Abstract
The baculovirus/insect cell system has proven to be a very powerful tool for the expression of several therapeutics. Nevertheless, these products sometimes suffer from reduced biological activity and unwanted side effects. Several studies have demonstrated that glycosylation can greatly influence the structure, function, half-life, antigenicity and immunogenicity of various glycoproteins. Yet, the glycosylation pattern of insect cell-derived products is not favourable for many applications. Especially the presence of core α1,3-linked fucose bears the risk of causing immediate hypersensitivity reactions in patients with allergy. In this study we evaluated the impact of fucose residues on the allergenic potential of an insect cell-expressed vaccine candidate. In order to block the GDP-L-fucose de novo synthesis pathway, we integrated the Pseudomonas aeruginosa GDP-6-deoxy-D-lyxo-4-hexulose reductase (RMD) gene into a baculovirus backbone. This virus was then used for the expression of soluble influenza A virus hemagglutinin. Expression studies showed that the co-expression of RMD did not influence the overall level of recombinant protein secretion. We confirmed the result of our strategy by analysing PNGase A-released N-glycans using MALDI-TOF-MS. In order to evaluate the biological impact of defucosylation of influenza HA we tested the binding activity of IgE derived from the sera of patients with allergy to the purified antigen. The nonfucosylated hemagglutinin showed a 10-fold decrease in IgE binding levels as compared to wildtype variants.
Keywords: Baculovirus, Fucose, Glycosylation, Insect cells, Vaccines
Introduction
Nowadays, several insect cell lines and a variety of baculovirus-based expression systems are available for the production of pharmaceutically relevant proteins. The most popular cell lines are Spodoptera frugiperda Sf9 cells [1] and Trichoplusia ni BTI-TN5B1-4 “High Five” cells [2]. Both these cell lines have been shown to be efficient in large scale production processes of vaccines and vaccine candidates such as the human papilloma virus vaccine CervarixTM [3], the influenza A virus hemagglutinin [4] and influenza A virus-like particles [5]. Whenever secreted proteins, such as the influenza A virus hemagglutinin, are produced in insect cells, High Five cells have shown to be more feasible for high yield expression [6, 7]. Yet, in terms of glycosylation, insect cell-derived proteins differ from mammalian cell-derived products. Insect cell lines lack the ability to provide complex type N-glycan structures and some insect specific structures represent possible immunogenic and allergenic epitopes. N-glycans found on insect cell-expressed proteins are mainly of a high mannose type or non-fucosylated and core-fucosylated tri-mannose structures [8]. Especially the core α1,3-linked fucose, that is most often accompanied by an α1,6-linked fucose, is known to be one of the most frequent individual glycan epitope structures inducing IgE-antibody production. The so-called carbohydrate cross-reactive determinant (CCD) is not exclusively present on insect cell-expressed proteins and was also identified from different types of allergens of plant or animal origin [9]. Hyaluronidases of the Apidae and Vespidae lineage and honeybee phospholipases A1 and A2 are glycosylated proteins found in insect venoms. They may cause the production of anti-CCD IgE after an insect sting [10-15]. A second group of allergens, where CCDs were identified are pollen. Carbohydrate cross-reactive determinants are described for tree and weed pollen, but they are most frequently found in grass pollen [16-19]. Anti-CCD IgE has further been observed in response to several vegetables, fruits and seeds [20-24]. Cross reactions between pollens and plant-derived foods are often caused by CCDs [25]. Anti-CCD IgE molecules bind to Fcε receptors that are present on mast cells. Subsequent cross-linking of IgE via the bound allergen, leads to mast cell activation, followed by the secretion of specific mediators, such as histamine, finally causing immediate allergic reactions.
In order to make insect cell-derived recombinant products safer and more attractive as vaccine candidates, systems for the production of non-fucosylated proteins have been developed. Changing the glycan structure by cell engineering of insect cells has been shown to be feasible for the expression of proteins with human-like glycan structures [26-31]. A major drawback with using such a setup is a metabolic stress for the transgenic cell line, leading to reduced growth characteristics and genetic instability as well as reduced yields of recombinantly produced proteins. Furthermore, the altered glycosylation pattern might influence the functionality of cellular proteins and have a wider impact on the robustness of the system. Alternatively, virus based engineering for modulating the N-glycan pattern of therapeutically relevant proteins has been shown to be feasible, e.g. for the production of human antibodies [32, 33]. Yet, the most promising application of the baculovirus insect cell system in the field of medical biotechnology is the production of vaccine candidates, especially of virus-like particles. Multi-subunit protein complexes, such as influenza virus-like particles are often difficult to produce in mammalian cell lines in sufficient yields. However, when insect cells are used, the impact of glycosylation must be tested and if necessary included in the vaccine design concept. This is the first study showing that the degree of fucosylation of insect cell-expressed influenza HA is relevant in terms of its allergic potential. Patient’s sera were tested for the level of IgE antibodies binding to wild type and low fucosylated HA produced in Sf9 and Hi5 cells.
Materials and Methods
All DNA manipulations were carried out essentially as summarised by Sambrook et al. [34]. DNA polymerase, Restriction enzymes, T4 DNA ligase and Calf Intestinal Alkaline Phosphatase were purchased from New England Biolabs (Ipswich, USA). All enzymes were used according to manufacturer’s recommendation. All primers and DNA oligos were synthesised by Integrated DNA Technologies (Leuven, Belgium).
Cells and viruses
Spodoptera frugiperda Sf9 cells (ATCC CRL-1711) [1] were grown in HyClone SFM4Insect media (Thermo Scientific, USA) supplemented with 3% fetal bovine serum (FCS) at 27°C using T-flasks. Trichoplusia ni BTI-TN5B1-4 “High Five” (“Hi5”) cells (ATCC CRL-10859) [2] were grown in IPL-41 medium (SAFC Biosciences, St. Louis, USA) containing yeast extract and a lipid mixture at 27°C using T-flasks. Recombinant Autographa californica nucleopolyhedroviruses were isolated and plaque purified by standard procedures. Viral titres were determined by plaque assay using 10-fold dilution series (n=3).
Cloning and generation of recombinant baculovirus
Viral gp64 promoter was PCR amplified using primers gp64-ClaI-for (5′-GAT GAT ATC GAT GTC GAC TGA GCG TCC GTG TT-3′) and gp64-BamHI-rev (5′- GAT GAT GGA TCC GGT GCT TGT GTG TTC CTT ATT G-3′). The product was digested with BamHI / ClaI and ligated into a pIDC vector cut with the same enzymes resulting in pIDC64.
Pseudomonas aeruginosa
GDP-6-deoxy-D-lyxo-4-hexulose reductase (RMD) (GenBank Accession No.: AAG08839.1) was codon optimised for the expression in insect cells and synthesized by GeneArt (Würzburg, Germany). RMD was digested with BamHI / XbaI and ligated in to pIDC64 cut with the same enzymes resulting in pIDC64-RMD.
A vector containing soluble trimeric Influenza A Hemagglutinin (A/California/04/09) consisting of signal peptide, ectodomain, T4 trimerization domain and 6x His-tag [35] was digested with BamHI / XbaI and the desired fragment was gel purified. The insert was subsequently ligated into a pACEBac1 vector cut with BamHI / XbaI resulting in pACEBac1-HA.
For creating dual-expression vectors pIDC64-RMD and pACEBac1-HA were fused in a cre-loxP mediated reaction according to manufacturer’s recommendations, resulting in pACEBac1-HA-RMD. Briefly, vectors were mixed in equal amounts and incubated with Cre recombinase for 1 h at 37°C. Vectors were subsequently purified, transformed in electorcompentent E.coli cells and incubated for at least 4 h at 37°C in SOC media. For selecting only that clones carrying the fused construct, cells were plated on LB-agar plates carrying different antibiotics, specific for the two initial vectors.
pACEBac1-HA and pACEBac1-HA-RMD were each inserted in the Tn7 site of a MultiBacY genome by transformation in DH10MultiBacY cells and virus was subsequently generated according to standard procedures resulting in AcHA and AcHA-RMD.
Expression and purification of Influenza HA
Hi5 and Sf9 cells were transferred to shaker flasks with supplemented IP-L41 or HyClone medium without FCS, respectively, two days prior infection and grown to a cell density of 2×106 cells / mL. For infection cells were diluted to 1×106 cells / mL with fresh medium in a total volume of 100 mL and infected with AcHA or AcHA-RMD at a Multiplicity of infection (MOI) of 5. All experiments were carried out in duplicates. For generating an expression profile, samples were taken 24 – 96 hours post infection (hpi). 96 hpi cellular supernatants were harvested by centrifugation at 1000 g for 10 min. In order to purify the expressed protein, the cleared supernatant was incubated with Ni-NTA Agarose (Qiagen, USA) for 2 h at 4°C while rotating at 10 rpm. The Agarose was loaded on Econo-columns (Biorad, USA) and washed with 2 column volumes of washing buffer (20 mM sodium phosphate, 0.5 M NaCl, 30 mM imidazole, pH 7.4). Bound HA was recovered by stepwise elution using elution buffer (20 mM sodium phosphate, 0.5 M NaCl, 500 mM imidazole, pH 7.4). For further experiments HA was concentrated, buffer was exchanged to phosphate buffered saline using Vivspin 6 centrifugal concentrators (Sartorius, Germany) and concentration was determined using NanoDrop (PeqLab, Germany).
SDS-PAGE and western blotting
Cellular supernatants (24 – 96 hpi, 5 μL each) were mixed with 2 x electrophoresis buffer containing 250 mM Tris/HCl, pH 6.8, 10% glycerol, 2% SDS, 100 mM DTT and 0.1% bromophenol blue. Proteins were separated by SDS-Page according to Laemmli [36] and electroblotted onto a PVDF membrane (GE Healthcare, USA). Detection was performed using a polyclonal anti-HA Cal09 mouse serum in TPBS (PBS + 0.05% Tween 20) and an anti-mouse IgG (γ-chain specific) alkaline phosphate conjugate (Sigma Aldrich, A1047). The blot was developed using NBT/BCIP (Promega, USA).
N-glycan analysis
N-glycan analyses were essentially performed as described in Rendic et al. [37]. Briefly, the band corresponding to Influenza HA was excised from a coomassie stained SDS-PAGE gel and cut into small pieces. After washing the gel pieces, they were treated with dithiothreitol and iodoacetamide solutions for modifying cysteine residues. The gel pieces were washed again and then subjected to trypsin digestion at 37°C over night. The resulting peptides were extracted with AcN:H2O:TFA solution (acetonitrile/water/trifluoroacetic acid; 666:333:1) and subsequently dried. For N-glycan release dry peptides were resuspended in 20 μl of 50 mM ammonium acetate (pH 5) buffer and subjected to PNGase A treatment at 37°C overnight. The released N-glycans were separated from peptides by using columns packed with LiChroprep RP 18 (25-40 μM) reversed phase resin (Merck, Germany) on top of Dowex 50WX8-400 ion exchange resin (Sigma, 217514). After equilibrating the column with 2% acetic acid, the sample was applied, the column was washed with 2% acetic acid and the flow-through containing N-glycans was collected. For further purification of the released N-glycans, columns were packed with Supelclean™ ENVI-Carb™ PGC material (Sigma Aldrich) on top of LiChroprep RP 18 (25-40 μM) reversed phase resin (Merck, Germany) and equilibrated with 2% acetic acid. The samples were loaded on the column and after washing with H2O, the N-glycans were eluted with 40% acetonitrile. The purified N-glycans were dried in a SpeedVac, finally resuspended in 5 μL deionised water and used for MALDI-TOF-MS analysis (on a Bruker Ultraflex MALDI-TOF/TOF in positive reflectron mode) using 6-aza-2-thiothymine (ATT) as matrix.
Enzyme-linked Immunosorbent Assay (ELISA)
96 well Maxisorp plates (Nunc, Roskilde, Denmark) were coated with 100 μL/well of purified influenza HA at a concentration of 2 μg/mL in coating buffer (50 mM sodium carbonate buffer, pH 7.4) and incubated at 4°C overnight. Plates were washed 5 times with TBST (TBS + 0.5% Tween20) and unspecific binding sites were blocked by incubating plates with 200 μL/well of blocking buffer (TBST, 3% milk powder). Plates were again washed (5x) with TBST and incubated with 100 μL/well of patients serum samples diluted 1:2 - 1:20 in dilution buffer (TBST, 0.5% BSA) at 4°C overnight followed by a subsequent washing step with TBST. For detection of bound IgE plates were incubated with 100 μL/well of AP-conjugated mouse anti-human IgE (BD PharminGen) diluted 1:100 in dilution buffer and incubated for 1 h in the dark. After one more round of washing, staining of ELISA plates was performed by incubation with 100 μL/well of AP substrate (1 SIGMAFAST™ p-Nitrophenyl phosphate Tablet set dissolved in 5 mL dH2O) (Sigma Aldrich, N1891). Plates were analysed by measuring OD405 at different time points. OD values were counted positive if they exceeded the mean OD of the negative controls by more than three standard deviations. As a positive control IgE binding to bromelain was tested in parallel.
Sera from 10 individuals suffering from inhalative allergies (tree and grass pollen) were included in this study. Diagnosis of allergy was based on routine diagnostic criteria: convincing case history and positive in vitro testing for allergen specific IgE. In addition these serum samples were tested positive of anti-CCD IgE. Blood samples from 3 healthy donors without any allergic symptoms or allergen-specific IgE antibodies were used as a control group. The study was performed under the Ethics Committee of the Medical University of Vienna (ethics committee approval number: 038/2009).
Results
Generation of fucose knock-down constructs
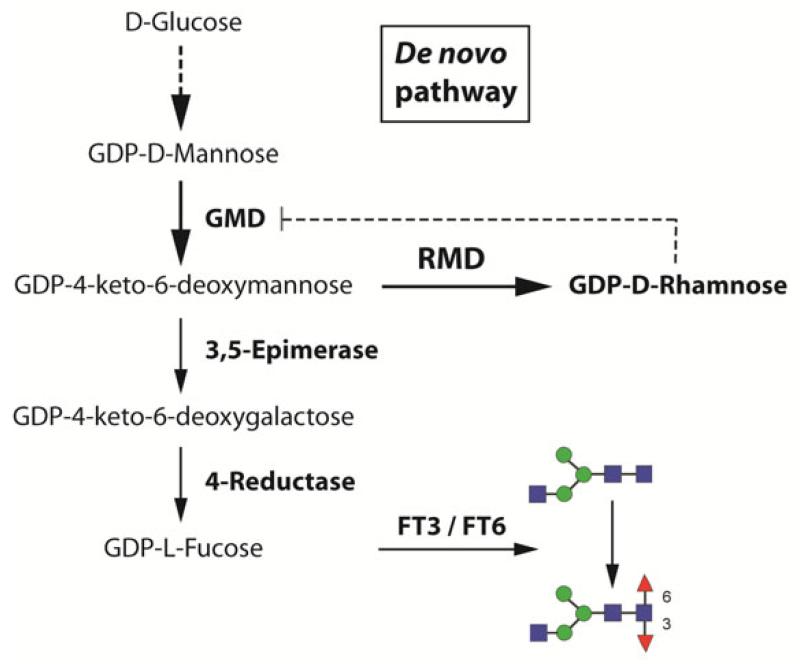
Previous studies have shown that the overexpression of Pseudomonas aeruginosa GDP-6-deoxy-D-lyxo-4-hexulose reductase (RMD) in stably transformed CHO cells leads to significantly reduced levels of fucosylation on secreted IgG antibodies [38]. This is achieved by the fact that RMD consumes a percursor of the de novo GDP-L-fucose synthesis pathway and converts it to the dead end product GDP-D-rhamnose (Fig. 1). The same effect was recently demonstrated for insect cell-derived expression [33].
Fig. 1. De novo GDP-L-fucose synthesis pathway.
In the classical insect cell pathway, GDP-D-mannose gets converted to GDP-L-fucose by a cascade of different enzymes. This precursor is then attached to the native N-glycan chain by using cellular fucosyltransferases (FT3 / FT6). The whole pathway can be blocked by the overexpression of Pseudomonas aeruginosa GDP-6-deoxy-D-lyxo-4-hexulose reductase (RMD) which converts GDP-4-keto-6-deoxymannose to the dead-end product GDP-D-rhamnose. This product, typically not present in vertebrate cells, may further block GMD by a negative feedback loop.
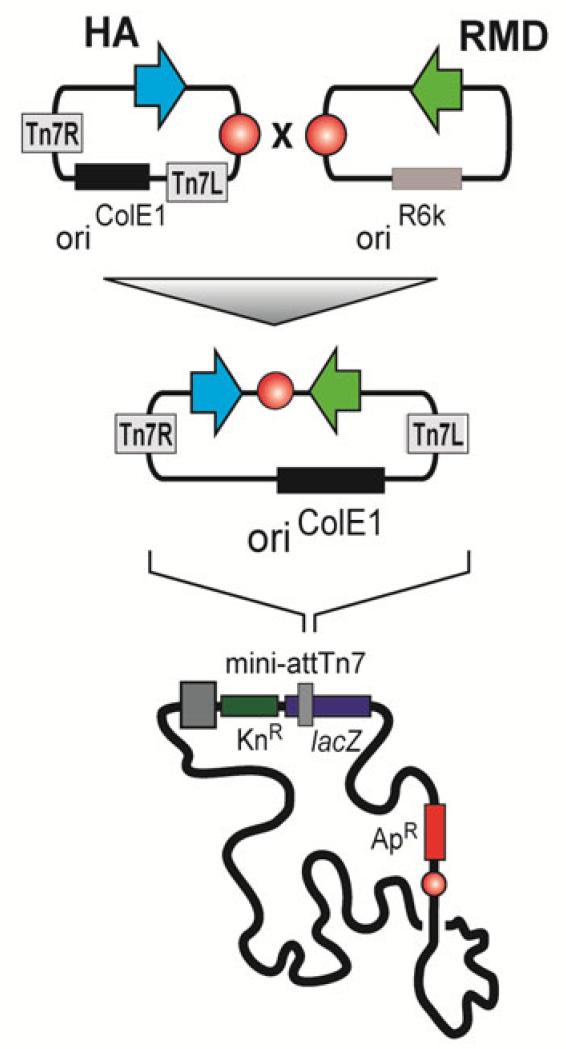
The baculovirus insect cell system usually employs late and very late promoters for transgene expression. In order to block the de novo GDP-L-fucose synthesis pathway we reconstructed the MultiBac donor vector pIDC. The original very late polyhedrin promoter was replaced by a modified viral gp64 tandem promoter containing both an immediate early promoter for expression beginning immediately after infection and a late promoter for continued expression in the late phase of infection. The RMD gene was codon optimised for the expression in insect cells and inserted in the hence generated pIDC64 vector. This setup guaranteed us that the pathway is already blocked when the desired product starts getting expressed. For being able to evaluate the impact of the fucose knock-down on a therapeutically relevant protein, we inserted a soluble Influenza A virus hemagglutinin (HA) [35] in the acceptor vector pACEBac1. In order to overcome the problem of inefficient co-infections, vectors coding for RMD and viral HA were fused in a cre-loxP mediated reaction and virus was generated (AcHA-RMD) (Fig. 2). Virus expressing viral HA alone served as a control (AcHA).
Fig. 2. Schematic representation of cloning procedure.
Open-reading frames coding for soluble influenza HA and Pseudomonas aeruginosa RMD were cloned in MultiBac acceptor vector pACEBac1 and donor vector pIDC64, respectively. For getting a dual expression construct, both vectors were combined using their loxP sites (red circles). The fused product was then integrated in the standard Tn7 site of a MultiBac backbone and virus was generated.
Production of non-fucosylated Influenza A virus HA
A variety of insect cell lines is available for the baculovirus driven protein expression. In our approach we decided to test cells derived from the Spodoptera frugiperda (Sf9) and Trichoplusia ni (HighFive) lineage. Sf9 cells and derivatives thereof, are the most widely used insect cells lines at the moment, yet HighFive cells are more suitable for the expression of secreted proteins.
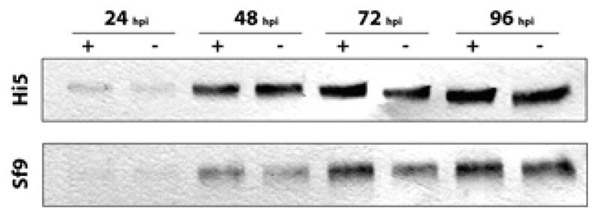
Sf9 and HighFive cells were infected with baculovirus clones expressing either influenza A virus hemagglutinin alone (AcHA) or in combination with RMD (AcHARMD) at an MOI of 5 respectively and samples were taken every 24 hours up to 4 days post infection (dpi). Expression levels of secreted HA were evaluated using western blot analysis. Fig. 3 shows that expression of HA in HighFive cells started 24 hours post infection (hpi) and was steadily increasing up to 96 hpi. Keeping infected cells in culture for a longer period did not make sense, because cells already started to lyse. The co-expression of Pseudomonas aeruginosa RMD did not significantly affect the expression levels of HA. Same results were obtained when using Sf9 cells, except that the overall levels of secreted HA were significantly lower as compared to HighFive cells.
Fig. 3. Impact of RMD on the overall expression level of influenza HA.
HighFive and Sf9 cells were infected with recombinant baculovirus expressing influenza HA (+) or influenza HA and RMD (−). Samples were taken every 24 h and the amount of secreted HA was measured by western blot analysis.
N-glycan analysis
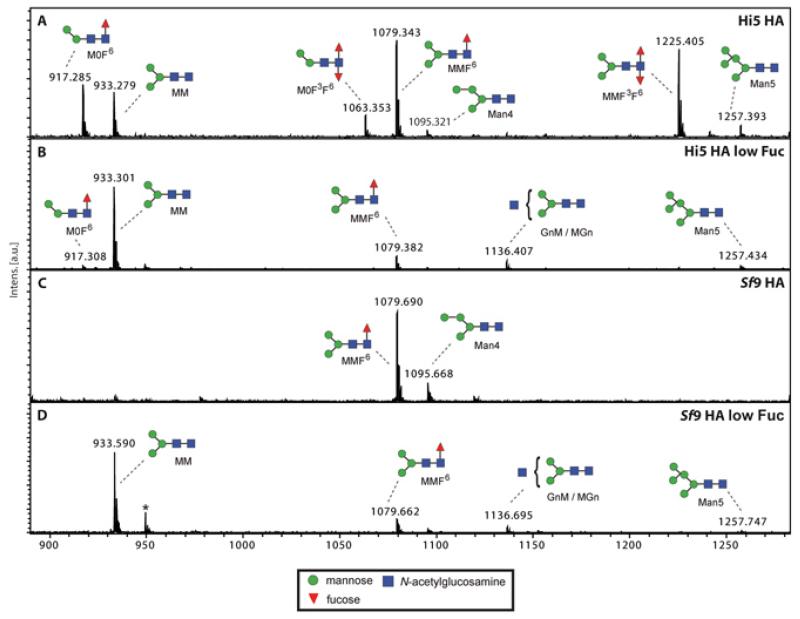
We analysed the N-glycosylation patterns of secreted HA expressed in Sf9 and HighFive insect cell lines, using mass spectrometry. Fig. 4 shows the MALDI-TOF MS spectra of PNGase A-released N-glycans. Influenza HA expressed in HighFive cells (Fig. 4A) carried mainly tri-mannose structures that were either non fucosylated (m/z 933.3) or, more dominant, single and double fucosylated (m/z 1079.3 and 1225.4). Structures with only two mannose residues carrying one or two fucose residues were found as well (m/z 917.3 and 1063.4). The co-expression of Pseudomonas aeruginosa RMD (Fig. 4B) leads to a total shift towards tri-mannose structures almost free from fucose (m/z 933.3). The glycan diversity of HA expressed in Sf9 cells (Fig. 4C) was much lower; almost exclusively tri-mannose structures carrying one fucose residue were found (m/z 1079.7). The N-glycans from HA expressed in Sf9 cells where fucosylation was blocked closely resembled the ones found in HighFive cells when RMD was present (Fig. 4D).
Fig. 4. MALDI-TOF-MS spectra of influenza HA N-glycans.
N-glycan structures found on HighFive cell expressed HA (A) mainly consist of single or double fucosylated tri-mannose structures, but fucosylated structures carrying only two mannose residues can be found as well. The co-expression of Pseudomonas aeruginosa RMD (B) leads to a shift of the dominant structures towards nonfucosylated tri-mannose structures. Using Sf9 cells for the expression of influenza HA (C), almost exclusively tri-mannose structures carrying a single fucose residue can be found. The results in the presence of RMD (D) closely resemble the one found for HighFive cells. Non-defined glycans are indicated by asterisks. Graphical representations of glycans (sodium adducts) are consistent with the nomenclature of the Consortium for Functional Glycomics.
IgE binding
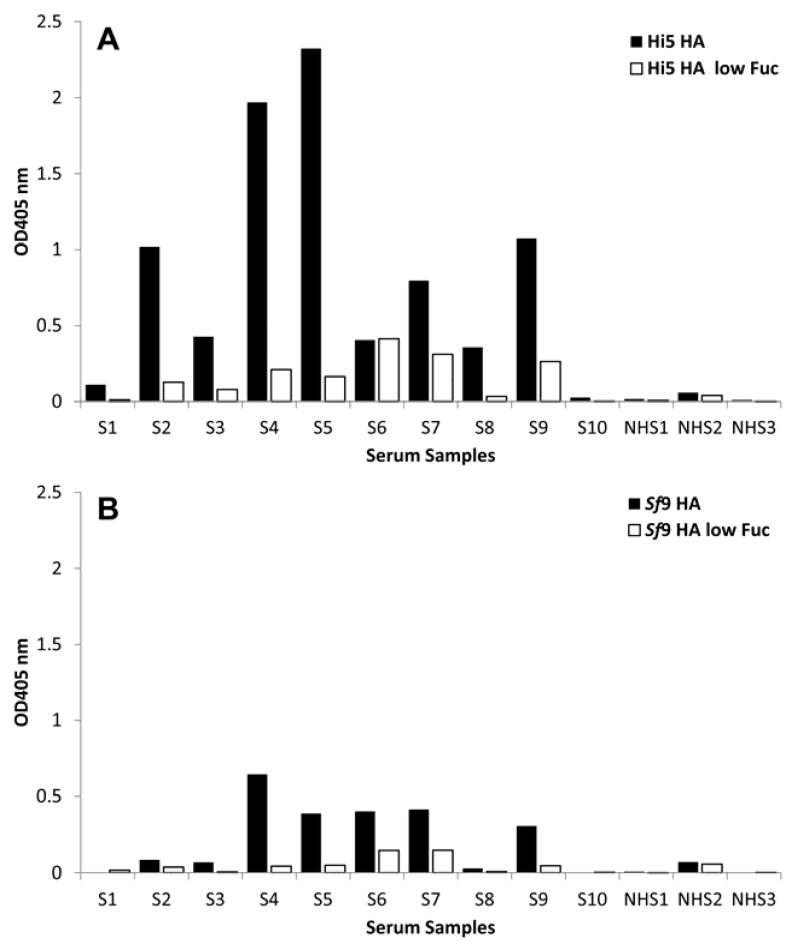
In order to evaluate the biological impact of defucosylation of influenza HA we tested the binding activity of the purified antigen to IgE derived from the sera of patients with allergy. 10 serum samples with a known reactivity towards CCDs and 3 serum samples from healthy donors were identified and an IgE ELISA performed. Fig. 5A shows the results of HA expressed in HighFive cells. 90% of the sera from patients with allergy showed reactivity with wildtype HA and 80% reacted to HA low fucose. With one exception IgE binding to influenza HA was considerably stronger as compared to HA low fucose. The strength of reactivity generally depended on the individual patient and varied within a 10-fold range. When RMD was co-expressed, the serum reactivity was drastically reduced. In 4 out of 10 samples the IgE binding levels dropped almost 10-fold and in 5 samples the binding was reduced at least 3-fold. Only one serum sample did not show a reduced reactivity with non-fucosylated HA. The results obtained with Sf9 cell-expressed HA (Fig. 5B) closely resembled the ones found for HA expressed by HighFive cells with the big difference that the overall reactivity was significantly lower. The sera from non-atopic controls displayed no or only low levels of binding activity to either HighFive HA, HighFive HA low Fuc, Sf9 HA and Sf9 HA low Fuc, respectively. Bromelain served as a positive control (data not shown). All experiments were carried out in triplicates.
Fig. 5. Binding of IgE antibodies from the sera of patients with allergy to influenza HA.
Influenza HA was expressed in HighFive (A) and Sf9 cells (B) and the binding levels of fucosylated (HA) and non-fucosylated variants (HA ΔFuc) to the sera of patients with allergy were measured in an IgE ELISA assay. The sera from 10 individual patients with allergy (S1 – S10) and from 3 healthy patients (NHS) were tested (n=3).
Discussion
The baculovirus insect cell system is a very powerful tool for the production of a wide range of therapeutically active proteins, especially vaccines. A major drawback so far is the altered N-glycosylation pattern, leading to either reduced activity or unwanted side effects. All insect cell-expressed proteins are fucosylated to varying degrees. While proteins expressed in Sf9 cells are mainly α1-6 fucosylated, HighFive cells often add fucose residues in an α1-6 and α1-3 orientation to the innermost N-acetylglucosamine [8]. Especially the latter modification can cause IgE binding to this so called carbohydrate cross-reactive determinant (CCD), further leading to a mast cell-derived histamine release [9]. This limits the use of insect cells for the production of therapeutics intended for human use, especially when they are secreted glycoproteins. In the past several attempts have been made to engineer the baculovirus insect cell system for improved N-glycosylation patterns. Many of them are based on the stable integration of glycozymes in the insect cell’s genome [27-31, 39]. This setup is on the one hand inflexible, because only one specific cell line can be used and on the other hand bears the risk of a metabolic overload, resulting in genetic instability. It therefore seems to be more suitable to generate virus based engineering strategies. It was shown previously that glycoengineering of proteins can be achieved by virally encoded co-expression of glycozymes [32]. In this study we focused on the elimination of unwanted side effects by knocking down the allergenic fucose. A previous study has shown the functionality of the Pseudomonas aeruginosa GDP-6-deoxy-D-lyxo-4-hexulose reductase (RMD) for the knock-down of fucosylation in stably transfected CHO cells [38]. Recently, a similar strategy was shown to be feasible in insect cells [33]. We codon optimised RMD for the expression in insect cells and cloned it in the MultiBac donor vector pIDC that has been modified to provide gp64 promoter driven gene expression. This tandem promoter enables RMD expression early in the infection cycle and guarantees that levels are still high enough for blocking the synthesis of GDP-L-fucose even in very late phases. In order to evaluate the efficiency of our approach for the production of a non-fucosylated, therapeutically relevant protein, we inserted an open reading frame encoding the soluble form of influenza A virus hemagglutinin (A/California/2009) [35] into the MultiBac acceptor vector pACEBac1. One advantage of MultiBac vectors is that simultaneous expression of two or more recombinant proteins from one baculovirus genome can be achieved by simple and straight forward cloning steps [32, 40-44].
Influenza HA was expressed in HighFive and Sf9 insect cells and expression profiles were monitored by western blot analysis up to 96 hpi. Fig. 3 shows that protein expression was steadily increasing as detected by western blot analysis, starting from 24 hpi. As already observed in previous studies, HighFive cells are much more suitable for the production of secreted proteins, because yields were significantly higher [6, 7, 45]. The co-expression of RMD gene did not significantly influence the level of HA production in both cell lines. This fact is very important when applying virus based engineering strategies for large scale production processes because industry always seeks for high protein quality on the one hand and highest possible yields on the other hand. MALDI-TOF-MS analyses revealed that HA expressed in HighFive cells showed the expected single and double fucosylated tri-mannose structures, as well as fucosylated structures carrying only two mannose residues (Fig. 4). The spectrum of Sf9 cell expressed HA only showed a single fucosylated trimannose structure. From the N-glycan point of view, Sf9 cells would be preferable for the production of high quality therapeutics, because they lack the highly immunogenic α1-3-linked fucose. Nevertheless, even an α1-6-linked fucose, that is present on most eukaryotic and insect cell expressed proteins, can have profound effects on protein functionality. Antibodies, for example, lacking α1-6-linked fucose have been shown to have an enhanced ability to support the ADCC (antibody dependent cellular cytotoxicity) response in vivo [38, 46, 47]. The co-expression of RMD in both cell lines leads to a shift of the dominant structures towards nonfucosylated tri-mannose structures. This together with the fact that RMD did not alter the overall transgene expression level showed the great potential of virus based glyco-engineering for the future. Nevertheless, there were still some residual amounts of fucose left. This could be explained by the presence of fucose in the insect cell media and the cell own salvage pathway for the production of GDP-L-fucose. By media optimization one could easily overcome this problem.
In order to evaluate the allergenic activity of the fucose knock-down, we evaluated the binding of fucosylated and non-fucosylated HA to IgE antibodies derived from the sera of patients with allergy. Fig. 5 shows the results of an IgE ELISA. Influenza HA expressed in HighFive cells using standard expression techniques displayed very high binding activity. In contrast, bound IgE levels were drastically reduced, when testing HA derived from the virus based knock-down. The same results were obtained when Sf9 cells were used, with the big difference that the overall reactivity was much lower. This difference in reactivity can be explained by the presence of α1-3-linked fucose that is only found on HighFive cell expressed proteins.
A type I allergic response is generally triggered by optimal cross-linking of IgE molecules that occupy high affinity IgE receptors on mast cells. The cross-linking process requires at least 2 epitopes on an allergen, but many glycoproteins in the molecular weight range of allergens are only monoglycosylated. This might be one of the reasons why the overall clinical significance of anti-CCD IgE has been challenged so far.
Here we could demonstrate that insect cell-derived proteins cause different reactions with sera from patients with allergy, depending whether they are fucosylated or not. Our data suggest that expression systems that avoid fucosylation of complex protein therapeutics are superior in terms of making vaccines. Although, the clinical significance of anti-CCD IgE is often not considered, there are unwanted side effects in patients with allergy when fucose structures, especially if α1-3-linked fucose, is present. Using a system that provides the possibility to produce proteins at high expression levels and at the same time minimizes the risk for unwanted side effects, would be a great benefit for the production process and the patients.
Acknowledgements
We would like to thank Dr. Imre Berger for providing us with the MultiBac vector system and Dr. Florian Krammer for providing us with soluble Influenza A HA constructs. This work was funded by grants from the Austrian Fonds zur Förderung der wissenschaftlichen Forschung (FWF; P 25092-B13 and F 4603).
Abbreviations
- CCD
cross-reactive carbohydrate determinant
- dpi
days post infection
- HA
influenza A virus hemagglutinin
- hpi
hours post infection
- RMD
Pseudomonas aeruginosa GDP-6-deoxy-D-lyxo-4-hexulose reductase
Footnotes
The authors declare no financial or commercial conflict of interest.
References
- [1].Summers MD, Smith GE. Texas Agricultural Experiment Station Bulletin. 1987;1555 [Google Scholar]
- [2].Wickham TJ, Nemerow GR. Optimization of growth methods and recombinant protein production in BTI-Tn-5B1-4 insect cells using the baculovirus expression system. Biotechnol Prog. 1993;9:25–30. doi: 10.1021/bp00019a004. [DOI] [PubMed] [Google Scholar]
- [3].Schiller JT, Castellsagué X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26(Suppl 10):K53–61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Treanor JJ, El Sahly H, King J, Graham I, et al. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok®) against influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine. 2011;29:7733–7739. doi: 10.1016/j.vaccine.2011.07.128. [DOI] [PubMed] [Google Scholar]
- [5].López-Macías C, Ferat-Osorio E, Tenorio-Calvo A, Isibasi A, et al. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine. 2011;29:7826–7834. doi: 10.1016/j.vaccine.2011.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Krammer F, Schinko T, Palmberger D, Tauer C, et al. Trichoplusia ni cells (High Five) are highly efficient for the production of influenza A virus-like particles: a comparison of two insect cell lines as production platforms for influenza vaccines. Mol Biotechnol. 2010;45:226–234. doi: 10.1007/s12033-010-9268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wilde M, Klausberger M, Palmberger D, Ernst W, Grabherr R. Tnao38, high five and Sf9-evaluation of host-virus interactions in three different insect cell lines: baculovirus production and recombinant protein expression. Biotechnol Lett. 2013 doi: 10.1007/s10529-013-1429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Altmann F, Staudacher E, Wilson I, März L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- [9].Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that crossreact with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981;68:356–364. doi: 10.1016/0091-6749(81)90133-0. [DOI] [PubMed] [Google Scholar]
- [10].Tretter V, Altmann F, Kubelka V, März L, Becker WM. Fucose alpha 1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int Arch Allergy Immunol. 1993;102:259–266. doi: 10.1159/000236534. [DOI] [PubMed] [Google Scholar]
- [11].Hemmer W, Focke M, Kolarich D, Wilson IB, et al. Antibody binding to venom carbohydrates is a frequent cause for double positivity to honeybee and yellow jacket venom in patients with stinging-insect allergy. J Allergy Clin Immunol. 2001;108:1045–1052. doi: 10.1067/mai.2001.120013. [DOI] [PubMed] [Google Scholar]
- [12].Prenner C, Mach L, Glössl J, März L. The antigenicity of the carbohydrate moiety of an insect glycoprotein, honey-bee (Apis mellifera) venom phospholipase A2. The role of alpha 1,3-fucosylation of the asparagine-bound N-acetylglucosamine. Biochem J. 1992;284(Pt 2):377–380. doi: 10.1042/bj2840377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kubelka V, Altmann F, März L. The asparagine-linked carbohydrate of honeybee venom hyaluronidase. Glycoconj J. 1995;12:77–83. doi: 10.1007/BF00731872. [DOI] [PubMed] [Google Scholar]
- [14].Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- [15].Seppälä U, Selby D, Monsalve R, King TP, et al. Structural and immunological characterization of the N-glycans from the major yellow jacket allergen Ves v 2: the N-glycan structures are needed for the human antibody recognition. Mol Immunol. 2009;46:2014–2021. doi: 10.1016/j.molimm.2009.03.005. [DOI] [PubMed] [Google Scholar]
- [16].Wicklein D, Lindner B, Moll H, Kolarich D, et al. Carbohydrate moieties can induce mediator release: a detailed characterization of two major timothy grass pollen allergens. Biol Chem. 2004;385:397–407. doi: 10.1515/BC.2004.044. [DOI] [PubMed] [Google Scholar]
- [17].Batanero E, Crespo JF, Monsalve RI, Martín-Esteban M, et al. IgE-binding and histamine-release capabilities of the main carbohydrate component isolated from the major allergen of olive tree pollen, Ole e 1. J Allergy Clin Immunol. 1999;103:147–153. doi: 10.1016/s0091-6749(99)70538-5. [DOI] [PubMed] [Google Scholar]
- [18].Andersson K, Lidholm J. Characteristics and immunobiology of grass pollen allergens. Int Arch Allergy Immunol. 2003;130:87–107. doi: 10.1159/000069013. [DOI] [PubMed] [Google Scholar]
- [19].Commins SP, Platts-Mills TA. Allergenicity of carbohydrates and their role in anaphylactic events. Curr Allergy Asthma Rep. 2010;10:29–33. doi: 10.1007/s11882-009-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Ree R, Cabanes-Macheteau M, Akkerdaas J, Milazzo JP, et al. Beta(1,2)-xylose and alpha(1,3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. J Biol Chem. 2000;275:11451–11458. doi: 10.1074/jbc.275.15.11451. [DOI] [PubMed] [Google Scholar]
- [21].Foetisch K, Westphal S, Lauer I, Retzek M, et al. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2003;111:889–896. doi: 10.1067/mai.2003.173. [DOI] [PubMed] [Google Scholar]
- [22].Paschke A, Kinder H, Zunker K, Wigotzki M, et al. Characterization of cross-reacting allergens in mango fruit. Allergy. 2001;56:237–242. doi: 10.1034/j.1398-9995.2001.056003237.x. [DOI] [PubMed] [Google Scholar]
- [23].Fahlbusch B, Rudeschko O, Schumann C, Steurich F, et al. Further characterization of IgE-binding antigens in kiwi, with particular emphasis on glycoprotein allergens. J Investig Allergol Clin Immunol. 1998;8:325–332. [PubMed] [Google Scholar]
- [24].Bublin M, Radauer C, Wilson IB, Kraft D, et al. Cross-reactive N-glycans of Api g 5, a high molecular weight glycoprotein allergen from celery, are required for immunoglobulin E binding and activation of effector cells from allergic patients. FASEB J. 2003;17:1697–1699. doi: 10.1096/fj.02-0872fje. [DOI] [PubMed] [Google Scholar]
- [25].Petersen A, Vieths S, Aulepp H, Schlaak M, Becker WM. Ubiquitous structures responsible for IgE cross-reactivity between tomato fruit and grass pollen allergens. J Allergy Clin Immunol. 1996;98:805–815. doi: 10.1016/s0091-6749(96)70130-6. [DOI] [PubMed] [Google Scholar]
- [26].Aumiller J, Hollister J, Jarvis D. A transgenic insect cell line engineered to produce CMP-sialic acid and sialylated glycoproteins. Glycobiology. 2003;13:497–507. doi: 10.1093/glycob/cwg051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Aumiller JJ, Mabashi-Asazuma H, Hillar A, Shi X, Jarvis DL. A new glycoengineered insect cell line with an inducibly-mammalianized protein N-glycosylation pathway. Glycobiology. 2012;22:417–428. doi: 10.1093/glycob/cwr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Breitbach K, Jarvis DL. Improved glycosylation of a foreign protein by Tn-5B1-4 cells engineered to express mammalian glycosyltransferases. Biotechnol Bioeng. 2001;74:230–239. doi: 10.1002/bit.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hollister JR, Shaper JH, Jarvis DL. Stable expression of mammalian beta 1,4-galactosyltransferase extends the N-glycosylation pathway in insect cells. Glycobiology. 1998;8:473–480. doi: 10.1093/glycob/8.5.473. [DOI] [PubMed] [Google Scholar]
- [30].Hollister J, Grabenhorst E, Nimtz M, Conradt H, Jarvis DL. Engineering the protein N-glycosylation pathway in insect cells for production of biantennary, complex N-glycans. Biochemistry. 2002;41:15093–15104. doi: 10.1021/bi026455d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jarvis DL, Kawar ZS, Hollister JR. Engineering N-glycosylation pathways in the baculovirus-insect cell system. Curr Opin Biotechnol. 1998;9:528–533. doi: 10.1016/s0958-1669(98)80041-4. [DOI] [PubMed] [Google Scholar]
- [32].Palmberger D, Wilson IBH, Berger I, Grabherr R, Rendic D. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0034226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mabashi-Asazuma H, Kuo CW, Khoo KH, Jarvis DL. A novel baculovirus vector for the production of non-fucosylated recombinant glycoproteins in insect cells. Glycobiology. 2013;24:325–340. doi: 10.1093/glycob/cwt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. New York: 1989. [Google Scholar]
- [35].Krammer F, Margine I, Tan GS, Pica N, et al. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One. 2012;7:e43603. doi: 10.1371/journal.pone.0043603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [37].Rendić D, Wilson IB, Lubec G, Gutternigg M, et al. Adaptation of the “ingel release method” to N-glycome analysis of low-milligram amounts of material. Electrophoresis. 2007;28:4484–4492. doi: 10.1002/elps.200700098. [DOI] [PubMed] [Google Scholar]
- [38].von Horsten HH, Ogorek C, Blanchard V, Demmler C, et al. Production of non-fucosylated antibodies by co-expression of heterologous GDP-6-deoxy-D-lyxo-4-hexulose reductase. Glycobiology. 2010;20:1607–1618. doi: 10.1093/glycob/cwq109. [DOI] [PubMed] [Google Scholar]
- [39].Jarvis DL, Summers MD. Glycosylation and secretion of human tissue plasminogen activator in recombinant baculovirus-infected insect cells. Mol Cell Biol. 1989;9:214–223. doi: 10.1128/mcb.9.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Berger I, Fitzgerald DJ, Richmond TJ. Baculovirus expression system for heterologous multiprotein complexes. Nat Biotechnol. 2004;22:1583–1587. doi: 10.1038/nbt1036. [DOI] [PubMed] [Google Scholar]
- [41].Bieniossek C, Richmond TJ, Berger I. MultiBac: multigene baculovirus-based eukaryotic protein complex production. Curr Protoc Protein Sci. 2008 doi: 10.1002/0471140864.ps0520s51. Chapter 5, Unit 5.20. [DOI] [PubMed] [Google Scholar]
- [42].Fitzgerald DJ, Berger P, Schaffitzel C, Yamada K, et al. Protein complex expression by using multigene baculoviral vectors. Nat Methods. 2006;3:1021–1032. doi: 10.1038/nmeth983. [DOI] [PubMed] [Google Scholar]
- [43].Trowitzsch S, Bieniossek C, Nie Y, Garzoni F, Berger I. New baculovirus expression tools for recombinant protein complex production. J Struct Biol. 2010;172:45–54. doi: 10.1016/j.jsb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- [44].Palmberger D, Klausberger M, Berger I, Grabherr R. MultiBac turns sweet. Bioengineered. 2013;4:78–83. doi: 10.4161/bioe.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Palmberger D, Rendić D, Tauber P, Krammer F, et al. Insect cells for antibody production: evaluation of an efficient alternative. J Biotechnol. 2011;153:160–166. doi: 10.1016/j.jbiotec.2011.02.009. [DOI] [PubMed] [Google Scholar]
- [46].Iida S, Kuni-Kamochi R, Mori K, Misaka H, et al. Two mechanisms of the enhanced antibody-dependent cellular cytotoxicity (ADCC) efficacy of nonfucosylated therapeutic antibodies in human blood. BMC Cancer. 2009;9:58. doi: 10.1186/1471-2407-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Satoh M, Iida S, Shitara K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin Biol Ther. 2006;6:1161–1173. doi: 10.1517/14712598.6.11.1161. [DOI] [PubMed] [Google Scholar]