Abstract
Indices of cardiovascular autonomic neuropathy (CAN) in experimental models of Type 1 diabetes mellitus (T1DM) are often contrary to clinical data. Here, we investigated whether a relatable insulin-treated model of T1DM would induce deficits in cardiovascular (CV) autonomic function more reflective of clinical results and if exercise training could prevent those deficits. Sixty-four rats were divided into four groups: sedentary control (C), sedentary T1DM (D), control exercise (CX), or T1DM exercise (DX). Diabetes was induced via multiple low-dose injections of streptozotocin and blood glucose was maintained at moderate hyperglycemia (9–17 mM) through insulin supplementation. Exercise training consisted of daily treadmill running for 10 weeks. Compared to C, D had blunted baroreflex sensitivity, increased vascular sympathetic tone, increased serum neuropeptide Y (NPY), and decreased intrinsic heart rate. In contrast, DX differed from D in all measures of CAN (except NPY), including heart rate variability. These findings demonstrate that this T1DM model elicits deficits and exercise-mediated improvements to CV autonomic function which are reflective of clinical T1DM.
1. Introduction
A common and serious complication of Type 1 diabetes mellitus (T1DM) is diabetic autonomic neuropathy [1, 2]. Cardiovascular autonomic neuropathy (CAN) is a subset of diabetic autonomic neuropathy characterized by impaired autonomic control of the cardiovascular (CV) system [3]. CAN is also consistently associated with increased mortality. For instance, CAN has been reported to increase the mortality of diabetic patients by a factor of 3.45 [4]. Clinically, the most common methods for assessing CAN are heart rate variability (HRV) analysis and baroreflex sensitivity (BRS) [3, 5, 6]. In T1DM, aspects of the baroreflex arc can be impaired [7], such that both baroreceptor activity and excitability are blunted [8, 9] and the aortic depressor nerves undergo axonal atrophy [8]. As well, autonomic efferents, primarily of the parasympathetic nervous system (PSNS), have decreased activity, reduced responsiveness, and decreased neurochemical activity in the heart [10, 11]. Impairment of central nervous system regions has also been reported as the limiting factor of BRS [12, 13]. Reduced heart rate variability (HRV) is often the earliest symptom of CAN [14]. Whether measured by time domain analysis or by frequency domain analysis and whether in clinical or experimental T1DM, HRV is consistently reported to be reduced in T1DM [5, 14–17].
Exercise has been demonstrated to be an effective means of improving deficits in HRV and BRS in both clinical and experimental T1DM [18–21]. Such improvements have been attributed to improved insulin sensitivity, increased endogenous antioxidant and anti-inflammatory mediators, and improved autonomic control of the CV system [22–24]. Despite similar reductions in HRV and BRS, there are marked differences in early-stage changes to other CV parameters between clinical and experimental T1DM [25]. Specifically, in clinical T1DM, increases in heart rate (HR) and blood pressure (BP) are commonly reported in early autonomic neuropathy [1, 3, 14, 24, 26–28]. In contrast, experimental STZ-induced T1DM is regularly associated with decreased BP and HR, beginning shortly after diabetes induction [15, 16, 29–31]. Due to these opposing initial changes in BP and HR, exercise training is often observed to produce contrasting outcomes on CV parameters in experimental and clinical T1DM, namely, increased BP and HR in experimental T1DM and decreased BP and HR in clinical T1DM [19, 25, 32–34]. As a result, both the increase and decrease of these CV factors are concurrently cited as exercise-mediated improvements to CAN with little consideration of the fact that the changes are opposed between these two contexts of T1DM [25]. This is important because if animal models do not accurately reproduce T1DM pathology, then the outcomes of experimental studies may not translate to the treatment of human CAN, as the mechanisms underlying the pathology and exercise modifications may differ.
Another important difference between experimental and clinical T1DM is the common omission of insulin treatment in experimental diabetes leading to severe hyperglycemia ranging from roughly 17 to 25 mM blood glucose concentrations ([BG]) [15, 16, 29, 30]. As the severity and duration of hyperglycemia have been shown to influence the degree of diabetic neuropathy, acute and steep elevations of [BG] in STZ-induced T1DM may not only cause early onset neuropathy to the PSNS but also cause acute neuropathy of the sympathetic nervous system (SNS) and directly affect the sinoatrial (SA) node. These changes may mediate the observed reduction in BP and HR that arise acutely in experimental T1DM and in late-stage clinical T1DM [2, 35–38]. Indeed, intensive insulin therapy has been shown to restore BP and HR to non-T1DM levels in STZ-induced T1DM rats [25, 39].
Yet, despite the use of insulin therapy in clinical T1DM, it is often the case that chronic, moderate hyperglycemia is maintained as a result of difficulties in regulating [BG] in response to dynamic influences on glycemic control, such as food intake and exercise [40, 41]. This is often resultant of a tendency to err on the side of moderate hyperglycemia in order to circumvent the acute discomfort and danger associated with hypoglycemic episodes, which occur more frequently with diabetic neuropathy due to the impairment of the glucagon response [40, 42, 43]. To address this, our laboratory established a model of T1DM using a multiple low-dose STZ-treatment and insulin therapy to replicate the moderate hyperglycemia observed in clinical T1DM [44]. In our previous studies that employed this model, we observed impairments in glucose tolerance, vascular responsiveness, cardiac function, and bone health, which were improved with high intensity aerobic exercise training [44–47].
The purpose of the current study was to investigate whether our model of multiple low-dose STZ-induced T1DM with insulin therapy would induce deficits in cardiovascular autonomic function more representative of clinical T1DM, and if high intensity aerobic training could prevent those deficits. We hypothesised that (1) our model of STZ-induced T1DM would elicit indices of CAN, including a blunted BRS (bradycardia and tachycardia response), lowered HRV and intrinsic heart rate, increased vascular sympathetic tone, and increased mean arterial pressure, and (2) high intensity aerobic exercise training would prevent or ameliorate the indications of CAN.
2. Materials and Methods
2.1. Ethics Approval
The protocols used in this investigation were approved by the University of Western Ontario Council on Animal Care and conformed to the guidelines of the Canadian Council on Animal Care.
2.2. Animals
Eight-week-old male Sprague-Dawley rats were obtained from Charles River Laboratories Canada (Saint-Constant, Quebec). The rats were housed in pairs and maintained on a 12-hour dark/light cycle at a constant temperature (20 ± 1°C) and relative humidity (50%). Rats were allowed access to standard rat chow and water ad libitum.
2.3. Experimental Groups
Sixty-four rats were randomly assigned to one of four groups as follows: (1) sedentary control (C, n = 16); (2) exercised control (CX, n = 16); (3) sedentary T1DM (D, n = 16); (4) exercised T1DM (DX, n = 16). All functional and blood endpoint measures were acquired 24 hours after the final exercise bout.
2.4. T1DM Induction and Insulin Dose
Upon arrival rats were acclimatized to the laboratory setting for five days. Subsequently, T1DM was induced over five consecutive days by multiple intraperitoneal (IP) injections of 20 mg/kg streptozotocin (STZ, Sigma-Aldrich) dissolved in a citrate buffer (0.1 M, pH 4.5). Diabetes was confirmed by blood glucose measurements greater than or equal to 18 mM on two consecutive days. If necessary, subsequent 20 mg/kg STZ injections were administered until diabetes was confirmed. Following the confirmation of diabetes, insulin pellets (1 pellet; 2 U insulin/day; Linplant, Linshin Canada, Inc., Toronto, Ontario, Canada) were implanted subcutaneously in the abdominal region. Insulin pellet doses were then monitored for 1 week and adjusted (±0.5 pellets) in order to obtain daily nonfasting blood glucose concentrations in the moderate hyperglycemic range of 9–17 mM. Insulin dose was determined by multiplying the total quantity of pellet implanted (0.5 pellet increments) by the amount of insulin released per pellet (2 units of insulin/day/pellet) divided by the body weight (Kg) of the rat.
2.5. Body Weight and Blood Glucose Concentration
Body weights and nonfasting blood glucose concentrations were obtained weekly. Blood was obtained from the saphenous vein by venous puncture with a 30-gauge needle and measured via Freestyle Lite Blood Glucose Monitoring System (Abbott Diabetes Care Inc., Mississauga, Ontario, Canada).
2.6. Intravenous Glucose Tolerance Test
Intravenous glucose tolerance tests (IVGTT) were performed on all animals prior to T1DM induction (pre-T1DM) and at the end of week 10 of the exercise training period. Rats were fasted for approximately 8 to 12 hours prior to the assay and did not perform exercise on the day of their IVGTT. A sterile-filtered dextrose solution (50% dextrose, 50% ddH2O) was injected (1 g/kg) into the lateral tail vein of the conscious rat. Following dextrose infusion, blood glucose was measured at 5 minutes, at 10 minutes, and then at 10-minute intervals thereafter until blood glucose levels plateaued.
2.7. Exercise Protocol
Prior to the initiation of the exercise training program, rats were familiarized with the exercise equipment on two consecutive days. The familiarization consisted of two 15-minute sessions of running at progressive treadmill speeds up to 30 meters per minute (m/min). The treadmill was a custom-built apparatus fabricated by the physical plant at University of Western Ontario and has been used in many previous studies [44–47]. The exercise training program consisted of 1 hour of motor-driven treadmill running per day at 27 m/min with a 6-degree incline, 5 days per week, for 10 weeks. The exercise intensity was determined based on earlier research that investigated oxygen uptake in rats at various treadmill speeds. The chosen intensity was found to represent approximately 75–85% VO2max [48, 49].
2.8. Preparative Surgery and Instrumentation
To achieve a surgical plane of anesthesia, rats were placed in an induction chamber circulating 4% isoflurane (96% O2). Once motor reflexes were undetectable, rats were transferred to a nosecone delivering 3% isoflurane (97% O2) and placed on a hot water pad (37°C). Rats were cannulated with saline-infused polyethylene (PE90) catheters in the right jugular vein and carotid artery and each catheter was attached to a three-way stopcock. The jugular vein catheter was used for drug infusions and the carotid artery catheter was connected in series with a pressure transducer (PX272, Edwards Life Sciences, Irvine, California, USA) for arterial blood pressure measurements.
At the end of the preparative surgery, rats were injected IP with a 25 mg/Kg “cocktail” of urethane (16 mg/mL) and α-chloralose (100 mg/mL), an anesthetic cocktail that has been shown to have the least inhibition of baseline CV control and autonomic function [50]. A total of 10 mL of urethane/α-chloralose was made, 5 mL of which was diluted to 50% with ddH2O and was used as needed to maintain anesthesia throughout data collection. Isoflurane anesthesia was gradually removed, whereby urethane/α-chloralose was the primary anesthesia used during data collection.
2.9. Basal Heart Rate, Systolic Blood Pressure, and Mean Arterial Pressure
Heart rate (HR), systolic blood pressure (SBP), and mean arterial pressure (MAP) were determined from the blood pressure pulse waveform and were collected while the rats were under urethane/α-chloralose anesthesia in the supine position. The pressure transducer was calibrated using a standard analog manometer. Data were obtained using a PowerLab data acquisition system, digitized, and recorded at 1000 Hz using the bundled LabChart 7 Pro software (ADInstruments, Colorado Springs, CO, USA).
2.10. Heart Rate Variability
Prior to drug infusions, 5 minutes of spontaneous electrocardiogram data was sampled at 1000 Hz and analyzed with LabChart HRV analysis software (ADInstruments). Time domain analysis of the standard deviation between normal peak pulses of the pressure pulse waveform (SDNN) was quantified as a measure of the total variability of the HR. Frequency domain analysis of the high frequency (HF) band of the Fast Fourier Transform (FFT) of the data was assessed as an index of parasympathetically mediated HRV.
2.11. Baroreflex Sensitivity
Baroreflex sensitivity (BRS) was assessed using the modified Oxford technique [51, 52]. The BRS was quantified using the slope of the linear regression line representing the linear portion of the sigmoidal heart rate-systolic blood pressure relationship (ΔHR {BPM}/ΔSBP {mmHg}−1) after rapid bolus injections (~5 s) of phenylephrine (PE, 12 μg/Kg, 10 μg/mL) and sodium nitroprusside (SNP, 60 μg/Kg, 110 μg/mL) dissolved in ddH2O. The rationale for this method is detailed by Studinger et al. (2007) [54]. For each drug, the catheter was first filled with a 0.2 mL volume to ensure accuracy of the drug dose. After a stable baseline was obtained, a bolus injection of SNP was rapidly infused and the reflex SNS mediated tachycardia response was measured. The analysis began at the onset of SBP decrease after SNP infusion and ended when SBP reached its nadir. This was followed by a saline flush to washout any remaining SNP in the catheter. After a stable baseline was reestablished, this same procedure was then followed using PE to measure PSNS mediated reflex bradycardia, except that analysis began at the onset of SBP increase and ended when SBP reached its zenith. Responses to PE and SNP were plotted separately and only regression lines (slopes) with correlation coefficients (r) ≥ 0.70 and p < 0.05 were accepted [53, 54].
2.12. Vascular Sympathetic Tone
To measure the sympathetic contribution to baseline vascular resistance, MAP was assessed before and after a bolus injection of the α-adrenergic receptor blocker, prazosin (85 μg/Kg, 500 μg/mL). Following this protocol, animals were euthanized via exsanguination while still under urethane/α-chloralose anesthesia.
2.13. Neuropeptide Y ELISA
To ensure physiological testing did not confound serum neuropeptide Y concentration [NPY] measurement, a subset of animals from each group did not undergo surgery for heart rate variability, baroreflex sensitivity, mean arterial pressure, or vascular sympathetic tone measurements. Rather, at the end of the 10-week exercise training period these animals were anesthetized via intraperitoneal injection of sodium pentobarbital (65 mg/kg) and blood serum samples for [NPY] measurement were collected upon euthanasia. Serum [NPY] was measured using an NPY ELISA kit (USCN Life Sciences Inc.) according to the manufacturer's instructions.
2.14. Intrinsic Heart Rate
A Langendorff preparation was used to measure intrinsic heart rate. Following the euthanasia of animals for blood [NPY] measurement, hearts were extracted and immediately arrested by placing them in ice cold Krebs-Henseleit buffer (KHB). Hearts were cannulated for unpaced retrograde aortic constant flow perfusion (15 mL/min) of coronary arteries with KHB (containing 120 mM NaCl, 4.63 M KCl, 1.17 mM KH2PO4, 1.25 mM CaCl2, 1.2 mM MgCl2, 20 mM NaHCO3, and 8 mM glucose gassed with 95% O2 and 5% CO2) that was maintained at 37°C [55]. Hearts were equilibrated for 30 min to determine baseline intrinsic heart rate.
2.15. Data Analysis and Statistics
Body weight and blood glucose concentrations were compared using a two-way repeated measures ANOVA, while endpoint measures were compared by two-way ANOVA, with the exception of endpoint insulin dose, which was compared using a one-tailed t-test. When significance was found, pairwise comparisons were made using the Fisher LSD post hoc test. Data are represented as mean ± standard error, with a significance level set at p < 0.05.
3. Results
3.1. Animal Characteristics
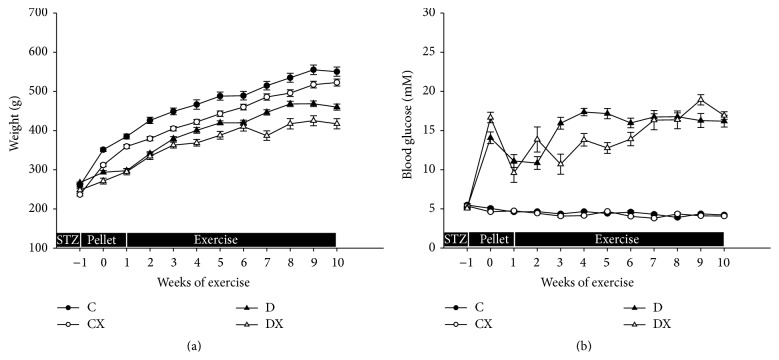
All groups increased in body weight over the course of the study (p < 0.05, Figure 1(a)). At the end of the study, the body weights of the T1DM groups (D and DX) were lower than non-T1DM groups (C and CX), and exercised groups (CX and DX) weighed less than their nonexercised counterparts (C and D; p < 0.05). Following the confirmation of diabetes, weekly [BG] was mostly maintained in the targeted range of 9–17 mM; however, the [BG] did move outside of this range periodically. The [BG] in the T1DM groups were elevated in comparison to the non-T1DM groups (p < 0.05; Figure 1(b)). Within the non-T1DM and T1DM groups, there was no difference in [BG] between nonexercised and exercised groups (C versus CX and D versus DX; p > 0.05).
Figure 1.
(a) Weekly body weights: C, sedentary control (n = 16); CX, control exercise (n = 16); D, sedentary T1DM (n = 15); DX, T1DM exercise (n = 12). (b) Weekly blood glucose concentrations: C (n = 16); CX (n = 15); D (n = 15); DX (n = 13). STZ, pellet, and exercise indicate the periods of STZ injection, insulin pellet implantation, and aerobic exercise, respectively. Significantly different groups (p < 0.05). Data are mean ± SE. There was significant difference in body weight between T1DM and non-T1DM groups, while a significant difference was evident between exercised and nonexercised groups. The blood glucose concentrations in the T1DM groups were significantly different than non-T1DM groups.
3.2. Intravenous Glucose Tolerance Test and Insulin Dosages
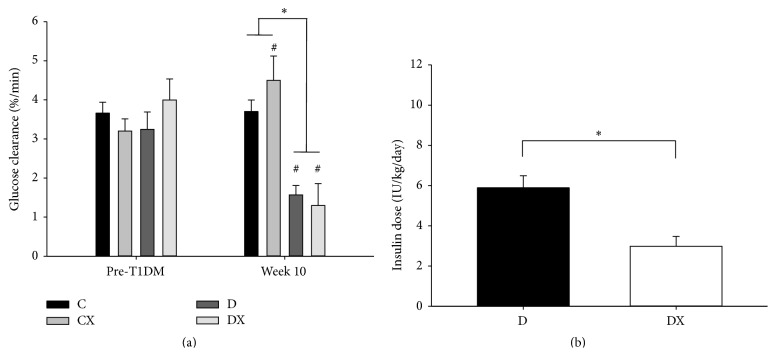
The glucose clearance rate (K G) of the diabetic groups (D and DX) decreased from pre-T1DM to week 10 of training (p < 0.05), whereas K G of the CX group increased (p < 0.05; Figure 2(a)). Both diabetic groups had significantly lower K G values than both the control groups (C and CX) at week 10 (p < 0.05; Figure 2(a)). However, there was not a significant interaction between diabetes and exercise on K G. The amount of insulin supplementation that the DX group received was significantly less than the amount the D group received at week 10 (p < 0.05; Figure 2(b)).
Figure 2.
(a) IVGTT glucose clearance rate (K G) values prior to T1DM induction (pre-T1DM) and week 10 of exercise training: C, sedentary control (n = 16); CX, control exercise (n = 15); D, sedentary T1DM (n = 15); DX, T1DM exercise (n = 15). (b) Insulin dosages at week 10: D (n = 16); DX (n = 16). ∗Significantly different groups (p < 0.05). #Significantly different from week 1. Data are mean ± SE.
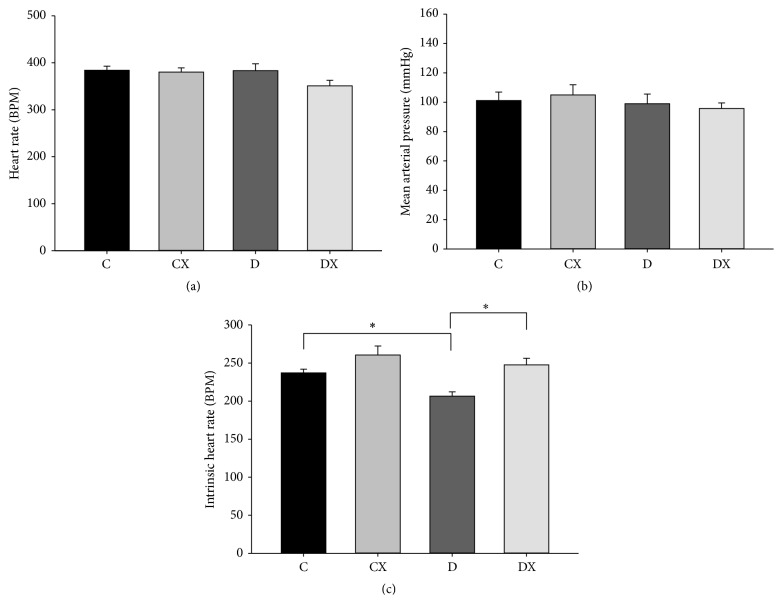
3.3. Mean Arterial Pressure, Heart Rate, and Intrinsic Heart Rate
For resting HR and MAP, there was not a significant difference between groups at week 10 (Figures 3(a) and 3(b), resp.). However, for the intrinsic heart rate (IHR), there was main effect of both exercise and T1DM, where T1DM decreased the IHR, while exercise increased IHR (p < 0.05, Figure 3(c)). Further, within the T1DM groups (D and DX), exercise increased IHR, while within the nonexercised groups (C and D) T1DM decreased IHR (p < 0.05).
Figure 3.
(a) Heart rate (beats per minute) and (b) mean arterial pressure at week 10. C, sedentary control (n = 7); CX, control exercise (n = 7); D, sedentary T1DM (n = 8); DX, T1DM exercise (n = 10). (c) Intrinsic heart rate (IHR) at week 10. C (n = 10); CX (n = 11); D (n = 12); DX (n = 9). ∗Significantly different groups (p < 0.05). Data are mean ± SE.
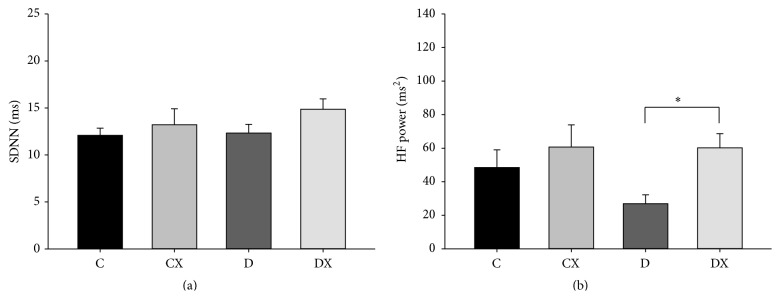
3.4. Heart Rate Variability
Total HRV at week 10, as measured by the standard deviation of the normal pulse wave peaks (SDNN), was not significantly different between groups (Figure 4(a)). However, there was a main effect of exercise on the HF contribution to HRV, where exercise increased HF HRV (p < 0.05, Figure 4(b)). Particularly, within the T1DM groups (D and DX), exercise increased HF HRV (p < 0.05).
Figure 4.
(a) Total HRV (SDNN) at week 10: C, sedentary control (n = 5); CX, control exercise (n = 7); D, sedentary T1DM (n = 8); DX, T1DM exercise (n = 8). (b) High Frequency (HF, parasympathetic) HRV component at week 10: C (n = 6); CX (n = 7); D (n = 8); DX (n = 8). ∗Significantly different groups (p < 0.05). Data are mean ± SE.
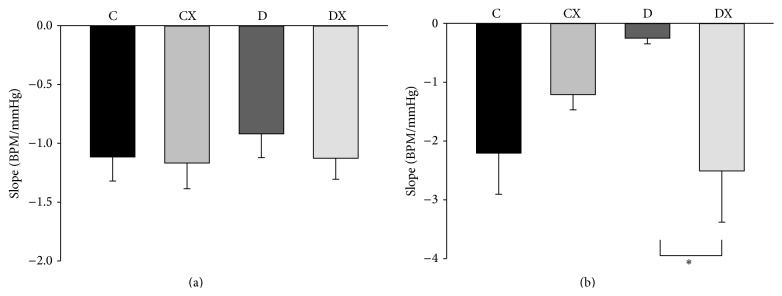
3.5. Baroreflex Sensitivity
In response to SNP infusion, there was not a significant difference between groups in the tachycardia BRS response (Figure 5(a)). However, a significant interaction between T1DM and exercise was observed for BRS during the bradycardia response to phenylephrine (p < 0.05, Figure 5(b)). More specifically, within the T1DM groups (D and DX) exercise prevented the reduction in BRS that was observed in the D group (p < 0.05).
Figure 5.
(a) Tachycardia baroreflex response sensitivity to sodium nitroprusside at week 10: C, sedentary control (n = 7); CX, control exercise (n = 6); D, sedentary T1DM (n = 6); DX, T1DM exercise (n = 10). (b) Bradycardia baroreflex response to phenylephrine at week 10: C (n = 7); CX (n = 5); D (n = 7); DX (n = 10). ∗Significantly different groups (p < 0.05). Data are mean ± SE.
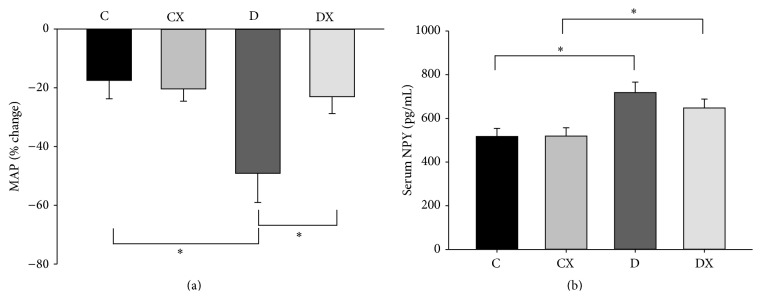
3.6. Vascular Sympathetic Tone and Serum NPY
An interaction between T1DM and exercise was observed for the prazosin-induced change in MAP (p < 0.05, Figure 6(a)). Within the nonexercised groups (C and D), T1DM resulted in an increased change in MAP (p < 0.05). Within the T1DM groups (D and DX) exercise prevented the increased change in MAP observed in the D group (p < 0.05). There was also a main effect of T1DM on [NPY] (p < 0.05, Figure 6(b)). Within the nonexercised groups (C and D) and exercised groups (CX and DX), serum [NPY] was increased by T1DM (p < 0.05).
Figure 6.
(a) Vascular sympathetic tone (VST) at week 10. This was determined by measuring the percent change in MAP after prazosin treatment at week 10: C, sedentary control (n = 8); CX, control exercise (n = 7); D, sedentary T1DM (n = 5); DX, T1DM exercise (n = 7). (b) Serum NPY at week 10: C (n = 6); CX (n = 6); D (n = 6); DX (n = 6). ∗Significantly different groups (p < 0.05). Data are mean ± SE.
4. Discussion
This study demonstrated that a multiple low-dose STZ model with moderate hyperglycemia, maintained using insulin therapy, produced deficits in cardiovascular autonomic function without inducing the resting bradycardia or hypotension typical of other STZ models. This study also showed that high intensity aerobic exercise training can prevent deficits of cardiovascular autonomic function caused by T1DM. Furthermore, because [BG] was held within a moderate hyperglycemic range, the observed exercise-mediated improvements to indications of CAN were independent of changes in [BG] and, instead, may primarily have been the result of improvements to other aspects of glucoregulation and/or the preservation of autonomic nervous system function.
Although we found time domain analysis of total HRV, as measured by the SDNN, did not demonstrate differences between groups, frequency domain analysis exposed a reduction in the HF power in the D group compared with the DX group. Since the HF power corresponds to the level of vagally mediated parasympathetic HRV, these results demonstrate not only the detrimental effects of T1DM on autonomic cardiac control but also the benefits of exercise training toward ameliorating those effects. These findings are similar to those of other experiments of both experimental [16, 21] and clinical diabetes [18, 56, 57]. For example, Mostarda et al. (2009) reported that STZ-induced T1DM reduced the HF component of HRV, which was improved by exercise [21]. Also, they found that the vagal tonus of the control exercised rats did not differ from sedentary controls [21]. Likewise, Chen et al. (2008) reported that children with T1DM who performed a high level of physical activity did not differ from controls in HRV; however, children with T1DM who had low level of physical activity had significantly reduced HRV compared to both active children with T1DM and non-T1DM children [18]. Thus, the current study provides support that exercise can be an effective means to improve HRV in T1DM.
Both tachycardia and bradycardia responses were studied in the context of BRS analysis in order to explore the control features related to unloading or loading of the baroreceptors, respectively. Some discrepancy exists between different experimental models of T1DM and their impact on BRS measures. Investigations using the hyperglycemic Non-Obese Diabetic (NOD) T1DM mouse model have shown elevations in BRS measures rather than attenuated responses [58]. In contrast, tachycardic-SNP and bradycardic-PE responses have been shown to be lower in STZ-induced T1DM hyperglycemic rats in comparison to non-T1DM controls [21] but were improved with exercise training [59]. In the current study, the slope of the hypotensive tachycardia response was not significantly different between any of the groups suggesting that responses to baroreceptor unloading are not affected by T1DM or exercise. However, T1DM reduced the bradycardia response to baroreceptor loading, which was nullified by concurrent exercise training. These findings are in line with previous reports demonstrating a bradycardia change in PE-BRS without an accompanying change in SNP-BRS [60], which was improved following aerobic exercise [61]. Discrepancies in BRS responses in T1DM models seem to be closely associated with both the duration and the severity of diabetes. A recent study examining the time-course of BRS changes in response to STZ-induced hyperglycemia reported that alteration of the SNP-BRS was not evident until 12 weeks of diabetes, while a change in PE-BRS was evident as early as 4 weeks after induction [62]. Interestingly, the animals in the aforementioned study were moderately hyperglycemic (16–18 mM), suggesting that the severity of the hyperglycemia may play a role in the progression of this neuropathy. This relationship has also been demonstrated in humans. Vinik and Ziegler (2007) reported that poor glycemic control [63] and duration of diabetes [64] play a central role in progression of cardiovascular autonomic neuropathy. Yet, it is not clear what role insulin therapy may play in the neuropathy. Insulin supplementation to STZ-induced T1DM rats can modify the changes in BRS sensitivity evident at 48 weeks of T1DM [65]. Indeed, in clinical T1DM patients, intensive therapy is well documented to slow the progression and delay the appearance of abnormal autonomic function [66].
However, the current study provides evidence that the ability of exercise to ameliorate cardiovascular autonomic dysfunction may be independent of its ability to reduce [BG], which challenges the direct relationship between [BG] and CAN suggested by previous studies [67–69]. The IVGTT performed at the conclusion of the 10-week exercise period demonstrated an increased glucose clearance rate (K G) and therefore glucose tolerance, in the CX group compared to the preexercise training period. However, in both the sedentary and exercise diabetic groups there was an equal decline in K G to nearly the same rate. This decrease was significantly different from pre-T1DM values and the week 10 values of the C and CX groups. While this would normally indicate that both of the diabetic groups developed equally impaired glucose tolerance, it was also the case that the DX group required approximately half of the dosage of exogenous insulin compared to the D group to maintain their [BG] in the 9–17 mM range. With double the insulin dose, it is likely that the total serum insulin over a given time during IVGTT would have been greater in the D than DX group, and with their K G being equal, that would indicate that there was a greater insulin sensitivity in the diabetic exercise group [70, 71]. Together, these IVGTT results demonstrate that exercise training improved glucose tolerance and insulin sensitivity [67]. Furthermore, since the [BG] of the diabetic groups in this study was held in a constant range, any abovementioned exercise-induced improvements to CV autonomic function would not have been mediated through a reduction in systemic [BG] but may have been the result of improvements in insulin sensitivity and glucose utilization [72, 73]. This should be borne in mind when considering the effects of diabetes and exercise on indices of CV autonomic function, such as HRV and BRS.
An alternative mechanism by which exercise can influence BRS was reported by Bernardi et al. (2011), who elucidated the importance of tissue oxygenation in T1DM [74]. They demonstrated that a reduced parasympathetic BRS in patients with T1DM was improved by both oxygen supplementation and deep breathing to the same degree, which indicated the increased respiration and oxygen delivery resultant of exercise could have been mediating increases in BRS. This led the authors to suggest that hypoxia in T1DM functionally restrains parasympathetic activity. However, reduced BRS could also be attributed to defects in the baroreceptors, baroreceptor afferent nerves, CNS structures, or efferent fibres of the baroreflex circuit [7, 8, 61]. In the present study, the finding that the tachycardia response of the baroreflex was unimpaired by T1DM, while the bradycardia response was, suggests that the afferent arm and central regulators of the baroreflex were not dysfunctional and that the observed decrement of baroreflex bradycardia may have been caused partly by alterations in efferent parasympathetic outflow [8, 29]. The smaller HF HRV in the D group is consistent with this interpretation.
Another interesting outcome of the current study was the alteration of sympathetic vasomotor control in the D group, which was also modified by concurrent exercise training. In this study, prazosin treatment resulted in a drop in MAP that was approximately twofold greater in the D group compared to the C and DX groups, which is indicative of a much greater sympathetic contribution to the maintenance of baseline vascular resistance [75, 76]. Similarly, Martinez-Nieves and Dunbar (1999) reported that male T1DM rats had a greater decrease in MAP after a bolus injection of prazosin compared to their control cohorts [77]. However, they postulated that an elevated prazosin response could be the result of increased α 1-adrenergic receptor sensitivity [77]. Yet, in this study, the finding that treatment with PE, an α 1-adrenergic receptor agonist, did not result in a greater peak SBP, nor a greater percent increase in SBP from baseline in the T1DM group (data not shown), argues against a receptor-based sensitivity mechanism and, rather, suggests that efferent sympathetic outflow may have been elevated in the D group. However, we cannot determine the mechanism that resulted in prazosin showing a preferential decrease in MAP in the D group versus DX or C based on the data in this study. Yet, in line with the current results, such elevations in resting sympathetic activity would make activation of the BRS response to SNP-induced hypotension more difficult.
The conclusion above regarding sympathetic hyperactivity in the D group is supported by measurements of neuropeptide Y [NPY] obtained in this study. [NPY] is coreleased with norepinephrine from perivascular and cardiac sympathetic nerve terminals during sympathetic activation [78, 79]. In clinical T1DM, a diabetes-related decrease in [NPY] is attributed to impaired sympathetic function, whereas increased [NPY] is attributed to sympathetic overactivity [79–81]. In the current study, serum [NPY] was greater in both of the T1DM groups in comparison to their control groups. This finding is consistent with elevated sympathetic outflow in clinical T1DM [81]. Interestingly, no major impact of exercise was observed on serum [NPY]. Thus, despite the ability of exercise to preserve reflex cardiac function in T1DM, hyperglycemia itself appears to have impacted basal vascular adrenergic activity in both T1DM groups. This observation is consistent with the sympathoexcitatory effect of hyperglycemia [82]. As both T1DM groups were maintained at equally elevated [BG], there may have been a correspondingly similar stimulation of peripheral sympathetic activation and NPY release [79, 82, 83].
Despite improvements by exercise training to deficits of cardiovascular autonomic function, no observable statistical differences in either MAP or HR were evident between any of the groups. Indeed, it has been shown that alterations in autonomic function occur before or without alterations in MAP and HR and are uncorrelated to changes in sympathetic tone [84]. The observed changes in basal sympathetic activity may assist in the maintenance of blood pressure, ventricular function, and cardiac output during the early stage of diabetes, which is supported by our findings that inhibition of sympathetic activity results in a greater decrease in MAP in diabetic rats than normal rats [85]. In that respect, we previously reported that although T1DM animals demonstrated significant alterations in myocardial dimensions and structure, measurements of cardiac performance (ejection fraction, fractional shortening, and cardiac output measurements) were unchanged [44].
To evaluate the heart rate of these animals without neural influence, we measured the beat rate of denervated hearts using the isolated Langendorff technique. We found that the IHR of the D group was lower than both C and DX groups, which would support the notion that decreased IHR masked the effects of sympathetic overactivity in the current study. Further, it supports evidence that STZ-induced diabetes may have a direct effect on heart rate by modifying the heart itself [86, 87]. Interestingly, in some studies, insulin therapy was only able to partially reverse bradycardia and it was shown that STZ-treatment itself could lengthen the action potential duration in the SA node, slowing the HR [88]. However, if hyperglycemia or STZ directly affected cardiac muscle or the SA node and caused a decreased IHR in the D group, it is also the case that exercise training rescued or prevented the deficit, as the IHR of the DX group was not different from the CX group. Thus, previous experimental T1DM studies that reported that STZ-induced bradycardia and hypotension were caused by CAN, and that exercise-induced normalization of HR and BP was evidence of improvements in autonomic function, may really have been observing changes in intrinsic cardiac function which were independent of autonomic control. Such changes could instead have been due to depressed sarcoplasmic reticulum function or impaired calcium handling [87, 89, 90]. Therefore, the direct effects of STZ on the heart and IHR require further examination and should be taken into consideration in future studies that investigate the autonomic regulation of CV function in STZ-induced T1DM models.
An important consideration regarding the design of the current study was the use of anesthetized rats. In order to accurately reflect cardiovascular parameters in such a state, we selected an anaesthetic regime that provides the lowest level of influence on baseline and reflexive CV control attainable in rodent models [50]. A light plane anesthesia 0.5–1.2 g/kg has been shown to maintain the integrity of the cardiovascular system, where higher doses of urethane (above 1.5 g/kg) can produce hypotension and bradycardia, as well as high rates of mortality [91, 92]. In the current study we used a minimal dose of 25 mg/kg, which was reported in previous studies by our laboratory to have little influence on neurovascular blood flow measures [93–95]. That being said, it cannot be determined to what extent, if at all, the autonomic nervous system was augmented by the urethane-chloralose treatment in comparison to conscious animals. Further work examining a comparison of our anesthesia regime with freely moving conscious animals (using telemetry devices) will better address this matter.
5. Conclusions
In this study, T1DM induced indications of parasympathetic withdrawal, sympathetic overactivity, and, despite a decreased IHR, no change in resting MAP or HR. However, concurrent exercise training with T1DM maintained the sensitivity of the parasympathetically mediated baroreflex bradycardia, prevented an increase in vascular sympathetic tone, maintained a higher bodyweight, and prevented a decrease in IHR. The ability of exercise training to preserve parasympathetic function in this model of T1DM indicates that the exercise-mediated improvements to parasympathetic function are independent of alterations in [BG]. However, the finding that [NPY] remained elevated suggests that hyperglycemia has a direct impact on adrenergic activity. Taken together, our T1DM model of progressive STZ induction and insulin treatment induced autonomic impairments similar to those observed in clinical T1DM and demonstrates the novelty of this model for investigating the effectiveness of high intensity aerobic exercise training as a means to prevent the progression of CAN in T1DM. Thus, although not examined in this study, the mechanisms that underlie the physiological changes caused by T1DM and exercise can be the focus of future investigations using this model.
Acknowledgments
This study was supported by the Canadian Institute of Health Research Grant (nos. CCT-83029 and 217532) and the Natural Sciences and Engineering Council Discovery Grant (RGPGP-2015-00059).
Abbreviations
- BG:
Blood glucose
- BP:
Blood pressure
- BRS:
Baroreflex sensitivity
- C:
Sedentary control
- CAN:
Cardiovascular autonomic neuropathy
- CX:
Exercised control
- CV:
Cardiovascular
- D:
Sedentary T1DM
- DX:
Exercised T1DM
- HF:
High frequency
- HR:
Heart rate
- HRV:
Heart rate variability
- IHR:
Intrinsic heart rate
- MAP:
Mean arterial pressure
- NPY:
Neuropeptide Y
- PE:
Phenylephrine
- SBP:
Systolic blood pressure
- SNP:
Sodium nitroprusside
- SNS:
Sympathetic nervous system
- STZ:
Streptozotocin
- T1DM:
Type 1 diabetes mellitus.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Kenneth N. Grisé contributed to the study design, data collection, data analysis, and writing. T. Dylan Olver, Matthew W. McDonald, Adwitia Dey, and Mao Jiang contributed to the study design and data collection. James C. Lacefield and Earl G. Noble facilitated the data collection. J. Kevin Shoemaker provided data analysis and writing. C. W. James Melling contributed to the study design, data analysis, and writing.
References
- 1.Vinik A. I., Maser R. E., Mitchell B. D., Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 2.Fazan S. V. P., De Vasconcelos C. C. A., Valencia M. M., Nessler R., Moore K. C. Diabetic peripheral neuropathies: a morphometric overview. International Journal of Morphology. 2010;28(1):51–64. [Google Scholar]
- 3.Kuehl M., Stevens M. J. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nature Reviews Endocrinology. 2012;8(7):405–416. doi: 10.1038/nrendo.2012.21. [DOI] [PubMed] [Google Scholar]
- 4.Maser R. E., Mitchell B. D., Vinik A. I., Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes. Diabetes Care. 2003;26(6):1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 5.Lishner M., Akselrod S., Avi V. M., Oz O., Divon M., Ravid M. Spectral analysis of heart rate fluctuations. A non-invasive, sensitive method for the early diagnosis of autonomic neuropathy in diabetes mellitus. Journal of the Autonomic Nervous System. 1987;19(2):119–125. doi: 10.1016/0165-1838(87)90005-1. [DOI] [PubMed] [Google Scholar]
- 6.Boysen A., Lewin M. A. G., Hecker W., Leichter H. E., Uhlemann F. Autonomic function testing in children and adolescents with diabetes mellitus. Pediatric Diabetes. 2007;8(5):261–264. doi: 10.1111/j.1399-5448.2007.00254.x. [DOI] [PubMed] [Google Scholar]
- 7.Li Y. Cardiovascular autonomic dysfunction in diabetes as a complication: cellular and molecular mechanisms. In: Wagner D., editor. Type 1 Diabetes Complications. 2011. [Google Scholar]
- 8.Salgado H. C., Fazan Júnior R., Fazan V. P., Da Silva V. J., Barreira A. A. Arterial baroreceptors and experimental diabetes. Annals of the New York Academy of Sciences. 2001;940(55):20–27. doi: 10.1111/j.1749-6632.2001.tb03663.x. [DOI] [PubMed] [Google Scholar]
- 9.Li Y.-L., Tran T. P., Muelleman R., Schultz H. D. Blunted excitability of aortic baroreceptor neurons in diabetic rats: involvement of hyperpolarization-activated channel. Cardiovascular Research. 2008;79(4):715–721. doi: 10.1093/cvr/cvn141. [DOI] [PubMed] [Google Scholar]
- 10.Maeda C. Y., Fernandes T. G., Timm H. B., Irigoyen M. C. Autonomic dysfunction in short-term experimental diabetes. Hypertension. 1995;26(6, part 2):1100–1104. doi: 10.1161/01.hyp.26.6.1100. [DOI] [PubMed] [Google Scholar]
- 11.Lund D. D., Subieta A. R., Pardini B. J., Chang K. S. K. Alterations in cardiac parasympathetic indices in STZ-induced diabetic rats. Diabetes. 1992;41(2):160–166. doi: 10.2337/diab.41.2.160. [DOI] [PubMed] [Google Scholar]
- 12.Gu H., Epstein P. N., Li L., Wurster R. D., Cheng Z. J. Functional changes in baroreceptor afferent, central and efferent components of the baroreflex circuitry in type 1 diabetic mice (OVE26) Neuroscience. 2008;152(3):741–752. doi: 10.1016/j.neuroscience.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Chen H.-Y., Wu J.-S., Chen J.-J., Cheng J.-T. Impaired regulation function in cardiovascular neurons of nucleus tractus solitarii in streptozotocin-induced diabetic rats. Neuroscience Letters. 2008;431(2):161–166. doi: 10.1016/j.neulet.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 14.Schönauer M., Thomas A., Morbach S., Niebauer J., Schönauer U., Thiele H. Cardiac autonomic diabetic neuropathy. Diabetes & Vascular Disease Research. 2008;5(4):336–344. doi: 10.3132/dvdr.2008.047. [DOI] [PubMed] [Google Scholar]
- 15.Howarth F. C., Jacobson M., Naseer O., Adeghate E. Short-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Experimental Physiology. 2005;90(2):237–245. doi: 10.1113/expphysiol.2004.029439. [DOI] [PubMed] [Google Scholar]
- 16.Schaan B. D., Dall'Ago P., Maeda C. Y., et al. Relationship between cardiovascular dysfunction and hyperglycemia in streptozotocin-induced diabetes in rats. Brazilian Journal of Medical and Biological Research. 2004;37(12):1895–1902. doi: 10.1590/s0100-879x2004001200016. [DOI] [PubMed] [Google Scholar]
- 17.Yang B., Chon K. H. Assessment of diabetic cardiac autonomic neuropathy in type I diabetic mice. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC '11); August 2011; Boston, Mass, USA. pp. 6560–6563. [DOI] [PubMed] [Google Scholar]
- 18.Chen S.-R., Lee Y.-J., Chiu H.-W., Jeng C. Impact of physical activity on heart rate variability in children with type 1 diabetes. Child's Nervous System. 2008;24(6):741–747. doi: 10.1007/s00381-007-0499-y. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen B. K., Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scandinavian Journal of Medicine and Science in Sports. 2006;16(supplement 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 20.Komine H., Sugawara J., Hayashi K., Yoshizawa M., Yokoi T. Regular endurance exercise in young men increases arterial baroreflex sensitivity through neural alteration of baroreflex arc. Journal of Applied Physiology. 2009;106(5):1499–1505. doi: 10.1152/japplphysiol.91447.2008. [DOI] [PubMed] [Google Scholar]
- 21.Mostarda C., Rogow A., Silva I. C. M., et al. Benefits of exercise training in diabetic rats persist after three weeks of detraining. Autonomic Neuroscience: Basic and Clinical. 2009;145(1-2):11–16. doi: 10.1016/j.autneu.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Golbidi S., Badran M., Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Experimental Diabetes Research. 2012;2012:16. doi: 10.1155/2012/941868.941868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriksen E. J., Saengsirisuwan V. Exercise training and antioxidants: relief from oxidative stress and insulin resistance. Exercise and Sport Sciences Reviews. 2003;31(2):79–84. doi: 10.1097/00003677-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Vinik A. I., Maser R. E., Ziegler D. Autonomic imbalance: prophet of doom or scope for hope? Diabetic Medicine. 2011;28(6):643–651. doi: 10.1111/j.1464-5491.2010.03184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hicks K. K., Seifen E., Stimers J. R., Kennedy R. H. Effects of streptozotocin-induced diabetes on heart rate, blood pressure and cardiac autonomic nervous control. Journal of the Autonomic Nervous System. 1998;69(1):21–30. doi: 10.1016/s0165-1838(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 26.Lafferty A. R., Werther G. A., Clarke C. F. Ambulatory blood pressure, microalbuminuria, and autonomic neuropathy in adolescents with type 1 diabetes. Diabetes Care. 2000;23(4):533–538. doi: 10.2337/diacare.23.4.533. [DOI] [PubMed] [Google Scholar]
- 27.Chillarón J. J., Sales M. P., Flores-Le-Roux J. A., et al. Insulin resistance and hypertension in patients with type 1 diabetes. Journal of Diabetes and its Complications. 2011;25(4):232–236. doi: 10.1016/j.jdiacomp.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Collado-Mesa F., Colhoun H. M., Stevens L. K., et al. Prevalence and management of hypertension in type 1 diabetes mellitus in Europe: the EURODIAB IDDM complications study. Diabetic Medicine. 1999;16(1):41–48. doi: 10.1046/j.1464-5491.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- 29.Dall'Ago P., Silva V. O. K., De Angelis K. L. D., Irigoyen M. C., Fazan R., Jr., Salgado H. C. Reflex control of arterial pressure and heart rate in short-term streptozotocin diabetic rats. Brazilian Journal of Medical and Biological Research. 2002;35(7):843–849. doi: 10.1590/s0100-879x2002000700013. [DOI] [PubMed] [Google Scholar]
- 30.Fazan R., Jr., Ballejo G., Salgado M. C. O., Moraes M. F. D., Salgado H. C. Heart rate variability and baroreceptor function in chronic diabetic rats. Hypertension. 1997;30(3, part 2):632–635. doi: 10.1161/01.hyp.30.3.632. [DOI] [PubMed] [Google Scholar]
- 31.Tomlinson K. C., Gardiner S. M., Hebden R. A., Bennett T. Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacological Reviews. 1992;44(1):103–150. [PubMed] [Google Scholar]
- 32.Lucini D., Pagani M. Exercise: should it matter to internal medicine? European Journal of Internal Medicine. 2011;22(4):363–370. doi: 10.1016/j.ejim.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Harthmann A. D., De Angelis K., Costa L. P., et al. Exercise training improves arterial baro- and chemoreflex in control and diabetic rats. Autonomic Neuroscience. 2007;133(2):115–120. doi: 10.1016/j.autneu.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 34.De Angelis K. L. D., Oliveira A. R., Dall'Ago P., et al. Effects of exercise training on autonomic and myocardial dysfunction in streptozotocin-diabetic rats. Brazilian Journal of Medical and Biological Research. 2000;33(6):635–641. doi: 10.1590/s0100-879x2000000600004. [DOI] [PubMed] [Google Scholar]
- 35.Harati Y. Diabetic neuropathies: unanswered questions. Neurologic Clinics. 2007;25(1):303–317. doi: 10.1016/j.ncl.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Orchard T. J., Lloyd C. E., Maser R. E., Kuller L. H. Why does diabetic autonomic neuropathy predict IDDM mortality? An analysis from the Pittsburgh Epidemiology of Diabetes Complications study. Diabetes Research and Clinical Practice. 1996;34(supplement 1):S165–S171. doi: 10.1016/s0168-8227(96)90025-x. [DOI] [PubMed] [Google Scholar]
- 37.De Angelis K. L., Schaan B. D., Maeda C. Y., Dall'Ago P., Wichi R. B., Irigoyen M. C. Cardiovascular control in experimental diabetes. Brazilian Journal of Medical and Biological Research. 2002;35(9):1091–1100. doi: 10.1590/s0100-879x2002000900010. [DOI] [PubMed] [Google Scholar]
- 38.Monckton G., Pehowich E. Autonomic neuropathy in the streptozotocin diabetic rat. Canadian Journal of Neurological Sciences. 1980;7(2):135–142. doi: 10.1017/s0317167100023519. [DOI] [PubMed] [Google Scholar]
- 39.Howarth F. C., Jacobson M., Shafiullah M., Adeghate E. Long-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Experimental Physiology. 2005;90(6):827–835. doi: 10.1113/expphysiol.2005.031252. [DOI] [PubMed] [Google Scholar]
- 40.Polonsky W. H., Davis C. L., Jacobson A. M., Anderson B. J. Hyperglycaemia, hypoglycaemia, and blood glucose control in diabetes: symptom perceptions and treatment strategies. Diabetic Medicine. 1992;9(2):120–125. doi: 10.1111/j.1464-5491.1992.tb01747.x. [DOI] [PubMed] [Google Scholar]
- 41.Valletta J. J., Chipperfield A. J., Clough G. F., Byrne C. D. Metabolic regulation during constant moderate physical exertion in extreme conditions in type 1 diabetes. Diabetic Medicine. 2012;29(6):822–826. doi: 10.1111/j.1464-5491.2011.03453.x. [DOI] [PubMed] [Google Scholar]
- 42.Taborsky G. J., Mundinger T. O. Minireview: the role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology. 2012;153(3):1055–1062. doi: 10.1210/en.2011-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox D. J., Gonder-Frederick L. A., Shepard J. A., Campbell L. K., Vajda K. A. Driving safety: concerns and experiences of parents of adolescent drivers with type 1 diabetes. Pediatric Diabetes. 2012;13(6):506–509. doi: 10.1111/j.1399-5448.2012.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melling C. W. J., Grisé K. N., Hasilo C. P., et al. A model of poorly controlled type 1 diabetes mellitus and its treatment with aerobic exercise training. Diabetes & Metabolism. 2013;39(3):226–235. doi: 10.1016/j.diabet.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Hall K. E., McDonald M. W., Grisé K. N., Campos O., Noble E. G., Melling C. W. J. he role of resistance and aerobic exercise training on insulin sensitivity measures in STZ-induced Type 1 diabetic rodents. Metabolism. 2013;62(10):1485–1494. doi: 10.1016/j.metabol.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Murias J. M., Grise K. N., Jiang M., Kowalchuk H., Melling C. W. J., Noble E. G. Acute endurance exercise induces changes in vasorelaxation responses that are vessel-specific. The American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2013;304(7):R574–R580. doi: 10.1152/ajpregu.00508.2012. [DOI] [PubMed] [Google Scholar]
- 47.Murias J. M., Dey A., Campos O. A., et al. High-intensity endurance training results in faster vessel-specific rate of vasorelaxation in type 1 diabetic rats. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059678.e59678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bedford T. G., Tipton C. M., Wilson N. C., Oppliger R. A., Gisolfi C. V. Maximum oxygen consumption of rats and its changes with various experimental procedures. Journal of Applied Physiology. 1979;47(6):1278–1283. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigues B., Figueroa D. M., Mostarda C. T., Heeren M. V., Irigoyen M.-C., De Angelis K. Maximal exercise test is a useful method for physical capacity and oxygen consumption determination in streptozotocin-diabetic rats. Cardiovascular Diabetology. 2007;6, article 38 doi: 10.1186/1475-2840-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Usselman C. W., Mattar L., Twynstra J., Welch I., Shoemaker J. K. Rodent cardiovascular responses to baroreceptor unloading: effect of plane of anaesthesia. Applied Physiology, Nutrition and Metabolism. 2011;36(3):376–381. doi: 10.1139/h11-029. [DOI] [PubMed] [Google Scholar]
- 51.Gribbin B., Pickering T. G., Sleight P., Peto R. Effect of age and high blood pressure on barorefiex sensitivity in man. Circulation Research. 1971;29(4):424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- 52.Hunt B. E., Fahy L., Farquhar W. B., Taylor J A. Quantification of mechanical and neural components of vagal baroreflex in humans. Hypertension. 2001;37(6):1362–1368. doi: 10.1161/01.hyp.37.6.1362. [DOI] [PubMed] [Google Scholar]
- 53.Rudas L., Crossman A. A., Morillo C. A., Halliwill J. R., Kari U. O., Kuusela T. A. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. The American Journal of Physiology—Heart and Circulatory Physiology. 1999;76(5, part 2):H1691–H1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- 54.Studinger P., Goldstein R., Taylor J. A. Mechanical and neural contributions to hysteresis in the cardiac vagal limb of the arterial baroreflex. The Journal of Physiology. 2007;583, part 3:1041–1048. doi: 10.1113/jphysiol.2007.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paroo Z., Haist J. V., Karmazyn M., Noble E. G. Exercise improves postischemic cardiac function in males but not females: consequences of a novel sex-specific heat shock protein 70 response. Circulation Research. 2002;90(8):911–917. doi: 10.1161/01.res.0000016963.43856.b1. [DOI] [PubMed] [Google Scholar]
- 56.Zoppini G., Cacciatori V., Gemma M. L., et al. Effect of moderate aerobic exercise on sympatho-vagal balance in Type 2 diabetic patients. Diabetic Medicine. 2007;24(4):370–376. doi: 10.1111/j.1464-5491.2007.02076.x. [DOI] [PubMed] [Google Scholar]
- 57.Chen S.-R., Lee Y.-J., Chiu H.-W., Jeng C. Impact of glycemic control, disease duration, and exercise on heart rate variability in children with type 1 diabetes mellitus. Journal of the Formosan Medical Association. 2007;106(11):935–942. doi: 10.1016/S0929-6646(08)60064-9. [DOI] [PubMed] [Google Scholar]
- 58.Gross V., Tank J., Partke H.-J., et al. Cardiovascular autonomic regulation in Non-Obese Diabetic (NOD) mice. Autonomic Neuroscience: Basic and Clinical. 2008;138(1-2):108–113. doi: 10.1016/j.autneu.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Silva K. A. D. S., Luiz R. D. S., Rampaso R. R., et al. Previous exercise training has a beneficial effect on renal and cardiovascular function in a model of diabetes. PLoS ONE. 2012;7(11):1–10. doi: 10.1371/journal.pone.0048826.e48826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murça T. M., Almeida T. C. S., Raizada M. K., Ferreira A. J. Chronic activation of endogenous angiotensin-converting enzyme 2 protects diabetic rats from cardiovascular autonomic dysfunction. Experimental Physiology. 2012;97(6):699–709. doi: 10.1113/expphysiol.2011.063461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jorge L., da Pureza D. Y., da Silva Dias D., Conti F. F., Irigoyen M.-C., De Angelis K. Dynamic aerobic exercise induces baroreflex improvement in diabetic rats. Experimental Diabetes Research. 2012;2012:5. doi: 10.1155/2012/108680.108680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong L.-Z., Chan Y.-C., Wang M.-F., et al. Modulation of baroreflex function by rosiglitazone in prediabetic hyperglycemic rats. Physiological Research. 2012;61(5):443–452. doi: 10.33549/physiolres.932304. [DOI] [PubMed] [Google Scholar]
- 63.Vinik A. I., Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi: 10.1161/circulationaha.106.634949. [DOI] [PubMed] [Google Scholar]
- 64.Ziegler D. Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes/Metabolism Reviews. 1994;10(4):339–383. doi: 10.1002/dmr.5610100403. [DOI] [PubMed] [Google Scholar]
- 65.Chang K. S., Lund D. D. Alterations in the baroreceptor reflex control of heart rate in streptozotocin diabetic rats. Journal of Molecular and Cellular Cardiology. 1986;18(6):617–624. doi: 10.1016/s0022-2828(86)80969-5. [DOI] [PubMed] [Google Scholar]
- 66.Pop-Busui R., Low P. A., Waberski B. H., et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the diabetes control and complications trial/epidemiology of diabetes interventions and complications study (DCCT/EDIC) Circulation. 2009;119(22):2886–2893. doi: 10.1161/circulationaha.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tancrede G., Rousseau-Migneron S., Nadeau A. Beneficial effects of physical training in rats with a mild streptozotocin-induced diabetes mellitus. Diabetes. 1982;31(5, part 1):406–409. doi: 10.2337/diab.31.5.406. [DOI] [PubMed] [Google Scholar]
- 68.Wegner J. A., Lund D. D., Overton J. M., Edwards J. G., Oda R. P., Tipton C. M. Select cardiovascular and metabolic responses of diabetic rats to moderate exercise training. Medicine & Science in Sports & Exercise. 1987;19(5):497–503. [PubMed] [Google Scholar]
- 69.Chipkinn S., Klugh S., Chasan-Taber L. Exercise and diabetes. Cardiology Clinics. 2001;19(3):489–505. doi: 10.1016/S0733-8651(05)70231-9. [DOI] [PubMed] [Google Scholar]
- 70.Tura A., Sbrignadello S., Succurro E., Groop L., Sesti G., Pacini G. An empirical index of insulin sensitivity from short IVGTT: validation against the minimal model and glucose clamp indices in patients with different clinical characteristics. Diabetologia. 2010;53(1):144–152. doi: 10.1007/s00125-009-1547-9. [DOI] [PubMed] [Google Scholar]
- 71.Hahn R. G., Ljunggren S., Larsen F., Nyström T. A simple intravenous glucose tolerance test for assessment of insulin sensitivity. Theoretical Biology and Medical Modelling. 2011;8(1, article 12) doi: 10.1186/1742-4682-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paulson J., Dennis J., Mathews R., Bow J. Metabolic effects of treadmill exercise training on the diabetic heart. Journal of Applied Physiology. 1992;73(1):265–271. doi: 10.1152/jappl.1992.73.1.265. [DOI] [PubMed] [Google Scholar]
- 73.Broderick T. L., Poirier P., Gillis M. Exercise training restores abnormal myocardial glucose utilization and cardiac function in diabetes. Diabetes/Metabolism Research and Reviews. 2005;21(1):44–50. doi: 10.1002/dmrr.479. [DOI] [PubMed] [Google Scholar]
- 74.Bernardi L., Rosengård-Bärlund M., Sandelin A., Mäkinen V. P., Forsblom C., Groop P.-H. Short-term oxygen administration restores blunted baroreflex sensitivity in patients with type 1 diabetes. Diabetologia. 2011;54(8):2164–2173. doi: 10.1007/s00125-011-2195-4. [DOI] [PubMed] [Google Scholar]
- 75.Rodríguez-Gómez I., Baca Y., Moreno J. M., et al. Role of sympathetic tone in BSO-induced hypertension in mice. American Journal of Hypertension. 2010;23(8):882–888. doi: 10.1038/ajh.2010.90. [DOI] [PubMed] [Google Scholar]
- 76.DeLorey D. S., Buckwalter J. B., Mittelstadt S. W., Anton M. M., Kluess H. A., Clifford P. S. Is tonic sympathetic vasoconstriction increased in the skeletal muscle vasculature of aged canines? The American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2010;299(5):R1342–R1349. doi: 10.1152/ajpregu.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez-Nieves B., Dunbar J. Vascular dilatatory responses to sodium nitroprusside (SNP) and alpha-adrenergic antagonism in female and male normal and diabetic rats. Proceedings of the Society for Experimental Biology and Medicine. 1999;222(2):90–98. doi: 10.1111/j.1525-1373.1999.10000.x.44433 [DOI] [PubMed] [Google Scholar]
- 78.Lundberg J. M., Franco-Cereceda A., Lacroix J. S., Pernow J. Neuropeptide Y and sympathetic neurotransmission. Annals of the New York Academy of Sciences. 1990;611:166–174. doi: 10.1111/j.1749-6632.1990.tb48930.x. [DOI] [PubMed] [Google Scholar]
- 79.Wahlestedt C., Hakanson R., Vaz C. A., Zukowska-Grojec Z. Norepinephrine and neuropeptide Y: vasoconstrictor cooperation in vivo and in vitro. The American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 1990;258(3):R736–R742. doi: 10.1152/ajpregu.1990.258.3.R736. [DOI] [PubMed] [Google Scholar]
- 80.Ejaz A., LoGerfo F. W., Pradhan L. Diabetic neuropathy and heart failure: role of neuropeptides. Expert Reviews in Molecular Medicine. 2011;13, article e26 doi: 10.1017/S1462399411001979. [DOI] [PubMed] [Google Scholar]
- 81.Perin P. C., Maule S., Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clinical and Experimental Hypertension. 2001;23(1-2):45–55. doi: 10.1081/CEH-100001196. [DOI] [PubMed] [Google Scholar]
- 82.Villafaña S., Huang F., Hong E. Role of the sympathetic and renin angiotensin systems in the glucose-induced increase of blood pressure in rats. European Journal of Pharmacology. 2004;506(2):143–150. doi: 10.1016/j.ejphar.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 83.Giugliano D., Marfella R., Coppola L., et al. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation. 1997;95(7):1783–1790. doi: 10.1161/01.cir.95.7.1783. [DOI] [PubMed] [Google Scholar]
- 84.Pagani M., Malfatto G., Pierini S., et al. Spectral analysis of heart rate variability in the assessment of autonomic diabetic neuropathy. Journal of the Autonomic Nervous System. 1988;23(2):143–153. doi: 10.1016/0165-1838(88)90078-1. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L., Xiong X.-Q., Fan Z.-D., Gan X.-B., Gao X.-Y., Zhu G.-Q. Involvement of enhanced cardiac sympathetic afferent reflex in sympathetic activation in early stage of diabetes. Journal of Applied Physiology. 2012;113(1):47–55. doi: 10.1152/japplphysiol.01228.2011. [DOI] [PubMed] [Google Scholar]
- 86.Malone M. A., Schocken D. D., Hanna S. K., Liang X., Malone J. I. Diabetes-induced bradycardia is an intrinsic metabolic defect reversed by carnitine. Metabolism. 2007;56(8):1118–1123. doi: 10.1016/j.metabol.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Penpargkul S., Fein F., Sonnenblick E., Scheur J. Sarcoplasmic from diabetic reticular rats function. Journal of Molecular and Cellular Cardiology. 1981;13(3):303–309. doi: 10.1016/0022-2828(81)90318-7. [DOI] [PubMed] [Google Scholar]
- 88.Howarth F. C., Al-Sharhan R., Al-Hammadi A., Qureshi M. A. Effects of streptozotocin-induced diabetes on action potentials in the sinoatrial node compared with other regions of the rat heart. Molecular and Cellular Biochemistry. 2007;300(1-2):39–46. doi: 10.1007/s11010-006-9366-5. [DOI] [PubMed] [Google Scholar]
- 89.Ganguly P. K., Pierce G. N., Dhalla K. S., Dhalla N. S. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. The American Journal of Physiology. 1983;244(6):E528–E535. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- 90.Ligeti L., Szenczi O., Prestia C. M., et al. Altered calcium handling is an early sign of streptozotocin-induced diabetic cardiomyopathy. International Journal of Molecular Medicine. 2006;17(6):1035–1043. [PubMed] [Google Scholar]
- 91.Severs W. B., Keil L. C., Klase P. A., Deen K. C. Urethane anesthesia in rats. Altered ability to regulate hydration. Pharmacology. 1981;22(4):209–226. doi: 10.1159/000137493. [DOI] [PubMed] [Google Scholar]
- 92.Field K. J., White W. J., Lang C. M. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Laboratory Animals. 1993;27(3):258–269. doi: 10.1258/002367793780745471. [DOI] [PubMed] [Google Scholar]
- 93.Olver T. D., McDonald M. W., Grisé K. N., Dey A., Allen M. D., Medeiros P. J., et al. Exercise training enhances insulin-stimulated nerve arterial vasodilation in rats with insulin-treated experimental diabetes. The American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2014;306(12):R941–R950. doi: 10.1152/ajpregu.00508.2013. [DOI] [PubMed] [Google Scholar]
- 94.Olver T. D., Mattar L., Grise K. N., et al. Glucose-stimulated insulin secretion causes an insulin-dependent nitric oxide-mediated vasodilation in the blood supply of the rat sciatic nerve. The American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2013;305(2):R157–R163. doi: 10.1152/ajpregu.00095.2013. [DOI] [PubMed] [Google Scholar]
- 95.Olver T. D., Grisé K. N., McDonald M. W., Dey A., Allen M. D., Rice C. L., et al. The relationship between blood pressure and sciatic nerve blood flow velocity in rats with insulin-treated experimental diabetes. Diabetes and Vascular Disease Research. 2014;11(4):281–289. doi: 10.1177/1479164114533357. [DOI] [PubMed] [Google Scholar]