Abstract
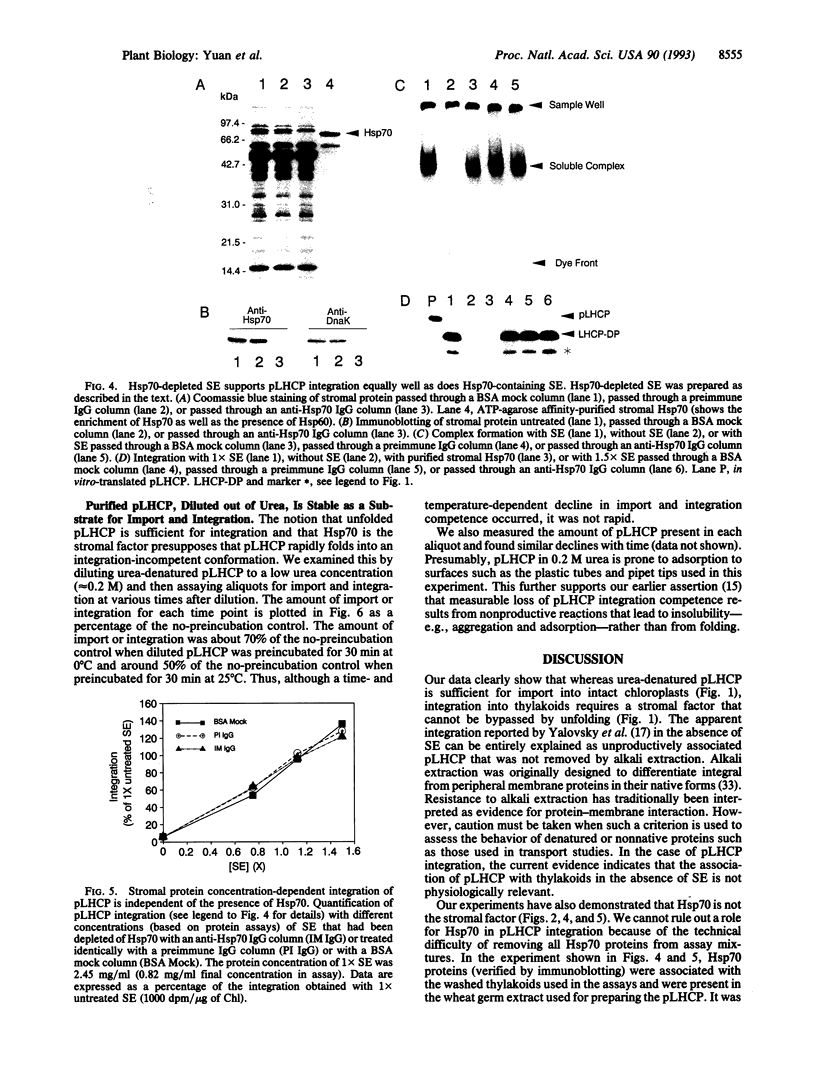
The light-harvesting chlorophyll a/b protein (LHCP) is an integral thylakoid membrane protein. It is made in the cytosol as a precursor (pLHCP), imported into chloroplasts, and subsequently integrated into thylakoids. Integration of pLHCP into thylakoids requires a stromal protein factor that functions in part to maintain the solubility and integration competence of pLHCP. Recently, it was reported that unfolded pLHCP was sufficient for integration and that the stromal factor, identified as the plastid Hsp70, was required only to prevent pLHCP refolding [Yalovsky, S., Paulsen, H., Michaeli, D., Chitnis, P. R. & Nechushtai, R. (1992) Proc. Natl. Acad. Sci. USA 89, 5616-5619]. Our studies, using more rigorous criteria for integration, show that unfolded pLHCP is not sufficient; stromal factor is an absolute requirement for integration. Furthermore, experiments with purified Hsp70 as well as Hsp70-depleted stromal extract demonstrate that Hsp70 is not the stromal factor. These results plus the finding that pLHCP diluted out of urea is relatively stable as a substrate for integration point to an additional role for the stromal factor in targeting and/or membrane translocation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Anderson J. M., Ryrie I. J. Transbilayer organization of the chlorophyll-proteins of spinach thylakoids. Eur J Biochem. 1982 Apr 1;123(2):465–472. doi: 10.1111/j.1432-1033.1982.tb19790.x. [DOI] [PubMed] [Google Scholar]
- Andino R., Rieckhof G. E., Baltimore D. A functional ribonucleoprotein complex forms around the 5' end of poliovirus RNA. Cell. 1990 Oct 19;63(2):369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchincloss A. H., Alexander A., Kohorn B. D. Requirement for three membrane-spanning alpha-helices in the post-translational insertion of a thylakoid membrane protein. J Biol Chem. 1992 May 25;267(15):10439–10446. [PubMed] [Google Scholar]
- Bochkareva E. S., Lissin N. M., Girshovich A. S. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature. 1988 Nov 17;336(6196):254–257. doi: 10.1038/336254a0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Cline K. Import of proteins into chloroplasts. Membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem. 1986 Nov 5;261(31):14804–14810. [PubMed] [Google Scholar]
- Cline K. Light-Harvesting Chlorophyll a/b Protein : Membrane Insertion, Proteolytic Processing, Assembly into LHC II, and Localization to Appressed Membranes Occurs in Chloroplast Lysates. Plant Physiol. 1988 Apr;86(4):1120–1126. doi: 10.1104/pp.86.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Cioppa G., Kishore G. M. Import of a precursor protein into chloroplasts is inhibited by the herbicide glyphosate. EMBO J. 1988 May;7(5):1299–1305. doi: 10.1002/j.1460-2075.1988.tb02944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Fulson D. R., Cline K. A Soluble Protein Factor is Required in Vitro for Membrane Insertion of the Thylakoid Precursor Protein, pLHCP. Plant Physiol. 1988 Dec;88(4):1146–1153. doi: 10.1104/pp.88.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Adam Z., Hoffman N. E. Deletion Mutants of Chlorophyll a/b Binding Proteins Are Efficiently Imported into Chloroplasts but Do Not Integrate into Thylakoid Membranes. Plant Physiol. 1992 May;99(1):247–255. doi: 10.1104/pp.99.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koll H., Guiard B., Rassow J., Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Antifolding activity of hsp60 couples protein import into the mitochondrial matrix with export to the intermembrane space. Cell. 1992 Mar 20;68(6):1163–1175. doi: 10.1016/0092-8674(92)90086-r. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A. Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5320–5324. doi: 10.1073/pnas.86.14.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lecker S., Lill R., Ziegelhoffer T., Georgopoulos C., Bassford P. J., Jr, Kumamoto C. A., Wickner W. Three pure chaperone proteins of Escherichia coli--SecB, trigger factor and GroEL--form soluble complexes with precursor proteins in vitro. EMBO J. 1989 Sep;8(9):2703–2709. doi: 10.1002/j.1460-2075.1989.tb08411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Topping T. B., Randall L. L. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9213–9217. doi: 10.1073/pnas.86.23.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. S., DeRocher A. E., Keegstra K., Vierling E. Identification of heat shock protein hsp70 homologues in chloroplasts. Proc Natl Acad Sci U S A. 1990 Jan;87(1):374–378. doi: 10.1073/pnas.87.1.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E. The amino acid sequence of the polypeptide segment which regulates membrane adhesion (grana stacking) in chloroplasts. J Biol Chem. 1983 Aug 25;258(16):9941–9948. [PubMed] [Google Scholar]
- Murakami H., Pain D., Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988 Dec;107(6 Pt 1):2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan L. A., Cline K. A stromal protein factor maintains the solubility and insertion competence of an imported thylakoid membrane protein. J Cell Biol. 1991 Feb;112(4):603–613. doi: 10.1083/jcb.112.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. J., Silhavy T. J. Heat-shock proteins DnaK and GroEL facilitate export of LacZ hybrid proteins in E. coli. Nature. 1990 Apr 26;344(6269):882–884. doi: 10.1038/344882a0. [DOI] [PubMed] [Google Scholar]
- Reed J. E., Cline K., Stephens L. C., Bacot K. O., Viitanen P. V. Early events in the import/assembly pathway of an integral thylakoid protein. Eur J Biochem. 1990 Nov 26;194(1):33–42. doi: 10.1111/j.1432-1033.1990.tb19423.x. [DOI] [PubMed] [Google Scholar]
- Schiebel E., Driessen A. J., Hartl F. U., Wickner W. Delta mu H+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991 Mar 8;64(5):927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Viitanen P. V., Doran E. R., Dunsmuir P. What is the role of the transit peptide in thylakoid integration of the light-harvesting chlorophyll a/b protein? J Biol Chem. 1988 Oct 15;263(29):15000–15007. [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. B., Bassford P. J., Jr The folding properties of the Escherichia coli maltose-binding protein influence its interaction with SecB in vitro. J Bacteriol. 1990 Jun;172(6):3023–3029. doi: 10.1128/jb.172.6.3023-3029.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S., Paulsen H., Michaeli D., Chitnis P. R., Nechushtai R. Involvement of a chloroplast HSP70 heat shock protein in the integration of a protein (light-harvesting complex protein precursor) into the thylakoid membrane. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5616–5619. doi: 10.1073/pnas.89.12.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Cline K., Theg S. M. Cryopreservation of chloroplasts and thylakoids for studies of protein import and integration. Plant Physiol. 1991 Apr;95(4):1259–1264. doi: 10.1104/pp.95.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]