Abstract
Background
Gly m 5 and Gly m 6 are known to induce severe reactions in soy-allergic patients. For birch pollen (BP)-allergic patients, the Bet v 1 homologous allergen Gly m 4 is also a potential trigger of generalized severe reactions upon soy consumption. Therefore, reliable component-resolved diagnosis of soy allergy is needed.
Methods
IgE reactivity from sera of 20 patients from a BP environment with reported soy allergy was assessed. Skin prick tests (SPT) with BP and soy drink were performed. Specific IgE for BP, soy, Bet v 1 and Gly m 4 was analyzed by ImmunoCAP. In addition, ISAC microarray profiling was performed.
Results
Nineteen of 20 patients were BP allergic (positive SPT and/or CAP results for BP extract and Bet v 1). Eighteen soy-allergic patients were tested positive with soy drink in SPT. Soy CAP results were negative in the majority of tests (15/20), whereas 19/20 sera had specific IgE to Gly m 4. In the microarray approach, 14/20 sera displayed Gly m 4-specific IgE, the additional 6 sera had IgE levels below 0.3 ISAC standardized units. The BP-negative serum had Gly m 5- and Gly m 6-specific IgE which correlated with positive soy ImmunoCAP.
Conclusions
Soy sensitization detected by SPT and Gly m 4 ImmunoCAP were in good qualitative agreement with ISAC results. Soy ImmunoCAP was only specific for Gly m 5 and Gly m 6 sensitization. Gly m 4 ImmunoCAP has a higher sensitivity than ImmunoCAP ISAC. In this patient cohort, Gly m 4 sensitization was linked to the development of severe and generalized allergic reactions upon soy consumption.
Keywords: Allergen microarray, Anaphylaxis, Food allergy, Gly m 4, PR-10 proteins, Soy allergy
Introduction
Component-resolved diagnosis provides a patient-specific sensitization profile. Especially for patients with anaphylactic reactions, this detailed information may help to identify marker allergens for severe versus mild symptoms. Ingestion of soybean (Glycine max) by atopic individuals can evoke generalized and life-threatening allergic symptoms. Among 7 already identified soy allergens (www.iuis.org), seed storage proteins Gly m 5 (7S globulin) and Gly m 6 (11S globulin) are associated with severe allergic reactions [1]. For birch pollen (BP)-allergic patients, Gly m 4 (Bet v 1 homologue) is considered as an allergen linked to crossreactivity and potentially severe symptoms [2–4].
The aim of the current study was to apply the allergen microarray diagnosis in a cohort of soy-allergic patients and to compare it to conventional in vitro and in vivo diagnosis.
Methods
Twenty patients (10 males/10 females) from a BP environment with reported soy allergy, ranging in age from 10–69 years (median: 36 years), were enrolled in the study (table 1). Skin prick tests (SPT) were performed with commercial BP extract (ALK-Abello, Linz, Austria) and with fresh soy drink (Alpro Soja; Alpro, Gent, Belgium). The sera were screened for total IgE and specific IgE for BP, soy, Bet v 1 and Gly m 4 by ImmunoCAP (Phadia AB, Upsala, Sweden). In addition, 30 ml of each serum sample were analyzed by an allergen microarray (ImmunoCAP ISAC 112; Phadia AB) according to the manufacturer’s instructions. GraphPad Pris (version 4.0) was used for statistics and graphs (GraphPad Software Inc., La Jolla, Calif., USA). The nonparametric Spearman correlation coefficient (r) and coefficient of determination (r2) were used to compare the Gly m 4-specific values obtained by the two testing systems.
Table 1.
Characterization of soy-allergic patients
| ID | Symptoms related with soy intake |
Age years/ sex |
SPT |
ImmunoCAP, kUA/l and (% specific IgE to total IgE) |
Total IgE kU/1 |
Microarray, ISU |
Symptoms with apple |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| soy drink | birch | soy | Gly m 4 | birch | Bet v 1 | Gly m 4 | Bet v 1 | Gly m 5 | Gly m 6 | Mal d 1 | |||||
| 1 | Ana | 37/f | ++ | +++ | 0 (0%) | 6.13 (7%) | 17.5 (21%) | 18.6 (22%) | 83.7 | 3.52 | 25.9 | 0 | 0 | 5.98 | OAS |
|
| |||||||||||||||
| 2 | Ap | 44/f | nd | +++ | 0 (0%) | 1.28 (5%) | 11.6 (45%) | 11.7 (45%) | 25.8 | 0.46 | 29.8 | 0 | 0 | 5.79 | none |
|
| |||||||||||||||
| 3 | Ana | 12/m | ++ | +++ | <0.35 (0%) | 1.02 (2%) | 3.23 (7%) | nd | 43.7 | 1.57 | 7.98 | 0 | 0 | 1.97 | none |
|
| |||||||||||||||
| 4 | Ana | 43/m | + | ++ | <0 (0%) | 2.31 (4%) | 7.05 (13%) | nd | 55.7 | 2.55 | 13.9 | 0 | 0 | 7.70 | OAS |
|
| |||||||||||||||
| 5 | OAS | 15/f | ++ | +++ | <0.35 (0%) | 18.6 (3%) | 37.1 (6%) | 34.5 (6%) | 596 | 4.08 | 24.8 | 0 | 0 | 16.8 | OAS |
|
| |||||||||||||||
| 6 | Ana | 10/m | ++ | +++ | <0.35 (0%) | 13.1 (6%) | 35.3 (17%) | nd | 212 | 2.11 | 12.4 | 0 | 0 | 4.20 | OAS |
|
| |||||||||||||||
| 7 | OAS | 30/m | +++ | +++ | 0.89 (0.4%) | 3.8 (2%) | 61.8 (24%) | 50.4 (20%) | 253 | 0.54 | 58.4 | 0 | 0 | 20.4 | OAS |
|
| |||||||||||||||
| 8 | Ana | 53/f | +++ | + | <0 (0%) | 0.24 (0%) | 22.8 (37%) | nd | 62.2 | 0.18 | 35.5 | 0 | 0 | 2.85 | OAS |
|
| |||||||||||||||
| 9 | OAS | 56/f | ++ | +++ | <0 (0%) | 0.95 | nd | nd | nd | 0.07 | 9.20 | 0 | 0 | 2.00 | OAS |
|
| |||||||||||||||
| 10 | Ana | 36/m | +++ | ++ | <0.35 (0%) | 20.9 (10%) | 58.5 (27%) | 54.2 (25%) | 217 | 6.34 | 24.9 | 0 | 0 | 8.16 | none |
|
| |||||||||||||||
| 11 | Ana | 44/f | +++ | +++ | 0.2 (0%) | 76.8 (8%) | >100 | >100 | 909 | 13.0 | 94.5 | 0 | 0 | 67.9 | OAS |
|
| |||||||||||||||
| 12 | Ana | 23/f | ++ | + | 0.06 (0%) | 5.21 (2%) | 51.4 (16%) | nd | 330 | 0.16 | 50.0 | 0 | 0 | 4.79 | OAS |
|
| |||||||||||||||
| 13 | OAS | 69/f | + | ++ | 0.17 (0.2%) | 2 (3%) | 27.7 (39%) | 25 (35%) | 71.2 | 0.23 | 11.3 | 0 | 0 | 13.4 | OAS |
|
| |||||||||||||||
| 14 | RC | 40/f | +++ | ++ | <0.35 (0%) | 5.51 (10%) | 26 (48%) | nd | 53.9 | 0.14 | 16.2 | 0 | 0 | 4.48 | OAS |
|
| |||||||||||||||
| 15 | OAS | 38/m | +++ | +++ | <0.35 (0%) | 1.84 (3%) | 16.2 (21%) | 21.8 (28%) | 77 | 1.07 | 24.2 | 0 | 0 | 7.02 | OAS |
|
| |||||||||||||||
| 16 | OAS | 36/f | ++ | +++ | <0.35 (0%) | 16.2 (10%) | 59.5 (38%) | 51.3 (32%) | 158 | 3.90 | 34.5 | 0 | 0 | 17.9 | OAS |
|
| |||||||||||||||
| 17 | Ana | 21/m | ++ | +++ | <0.35 (0%) | 7.65 (5%) | 23.5 (16%) | nd | 150 | 1.40 | 36.2 | 0 | 0 | 8.34 | OAS |
|
| |||||||||||||||
| 18 | Ana | 66/m | + | + | 0.78 (0.2%) | 7.76 (3%) | nd | 65.8 (24%) | 278 | 1.54 | 32.4 | 0 | 0 | 7.60 | none |
|
| |||||||||||||||
| 19 | Ana | 13/m | nd | nd | 55.7 (32%) | 0.02 (1%) | nd | <0.35 | 1.718 | 0.12 | 0.3 | 6.0 | 11.5 | 0.08 | none |
|
| |||||||||||||||
| 20 | OAS, Rh | 41/m | +++ | +++ | <0.35 (0%) | 3.86 (8%) | 10.8 (21%) | 12.1 (24%) | 50.6 | 0.89 | 8.40 | 0 | 0 | 2.03 | OAS |
Nd = Not determined; + = test wheal ≥1/2 wheal size of histamine control; ++ = test wheal = histamine wheal; +++ = test wheal > histamine wheal; Ana = anaphylaxis; Ap = abdominal pain; OAS = oral allergy syndrome; RC = rhinoconjunctivitis; Rh = rhinitis. ISAC IgE values below the threshold level of 0.3 ISU and ImmunoCAP IgE values below the threshold level of 0.1 kUA/l are shown in italics.
Results
All patients displayed food-allergic symptoms upon ingestion of soy drinks. In addition, 1 patient had symptoms with cooked soy beans. Nineteen of those were BP allergic (positive SPT and/or CAP results for BP extract and Bet v 1; table 1). Thirty percent of the patients (6/20) displayed rather mild symptoms (oral allergy syndrome, OAS) whereas 70% had generalized symptoms (table 1). Twenty-five percent (5/20) of the patients had no problems with other plant foods and 75% (15/20) had OAS to a range of fruits, primarily apples and other Rosaceae fruits, vegetables and nuts. Severe symptoms occurred in 2 patients upon ingestion of celery and carrot, respectively (data not shown). Eighteen soy-allergic patients were tested positive for soy drink in SPT. CAP results for soy-specific IgE were negative in the majority of tests (15/20) whereas 19/20 sera had specific IgE to Gly m 4.
In the microarray approach, 14/20 sera displayed Gly m 4-specific IgE levels, and the remaining 6 sera had IgE levels below 0.3 ISAC standardized units (ISU; table 1).
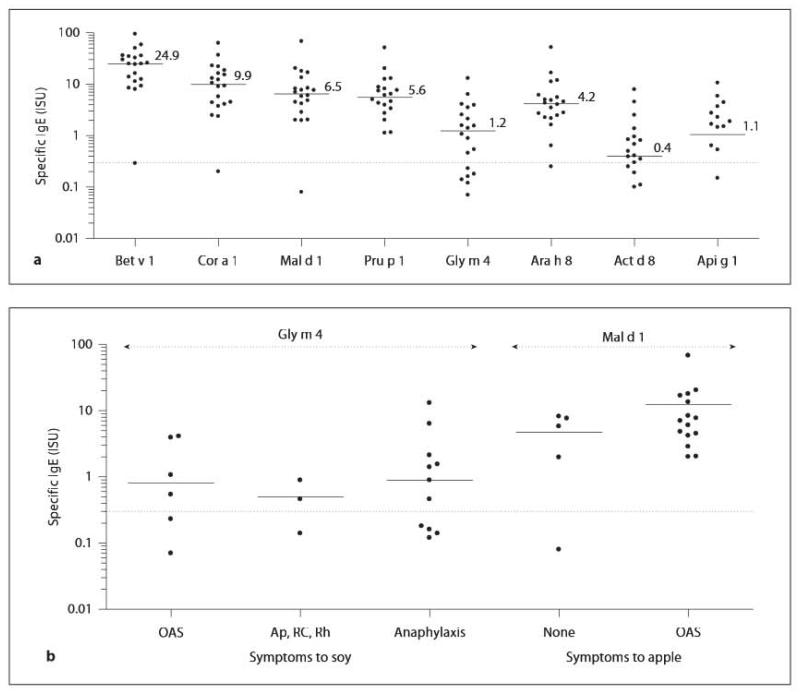
Furthermore, all serum samples showed specific IgE antibodies to related food allergens, Cor a 1.04, Mal d 1, Pru p 1, Ara h 8, Act d 8 and Api g 1, with varying concentrations (fig. 1a). In contrast, serum from patient No. 19 without BP allergy had detectable IgE levels to Gly m 5 and Gly m 6 in the ImmunoCAP ISAC, which correlated with positive soy ImmunoCAP results, whereas IgE levels to Bet v 1 and Bet v 1-related food allergens were below the threshold level (table 1). Additionally, this serum exhibited IgE antibodies to Ara h 1, Ara h 2 and Ara h 3, Cor a 9 as well as Cor a 8, Ara h 9, Jug r 3 and Pru p 3 (data not shown).
Fig. 1.
a Specific IgE recognition of Bet v 1 and related food allergens, Cor a 1.04 (hazelnut), Mal d 1 (apple), Pru p 1 (peach), Gly m 4 (soy), Ara h 8 (peanut), Act d 8 (kiwifruit), Api g 1 (celeriac), determined in sera from 20 soy-allergic patients. Specific IgE values are given in ISAC standardized units (ISU). Median values are indicated. b Specific Gly m 4 and Mal d 1 IgE values (ISU) and soy- and apple-related allergic symptoms, respectively. Ap = Abdominal pain; RC = rhinoconjunctivitis; Rh = rhinitis.
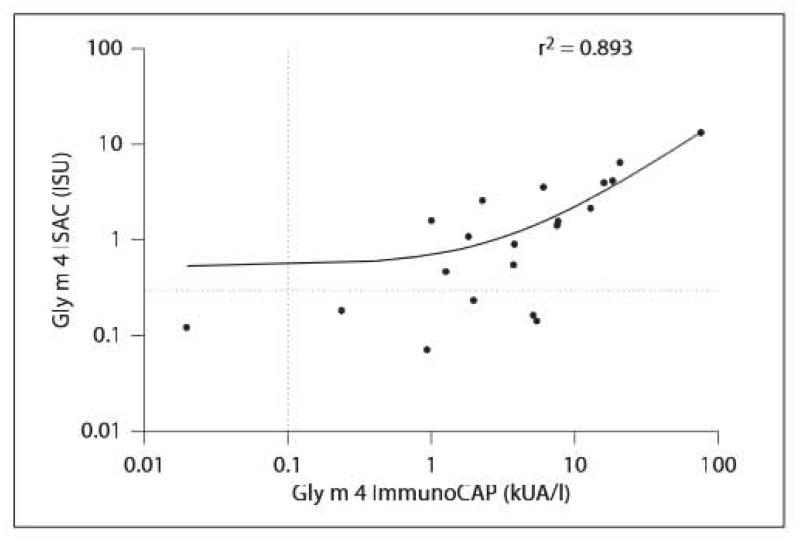
There was a strong correlation (r = 0.95, p < 0.0001) between the Gly m 4 results measured by ImmunoCAP and ImmunoCAP ISAC (fig. 2).
Fig. 2.
Linear regression analysis of Gly m 4-specific IgE measured by Gly m 4 ImmunoCAP and ISAC in the soy-allergic patients (n = 20). Dotted lines indicate 0.1 kUA/l cutoff level for ImmunoCAP and 0.3 ISU for ImmunoCAP ISAC 112.
Regarding ISU values to Gly m 4 and related food allergens, differences were observed (fig 1a). In the microarray analysis, the highest values were measured with Bet v 1, ranging from 0.3 to 94.5 ISU (median 24.9). Specific IgE levels to Cor a 1 ranged from 0.2 to 62.7 ISU (median 9.9), followed by Mai d 1-specific ISU values between 0.1 and 67.9 ISU (median 6.5) and Pru p 1 with values from 1.1 to 51 ISU. In contrast, Gly m 4 levels ranged from 0.1 to 13 ISU (median 1.2) and Api g 1-specific ISU values from 0.2 to 10.6 ISU (median 1.1). However, no association between severity of allergic symptoms and increasing specific IgE levels could be observed for Gly m 4 (fig. 1b). Reversely, specific Mal d 1 levels were generally higher compared to Gly m 4 levels (fig. 1b). Again, no significant association (p > 0.05) between IgE levels and presence of symptoms (OAS vs. no symptoms) was observed.
Discussion
Taken together, in our patient cohort, SPT with soy drink and Gly m 4 ImmunoCAP proved highly reliable to detect Gly m 4 sensitization. Soy extract ImmunoCAP was highly specific for Gly m 5 and Gly m 6, whereas Gly m 4-specific IgE was neglected by this reagent. Furthermore, sensitization to Gly m 4, the Bet v 1 homologous allergen, tends to induce moderate to rather severe food-allergic symptoms compared to other Bet v 1-related food allergens, such as Mal d 1 [2, 5]. When performing the allergen microarray analysis, there was a highly significant correlation of quantitative results with the Gly m 4 ImmunoCAP (fig. 2). However, differences around the threshold level were detectable, demonstrating that Gly m 4 ImmunoCAP has a higher sensitivity (95%) than ImmunoCAP ISAC (70%), resulting in a lower quantitative agreement with a cutoff of 0.1 kUA/l for ImmunoCAP and of 0.3 ISU for ImmunoCAP ISAC.
In addition, this new approach provided individual IgE binding profiles. Bet v 1-related food allergens are known to induce rather mild local reactions, which were also observed in these BP-allergic patients. For example Mal d 1 sensitization was linked with either no or mild allergic reactions. In contrast, Gly m 4 sensitization was related to severe, generalized symptoms in most of the patients in the absence of Gly m 5 and Gly m 6 sensitization, respectively. The abundance of total soy proteins, including Gly m 4, in soy-containing food could contribute to this activity as well as the food matrix of these foods, which is different to fruits and raw vegetables.
In summary, for the diagnosis of soy allergy, the microarray approach provided additional information to conventional methods [6, 7], However, regarding assay sensitivity, improvement should be considered for certain allergens such as Gly m 4.
Acknowledgments
The study was supported by the ‘Österr. Wirtschaftskammerpreis 2010’ and by grant SFB F46-B19 (K.H.-S.) from the Austrian Science Fund. We want to thank Daniel Ebner for his excellent technical assistance.
References
- 1.Holzhauser T, Wackermann O, Ballmer-Weber BK, Bindslev-Jensen C, Scibilia J. Soybean (Glycine max) allergy in Europe: Gly m 5 (beta-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. J Allergy Clin Immunol. 2009;123:452–458. doi: 10.1016/j.jaci.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Kleine-Tebbe J, Vogel L, Crowell DN, Haustein UF, Vieths S. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1-related PR-10 protein in soybean, SAM22. J Allergy Clin Immunol. 2002;110:797–804. doi: 10.1067/mai.2002.128946. [DOI] [PubMed] [Google Scholar]
- 3.van Zuuren EJ, Terreehorst I, Tupker RA, Hiemstra PS, Akkerdaas JH. Anaphylaxis after consuming soy products in patients with birch pollinosis. Allergy. 2010;65:1348–1349. doi: 10.1111/j.1398-9995.2010.02357.x. [DOI] [PubMed] [Google Scholar]
- 4.Vissers YM, Jansen AP, Ruinemans-Koerts J, Wichers HJ, Savelkoul HF. IgE componenl-resolved allergen profile and clinical symptoms in soy and peanut allergic patients. Allergy. 2011;66:1125–1127. doi: 10.1111/j.1398-9995.2011.02575.x. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Rivas M, Bolhaar S, Gonzalez-Mancebo E, Asero R, van Leeuwen A. Apple allergy across Europe: how allergen sensitization profiles determine the clinical expression of allergies to plant foods. J Allergy Clin Immunol. 2006;118:481–488. doi: 10.1016/j.jaci.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Jahn-Schmid B, Harwanegg C, Hiller R, Bohle B, Ebner C. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003;33:1443–1449. doi: 10.1046/j.1365-2222.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 7.Gadisseur R, Chapelle JP, Cavalier E. A new tool in the field of in-vitro diagnosis of allergy: preliminary results in the comparison of ImmunoCAP© 250 with the ImmunoCAP© ISAC. Clin Chem Lab Med. 2011;49:277–280. doi: 10.1515/CCLM.2011.052. [DOI] [PubMed] [Google Scholar]