Abstract
Alhagi maurorum (camel thorn plant) is a promising medicinal plant due to the presence of flavonoids and phenolic compounds as major contents of its constituents. No previous study has been conducted before on A. maurorum extracts as an antioxidative stress and/or antidiabetic herb in STZ-induced DM in rats. Therefore, four groups of rats were allocated as control (C), STZ-induced DM (D), and STZ-induced DM supplemented with 300 mg/kg BW of either aqueous extract (WE) or ethanolic extract (EE) of A. maurorum. The plasma levels of glucose, TG, TC, LDL-C and VLDL-C, MDA, and bilirubin and the activities of transaminases and GR were significantly increased in the diabetic group. Also, diabetic rats showed severe glucose intolerance and histopathological changes in their livers. In addition, levels of insulin, total proteins, GSH, and HDL-C and the activities of SOD, GPx, and GST were significantly decreased in the diabetic rats compared to those of the control group. The ingestion of A. maurorum extracts lowered the blood glucose levels during the OGTT compared to the diabetic rats and restored all tested parameters to their normal levels with the exception of insulin level that could not be restored. It is concluded that A. maurorum extracts decreased elevated blood glucose levels and hyperlipidemia and suppressed oxidative stress caused by diabetes mellitus in rats.
1. Introduction
The incidence of diabetes mellitus has been increased annually all over the world and the number of diabetic patients will jump from 382 million patients in year 2013 to 592 million in year 2035 [1]. The majority of diabetic patients are non-insulin-dependent and relatively small proportions (7–10%) of diabetic patients have insulin-dependent diabetes (T1D) [2]. Type 1 diabetes (T1D) is a chronic disease that results from an autoimmune destruction of β-cells of the pancreas. Therefore, insulin deficiency and hyperglycemia are the main outcomes of T1D [3]. This may generate an array of disturbances in glucose and lipid homeostasis resulting in hyperglycemia and dyslipidemia [4]. Persistent hyperglycemia in diabetes causes increased production of oxygen free radicals from autoxidation of glucose [5] and glycosylation of protein [6] which lead to oxidative stress which is associated with several health complications including antipathies, cardiovascular disorders, blindness, renal failure, neuropathies, and cancers [3, 7].
Recently, drug formulation from natural herbs, for treatment of diabetes mellitus drugs and other diseases, attracted the attention of many researchers [8]. Alhagi maurorum (Leguminosae) also called camel thorn plant or aqool is a favorable food for camels. It is widely distributed in Asia, the Middle East, Europe, and Africa [9]. It has been used as diaphoretic, diuretic, expectorant, and ulcer treatment [10]. Oil from its leaves was used for rheumatoid treatment and as laxative [11, 12]. Water extract of the roots is used to enlarge the ureter and to remove the kidney stones, whereas the methanolic extract is used as an antidiarrheal agent [13] and as herbal cough syrup [12]. A. maurorum species contains fatty acids and sterols, flavonoids, coumarins, alkaloids, and vitamins. In addition, six main flavonoid glycosides were isolated from the ethanolic extract of A. maurorum [10]. Moreover, A. maurorum roots contain lupeol [11], which is used as an antiangiogenic, antioxidative, and anti-inflammatory agent [14, 15].
Streptozotocin-treated rats developed clinical features and signs, which are similar to those found in type 1 diabetes mellitus [16]. To the best of our knowledge, no previous studies have been conducted before to investigate the antidiabetic effects of ethanolic and aqueous extracts of A. maurorum through determination of blood glucose level, free radicals, antioxidant enzymes, and lipid profile in STZ-induced diabetic rats. Therefore, the present study was undertaken to investigate the effectiveness of A. maurorum extracts in STZ-induced diabetic rats and to evaluate their therapeutic potential for treatment of diabetes mellitus.
2. Methods and Materials
2.1. Preparation of Ethanolic and Aqueous Extracts of Alhagi maurorum
Camel thorn plant (A. maurorum) was collected from Wadi El Natrun region (Egypt) after getting the agreement of the Director of Wilderness Areas in El-Beheira Governorate and authentication by Salama El Darer, Professor of Plant Ecology, Botany and Microbiology Department, Faculty of Science, Alexandria University. We confirm that no specific permission was required for collection this plant from Wadi El Natrun region because it is a desert. Moreover, we confirm that this field study did not cause any danger for any plant in this area. The aerial parts of A. maurorum were collected, washed three times with tap water and two times with distilled water, dried in the shade, and milled to fine powder by Wiley mill (Model 4-GMI, Germany). Ground plant (100 g) was refluxed with 1 liter of 70% ethanol or with 1 liter of double distilled water for 1 hour. After filtration, solvents were removed under reduced pressure at 40°C using rotary evaporator (Buchi, Model 462, Germany) and freeze dried by lyophilizer to obtain the dried extracts. Aqueous suspensions were prepared from ethanolic or aqueous extracts and administered to rats orally.
2.2. Determination of Total Phenolic and Flavonoid Contents of Alhagi maurorum Extracts
Total phenolic contents of ethanolic and aqueous extracts of A. maurorum were determined by the Folin-Ciocalteu method [17]. Gallic acid (0–0.9 mg/dL) was used as standard for phenolic compounds. The data were expressed as milligram gallic acid equivalents/g lyophilized powder. The total flavonoid content was determined using aluminum chloride colorimetric method as described earlier [18]. Rutin (0–20 mg/L) was used as a standard for flavonoid. The results were expressed as milligram rutin equivalents/g lyophilized powder.
2.3. Induction of Diabetes Mellitus by Streptozotocin
Type 1 diabetes mellitus was induced in rats by the intraperitoneal injection of freshly prepared streptozotocin (STZ) at a dose of 45 mg/kg dissolved in 0.1 M citrate buffer solution BW [19]. Three days after the STZ injection, the blood was withdrawn from the tail vein, and the glucose level was determined. Rats were diabetic when their fasting blood glucose levels were more than 200 mg/dL.
2.4. Experimental Design
Forty healthy, mature male Albino rats were provided by the Animal House of the Faculty of Medicine, Alexandria University, Egypt. The average weight of rats was 140 g and maintained in wire-bottomed cages. Rats had free access to food and water and were kept at 25 ± 2°C and 50–60% humidity. The protocol of animals handling was approved by the ethical guidelines prescribed by the MRI, Alexandria University, Alexandria, Egypt. After induction of diabetes, the diabetic rats were randomly allocated to 3 groups (10 rats each): (1) diabetic group (D-group); (2) diabetic group receiving 300 mg/kg BW water extract of A. maurorum (WE-group); and (3) diabetic group receiving 300 mg/kg BW ethanolic extract of A. maurorum (EE-group). Ten rats received distilled water and were used as control group (C-group). The A. maurorum extracts were suspended in distilled water and administered orally as a daily dose for four weeks. Rats were fasted overnight and euthanized by cervical dislocation. Blood samples were collected in EDTA-coated tubes. Livers and pancreases were removed, washed with cold saline, and stored at −80°C.
2.5. Oral Glucose Tolerance Test and Assay of Biochemical Parameters
Briefly, after overnight fasting, rats were intragastrically loaded with glucose (2 g/kg). Blood samples were withdrawn from the tail vein at 0, 30, 60, 90, and 120 minutes and the blood glucose levels were determined using commercial kit as described by Tietz [20]. Nonradioactive assay was used for determination of insulin level in plasma of rats according to the manufacturer's protocol [21]. Triglyceride, total cholesterol, LDL-C and HDL-C, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin were measured in the plasma using commercial kits from Bio-System Company (Egypt). The protein content was determined according to the method of Lowry et al. [22].
2.6. Evaluation of Oxidative Stress Markers
Lipid peroxidation was evaluated by measuring thiobarbituric acid reactive substances (TBARS) according to the method of Draper and Hadley [23]. Reduced glutathione was measured spectrophotometrically as described by Shaikh et al. [24]. Liver superoxide dismutase was estimated according to the method of S. Marklund and G. Marklund [25]. Glutathione peroxidase activity in the liver supernatant was measured according to the method of Flohe and Gunzler [26]. The hepatic activity of glutathione reductase was assayed according to the method of Smith et al. [27]. The glutathione-S-transferase activity was measured according to the method of Habig et al. [28].
2.7. Histological Analysis
Specimens of pancreas and liver tissues of the different groups were immediately fixed in 10% formalin and then treated with conventional grade of alcohol and xylene. For histopathological examination, 6 μM specimens thicknesses of both pancreas and livers were stained with hematoxylin and eosin (H&E) stains[29].
2.8. Statistical Analyses
Statistical analysis was performed using SPSS software package (Version 17.0). The data were analyzed using one-way analysis of variance (ANOVA) and the differences between means of all groups were tested using Least Significant Difference (LSD). Probability value less than 0.05 was considered statistically significant.
3. Results
3.1. The Total Flavonoids and Phenolic Contents of Alhagi Extracts
The results of the present study showed that concentrations of the total phenolic and flavonoid compounds in the aqueous extract of A. maurorum were gallic acid (90.87 ± 1.5 mg) and rutin (5.20 ± 0.24 mg) equivalent/100 g dried-weight, respectively, while the contents of the ethanolic extract of A. maurorum from these components were gallic acid (105.16 ± 2.6 mg) and rutin (6.16 ± 0.27 mg) equivalent/100 g dried-weight, respectively.
3.2. Alhagi Extracts and Biochemical Parameters
Activities of ALT and AST and the level of the total bilirubin were significantly increased (P ≤ 0.05) in diabetic rats compared to those of control group. Water and ethanolic extracts of A. maurorum exhibited significantly improved hepatic function of the diabetic rats (Table 1). The fasting blood glucose levels of diabetic rats were significantly (P ≤ 0.05) higher than those of the control group. However, administration of water and/or ethanolic extract of A. maurorum to the diabetic group significantly (P ≤ 0.001) restored their fasting blood glucose levels to the control value. Plasma insulin levels were significantly (P ≤ 0.05) decreased in the diabetic group compared to the control group. However, WE and EE did not restore the plasma insulin levels in diabetic groups to the normal level (Figure 1).
Table 1.
Changes in levels of liver function markers, blood glucose levels, and lipid profile in plasma of diabetic rats treated with either water or ethanolic extract of Alhagi maurorum.
| Parameters | Animals treatments | |||
|---|---|---|---|---|
| Control | STZ-group | STZ-WE-group | STZ-EE-group | |
| AST (U/L) | 18.67 ± 1.41c | 503.46 ± 29.61a | 24.33 ± 2.62b | 21.57 ± 1.23b |
| ALT (U/L) | 6.141 ± .079c | 103.63 ± 12.563a | 9.64 ± 1.479b | 8.62 ± 1.216b |
| Bilirubin (mg/dL) | 0.51 ± 0.03c | 6.982 ± .18a | 0.43 ± 0.04b | 0.72 ± 0.04b |
| Cholesterol (mg/dL) | 104.76 ± 7.6c | 203.14 ± 9.03a | 63.92 ± 4.76b | 90.47 ± 7.90b |
| LDL-C (mg/dL) | 83.58 ± 7.01c | 173.18 ± 8.47a | 54.60 ± 6.41b | 56.51 ± 4.88b |
| HDL-C (mg/dL) | 88.26 ± 8.15c | 40.246 ± 1.774a | 73.965 ± 6.927b | 63.499 ± 7.549b |
| LDL/HDL ratio | 1.1 ± 0.14c | 4.35 ± 0.23a | 0.76 ± 0.08b | 0.99 ± 0.13b |
| VLDL-C (mg/dL) | 24.37 ± 2.15c | 97.52 ± 12.51a | 10.14 ± 1.60b | 16.70 ± 2.24b |
| Triglyceride (mg/dL) | 121.83 ± 10.7c | 487.60 ± 62.57a | 50.68 ± 8.02c | 83.52 ± 11.21b |
| Glucose (mg/dL) | 90.81 ± 5.36c | 437.6 ± 14.10a | 106.83 ± 8.57b | 82.24 ± 7.09b |
Values are expressed as mean ± SE of 10 rats in each group.
abcdMean values within a row not sharing the same superscript letters were significantly different, P < 0.05.
Figure 1.
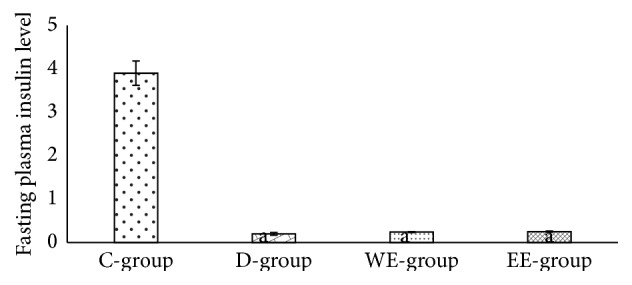
The fasting insulin level in diabetic rats treated with 300 mg/kg BW of either water or ethanolic extract of Alhagi maurorum.
The time-course changes in the blood glucose levels during the oral glucose tolerance test (0–120 min) of all groups were shown in Figure 2. The blood glucose levels reached the maximum after 30 minutes of administration of 2 g glucose/kg BW and then reduced to initial levels within 2 hours in all groups except diabetic rats that showed severe glucose intolerance throughout the experimental period (0–120 min). However, treatment of diabetic rats with 300 mg/kg BW of either water or ethanolic extracts of A. maurorum significantly (P ≤ 0.05) lowered their blood glucose levels to the normal level (Figure 2).
Figure 2.
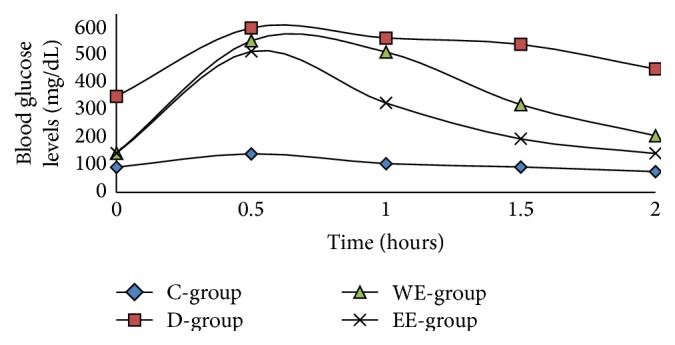
Changes in blood glucose level during oral glucose tolerance test (OGTT).
Plasma levels of triglycerides (TG), total cholesterol (TC), LDL-cholesterol (LDL-C), and VLDL-cholesterol (VLDL-C) were increased in diabetic rats compared to the control rats (Table 1). On the other hand, HDL-cholesterol (HDL-C) level was decreased in the diabetic rats compared to control group. Oral administration of either water or ethanolic extracts of A. maurorum resulted in significant (P ≤ 0.05) decreases in the levels of TG, TC, LDL-C, and VLDL-C compared to the diabetic group and increased HDL-C concentration in both WE- and EE-treated diabetic groups compared to nontreated diabetic group (Table 1).
3.3. Alhagi Extracts and Oxidative Stress
The level of MDA and the activity of GR significantly (P ≤ 0.05) increased in hepatic tissues of diabetic rats compared to control group (Table 2). However, the hepatic content of GSH and activities of SOD, GPx, and GST were significantly (P ≤ 0.05) decreased in diabetic rats compared to control group (Table 2). Both WE and EE treatments significantly (P ≤ 0.05) reduced hepatic MDA level and GR activity compared to the diabetic group. Also, treatments of diabetic rats with either WE or EE improved the antioxidant status of hepatic tissues by increasing GSH level and the activities of SOD, GPx, and GST compared to the diabetic group (Table 2).
Table 2.
Changes in level of free radicals and activities of antioxidant enzymes in liver of diabetic rats treated with either water or ethanolic extract of Alhagi maurorum.
| Parameters | Animals treatments | |||
|---|---|---|---|---|
| Control group | STZ-group | STZ-WE-group | STZ-EE-group | |
| MDA (nmoles/g tissue) | 551.20 ± 64.29a | 1222.40 ± 101.9d | 845.00 ± 31.32b | 647.80 ± 52.73c |
| GSH (nmoles/g tissue) | 876.35 ± 57.55d | 424.15 ± 48.34a | 1103.66 ± 105b | 1317.82 ± 133.8c |
| GPx (mU/mg protein) | 634.12 ± 61.31d | 355.28 ± 41.68a | 474.50 ± 25.37b | 506.19 ± 21.51c |
| GR (mU/mg protein) | 15.04 ± 0.53b | 23.35 ± 1.85a | 13.62 ± 1.09b | 15.98 ± 0.96b |
| GST (mU/mg protein) | 17.95 ± 1.16b | 13.81 ± 0.45a | 17.05 ± 1.67b | 15.57 ± 1.01d |
| SOD (U/mg protein) | 216.57 ± 19.01b | 139.94 ± 7.39a | 197.79 ± 32.37b | 205.50 ± 16.6b |
Values are expressed as mean ± SE of 10 rats in each group.
abcdMean values within a row not sharing the same superscript letters were significantly different, P < 0.05.
3.4. Histopathological Studies
3.4.1. Pancreatic Tissues
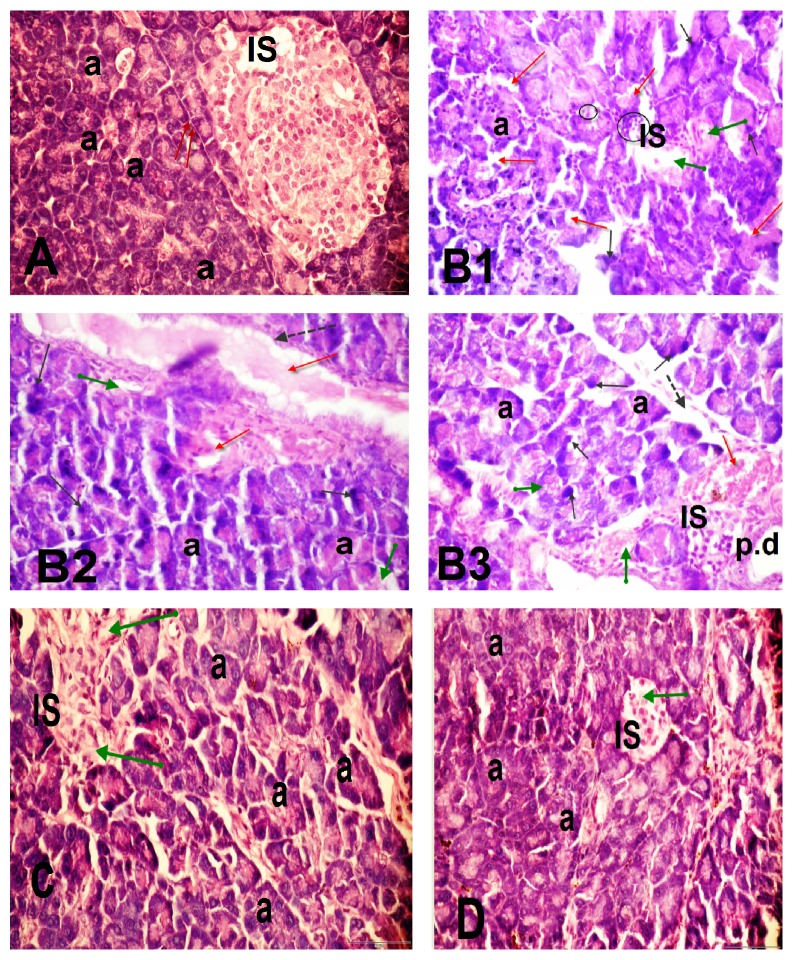
Control rats reveal normal pancreatic architecture; the closely packed pancreatic acini were composed of pyramidal shaped cells with rounded nuclei (a), the pale-stained normal islets of Langerhans (IS) scattered between acini with well-preserved cytoplasm, and nucleus normal interlobular connective tissue septa (red arrow), Figure 3(A): Figures 3(B1), 3(B2), and 3(B3) of STZ-diabetic group of rats showing disturbance of the acini pattern structure, pyknotic nuclei of some acini cells (black arrow) with severe damage; dilation, thickening, and congestion of the blood vessels (dotted arrow and red arrow); and vacuolated acini (green arrow). Islets with irregular outline, vacuolated cytoplasm (circle), and degeneration of β-islet cells (green arrow) inflammatory cells infiltrate around the pancreatic duct (p.d) (Figures 3(C) and 3(D)): STZ + WE- and STZ + EE-treated rats showing slight histological alterations of the pancreatic acini only.
Figure 3.
Light micrographs of pancreatic sections of the following. (A) Control rats revealed normal pancreatic architecture; the closely packed pancreatic acini composed of pyramidal shaped cells with rounded nuclei (a), the pale-stained normal islets of Langerhans (IS) scattered in between acini with well-preserved cytoplasm, and nucleus normal interlobular connective tissue septa (red arrow). B1, B2, and B3 represent STZ-diabetic group of rats and showed disturbance of the acinar pattern structure, pyknotic nuclei of some acini cells (black arrow) with severe damage; dilation and thickening of blood vessels (dashed arrow) and congestion of the blood vessels (red arrow) and vacuolated acini (green arrow). Islets with irregular outline, vacuolated cytoplasm (circle), and degeneration of β-islet cells (green arrow). C and D represent STZ + WE- and STZ + EE-treated rats and showed a slight reduction in the histological alterations of the pancreatic acini only. H&E, 400x.
3.4.2. Hepatic Tissues
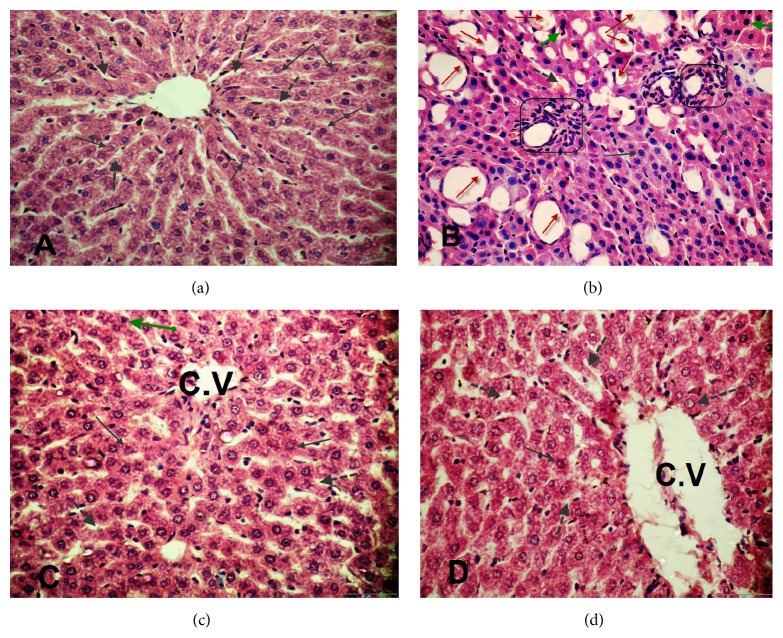
The histological examinations of hepatic tissues are represented in Figure 4. The light micrographs of liver tissues demonstrated normal architecture of hepatic cells and central vein and normal blood sinusoids in the control group (Figure 4(a)), while STZ-diabetic rats revealed severe pathological changes including congestion and dilation of hepatic sinusoids. Moreover, portal areas showed hyperplasia in the biliary epithelium and wall thickness of hepatic arteries. Focal aggregations of lymphocytes were also noticed in diabetic rats. The hepatic cells revealed degenerative and necrotic changes. Also, diffuse vacuolar, hydropic degeneration, and hypertrophied Kupffer cells were seen in diabetic rats (Figure 4(b)). However, livers of diabetic rats treated with A. maurorum extracts markedly reduced and attenuated the histological changes from severe to moderate alterations (Figures 4(c) and 4(d)).
Figure 4.
Paraffin sections stained by hematoxylin and eosin for histopathological examination of hepatocytes of rats: (a) liver tissue of control showing normal structure, central vein (C.V), normal arrangement of hepatic cords, normal blood sinusoids (⇢), and hepatocytes (→); (b) liver tissue of diabetic rats (STZ) showing hepatocyte vacuolization and fatty changes (red arrow), necrosis (green arrow), dilation of hepatic sinusoids (⇢) also, bile duct and portal vein (□), and cell infiltration (○); (c and d) liver tissue of diabetic rats + WE and diabetic rats + EE extracts of Alhagi showing normal structure, central vein (C.V), normal arrangement of hepatic cords, normal blood sinusoids and hepatocytes, few necroses, less degenerative changes, and vacuolization. H&E, 400x.
4. Discussion
From ancient times, diabetic patients have used medicinal plants to maintain blood glucose level [30]. In this regard, the present study is extended to show the influence of A. maurorum extracts on blood glucose level, oxidative stress, and lipid profile in STZ-induced diabetic rats [31]. Diabetes mellitus type 1 is caused due to lack of insulin secretion [32]. Consistent with this finding, the present study showed a significant reduction in insulin levels in diabetic rats with no recovery after their treatments with either water or ethanolic extracts of A. maurorum. The hypoglycemic effects of A. maurorum extract were not attributed to regeneration of β-cells or to increase of insulin secretion. This finding was confirmed by the histological analysis of pancreatic tissue since diabetic rats showed a reduction in numbers of islets and degeneration of β-cells. In agreement with the present study, a selective necrosis of β-cells islets of Langerhans of STZ-treated rats has been found [33]. It has been found a variable changes in nuclei of islets of pancreas in diabetic rats and some of them appeared as pyknotic nuclei due to condensation and shrinkage of the nuclear materials [34], and this is in agreement with the finding of the present study. However, treatment of diabetic rats with A. maurorum extracts showed a slight attenuation in pancreatic acini only.
It is known that hyperglycemia in both animals and humans with type 1 diabetes results from the increase in hepatic glucose output and the decrease in peripheral glucose utilization [35]. Because A. maurorum extracts do not affect β-cells regeneration or insulin secretion, the hypoglycemic effects of these extracts may be due to the presence of gallic acid and rutin as major constituents of these extracts. Interestingly, gallic acid increased glucose uptake and enhanced the translocation of GLUT4 at concentrations comparable to the amount of gallic acid. The hypoglycemic effects of A. maurorum extracts may be due to presence of phenolic compounds in these extracts. Supporting our finding, it has been found that gallic acid increased glucose uptake via different mechanisms [36]. In addition, it has been reported that quercetin, a flavonoid of A. maurorum, increased glucose uptake and increased GLUT4 translocation [37]. In addition, it has been found that rutin was served as a potential agent for glycemic control through enhancement of insulin-dependent receptor kinase activity, thereby inducing the insulin signaling pathway causing increased GLUT4 translocation and increased glucose uptake [38]. In skeletal muscle, rutin significantly increases intracellular calcium concentration which may induce glucose transporter-4 (GLUT-4) translocation with consequent glucose uptake [39].
The liver diseases are more prevalent in the diabetic population [40]. The activities of AST and ALT and the level of bilirubin were increased in diabetic rats. However, treatment of STZ-induced diabetes in rats with either water or ethanolic extract of A. maurorum reduced AST and ALT activities and total bilirubin levels compared to diabetic rats which are consistent with the finding of Shaker et al. [41]. The hepatoprotective effect of A. maurorum extracts may be due to the presence of flavone structures in the ethanolic extract [42].
Both hypertriglyceridemia and low level of HDL are the most common lipid abnormalities related to diabetes mellitus [43]. In the present study, the diabetic rats exhibited hypertriglyceridemia, hypercholesterolemia, elevated LDL-C, elevated VLDL-C, and reduced HDL-C levels compared to the control group. However, treatment of diabetic rats with either water or ethanolic extracts improved LDL/HDL ratio and lowered TG, TC, and VLDL-C levels. This improvement might be due to the presence of lupeol, a component of A. maurorum extract, which plays an important role in normalization of lipid profile [15].
The elevated levels of oxidative stress in diabetic animals are due to autoxidation of glucose, protein glycation, lipid peroxidation, and low activities of antioxidant enzymes [44]. Consistent with this finding, the present study showed that increased MDA level, decreased GSH level, and decreased activities of antioxidant enzymes, such as SOD, GPx, and GST, were seen in livers of the STZ-induced diabetic rats. These results are in agreement with other previous study which showed that glutathione level was decreased in different phases of diabetes [45]. The mechanism of enhancing of oxidative stress might be due to protein glycation and inhibition of antioxidant enzymes activities (superoxide dismutase and glutathione peroxidase) [46]. In addition, it has been found that flaxseed oil diet upregulated expression and induced activities of CAT and SOD and the protein expression of GPx, whereas fish oil diet induced both the activity and expression of CAT in liver of streptozotocin-nicotinamide induced diabetic rats [47]. In the current study, A. maurorum extracts alleviated oxidative stress by inducing the activities of antioxidant enzymes (GPx and GST) that were inhibited in diabetic rats. These results are in agreement with other study which reported that the ethanolic extracts of A. maurorum ameliorate the oxidative stress by increasing the level of glutathione and decreasing the MDA level [41]. Moreover, the antioxidative effect of A. maurorum might be due to the presence of flavonoid compounds [48] such as quercetin, which protects human intestinal cells and hemoglobin against oxidative stress attack [49].
The depletion of GSH level in diabetic rats might be due to its utilization to alleviate the oxidative stress in diabetes [50]. Therefore, the increased activity of GR in the diabetic rats was to compensate the decreased GSH levels through reduction of the oxidized glutathione (GSSG), which might be increased due to the presence of high levels of free radicals in DM. On the other hand, treatment of diabetic rats with A. maurorum extracts elevated the GSH levels and restored the activity of glutathione reductase in diabetic rats to its normal levels. The liver is frequently damaged during diabetes, as a consequence of increased levels of oxidative stress and dysregulation of immune function [16]. The degenerative changes in the histology of liver with abnormal localization and infiltration of hepatocytic nuclei were found in STZ-induced diabetes. On the other hand, livers of the diabetic rats that were treated with A. maurorum extracts revealed that most of these changes were attenuated from severe to moderate alterations which are in agreement with the finding of Alqasoumi et al. [51].
In conclusion, A. maurorum extracts decreased blood glucose levels and increased antioxidant enzymes activities in diabetic rats. In addition, A. maurorum extracts suppressed the level of free radicals and dyslipidemia in diabetic rats and consequently could alleviate complications of TD. Further studies to isolate the active components of A. maurorum are needed for treatment of diabetes mellitus.
Abbreviations
- DM:
Diabetes mellitus
- SOD:
Superoxide dismutase
- GPx:
Glutathione peroxidase
- GR:
Glutathione reductase
- GST:
Glutathione-S-transferase
- TG:
Triacylglycerol
- TC:
Total cholesterol
- LDL-C:
Low density lipoprotein-cholesterol
- HDL-C:
High density lipoprotein-cholesterol
- VLDL-C:
Very low density lipoprotein-cholesterol
- ROS:
Reactive oxygen species
- MDA:
Malondialdehyde
- STZ:
Streptozotocin
- ALT:
Alanine aminotransferase
- AST:
Aspartate aminotransferase
- OGTT:
Oral glucose tolerance test.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 6th. Brussels, Belgium: International Diabetes Federation; 2013. [PubMed] [Google Scholar]
- 2.Rochette L., Zeller M., Cottin Y., Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochimica et Biophysica Acta—General Subjects. 2014;1840(9):2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(supplement 1):s43–s48. [PubMed] [Google Scholar]
- 4.Newairy A.-S. A., Mansour H. A., Yousef M. I., Sheweita S. A. Alterations of lipid profile in plasma and liver of diabetic rats: effect of hypoglycemic herbs. Journal of Environmental Science and Health—Part B: Pesticides, Food Contaminants, and Agricultural Wastes. 2002;37(5):475–484. doi: 10.1081/pfc-120014877. [DOI] [PubMed] [Google Scholar]
- 5.Hunt J. V., Smith C. C. T., Wolff S. P. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990;39(11):1420–1424. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]
- 6.Wolff S. P., Dean R. T. Glucose autoxidation and protein modification: the potential role of ‘autoxidative glycosylation’ in diabetes. Biochemical Journal. 1987;245(1):243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansour H. A., Newairy A.-S. A., Yousef M. I., Sheweita S. A. Biochemical study on the effects of some Egyptian herbs in alloxan-induced diabetic rats. Toxicology. 2002;170(3):221–228. doi: 10.1016/S0300-483X(01)00555-8. [DOI] [PubMed] [Google Scholar]
- 8.Sheweita S. A., Newairy A. A., Mansour H. A., Yousef M. I. Effect of some hypoglycemic herbs on the activity of phase I and II drug-metabolizing enzymes in alloxan-induced diabetic rats. Toxicology. 2002;174(2):131–139. doi: 10.1016/s0300-483x(02)00048-3. [DOI] [PubMed] [Google Scholar]
- 9.Loizzo M. R., Rashed K., Said A., Bonesi M., Menichini F., Tundis R. Antiproliferative and antioxidant properties of Alhagi maurorum Boiss (Leguminosae) aerial parts. Industrial Crops and Products. 2014;53:289–295. doi: 10.1016/j.indcrop.2013.12.049. [DOI] [Google Scholar]
- 10.Awaad Amani A. S., Maitland D. J., Soliman G. A. Antiulcerogenic activity of Alhagi maurorum . Pharmaceutical Biology. 2006;44(4):292–296. doi: 10.1080/13880200600714160. [DOI] [Google Scholar]
- 11.Laghari A. H., Memon S., Nelofar A., Khan K. M. Alhagi maurorum: a convenient source of lupeol. Industrial Crops and Products. 2011;34(1):1141–1145. doi: 10.1016/j.indcrop.2011.03.031. [DOI] [Google Scholar]
- 12.Laghari A. H., Memon S., Nelofar A., et al. A new flavanenol with urease-inhibition activity isolated from roots of manna plant camelthorn (Alhagi maurorum) Journal of Molecular Structure. 2010;965(1–3):65–67. doi: 10.1016/j.molstruc.2009.11.039. [DOI] [Google Scholar]
- 13.Atta A. H., Mouneir S. M. Antidiarrhoeal activity of some Egyptian medicinal plant extracts. Journal of Ethnopharmacology. 2004;92(2-3):303–309. doi: 10.1016/j.jep.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 14.You Y.-J., Nam N.-H., Kim Y., Bae K.-H., Ahn B.-Z. Antiangiogenic activity of lupeol from Bombax ceiba . Phytotherapy Research. 2003;17(4):341–344. doi: 10.1002/ptr.1140. [DOI] [PubMed] [Google Scholar]
- 15.Sudhahar V., Kumar S. A., Mythili Y., Varalakshmi P. Remedial effect of lupeol and its ester derivative on hypercholesterolemia-induced oxidative and inflammatory stresses. Nutrition Research. 2007;27(12):778–787. doi: 10.1016/j.nutres.2007.09.012. [DOI] [Google Scholar]
- 16.Park J.-H., Jung J.-H., Yang J.-Y., Kim H.-S. Olive leaf down-regulates the oxidative stress and immune dysregulation in streptozotocin-induced diabetic mice. Nutrition Research. 2013;33(11):942–951. doi: 10.1016/j.nutres.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Meda A., Lamien C. E., Romito M., Millogo J., Nacoulma O. G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry. 2005;91(3):571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 18.Chang C.-C., Yang M.-H., Wen H.-M., Chern J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. Journal of Food and Drug Analysis. 2002;10(3):178–182. [Google Scholar]
- 19.Liu X., Hering B. J., Brendel M. D., Bretzel R. G. The effect of streptozotocin on the function of fetal porcine and rat pancreatic (pro-)islets. Experimental and Clinical Endocrinology. 1994;102(5):374–379. doi: 10.1055/s-0029-1211307. [DOI] [PubMed] [Google Scholar]
- 20.Tietz N. W. Clinical Chemistry and Molecular Diagnostics. 4th. Philadelphia, Pa, USA: W.B. Saunders Company; 2006. [Google Scholar]
- 21.Weyer C., Hanson R. L., Tataranni P. A., Bogardus C., Pratley R. E. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49(12):2094–2101. doi: 10.2337/diabetes.49.12.2094. [DOI] [PubMed] [Google Scholar]
- 22.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurements with Folin-phenol reagent. The Journal of Biological Chemistry. 1951;193:265–270. [PubMed] [Google Scholar]
- 23.Draper H. H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods in Enzymology. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-I. [DOI] [PubMed] [Google Scholar]
- 24.Shaikh Z. A., Vu T. T., Zaman K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicology and Applied Pharmacology. 1999;154(3):256–263. doi: 10.1006/taap.1998.8586. [DOI] [PubMed] [Google Scholar]
- 25.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry. 1974;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 26.Flohe L., Gunzler W. A. Assays of glutathione peroxidase. Methods in Enzymology. 1984;105:114–121. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 27.Smith I. K., Vierheller T. L., Thorne C. A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid) Analytical Biochemistry. 1988;175(2):408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- 28.Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 29.Drury R. A., Wallington E. A., Carleton S. Histological Techniques. 5th. London, UK: Oxford University Press; 1980. [Google Scholar]
- 30.Antu K. A., Riya M. P., Mishra A., Sharma S., Srivastava A. K., Raghu K. G. Symplocos cochinchinensis attenuates streptozotocin-diabetes induced pathophysiological alterations of liver, kidney, pancreas and eye lens in rats. Experimental and Toxicologic Pathology. 2014;66(7):281–291. doi: 10.1016/j.etp.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Dufrane D., van Steenberghe M., Guiot Y., Goebbels R.-M., Saliez A., Gianello P. Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and β-cell plasticity. Transplantation. 2006;81(1):36–45. doi: 10.1097/01.tp.0000189712.74495.82. [DOI] [PubMed] [Google Scholar]
- 32.Ke Y. D., Delerue F., Gladbach A., Götz J., Ittner L. M. Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer's disease. PLoS ONE. 2009;4(11) doi: 10.1371/journal.pone.0007917.e7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waer H. F. Cytological and histochemical studies in rat liver and pancreas during progression of streptozotocin induced diabetes and possible protection of certain natural antioxidants. Journal of Nutrition & Food Sciences. 2012;2, article 165 doi: 10.4172/2155-9600.1000165. [DOI] [Google Scholar]
- 34.Arulselvan P., Subramanian S. P. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic β-cells in experimental diabetes in rats. Chemico-Biological Interactions. 2007;165(2):155–164. doi: 10.1016/j.cbi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Qi L.-W., Liu E.-H., Chu C., Peng Y.-B., Cai H.-X., Li P. Anti-diabetic agents from natural products—an update from 2004–2009. Current Topics in Medicinal Chemistry. 2010;10(4):434–457. doi: 10.2174/156802610790980620. [DOI] [PubMed] [Google Scholar]
- 36.Naowaboot J., Pannangpetch P., Kukongviriyapan V., Prawan A., Kukongviriyapan U., Itharat A. Mulberry leaf extract stimulates glucose uptake and GLUT4 translocation in rat adipocytes. The American Journal of Chinese Medicine. 2012;40(1):163–175. doi: 10.1142/s0192415x12500139. [DOI] [PubMed] [Google Scholar]
- 37.Dhanya R., Arun K. B., Syama H. P., et al. Rutin and quercetin enhance glucose uptake in L6 myotubes under oxidative stress induced by tertiary butyl hydrogen peroxide. Food Chemistry. 2014;158:546–554. doi: 10.1016/j.foodchem.2014.02.151. [DOI] [PubMed] [Google Scholar]
- 38.Hsu C.-Y., Shih H.-Y., Chia Y.-C., et al. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Molecular Nutrition and Food Research. 2014;58(6):1168–1176. doi: 10.1002/mnfr.201300691. [DOI] [PubMed] [Google Scholar]
- 39.Kappel V. D., Zanatta L., Postal B. G., Silva F. R. M. B. Rutin potentiates calcium uptake via voltage-dependent calcium channel associated with stimulation of glucose uptake in skeletal muscle. Archives of Biochemistry and Biophysics. 2013;532(2):55–60. doi: 10.1016/j.abb.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Levinthal G. N., Tavill A. S. Liver disease and diabetes mellitus. Clinical Diabetes. 1999;17(4, article 14) [Google Scholar]
- 41.Shaker E., Mahmoud H., Mnaa S. Anti-inflammatory and anti-ulcer activity of the extract from Alhagi maurorum (camelthorn) Food and Chemical Toxicology. 2010;48(10):2785–2790. doi: 10.1016/j.fct.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad S., Riaz N., Saleem M., Jabbar A., Nisar-Ur-Rehman, Ashraf M. Antioxidant flavonoids from Alhagi maurorum . Journal of Asian Natural Products Research. 2010;12(2):138–143. doi: 10.1080/10286020903451724. [DOI] [PubMed] [Google Scholar]
- 43.O'Brien T., Nguyen T. T., Zimmerman B. R. Hyperlipidemia and diabetes mellitus. Mayo Clinic Proceedings. 1998;73(10):969–976. doi: 10.4065/73.10.969. [DOI] [PubMed] [Google Scholar]
- 44.Giugliano D., Ceriello A., Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19(3):257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 45.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clinical Interventions in Aging. 2007;2(2):219–236. [PMC free article] [PubMed] [Google Scholar]
- 46.Adachi T., Ohta H., Hayashi K., Hirano K., Marklund S. L. The site of nonenzymic glycation of human extracellular-superoxide dismutase in vitro. Free Radical Biology and Medicine. 1992;13(3):205–210. doi: 10.1016/0891-5849(92)90016-a. [DOI] [PubMed] [Google Scholar]
- 47.Jangale N. M., Devarshi P. P., Dubal A. A., et al. Dietary flaxseed oil and fish oil modulates expression of antioxidant and inflammatory genes with alleviation of protein glycation status and inflammation in liver of streptozotocin-nicotinamide induced diabetic rats. Food Chemistry. 2013;141(1):187–195. doi: 10.1016/j.foodchem.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Singh V. P., Yadav B., Pandey V. B. Flavanone glycosides from Alhagi pseudalhagi . Phytochemistry. 1999;51(4):587–590. doi: 10.1016/s0031-9422(99)00010-2. [DOI] [PubMed] [Google Scholar]
- 49.Asgary S., Naderi G., Sarrafzadegan N., et al. Anti-oxidant effect of flavonoids on hemoglobin glycosylation. Pharmaceutica Acta Helvetiae. 1999;73(5):223–226. doi: 10.1016/S0031-6865(98)00025-9. [DOI] [PubMed] [Google Scholar]
- 50.Coskun O., Kanter M., Korkmaz A., Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacological Research. 2005;51(2):117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Alqasoumi S. I., Al-Rehaily A. J., AlSheikh A. M., Abdel-Kader M. S. Evaluation of the hepatoprotective effect of Ephedra foliate, Alhagi maurorum, Capsella bursa-pastoris and Hibiscus sabdariffa against experimentally induced liver injury in rats. Natural Product Sciences. 2008;14(2):95–99. [Google Scholar]