality and utility of a vaccine candidate.
2. Methods
2.1. Ethics Statement
Research was conducted under an IACUC approved protocol in compliance with the Animal Welfare Act, PHS Policy, and other Federal statutes and regulations relating to animals and experiments involving animals. The IACUC committee approving this protocol is the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) IACUC. The facility where this research was conducted, USAMRIID, is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International and adheres to principles stated in the 8th Edition of the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011.
2.2. Animals, Vaccinations, and Viral Challenge
C57BL/6 mice were obtained from NCI Charles River. Mice between 8 and 12 weeks of age were vaccinated with 100 μl via the intramuscular (IM) route, in the caudal thigh. All mice in each study were female and age-matched and therefore were inherently randomized. For studies involving CD8-deficient animals (Jackson Laboratory strain 002665) and CD4-deficient animals (Jackson Laboratory strain 002663), C57BL/6J (Jackson Laboratory strain 000664) mice were used as controls.
Animals were monitored at least once daily by technical staff members who were blinded to the study aims. Animal status was evaluated according to an Intervention Scoresheet approved by USAMRIID IACUC. Monitoring increased to three times daily if the animals were given a score of three or four. Euthanization was by CO2 inhalation followed by confirmatory cervical dislocation. Analgesics and anesthetics were not used in this study, and animals were euthanized for humane purposes if they reached a score of five or more, which would be indicated if the animals exhibited ruffled fur, weakness, unresponsiveness, and/or difficulty walking. Otherwise, animals were euthanized on day 14 of the study. For all survival studies, control groups included animals vaccinated with saline and/or adjuvant alone.
MPLA (MPLA-SM; extracted from LPS produced by Salmonella minnesota R595) and CpG (CpG 2395 Class C, vac-2395-1; 5′-tcgtcgttttcggcgc:gcgccg-3′) were from Invivogen and polyICLC (Hiltonol) was provided by Oncovir, Inc.; these adjuvants were diluted with sterile saline after resuspension in DMSO (MPLA) or water (CpG). Alhydrogel was from Brenntag (CAS #21645-51-2, 10 mg/ml stock) and was diluted with sterile PBS. VLPs were manufactured by Paragon Bioservices and were produced by transfecting HEK293F cells with Ebola Zaire virus GP and VP40 genes in pWRG expression vectors, essentially as previously described (Swenson et al., 2004). VLP were irradiated at 1e6 rad to ensure sterility and contained less than 25 EU/ml endotoxin and less than 10 colony forming units of bacteria per vaccination. Vaccines were administered IM two times, with 3 weeks between vaccinations. A challenge dose of 1000 pfu of mouse-adapted (ma-) Ebola virus was administered via the intraperitoneal (IP) route (Bray et al., 1998). The mouse model of Ebola virus challenge is a well-documented small animal model of Ebola virus challenge and recapitulates some of the symptoms of human Ebola virus infection. It has been used to evaluate multiple vaccines and therapeutics developed against filoviruses.
2.3. Adoptive Transfer Studies
C57BL/6 mice were vaccinated two times with three weeks between vaccinations. Four weeks after the second vaccination, serum and splenocytes were harvested. Negatively selected (“untouched”) T cells (Miltenyi Biotech, 130-095-130), CD4 T cells (Miltenyi Biotech, 130-104-454), or CD8 T cells (Miltenyi Biotech, 130-104-075) were isolated using magnetic separation in accordance with the manufacturer's instructions. Cell purity was universally greater than 90% and on average 94%. Cells and serum were combined prior to injection IP into recipient mice. Twenty-four hours after transfer, mice were challenged IP with 1000 pfu of ma-EBOV.
2.4. Antibody Assays
Antibody titers were determined using an ELISA. Two μg/ml of recombinant Ebola virus GP was plated in a flat bottom 96 well plate overnight. Plates were incubated with blocking buffer (5% milk, 0.05% Tween in PBS) for 2 h, and then serum samples were added to plates. The standard protocol used half log dilutions starting at a 1:100 dilution. After 2 h, plates were washed with PBS + 0.05% Tween and secondary antibody was added at a 0.6 μg/ml. Secondary antibodies included goat anti-mouse IgG-HRP (Southern Biotech 1030–05), IgG1-HRP (Southern Biotech 1070–05), IgG2c-HRP (Southern Biotech 1079–05), and IgG3-HRP (Southern Biotech 1100–05). One hour later, plates were washed and exposed using Sure Blue TMB 1-component substrate and stop solution (KPL), and the absorbance at 450 nm was recorded. Serum from unvaccinated animals was used to establish background and titers were defined as the serum dilution resulting in an absorbance greater than 0.2, where background was universally less than 0.2. Serum from animals previously determined to contain anti-GP antibody was included in each assay to serve as a positive control.
2.5. Pseudovirion Neutralization Assay
The pseudovirion neutralization assay (PsVNA) used to detect neutralizing antibodies in sera was essentially described previously; it uses a replication-restricted, recombinant vesicular stomatitis virus (rVSV*ΔG) expressing luciferase, which is pseudotyped with the Ebola GP (Kikwit) (Martins et al., 2015a). Briefly, heat-inactivated mouse sera was first diluted 1:20, followed by five-fold serial dilutions that were mixed with an equal volume of Eagle's minimum essential medium with Earle's salts and 10% fetal bovine sera (FBS) containing 4000 fluorescent focus units (FFU) of EBOV-95 pseudovirions and 10% guinea pig complement (Cedarlane). This mixture was incubated overnight at 4 °C. Following this incubation, 50 μl was inoculated onto Vero cell monolayers in a clear bottom, black-walled 96-well plate in duplicate. Plates were incubated at 37 °C for 18–24 h. The media was discarded and cells were lysed according to the luciferase kit protocol (Promega #E2820). A Tecan M200 Pro was used to acquire luciferase data. The values were graphed using GraphPad Prism software and used to calculate the percent neutralization using cells alone and pseudovirions alone, using the minimum and maximum signals, respectively. Maximum signal was controlled on a per plate basis to reduce signal variation. The curves generated were interpolated to obtain PsVNA 50 and 80% neutralization titers.
2.6. T Cell Assays — Intracellular Cytokine Staining
Mice were euthanized by CO2 inhalation at various time points after vaccination and splenocytes were harvested for T cell assays. After red blood cell lysis, cells were cultured at 10e6 cells/ml in complete media (90% RPMI 1640, 10% FBS, 20 mM Hepes, 1% Pen/strep, 0.05 mM BME) with 10 U/ml mouse recombinant IL2, 1 μg/ml mouse CD49d (BD #553,314), 1 μg/ml mouse CD28 (BD 553295), and 1 × protein transport inhibitor cocktail (eBioscience #00–4980). 1e6 cells were plated in each well of a 96 well plate and were stimulated with either cell stimulation cocktail (eBioscience 00-4970-93) as a staining control, DMSO, or Ebola virus peptides at 2 μg/ml. Ebola virus GP peptide WIPYFGPAAEGIYTE (WE15) was utilized for experimental samples as it had previously been shown to elicit a detectable T cell response in C57BL/6 mice (Olinger et al., 2005, Shedlock et al., 2013).
Six hours after stimulation, cells were washed in PBS + 10% FBS. Live/Dead aqua (Invitrogen) was used to identify viable cells by incubation for 10 min at 4 °C, and Fc Block (Miltenyi) was used to prevent non-specific antibody binding. After washing, surface antibodies CD3-FITC (BD clone 145-2C11), CD8-APC-H7 (BD clone 53–6.7), CD4-PacBlue (BD clone RM4-5) were incubated with samples for 20′ at 4C, and then cells were washed again and fixed with 3.7% paraformaldehyde overnight. Cells were then permeabilized with perm/wash (eBioscience 00-8333-56) and stained with IFNγ-APC (BD clone XMG1.2), IL2-PECy7 (BD clone JES6-5H4), and TNFα-PE (BD clone MP6-XT22) antibodies. Cells were run on the Canto II flow cytometer, and analysis was conducted using FlowJo software. Isotype controls and minus one controls were used to define populations.
2.7. T Cell Assays — Sorting of T Cell Memory Populations
Four weeks after the second vaccination, splenocytes were harvested from C57BL/6 mice and subjected to red blood cell lysis. Untouched T cells were isolated by magnetic bead separation, in accordance with the manufacturer's instructions (Miltenyi Biotech, 130-095-130). Cells were stained with CD44 (BD Clone IM7) and CD62L (L-Selectin, BD Clone MEL-14) to identify central and effector memory cell populations, as well as Live/Dead aqua (Invitrogen) (Henao-Tamayo et al., 2010, Hikono et al., 2007, Lefrancois and Masopust, 2002, Krishnan et al., 2007). Cells were then washed two times and sorted on the BD Aria II. Purity of sorted populations was confirmed on the BD Canto II and cell purity was universally greater than 95% for each T cell sub-population added to culture.
Feeder cells were isolated from splenocytes of naïve C57BL/6 mice. CD3 + T cells were depleted using magnetic bead isolation (Miltenyi Biotech 130-094-973) in accordance with the manufacturer's instructions. Purity was confirmed on the BD Canto II and cells were universally greater than 95% CD3 negative.
Feeder cells were plated at 5e5 cells/well in 100 μl of media with 20 U/ml mouse recombinant IL2, 2 μg/ml mouse CD49d (BD Clone 9C10), 2 μg/ml mouse CD28 (BD Clone 37.51), and 0.2 μM GP. Sorted T cells were added at 1e5-2e5 cells/well in 100 μl. Cells were cultured for four days; on the third day, 50 μl/well of media with 40 U/ml of IL2 was added to each well. On the fourth day of culture, cells were pelleted and subjected to intracellular cytokine staining, as described above.
2.8. ELISPOT Assay
MabTech Mouse IFNγ ELISPOT PLUS kit (3321-2HW-Plus) was used for evaluation of IFNγ production. Cells were pre-plated overnight with capture antibody, as per the manufacturer's instructions. Splenocytes were isolated from vaccinated animals and subjected to red blood cell lysis. Cells were then resuspended at 2e6/ml and 100 μl of cells was combined with 100 μl of stimulation master mix. Master mixes included 20 U/ml mouse recombinant IL2, 2 μg/ml mouse CD49d (BD 553314), 2 μg/ml mouse CD28 (BD 553295), and one of the following stimulants: 4 μg/ml WE15 peptide, 0.2 μM GP, or 4 μg/ml DMSO. Cells were incubated in ELISPOT plates for 16 h at 37 °C, and the ELISPOT assay was conducted as per the manufacturer's instructions. Plates were analyzed using the CTL ImmunoSpot reader. Values were calculated by averaging duplicate wells and then subtracting the average of the unstimulated duplicate wells for each animal.
2.9. Popliteal Lymph Node Isolation for Transcriptomic Analysis
Popliteal lymph nodes (LN) were isolated from vaccinated mice seven days after vaccination. LN were filtered on a 70 μm filter and resuspended in PBS. Untouched T cells were isolated by magnetic bead separation, in accordance with the manufacturer's instructions (Miltenyi Biotech, 130-095-130). Cells were then lysed with buffer RLT and RNA was isolated using the Qiagen RNeasy Mini Kit, according to the manufacturer's instructions (Qiagen 74104). RNA from 3 to 4 animals per vaccination group was pooled and analyzed using the SABiosciences PCR Array (T cell and B cell activation array, PAMM-053Z) on an ABI 7900 HT real time PCR instrument, in triplicate. Ct values were normalized to housekeeping genes and the fold difference in expression of genes in the T cells of animals vaccinated with VLP and polyICLC vs. VLP alone was determined.
2.10. Popliteal Lymph Isolation and Identification of T Follicular Helper Cell Populations
Popliteal LN were isolated from vaccinated mice 7 days after vaccination. LN were filtered on a 70 μm filter and resuspended in PBS. Cells were plated in 96 well plates and viable cells were identified using Invitrogen's Live/Dead aqua dye. T follicular helper (Tfh) cell populations were identified by surface staining with CD3, CD4, CXCR5, ICOS, and PD1 as well as intracellular staining for Bcl6.
CXCR5 was identified by incubating cells with purified rat anti-mouse CXCR5 (BD Clone 2G8) at 1:100 in buffer A (PBS + 0.5% BSA + 0.1% sodium azide supplemented with 2% normal mouse serum (NMS) and 2% FCS) for 1 h. Cells were then washed two times and incubated with goat anti-rat (H + L)-biotin (Jackson ImmunoResearch, 112-067-003) in buffer A for 30 min. Cells were then washed twice and incubated with streptavidin-PECy7 (BD 557598), anti-CD3-V450 (BD Clone 500A2), anti-CD4-PerCyCy5.5 (BD Clone RM4-5), anti-PD1-APC (eBioscience Clone RMP1-30), and anti-ICOS-PE (BD Clone 7E.17G9) in buffer A for a further 30 min. After two more washes, cells were incubated with BD Phosflow Lyse/Fix buffer (BD 558049) for 12 min at 37 °C, washed two times, and incubated with anti-Bcl6-Alexa488 (BD Clone K112-91) for 1 h in Perm/Wash Buffer I (BD 557885). Cells were washed two times and examined on the BD FACS Canto II.
2.11. Statistical Analysis
Statistical analyses were performed using SAS Version 9.4 (2012 SAS Institute, Cary, NC). Continuous variables were screened for normality and homogeneity of variance. IgG, IgG1, IgG2c, IgG3, T-cell responses, cytokines, chemokines, and splenocytes were analyzed by nonparametric methods. In instances where multiple group comparisons using nonparametric methods were required, Kruskal–Wallis tests were initially used followed by the Dwass, Steel, Critchlow-Fligner (DSCF) multiple pairwise comparison procedure to control the familywise error rate. In instances where only two-sample comparisons were required, Mann–Whitney–Wilcoxon rank-sum tests were used, and, where required, stepdown Bonferroni corrections were used to control the familywise error rate. Neutralizing antibody titers met assumption of normality and homogeneity of variance and were analyzed using one-way analysis of variance (ANOVA) with post-hoc Tukey's studentized range tests for pairwise comparisons. Percentage surviving between groups was compared using Fisher's exact tests. In instances where multiple pairwise survival comparisons were required, stepdown Bonferroni corrections were used to control the familywise error rate. Cochran-Armitage test for trend was used to evaluate survival across groups with increasing time to challenge. For IgG and IgG1, values below the lower limits of detection (LLOD) (100) were set to a value equal to the LLOD divided by the square root of 2 (100/√2). For IgG2c and IgG3, values below the lower limits of detection (LLOD) (10) were set to a value equal to the LLOD divided by the square root of 2 (10/√2). For neutralizing antibody titers, values below the lower limits of detection (LLOD) (40) were set to a value equal to the LLOD divided by the square root of 2 (40/√2).
3. Results
3.1. Adjuvants can Enhance the Duration of Vaccine-Mediated Protection
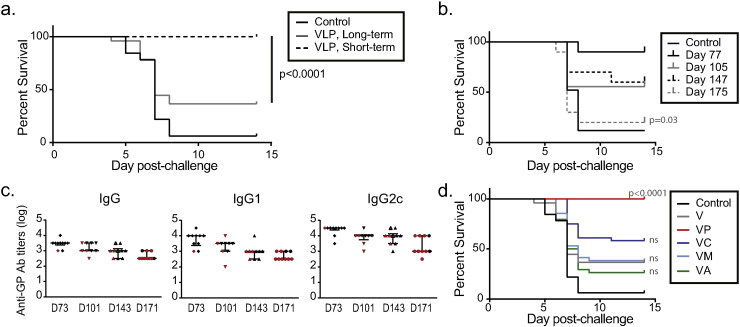
We previously demonstrated that VLP expressing GP and VP40 of Ebola Zaire can confer protection from ma-EBOV challenge in C57BL/6 mice. Inclusion of adjuvant provided vaccine dose-sparing, but the durability of the protective immune response was unclear (Martins et al., 2014). To test the efficacy of VLP vaccination in a durable challenge model, we vaccinated mice two times with 10 μg of VLP, a dose level that is protective when challenge occurs 4 weeks after vaccine boost. We then challenged the animals 22 weeks after the vaccine boost. One hundred percent of animals challenged on the short-term schedule survived challenge, as anticipated; however, only 37.5% (24/64) of animals challenged 22 weeks after vaccination survived (Fig. 1a) (p < 0.0001). Using a serial challenge strategy, we vaccinated animals as before and then challenged 8 weeks, 12 weeks, 18 weeks, or 22 weeks after the second vaccination. We observed that protection declined as the length of time from vaccination to challenge increased (Fig. 1b) (p = 0.0046). This decline was concurrent with a drop in antibody titer, and significant differences between challenge days, regardless of survival status, were found for IgG (p = 0.0002), IgG1 (p = 0.0005), and IgG2c (p = 0.0009). The sample sizes were insufficient to compare survivors and non-survivors within each challenge day, however (Fig. 1c).
Fig. 1.
Adjuvants impact the durability of protection conferred by eVLP vaccination.
(a) C57BL/6 mice were vaccinated IM two times with 10 μg of eVLP and challenged four (short-term) weeks or twenty-two (long-term) weeks after the vaccine boost. Data in A are pooled from 8 individual studies with 6–10 animals/group. Fisher's exact test: survival in the short-term group was significantly higher than in the long-term group (p < 0.0001). (b) C57BL/6 mice were vaccinated IM two times with 10 μg of eVLP and challenged at the indicated days after the second vaccination. n = 9 or 10/group. Cochran-Armitage test: percentage surviving declined as time to challenge increased (p = 0.0046). There was a significant difference (p = 0.03) between survival on Day 77 and Day 175. (c) Serum samples collected from animals in (B) one week prior to challenge were subjected to an ELISA for the evaluation of anti-GP IgG, IgG1, and IgG2c antibody titers. Red symbols indicate titers of animals that succumbed to challenge while black indicate titers of survivors; red symbols with black outlines indicate that one of these two animals succumbed to challenge, but animal tags were indeterminate after challenge. Median and IQR shown. (d) C57BL/6 mice were vaccinated two times (IM) with VLP, with or without the indicated adjuvants. Animals were challenged twenty-two weeks after the vaccine boost. Data in D are pooled from at least 4 separate studies with a total of at least 35 animals per group. P-values comparing VLP alone to vaccination with VLP and adjuvant are shown, calculated using Fisher's exact tests with stepdown Bonferroni correction. V = VLP, VP = VLP + PolyICLC, VC = VLP + CpG, VM = VLP + MPLA, and VA = VLP + alhydrogel.
We next evaluated whether inclusion of adjuvants could augment durable protection. Using data from short-term challenges, we identified the dose level of adjuvant that could provide dose-sparing on the short term schedule. We then evaluated the efficacy of that dose level on VLP vaccination under the durable protection model. C57BL/6 mice were vaccinated with 10 μg of VLP alone or in combination with 10 μg of polyICLC, MPLA, CpG ODN, or 10% alhydrogel. As shown in Fig. 1d, inclusion of polyICLC rescued VLP-mediated durable protection, resulting in 100% survival, and this was a significant increase compared to survival after vaccination with VLP alone (p < 0.0001). Inclusion of CpG resulted in an increase in survival compared to VLP alone, but this was not significant (p = 0.179). Inclusion of MPLA or alhydrogel had no impact on survival. These data provided a clear basis by which effective and ineffective vaccination regimens could be evaluated, permitting the identification of correlates of immune protection.
3.2. Adjuvants Associated with Durable Protection Elicit a Th1 IgG Response
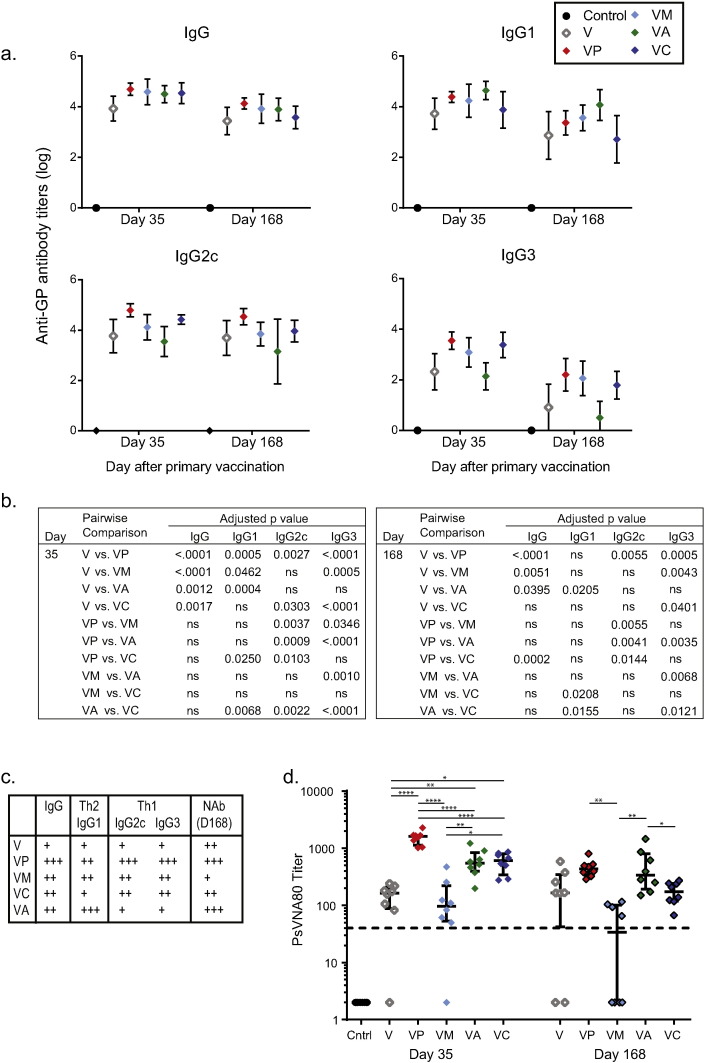
Antigen-specific IgG titers are frequently used as a correlate of vaccine-mediated protection, including in some EBOV challenge models (Wong et al., 2012). The efficacy of the rVSV-ZEBOV vaccine appears to be highly dependent upon antibody, for example, and anti-GP antibodies have been used successfully as therapeutics in NHP models of infection (Wong et al., 2012, Wong et al., 2014, Qiu et al., 2012, Dye et al., 2012). In the present study, all four adjuvants significantly enhanced anti-GP IgG antibody titers by Day 35 when compared to vaccination with VLP alone, with p-values less than 0.0017 (Fig. 2a,b). By Day 168, 1 week prior to challenge, titers after vaccination with polyICLC, MPLA, or alhydrogel were still significantly higher than titers after vaccination with VLP alone (p < 0.04), but titers from animals vaccinated with VLP and CpG were comparable to those of animals vaccinated with VLP alone.
Fig. 2.
Adjuvants have variable impact on IgG subclasses and antibody neutralization.
(a) Serum was collected 14 days and 147 days after the vaccine boost (days 35 and 168, respectively) and evaluated for anti-GP IgG, IgG1, IgG2c, and IgG3 levels using an ELISA. Data shown are pooled from at least two separate experiments per group. (b) Pairwise comparison using DSCF multiple pairwise comparison was used and p values greater than 0.05 are shown as “ns”. (c) Summary of results shown in A, B and D. (d) Neutralizing antibody titers were evaluated using the PsVNA, with titers giving 80% neutralization shown; median and IQR shown. Samples within each group were selected randomly from three separate studies for evaluation in the assay. Pairwise comparison using post-hoc Tukey's studentized range test procedure was used to evaluate differences between groups at both days 35 and day 168, where “*” indicates 0.01 < p < 0.05, “**” indicates 0.001 < p < 0.01, “***” indicates 0.0001 < p < 0.001, and “****” indicates p < 0.0001.
The subclass of IgG significantly impacts the function of the antibody. In C57BL/6 mice, a Th2 skewed immune response is marked by higher IgG1 titers while a Th1 skewed immune response is marked by high IgG2c and IgG3 titers; these isotypes were therefore selected for analysis (Finkelman et al., 1988, Snapper and Paul, 1987). There were many statistically significant differences between vaccination groups (Fig. 2b), and the overall trends are presented in Fig. 2c. Mice vaccinated with VLP and alhydrogel had significantly higher IgG1 titers than mice vaccinated VLP alone or VLP with CpG, and this was true at both time points. In contrast, these same mice had the lowest IgG2c and IgG3 titers, in keeping with the published observation that alhydrogel elicits a Th2 skewed immune response. In contrast, animals vaccinated with VLP and polyICLC, the vaccination regimen that conferred protection, had the highest IgG2c titers of all groups at both time points (p < 0.015).
In order to determine whether antibody neutralization was impacted by the inclusion of adjuvants, we evaluated neutralization using the PsVNA, which measures neutralization of pseudoparticles expressing Ebola GP. At the early time point, serum from animals vaccinated with VLP and polyICLC had significantly higher NAb titers as compared to VLP alone or VLP administered with any other adjuvant (p < 0.0001) (Fig. 2d). At the late time point, vaccination with VLP and alhydrogel induced the highest NAb titers on average, despite the fact that alhydrogel had no beneficial impact on survival. Titers in animals vaccinated with VLP and alhydrogel were significantly higher than those of VLP with MPL or CpG on day 168 (p < 0.04).
3.3. Adjuvants Associated with Durable Protection Elicit Antigen-Specific CD4 and CD8 T Cell Responses
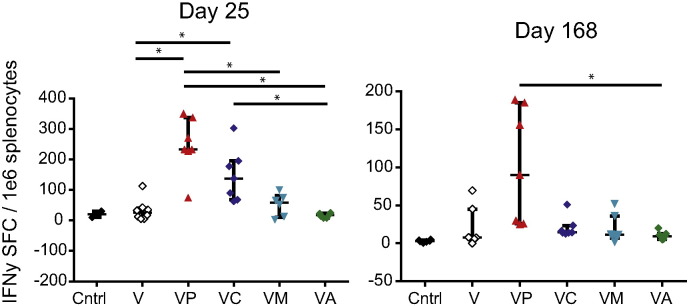
VLP-mediated protection from Ebola virus challenge has been associated with the presence of antigen-specific T cell responses as well as antibody. To evaluate the impact of the adjuvants on overall IFNγ T cell responses, we euthanized mice 4 days after the vaccine boost and evaluated IFNγ production via ELISPOT analysis. For the antigen, we utilized a known CD4 and CD8 T cell epitope, the peptide WE15, as described in the Methods section (Fig. 3a). Additionally, we used full-length recombinant GP as the antigen in a duplicate set of experiments, and we observed the same pattern of IFNγ production regardless of the antigen used (Fig. 3a and Supplemental Fig. 1).
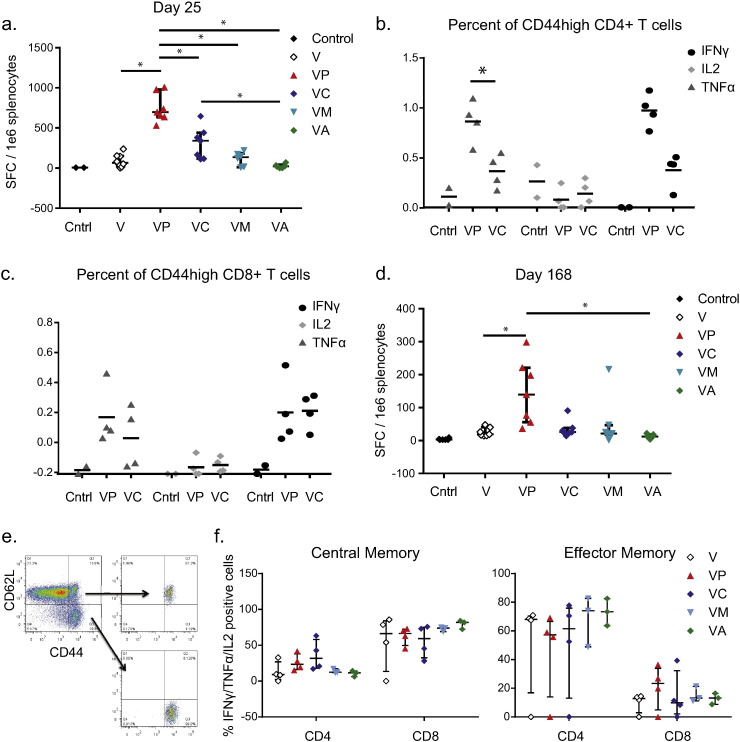
Fig. 3.
Effective adjuvants increase the frequency of antigen-specific T cells.
(a) IFNγ ELISPOT analysis of splenocytes from C57BL/6 mice vaccinated two times with indicated vaccine and adjuvant combination. Splenocytes were collected on day 4 after the second vaccination. Data is pooled from four separate experiments each containing 2–3 mice per group; median with IQR shown. (B&C) Frequency of IFNγ +, IL2 +, or TNFα + cells after vaccination with VLP and polyICLC or VLP and CpG; median shown. Gating is on viable T cells and then CD4 + CD44high T cells (b) or CD8 + CD44high T cells (c). (d) IFNγ ELISPOT analysis of splenocytes from C57BL/6 mice vaccinated with indicated vaccine and adjuvant combination. Animals received the standard two vaccinations and then received a third vaccine boost 22 weeks after the second vaccination. Splenocytes were collected 4 days later. Data is pooled from two separate experiments each containing 3–4 mice per group; median with IQR shown. (e) Four weeks after the second vaccination, splenocytes were collected from animals and T cells were isolated using negative bead selection. T cells were then sorted to collect central memory, effector memory, and CD44int/low cell populations. Gating strategy is shown. (f) Sorted T cells were cultured with peptide-exposed, T cell-depleted, naïve splenocytes and the frequency of cytokine-positive CD4 and CD8 T cells in each sorted population was quantified after 5 days of culture. Gating is on CD4 or CD8 T cells and data shown are the frequency of cells expressing IFNγ, TNFα, or IL2; median is shown. “*” indicates 0.01 < p < 0.05.
ELISPOT data indicated that polyICLC induced the highest frequency of IFNγ producing cells, which was significantly higher than the frequency of cells elicited by VLP vaccination alone or with any other adjuvants (p < 0.04) (Fig. 3a). Vaccination with CpG and VLP elicited the second highest IFNγ response, which was approximately half that observed with VLP and polyICLC. To determine whether the response observed after vaccination with VLP and polyICLC or CpG was CD4 or CD8 T cell mediated, we performed intracellular cytokine staining on splenocytes on day 4 after the vaccine boost. Cells from animals vaccinated with VLP and polyICLC or VLP and CpG were evaluated and, in keeping with the ELISPOT results, we observed that polyICLC elicited higher frequencies of antigen-specific IFNγ-producing cells than CpG, though this was not significant, as well as higher frequencies of TNFα-producing CD4 T cells (p = 0.0209) (Fig. 3b,c).
In order to evaluate the persistence of this T cell response, we vaccinated mice two times, and then we left them to rest for 22 weeks. At that time, when we would normally challenge for the durable challenge model, we administered a third vaccine boost and then euthanized animals 4 days later. We performed ELISPOT analysis on splenocytes from these animals and observed, as anticipated, a lower overall frequency of IFNγ-producing cells as compared to Day 4 after the boost (Fig. 3d). However, animals vaccinated with VLP and polyICLC still had significantly higher frequencies of antigen-specific cells as compared to animals vaccinated with VLP alone or VLP with alhydrogel (p < 0.05).
We next hypothesized that the inclusion of adjuvants may impact not only the frequency of antigen-specific T cells, but also the memory phenotype. The frequency of antigen-specific T cells in vaccinated mice is quite low after the peak response. We therefore developed an assay to sort and culture memory T cells. Four weeks after vaccination, we purified and cultured the CD44highCD62L + (central memory), CD44highCD62L- (effector memory), and CD44low/int (antigen-inexperienced) cell populations using FACS (Fig. 3e). We evaluated the frequency of antigen-specific CD4 vs. CD8 T cells that expanded from each sorted population and found that while CD44low/int cells failed to expand in response to antigen-pulsed feeder cells, both central and effector memory cell populations did expand (Fig. 3f). Regardless of the inclusion of adjuvant, there was a strong bias toward central memory cells being predominantly CD8 T cells while effector memory cells were predominantly CD4 T cells. No clear difference between the adjuvants was observed. Additionally, no clear impact on the skewing of the central vs. effector memory populations by adjuvants was observed in either CD4 or CD8 T cells.
Overall our studies found that the frequency of antigen-specific T cells appeared to correlate with efficacy, but the specific role of CD4 and CD8 T cells was unclear. To further understand the relevance of CD8 T cells, we examined vaccination in CD8-deficient mice.
3.4. CD8 T Cells are not Required for VLP-Mediated Protection, with or without Adjuvants
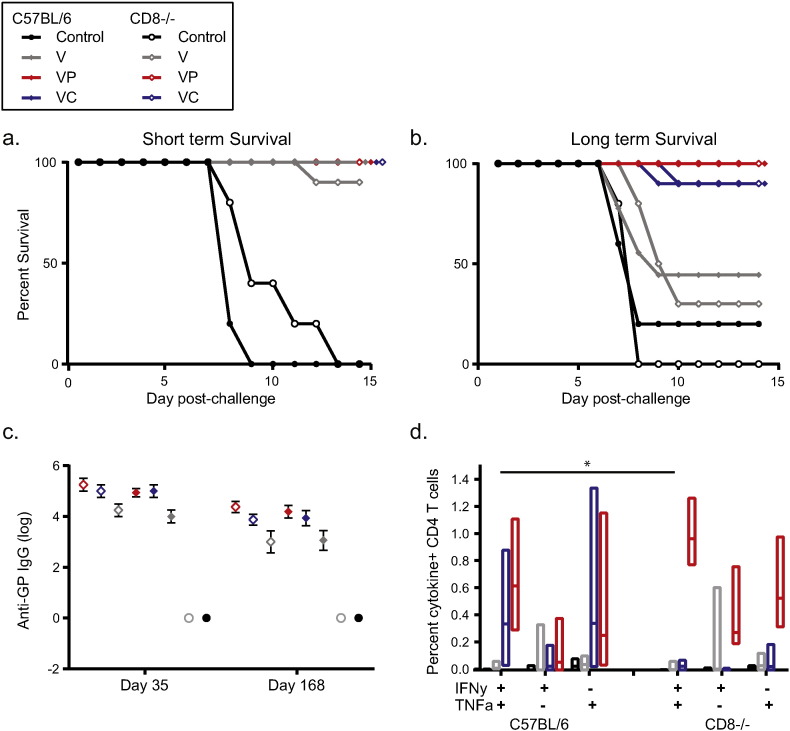
Protection against some pathogens, including HIV-1, malaria, and even influenza, is associated not only with antibody responses but with the frequency of Th1 profile, cytotoxic T cell responses (Watkins, 2008, Doll and Harty, 2014, Sridhar et al., 2013, Mendoza et al., 2013, Quinn et al., 2013a). CD8 T cell responses may also be critical for protection mediated by the adenovirus-based Ebola vaccine (Sullivan et al., 2011), although other filovirus vaccines do not appear to require an antigen-specific CD8 T cell response (Rao et al., 2002, Marzi et al., 2013, Wong et al., 2012). To evaluate whether the presence of antigen-specific CD8 T cells is necessary for VLP-mediated durable protection, we vaccinated C57BL/6J mice and CD8-deficient mice with VLP, with or without polyICLC or CpG. Animals were challenged four weeks or twenty-two weeks after the vaccine boost. The lack of CD8 T cells had no effect on animal survival or on anti-GP IgG titers. All vaccinated animals survived short term challenge, with the exception of one CD8 deficient animal vaccinated with VLP alone (Fig. 4a). Additionally, in the durable challenge model, all (10/10) animals vaccinated with VLP and polyICLC and 90% (9/10) of animals vaccinated with VLP and CpG survived, as did 44% (4/9) of C57BL/6J animals vaccinated with VLP alone and 30% (3/10) of CD8-deficient animals vaccinated with VLP alone (Fig. 4b). Notably, it appears that CpG was more effective in this C57BL/6J strain than in the C57BL/6 mice used for other studies; nonetheless, the trends between the CD8-deficient animals and the control animals were comparable.
Fig. 4.
CD8 T cell deficiency does not impact short term or long term survival of vaccinated C57BL/6J mice.
(a) Mice were treated IM twice with saline, VLP, VLP and polyICLC, or VLP and CpG. Four weeks after the second vaccination, mice were challenged. Closed symbols represent wild type C57BL/6J mice and open symbols indicate CD8-deficient mice. (b) Mice were vaccinated on the same schedule as in A, but challenge occurred 22 weeks after the second vaccination. (c) Two weeks after the second vaccination and 1 week prior to challenge, blood was collected from vaccinated animals and evaluated for anti-GP IgG antibody titers. (D) A subset of vaccinated animals was euthanized 4 days after the vaccine boost to evaluate CD4 + T cell responses. Median response of C57BL/6J mice and CD8-deficient mice is shown. N = 8–10 per treated group for A–C and D presents data pooled from two separate evaluations of 4–8 mice per group each. “*” indicates 0.005 < p < 0.05.
To determine whether a compensatory immune response was accounting for survival in the CD8-deficient animals, we examined antigen-specific antibody and CD4 T cell frequencies. Anti-GP IgG titers between CD8-deficient animals and C57BL/6J animals did not differ at either time point (Fig. 4c). Additionally, there were minimal differences in the frequency of antigen-specific CD4 T cells between mouse strains (Fig. 4d). These data suggest that the survival observed in the CD8-deficient animals was not attributable to a compensatory increase in the CD4 T cell population or an enhanced antibody response due to potentially higher frequencies of helper CD4 T cells, and they imply that CD8 T cells are not required for VLP-mediated protection in the mouse model.
3.5. Adjuvants Associated with Durable Protection Enhance Tfh Cell Frequency
In our study, the protective vaccination regimen resulted in an increase in the frequency of antigen-specific, cytokine-positive CD4 T cells (Fig. 3b). Additionally, CD4-deficient animals did not survive upon vaccination with VLP alone (p < 0.0001 comparing survival of C57BL/6J vs CD4 −/−) and these animals were largely incapable of mounting an IgG response upon VLP vaccination, suggesting that antibody class switch recombination was T cell dependent (Supplemental Fig. 2).
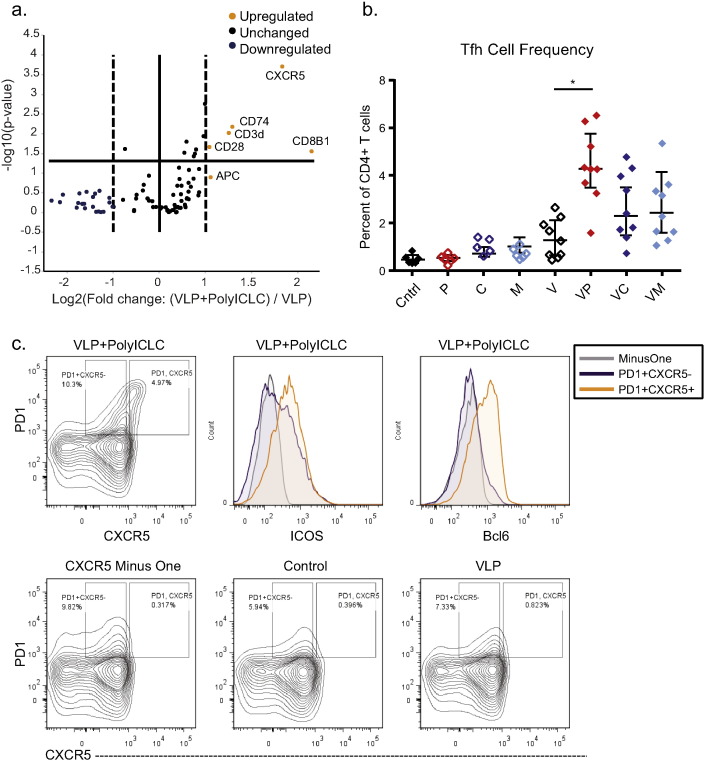
In order to take a more global look at factors differentiating T cells after vaccination with VLP as compared to VLP and polyICLC, we vaccinated mice and isolated draining popliteal LN seven days after vaccination. Untouched T cells were isolated from the lymph nodes using bead depletion, and RNA was isolated, pooled, and subjected to analysis using the SABioscience T-cell and B-cell Activation Array (PAMM-053Z). Five transcripts were up-regulated more than two-fold in T cells from animals vaccinated with VLP and polyICLC as compared to VLP alone, with p values less than 0.05 (Fig. 5a). CXCR5 was one of these transcripts, and it was increased with a p-value of 0.0002. CXCR5 is upregulated in T follicular helper (Tfh) cells, which are critical for B cell class switch recombination.
Fig. 5.
Tfh frequencies are increased by adjuvants associated with protection from challenge.
C57BL/6 mice were vaccinated a single time IM with VLP, with or without adjuvant. Seven days after vaccination, the draining popliteal LN was isolated. (a) Untouched T cells were isolated from the LN (n = 3 or 4/group). RNA was isolated from the purified T cells and pooled for each vaccination group. Samples were then evaluated using the SABiosciences T and B cell activation PCR array in triplicate. For the volcano plot, the fold difference is the average of triplicate, and the p value was calculated comparing animals vaccinated with VLP to those vaccinated with VLP and polyICLC. (b) Seven days after vaccination, cells from the draining LN were evaluated for Tfh populations. Cells were gated on viable lymphocytes after doublet exclusion, and then on CD4 + T cells expressing CXCR5 and PD1. Median and IQR shown. (c) CD3 + CD4 + CXCR5 + PD1 + cell population in mice vaccinated with VLP and polyICLC. ICOS and Bcl6 expression of this population is shown. Data shown are pooled from three separate vaccination experiments. Comparisons between animals receiving VLP with or with adjuvant are shown, where “*” indicates 0.01 < p < 0.05 (Kruskal–Wallis test).
To explore the impact of adjuvants on Tfh expansion, we vaccinated animals with adjuvant alone, VLP alone, or VLP in combination with each of the TLR agonist adjuvants. Seven days after vaccination, we euthanized mice and examined the frequency of Tfh cells (CXCR5+ PD1+) in the draining popliteal LN. These cells expressed elevated ICOS and Bcl6 in comparison to CXCR5 − cells. Animals vaccinated with VLP and polyICLC had significantly higher frequencies of this cell population than those vaccinated with VLP alone (p = 0.0276), while animals vaccinated with VLP in combination with either MPLA or CpG exhibited intermediate frequencies of Tfh cells (Fig. 5b-c). These data point to a critical helper role for CD4 T cells in durable vaccination, which may account for the robust IgG responses observed in this model.
3.6. Adoptive Transfer of T Cells and Serum from Vaccinated Animals Confers Protection from Challenge
Having established that T cell responses were impacted by inclusion of protective adjuvants both in terms of cytokine-producing antigen-specific T cells and the frequency of Tfh, we examined whether adoptive transfer of T cells from vaccinated animals into naïve mice could confer protection from Ebola virus challenge. Cells and serum were collected from vaccinated animals 4 weeks after the second vaccination. T cell subsets were isolated by negative depletion so as to minimize inadvertent activation of T cells prior to transfer. Cells and serum, alone or in combination, were transferred into naïve mice (IP) 24 h prior to virus challenge.
As shown in Table 1, transfer of cells or serum from animals vaccinated with VLP alone had no impact on the survival of the recipient animals. Additionally, transfer of serum alone or splenocytes alone from animals vaccinated with VLP and polyICLC had no impact on the survival of the recipient animals. However, combining serum with cells from animals vaccinated with VLP and polyICLC was capable of rescuing survival, and transfer of as few as 5e6 splenocytes from vaccinated animals rescued survival in ~ 90% (9/10) of recipient animals (Table 1). To determine whether T cells were important for the cellular aspect of conferred protection, 5e6 T cells from VLP and polyICLC-vaccinated animals were transferred to naïve animals, with or without serum (Table 2). Not surprisingly, the T cells alone were unable to confer protection from challenge; however, 89% (8/9) of animals receiving serum and 5e6 T cells survived challenge. Again, transfer of cells and serum from animals vaccinated with VLP alone was not protective.
Table 1.
Adoptive transfer of bulk splenocytes and serum from vaccinated animals to naïve animals.
| Group | Transfer group (donor animals) | Number of cells | Cell type | Serum (μl) | Number of animalsa | Percent survival | Challenge material | Challenge dose level |
|---|---|---|---|---|---|---|---|---|
| 1 | PolyICLC + VLP | 5e6 | Splenocytes | 200 | 10a | 90 | Ma-EBOV | 1000 pfu |
| 2 | 20e6 | Splenocytes | 200 | 24a | 83 | |||
| 3 | 20e6 | Splenocytes | n/a | 10a | 0 | |||
| 4 | n/a | n/a | 200 | 5 | 0 | |||
| 5 | VLP | 5e6 | Splenocytes | 200 | 5 | 0 | ||
| 6 | 20e6 | Splenocytes | 200 | 5 | 0 | |||
| 7 | 20e6 | Splenocytes | 500 | 3 | 0 | |||
| 8 | n/a | n/a | n/a | n/a | 60a | 0 |
Pooled from multiple iterations with n = 4 to 10 per iteration. Fisher's exact test with Bonferroni correction, pairwise comparisons were used to evaluate Group 1 vs. 2 (p = 0.3819), 1 vs. 4 (p = 0.006), 1 vs. 5 (p = 0.006), 2 vs. 3 (p = 0.0005), and 2 vs. 6 (0.0044).
Table 2.
Adoptive transfer of purified T cells from vaccinated animals to naïve animals.
| Group | Transfer group (donor animals) | Number of cells | Cell type | Serum (μl) | Number of animalsa | Percent survival | Challenge material | Challenge dose level |
|---|---|---|---|---|---|---|---|---|
| 1 | PolyICLC + VLP | 5e6 | T cells | 200 | 9a | 89 | Ma-EBOV | 1000 pfu |
| 2 | 5e6 | T cells | n/a | 10a | 0 | |||
| 3 | 4e6 | CD4 + T cells | 200 | 25a | 92 | |||
| 4 | 4e6 | CD8 + T cells | 200 | 9a | 0 | |||
| 5 | 12e6 | CD8 + T cells | 200 | 5 | 60 | |||
| 6 | VLP | 5e6 | T cells | 200 | 5 | 0 | ||
| 7 | CpG + VLP | 4e6 | CD4 + T cells | 200 | 5 | 80 |
Pooled from multiple iterations with n = 4 to 10 per iteration. Fisher's exact test with Bonferroni correction, pairwise comparisons were used to evaluate Group 1 vs. 2 (p = 0.0005), 3 vs. 4 (p = 0.0005), 4 vs. 5 (p = 0.055), 7 vs. 3 (p = 0.4335), and 1 vs. 6 (0.009).
Data obtained from vaccination studies with CD8-deficient animals (Fig. 4) and the statistically significant increase in CD4 effector cells upon vaccination with VLP and polyICLC as compared to VLP and CpG (Fig. 3) led us to hypothesize that CD4 T cells would be critical for protection from challenge. To test this hypothesis, 4e6 CD4 or CD8 T cells from animals vaccinated with VLP and polyICLC were transferred into naïve animals. Ninety-two percent of animals receiving CD4 T cells and serum survived challenge while none of the animals receiving CD8 T cells and serum survived. To determine whether increasing the number of transferred CD8 T cells would impact the result, 12e6 CD8 T cells were transferred with serum; this combination protected 60% (3/5) of recipient animals (Table 2). These data suggest that the frequency of transferred cells is critical for mediating protection. Finally, to determine whether CD4 T cells from animals vaccinated with VLP and CpG could confer protection, serum and 4e6 CD4 T cells were transferred into naïve animals. Eighty percent of recipient animals survived challenge (Table 2).
4. Discussion
Protein-based vaccines offer a safe and effective means to achieve protection from a variety of pathogens, but their poor immunogenicity makes inclusion of an adjuvant imperative. In addition to enhancing immunogenicity, adjuvants can provide dose sparing and impact the required vaccination schedule. In this study, we examined the impact of adjuvants on the durability of a model protein-based vaccine, the filovirus VLP.
Of the adjuvants tested in this system, polyICLC had the most beneficial impact on durable protection. However, vaccination with VLP and polyICLC impacted nearly every immune parameter tested, including NAb titers, Th1-skewed antibody titers, CD8 T cell responses, CD4 T cell responses, and Tfh cell responses. To delineate the relative importance of these immune parameters, we compared effects elicited by polyICLC to those elicited by the less effective antigen and adjuvant combinations.
In terms of antibody response, a Th1-skewed antibody response correlated with protection. Vaccination with VLP and polyICLC yielded significantly higher IgG2c titers than vaccination with any other vaccine and adjuvant combination. In contrast, vaccination with alhydrogel and VLP, which had no impact on survival, resulted in a Th2 skewed antibody response. To further examine the impact of antibody on survival, NAb titers were examined. NAb are associated with protection in several vaccination models (Roy et al., 2015, van Gils and Sanders, 2014, Kok et al., 2014, Plotkin, 2010), but data regarding the importance of NAb in EBOV protection are conflicting (Wong et al., 2012, Sullivan et al., 2009, Audet et al., 2014, Oswald et al., 2007, Grant-Klein et al., 2015, Lee et al., 2008, Bale et al., 2012, Martins et al., 2015a, Agnandji et al., 2015). Interestingly, NAb titers had no relationship to survival in this durable protection model. Both polyICLC and alhydrogel substantially impacted NAb titers and improved survival on a short term timescale; however, only polyICLC had a beneficial impact on durable protection. This examination of antibody feeds into a larger discussion on the role of neutralization and the definition of neutralizing antibody. The assay utilized in this study includes supplementation with complement, which complicates the definition of neutralization. Despite this fact, neutralization still did not correlate with survival while IgG2c titers did, suggesting an under-appreciated role for non-neutralizing antibody in controlling infection under a durable protection model.
Previous work has demonstrated that CD4 T cells may be important for Ebola-mediated protection (Wong et al., 2012, Rao et al., 2002). However, dissecting the role of CD4 T cells in protective immunity is challenging due to their impact on the development of high affinity antibody responses and effective CD8 T cell responses (Sant and McMichael, 2012). CD4 T cells also have an effector function and are critical for immune cell recruitment during infection (Soghoian and Streeck, 2010, McKinstry et al., 2012, Brown et al., 2012, Johnson et al., 2015). Recently, an appreciation for the importance of CD4 T cells in viral infection has grown, particularly in the context of influenza vaccination (Zens and Farber, 2015). Antigen-specific CD4 T cells were associated with lower viremia during human influenza infection (Wilkinson et al., 2012), and, in nonhuman primates, CD4 T cell populations were important in Hepatitis A clearance (Zhou et al., 2012). Because of the complex role of CD4 T cells in the immune response, the impact of adjuvants on CD4 T cell responses has also been extensively examined (Baumgartner and Malherbe, 2010, McAleer and Vella, 2010). Both TLR agonist adjuvants and aluminum-containing adjuvants have been associated with CD4 T cell efficacy (Monaci et al., 2015, Sokolovska et al., 2007).
Three compelling sets of data from our studies suggest that, in combination with a Th1-skewed antibody response, CD4 T cells correlate with VLP-mediated protection under a durable protection murine model. First, the frequency of IFNγ-producing T cells was significantly higher in the protective adjuvant vaccination (polyICLC + VLP) than the partially protective (CpG + VLP) or unadjuvanted vaccination, and these cells were predominantly CD4 T cells. Second, transcriptomic analysis of T cells from animals vaccinated with the protective vaccine revealed up-regulation of CXCR5 as compared to vaccination with VLP alone. This observation led us to examine the frequency of Tfh cells in the draining lymph node, where we observed that polyICLC significantly enhanced the frequency of Tfh cells over the suboptimal vaccinations. Third, adoptive transfer of CD4 T cells from animals vaccinated with VLP and polyICLC, in combination with serum, was protective at cell frequencies as low as 4e6 whereas three times as many CD8 T cells were required for comparable protection. In combination, these data suggest that it is not simply the impact of CD4 T cells on B cell maturation or CD8 T cell development that makes CD4 T cells critical for vaccine-mediated protection, but that the actual presence of antigen-specific CD4 T cells is relevant for protection from challenge.
The data presented here inform broadly on how the tested adjuvants impact the immune response to a protein antigen. Moreover, by testing these adjuvants in a challenge model, the data inform on the specific requirements for long term protection from ma-EBOV challenge. Describing the immune profile of these canonical TLR agonists may help direct adjuvant discovery work targeted at achieving long-lasting, vaccine-mediated protection.
Contributions
KM developed study design, implemented assays, analyzed data, and wrote manuscript. CC implemented some assays and contributed to writing. SS, SK, JS, JB, and SvT conducted experiments, and SS and SK did data analysis. SN did statistical analysis. SB contributed to project development.
Conflicts of Interest
None.
Acknowledgments
PolyICLC (Hiltonol) was generously provided by Dr. Andres Salazar of Oncovir, Inc.
Dr. Jeffrey Scott Hale of Emory University kindly assisted in the optimization of Tfh cell identification.
This study was supported with funding from the Department of Defense DTRA project number CB2630 and with support from the Oak Ridge Institute for Science and Education. Funding sources did not contribute to the development of assays or the writing of this manuscript, and authors were not directly compensated to write this manuscript. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.11.041.
Appendix A. Supplementary data
Supplemental Fig. 1 Effective adjuvants increase the frequency of whole glycoprotein-specific T cells.
IFNγ ELISPOT analysis of splenocytes from C57BL/6 mice vaccinated two times with indicated vaccine and adjuvant combination. Splenocytes were collected on day 4 or day 147 after the second vaccination, which occurred on day 21. Data is pooled from two separate experiments each containing 3–4 mice per group, as described in Fig. 3a and d. Recombinant Ebola GP was used as antigen. Mean and IQR shown. Comparisons between animals receiving VLP with or with adjuvant are shown, where “*” indicates 0.01 < p < 0.05 (Kruskal–Wallis test).
Supplemental Fig. 2 CD4-deficient mice are not protected from challenge after vaccination with VLP.
(A) Mice were treated IM twice with saline or VLP. Four weeks after the second treatment, mice were challenged IP with 1000 pfu of ma-EBOV. Closed symbols represent wild type C57BL/6 mice and open symbols indicated CD4-deficient mice. (B) Two weeks after the second vaccination and one week prior to challenge, blood was collected from vaccinated animals and evaluated for anti-GP IgG antibody titers. N = 5–10/group with survival data shown pooled from two separate studies.
References
- Agnandji S.T., Huttner A., Zinser M.E., Njuguna P., Dahlke C., Fernandes J.F., Yerly S., Dayer J.A., Kraehling V., Kasonta R., Adegnika A.A., Altfeld M., Auderset F., Bache E.B., Biedenkopf N., Borregaard S., Brosnahan J.S., Burrow R., Combescure C., Desmeules J., Eickmann M., Fehling S.K., Finckh A., Goncalves A.R., Grobusch M.P., Hooper J., Jambrecina A., Kabwende A.L., Kaya G., Kimani D., Lell B., Lemaitre B., Lohse A.W., Massinga-Loembe M., Matthey A., Mordmuller B., Nolting A., Ogwang C., Ramharter M., Schmidt-Chanasit J., Schmiedel S., Silvera P., Stahl F.R., Staines H.M., Strecker T., Stubbe H.C., Tsofa B., Zaki S., Fast P., Moorthy V., Kaiser L., Krishna S., Becker S., Kieny M.P., Bejon P., Kremsner P.G., Addo M.M., Siegrist C.A. Phase 1 trials of rVSV ebola vaccine in Africa and Europe — preliminary report. N. Engl. J. Med. 2015 doi: 10.1056/NEJMoa1502924. (ePub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappab by Toll-Like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Audet J., Wong G., Wang H., Lu G., Gao G.F., Kobinger G., Qiu X. Molecular characterization of the monoclonal antibodies composing zmab: a protective cocktail against Ebola virus. Sci. Rep. 2014;4:6881. doi: 10.1038/srep06881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale S., Dias J.M., Fusco M.L., Hashiguchi T., Wong A.C., Liu T., Keuhne A.I., Li S., Woods V.L., Jr., Chandran K., Dye J.M., Saphire E.O. Structural basis for differential neutralization of ebolaviruses. Viruses. 2012;4:447–470. doi: 10.3390/v4040447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner C.K., Malherbe L.P. Regulation of CD4 T-cell receptor diversity by vaccine adjuvants. Immunology. 2010;130:16–22. doi: 10.1111/j.1365-2567.2010.03265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran J. Safety and immunogenicity of a new Hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients. Expert. Opin. Biol. Ther. 2008;8:235–247. doi: 10.1517/14712598.8.2.235. [DOI] [PubMed] [Google Scholar]
- Bohannon J.K., Hernandez A., Enkhbaatar P., Adams W.L., Sherwood E.R. The immunobiology of Toll-Like receptor 4 agonists: from endotoxin tolerance to immunoadjuvants. Shock. 2013;40:451–462. doi: 10.1097/SHK.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M., Davis K., Geisbert T., Schmaljohn C., Huggins J. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J. Infect. Dis. 1998;178:651–661. doi: 10.1086/515386. [DOI] [PubMed] [Google Scholar]
- Brown D.M., Lee S., Garcia-Hernandez Mde L., Swain S.L. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J. Virol. 2012;86:6792–6803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner R., Jensen-Jarolim E., Pali-Scholl I. The ABC of clinical and experimental adjuvants—a brief overview. Immunol. Lett. 2010;128:29–35. doi: 10.1016/j.imlet.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caproni E., Tritto E., Cortese M., Muzzi A., Mosca F., Monaci E., Baudner B., Seubert A., De Gregorio E. MF59 and Pam3CSK4 boost adaptive responses to influenza subunit vaccine through an IFN type I-independent mechanism of Action. J. Immunol. 2012;188:3088–3098. doi: 10.4049/jimmunol.1101764. [DOI] [PubMed] [Google Scholar]
- Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll K.L., Harty J.T. Correlates of protective immunity following whole sporozoite vaccination against malaria. Immunol. Res. 2014;59:166–176. doi: 10.1007/s12026-014-8525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye J.M., Herbert A.S., Kuehne A.I., Barth J.F., Muhammad M.A., Zak S.E., Ortiz R.A., Prugar L.I., Pratt W.D. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5034–5039. doi: 10.1073/pnas.1200409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein M.H., Levin M.J., Chatterjee A., Chakhtoura N., Takacs P., Catteau G., Dessy F.J., Moris P., Lin L., Struyf F., Dubin G., Group, H. P. V. S. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: follow-up through month 48 in a Phase III randomized study. Hum. Vaccines Immunother. 2014;10:3455–3465. doi: 10.4161/hv.36117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein M.H., Takacs P., Chatterjee A., Sperling R.S., Chakhtoura N., Blatter M.M., Lalezari J., David M.P., Lin L., Struyf F., Dubin G., Group, H. P. V. S. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: end-of-study analysis of a Phase III randomized trial. Hum. Vaccines Immunother. 2014;10:3435–3445. doi: 10.4161/hv.36121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F.D., Katona I.M., Mosmann T.R., Coffman R.L. IFN-Gamma Regulates the isotypes of Ig secreted during in vivo humoral immune responses. J. Immunol. 1988;140:1022–1027. [PubMed] [Google Scholar]
- Grant-Klein R.J., Altamura L.A., Badger C.V., Bounds C.E., Van Deusen N.M., Kwilas S.A., Vu H.A., Warfield K.L., Hooper J.W., Hannaman D., Dupuy L.C., Schmaljohn C.S. Codon-optimized Filovirus DNA Vaccines Delivered by Intramuscular Electroporation Protect Cynomolgus Macaques from Lethal Ebola and Marburg Virus Challenges. Hum. Vaccines Immunother. 2015;11(8):1991–2004. doi: 10.1080/21645515.2015.1039757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K. Aluminum compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 1998;32:155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- Guven E., Duus K., Laursen I., Hojrup P., Houen G. Aluminum hydroxide adjuvant differentially activates the three complement pathways with major involvement of the alternative pathway. PLoS One. 2013;8:e74445. doi: 10.1371/journal.pone.0074445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M.C., Crespo M.P., Abraham W., Moynihan K.D., Szeto G.L., Chen S.H., Melo M.B., Mueller S., Irvine D.J. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Invest. 2015;125:2532–2546. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann G., Battiany J., Poeck H., Wagner M., Kerkmann M., Lubenow N., Rothenfusser S., Endres S. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur. J. Immunol. 2003;33:1633–1641. doi: 10.1002/eji.200323813. [DOI] [PubMed] [Google Scholar]
- Henao-Tamayo M.I., Ordway D.J., Irwin S.M., Shang S., Shanley C., Orme I.M. Phenotypic definition of effector and memory T-lymphocyte subsets in mice chronically infected with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 2010;17:618–625. doi: 10.1128/CVI.00368-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikono H., Kohlmeier J.E., Takamura S., Wittmer S.T., Roberts A.D., Woodland D.L. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8 + T cells. J. Exp. Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenesch H. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine. 2002;20(Suppl. 3):S34–S39. doi: 10.1016/s0264-410x(02)00169-x. [DOI] [PubMed] [Google Scholar]
- Hutchison S., Benson R.A., Gibson V.B., Pollock A.H., Garside P., Brewer J.M. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012;26:1272–1279. doi: 10.1096/fj.11-184556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., O'Hagan D.T., Singh M. The long-term potential of biodegradable poly(lactide-co-glycolide) microparticles as the next-generation vaccine adjuvant. Expert Rev. Vaccines. 2011;10:1731–1742. doi: 10.1586/erv.11.126. [DOI] [PubMed] [Google Scholar]
- Johnson S., Eller M., Teigler J.E., Maloveste S.M., Schultz B.T., Soghoian D.Z., Lu R., Oster A.F., Chenine A.L., Alter G., Dittmer U., Marovich M., Robb M.L., Michael N.L., Bolton D., Streeck H. Cooperativity of HIV-specific cytolytic CD4 + T cells and CD8 + T cells in control of HIV viremia. J. Virol. 2015;89(15):7494–7505. doi: 10.1128/JVI.00438-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmuller K., Espinosa D.A., Trager L., Stoyanov C., Salazar A.M., Pokalwar S., Singh S., Dutta S., Ockenhouse C.F., Zavala F., Seder R.A. Full-length P. falciparum circumsporozoite protein administered with poly-ICLC or GLA/SE elicits potent antibody and CD4 + T cell immunity and protection in Mice. Infect. Immun. 2012;81(3):789–800. doi: 10.1128/IAI.01108-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok T., Gaeguta A., Finnie J., Gorry P.R., Churchill M., Li P. Designer antigens for elicitation of broadly neutralizing antibodies against HIV. Clin. Transl. Immunol. 2014;3:e24. doi: 10.1038/cti.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan L., Gurnani K., Dicaire C.J., van Faassen H., Zafer A., Kirschning C.J., Sad S., Sprott G.D. Rapid clonal expansion and prolonged maintenance of memory CD8 + T cells of the effector (CD44highCD62Llow) and central (CD44highCD62Lhigh) phenotype by an archaeosome adjuvant independent of TLR2. J. Immunol. 2007;178:2396–2406. doi: 10.4049/jimmunol.178.4.2396. [DOI] [PubMed] [Google Scholar]
- Lee J.E., Fusco M.L., Hessell A.J., Oswald W.B., Burton D.R., Saphire E.O. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature. 2008;454:177–182. doi: 10.1038/nature07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L., Masopust D. T cell immunity in lymphoid and non-lymphoid tissues. Curr. Opin. Immunol. 2002;14:503–508. doi: 10.1016/s0952-7915(02)00360-6. [DOI] [PubMed] [Google Scholar]
- Levy H.B., Baer G., Baron S., Buckler C.E., Gibbs C.J., Iadarola M.J., London W.T., Rice J. A modified polyriboinosinic-polyribocytidylic acid complex that induces interferon in primates. J. Infect. Dis. 1975;132:434–439. doi: 10.1093/infdis/132.4.434. [DOI] [PubMed] [Google Scholar]
- Longhi M.P., Trumpfheller C., Idoyaga J., Caskey M., Matos I., Kluger C., Salazar A.M., Colonna M., Steinman R.M. Dendritic cells require a systemic type I interferon response to mature and induce CD4 + Th1 immunity with poly IC as adjuvant. J. Exp. Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Mckee A.S., Munks M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J.D., Fearon K., Abbate C., Subramanian S., Yee P., Gregorio J., Coffman R.L., Van Nest G. Identification of a novel cpg DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J. Leukoc. Biol. 2003;73:781–792. doi: 10.1189/jlb.1202630. [DOI] [PubMed] [Google Scholar]
- Martins K.A., Steffens J.T., van Tongeren S.A., Wells J.B., Bergeron A.A., Dickson S.P., Dye J.M., Salazar A.M., Bavari S. Toll-like receptor agonist augments virus-like particle-mediated protection from Ebola virus with transient immune activation. PLoS One. 2014;9:e89735. doi: 10.1371/journal.pone.0089735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins K., Carra J.H., Cooper C.L., Kwilas S.A., Robinson C.G., Shurtleff A.C., Schokman R.D., Kuehl K.A., Wells J.B., Steffens J.T., van Tongeren S.A., Hooper J.W., Bavari S. Cross-protection conferred by filovirus virus-like particles containing trimeric hybrid glycoprotein. Viral Immunol. 2015;28:62–70. doi: 10.1089/vim.2014.0071. [DOI] [PubMed] [Google Scholar]
- Martins K.A., Bavari S., Salazar A.M. Vaccine adjuvant uses of poly-ic and derivatives. Expert Rev. Vaccines. 2015;14:447–459. doi: 10.1586/14760584.2015.966085. [DOI] [PubMed] [Google Scholar]
- Marzi A., Engelmann F., Feldmann F., Haberthur K., Shupert W.L., Brining D., Scott D.P., Geisbert T.W., Kawaoka Y., Katze M.G., Feldmann H., Messaoudi I. Antibodies are necessary for rvsv/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc. Natl. Acad. Sci. U. S. A. 2013;110:1893–1898. doi: 10.1073/pnas.1209591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleer J.P., Vella A.T. Educating CD4 T cells with vaccine adjuvants: lessons from lipopolysaccharide. Trends Immunol. 2010;31:429–435. doi: 10.1016/j.it.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry K.K., Strutt T.M., Kuang Y., Brown D.M., Sell S., Dutton R.W., Swain S.L. Memory CD4 + T cells protect against influenza through multiple synergizing mechanisms. J. Clin. Invest. 2012;122:2847–2856. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza D., Migueles S.A., Rood J.E., Peterson B., Johnson S., Doria-Rose N., Schneider D., Rakasz E., Trivett M.T., Trubey C.M., Coalter V., Hallahan C.W., Watkins D., Franchini G., Lifson J.D., Connors M. Cytotoxic capacity of SIV-specific CD8(+) T cells against primary autologous targets correlates with immune control in SIV-infected rhesus macaques. PLoS Pathog. 2013;9:e1003195. doi: 10.1371/journal.ppat.1003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaci E., Mancini F., Lofano G., Bacconi M., Tavarini S., Sammicheli C., Arcidiacono L., Giraldi M., Galletti B., Rossi Paccani S., Torre A., Fontana M.R., Grandi G., De Gregorio E., Bensi G., Chiarot E., Nuti S., Bagnoli F., Soldaini E., Bertholet S. MF59- and Al(OH)3-Adjuvanted Staphylococcus aureus (4C-Staph) Vaccines Induce Sustained Protective Humoral and Cellular Immune Responses, with a Critical Role for Effector CD4 T Cells at Low Antibody Titers. Front. Immunol. 2015;6:439. doi: 10.3389/fimmu.2015.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J.J., Suh H., Li A.V., Ockenhouse C.F., Yadava A., Irvine D.J. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes M.M., Tytell A.A., Lampson G.P., Field A.K., Hilleman M.R. Inducers of interferon and host resistance. VI. Antiviral efficacy of poly I:C in animal models. Proc. Soc. Exp. Biol. Med. 1969;132:776–783. doi: 10.3181/00379727-132-34308. [DOI] [PubMed] [Google Scholar]
- O'Hagan D.T., Fox C.B. New generation adjuvants — From empiricism to rational design. Vaccine. 2015;33(Suppl. 2):B14–B20. doi: 10.1016/j.vaccine.2015.01.088. [DOI] [PubMed] [Google Scholar]
- Olinger G.G., Bailey M.A., Dye J.M., Bakken R., Kuehne A., Kondig J., Wilson J., Hogan R.J., Hart M.K. Protective cytotoxic T-cell responses induced by Venezuelan equine encephalitis virus replicons expressing Ebola virus proteins. J. Virol. 2005;79:14189–14196. doi: 10.1128/JVI.79.22.14189-14196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald W.B., Geisbert T.W., Davis K.J., Geisbert J.B., Sullivan N.J., Jahrling P.B., Parren P.W., Burton D.R. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLoS Pathog. 2007;3:e9. doi: 10.1371/journal.ppat.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret R., Sierro S.R., Botelho N.K., Corgnac S., Donda A., Romero P. Adjuvants that improve the ratio of antigen-specific effector to regulatory T cells enhance tumor immunity. Cancer Res. 2013;73:6597–6608. doi: 10.1158/0008-5472.CAN-13-0875. [DOI] [PubMed] [Google Scholar]
- Pitoiset F., Vazquez T., Bellier B. Enveloped virus-like particle platforms: Vaccines of the future? Expert Rev. Vaccines. 2015:1–3. doi: 10.1586/14760584.2015.1046440. [DOI] [PubMed] [Google Scholar]
- Plotkin S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Audet J., Wong G., Pillet S., Bello A., Cabral T., Strong J.E., Plummer F., Corbett C.R., Alimonti J.B., Kobinger G.P. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci. Transl. Med. 2012;4:138ra81. doi: 10.1126/scitranslmed.3003876. [DOI] [PubMed] [Google Scholar]
- Quinn K.M., Da Costa A., Yamamoto A., Berry D., Lindsay R.W., Darrah P.A., Wang L., Cheng C., Kong W.P., Gall J.G., Nicosia A., Folgori A., Colloca S., Cortese R., Gostick E., Price D.A., Gomez C.E., Esteban M., Wyatt L.S., Moss B., Morgan C., Roederer M., Bailer R.T., Nabel G.J., Koup R.A., Seder R.A. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8 + T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 2013;190:2720–2735. doi: 10.4049/jimmunol.1202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn K.M., Yamamoto A., Costa A., Darrah P.A., Lindsay R.W., Hegde S.T., Johnson T.R., Flynn B.J., Lore K., Seder R.A. Coadministration of polyinosinic:polycytidylic acid and immunostimulatory complexes modifies antigen processing in dendritic cell subsets and enhances HIV gag-specific T cell immunity. J. Immunol. 2013;191:5085–5096. doi: 10.4049/jimmunol.1301730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M., Bray M., Alving C.R., Jahrling P., Matyas G.R. Induction of immune responses in mice and monkeys to Ebola virus after immunization with liposome-encapsulated irradiated Ebola virus: protection in mice requires CD4(+) T cells. J. Virol. 2002;76:9176–9185. doi: 10.1128/JVI.76.18.9176-9185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C.J., Brey R.N., Mantis N.J., Mapes K., Pop I.V., Pop L.M., Ruback S., Killeen S.Z., Doyle-Meyers L., Vinet-Oliphant H.S., Didier P.J., Vitetta E.S. Thermostable ricin vaccine protects rhesus macaques against aerosolized ricin: Epitope-specific neutralizing antibodies correlate with protection. Proc. Natl. Acad. Sci. U. S. A. 2015;112:3782–3787. doi: 10.1073/pnas.1502585112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant A.J., McMichael A. Revealing the role of CD4(+) T cells in viral immunity. J. Exp. Med. 2012;209:1391–1395. doi: 10.1084/jem.20121517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R., Reed S.G., O'Hagan D., Malyala P., D'Oro U., Laera D., Abrignani S., Cerundolo V., Steinman L., Bertholet S. Gaps in knowledge and prospects for research of adjuvanted vaccines. Vaccine. 2015;33(Suppl. 2):B40–B43. doi: 10.1016/j.vaccine.2015.03.057. [DOI] [PubMed] [Google Scholar]
- Shedlock D.J., Aviles J., Talbott K.T., Wong G., Wu S.J., Villarreal D.O., Myles D.J., Croyle M.A., Yan J., Kobinger G.P., Weiner D.B. Induction of broad cytotoxic T cells by protective DNA vaccination against Marburg and Ebola. Mol. Ther. 2013;21:1432–1444. doi: 10.1038/mt.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C.M., Paul W.E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Soghoian D.Z., Streeck H. Cytolytic CD4(+) T cells in viral immunity. Expert Rev. Vaccines. 2010;9:1453–1463. doi: 10.1586/erv.10.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovska A., Hem S.L., Hogenesch H. Activation of dendritic cells and induction of CD4(+) T cell differentiation by aluminum-containing adjuvants. Vaccine. 2007;25:4575–4585. doi: 10.1016/j.vaccine.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Sridhar S., Begom S., Bermingham A., Hoschler K., Adamson W., Carman W., Bean T., Barclay W., Deeks J.J., Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013;19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- Stahl-Hennig C., Eisenblatter M., Jasny E., Rzehak T., Tenner-Racz K., Trumpfheller C., Salazar A.M., Uberla K., Nieto K., Kleinschmidt J., Schulte R., Gissmann L., Muller M., Sacher A., Racz P., Steinman R.M., Uguccioni M., Ignatius R. Synthetic double-stranded rnas are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 2009;5:e1000373. doi: 10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen F., Kinjo T., Bode C., Klinman D.M. TLR-based immune adjuvants. Vaccine. 2011;29:3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N.J., Martin J.E., Graham B.S., Nabel G.J. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat. Rev. Microbiol. 2009;7:393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N.J., Hensley L., Asiedu C., Geisbert T.W., Stanley D., Johnson J., Honko A., Olinger G., Bailey M., Geisbert J.B., Reimann K.A., Bao S., Rao S., Roederer M., Jahrling P.B., Koup R.A., Nabel G.J. CD8 + cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat. Med. 2011;17:1128–1131. doi: 10.1038/nm.2447. [DOI] [PubMed] [Google Scholar]
- Surquin M., Tielemans C., Nortier J., Jadoul M., Peeters P., Ryba M., Roznovsky L., Doman J., Barthelemy X., Crasta P.D., Messier M., Houard S. Anti-HBs antibody persistence following primary vaccination with an investigational AS02(v)-adjuvanted hepatitis B vaccine in patients with renal insufficiency. Hum. Vaccines. 2011;7:913–918. doi: 10.4161/hv.7.9.16225. [DOI] [PubMed] [Google Scholar]
- Swenson D.L., Warfield K.L., Kuehl K., Larsen T., Hevey M.C., Schmaljohn A., Bavari S., Aman M.J. Generation of Marburg virus-like particles by co-expression of glycoprotein and matrix protein. FEMS Immunol. Med. Microbiol. 2004;40:27–31. doi: 10.1016/S0928-8244(03)00273-6. [DOI] [PubMed] [Google Scholar]
- Swenson D.L., Warfield K.L., Negley D.L., Schmaljohn A., Aman M.J., Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine. 2005;23:3033–3042. doi: 10.1016/j.vaccine.2004.11.070. [DOI] [PubMed] [Google Scholar]
- Swenson D.L., Wang D., Luo M., Warfield K.L., Woraratanadharm J., Holman D.H., Dong J.Y., Pratt W.D. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin. Vaccine Immunol. 2008;15:460–467. doi: 10.1128/CVI.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson D.L., Warfield K.L., Larsen T., Alves D.A., Coberley S.S., Bavari S. Monovalent virus-like particle vaccine protects guinea pigs and nonhuman primates against infection with multiple Marburg viruses. Expert Rev. Vaccines. 2008;7:417–429. doi: 10.1586/14760584.7.4.417. [DOI] [PubMed] [Google Scholar]
- Trumpfheller C., Caskey M., Nchinda G., Longhi M.P., Mizenina O., Huang Y., Schlesinger S.J., Colonna M., Steinman R.M. The microbial mimic poly IC induces durable and protective CD4 + T cell immunity together with a dendritic cell targeted vaccine. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gils M.J., Sanders R.W. In vivo protection by broadly neutralizing HIV antibodies. Trends Microbiol. 2014;22:550–551. doi: 10.1016/j.tim.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Verthelyi D., Ishii K.J., Gursel M., Takeshita F., Klinman D.M. Human peripheral blood cells differentially recognize and respond to two distinct CPG motifs. J. Immunol. 2001;166:2372–2377. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- Wang Y., Cella M., Gilfillan S., Colonna M. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J. Immunol. 2010;184:2751–2755. doi: 10.4049/jimmunol.0903201. [DOI] [PubMed] [Google Scholar]
- Warfield K.L., Bosio C.M., Welcher B.C., Deal E.M., Mohamadzadeh M., Schmaljohn A., Aman M.J., Bavari S. Ebola virus-like particles protect from lethal Ebola virus infection. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15889–15894. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield K.L., Swenson D.L., Negley D.L., Schmaljohn A.L., Aman M.J., Bavari S. Marburg virus-like particles protect guinea pigs from lethal Marburg virus infection. Vaccine. 2004;22:3495–3502. doi: 10.1016/j.vaccine.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Warfield K.L., Swenson D.L., Olinger G.G., Kalina W.V., Aman M.J., Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J. Infect. Dis. 2007;196(Suppl. 2):s430–s437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- Watkins D.I. The hope for an HIV vaccine based on induction of CD8 + T lymphocytes–a review. Mem. Inst. Oswaldo Cruz. 2008;103:119–129. doi: 10.1590/s0074-02762008000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson T.M., Li C.K., Chui C.S., Huang A.K., Perkins M., Liebner J.C., Lambkin-Williams R., Gilbert A., Oxford J., Nicholas B., Staples K.J., Dong T., Douek D.C., Mcmichael A.J., Xu X.N. Preexisting influenza-specific CD4 + T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- Wong G., Richardson J.S., Pillet S., Patel A., Qiu X., Alimonti J., Hogan J., Zhang Y., Takada A., Feldmann H., Kobinger G.P. Immune parameters correlate with protection against Ebola virus infection in rodents and nonhuman primates. Sci. Transl. Med. 2012;4:158ra146. doi: 10.1126/scitranslmed.3004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Audet J., Fernando L., Fausther-Bovendo H., Alimonti J.B., Kobinger G.P., Qiu X. Immunization with vesicular stomatitis virus vaccine expressing the Ebola glycoprotein provides sustained long-term protection in rodents. Vaccine. 2014;32:5722–5729. doi: 10.1016/j.vaccine.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zens K.D., Farber D.L. Memory CD4 T cells in influenza. Curr. Top. Microbiol. Immunol. 2015;386:399–421. doi: 10.1007/82_2014_401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Callendret B., Xu D., Brasky K.M., Feng Z., Hensley L.L., Guedj J., Perelson A.S., Lemon S.M., Lanford R.E., Walker C.M. Dominance of the CD4(+) T helper cell response during acute resolving hepatitis A virus infection. J. Exp. Med. 2012;209:1481–1492. doi: 10.1084/jem.20111906. [DOI] [PMC free article] [PubMed] [Google Scholar]