Abstract
Sentence comprehension requires the integration of both syntactic and semantic information, the acquisition of which seems to have different trajectories in the developing brain. Using functional magnetic resonance imaging, we examined the neural correlates underlying syntactic and semantic processing during auditory sentence comprehension as well as its development in preschool children by manipulating case marking and animacy hierarchy cues, respectively. A functional segregation was observed within Broca's area in the left inferior frontal gyrus for adults, where the pars opercularis was involved in syntactic processing and the pars triangularis in semantic processing. By contrast, five-year-old children sensitive to animacy hierarchy cues showed diffuse activation for semantic processing in the left inferior frontal and posterior temporal cortices. While no main effect of case marking was found in the left fronto-temporal language network, children with better syntactic skills showed greater neural responses for syntactically complex sentences, most prominently in the posterior superior temporal cortex. The current study provides both behavioral and neural evidence that five-year-old children compared to adults rely more on semantic information than on syntactic cues during sentence comprehension, but with the development of syntactic abilities, their brain activation in the left fronto-temporal network increases for syntactic processing.
Keywords: fMRI, Syntax, Semantics, Language development, Pars opercularis, Pars triangularis
Highlights
-
•
Adults showed a functional segregation in Broca's area for syntax and semantics.
-
•
Brodmann Area (BA) 44 was involved in syntactic and BA 45 in semantic processing.
-
•
Preschoolers relied more on semantic animacy than on syntactic case marking cues.
-
•
Children showed adult-like left fronto-temporal activation for semantic processing.
-
•
The left fronto-temporal activation for syntax correlated with syntactic abilities.
Introduction
An essential aspect of sentence comprehension is to understand the relations between words in a string, such as the agent–patient relation which determines who is doing what to whom. Humans make use of several cues carried by the components to determine which participant in a sentence is the actor of an action expressed by the verb, thereby helping with interpretation. Take an English sentence “she plays the piano” for example. One could use several cues in the sentence to identify she as the agent to play and the piano as the patient to be played, such as (1) animacy hierarchy—an animate noun she is more likely to act upon an inanimate noun the piano; (2) “subject–verb–object” word order—the noun before the verb (i.e., she) is the subject, and the noun after the verb (i.e., the piano) is the object; (3) case marking—the first noun she is a subject pronoun but not an object pronoun (i.e., her). While it seems to be an automatic process for adults to interpret sentences by assigning different weights to the available cues (MacWhinney et al., 1984), sentence comprehension is nonetheless a challenging task for the developing cognitive system (Bates et al., 1984).
According to the competition model, the age of acquisition of a sentential cue is determined by its cue validity, which is jointly influenced by cue availability and cue reliability in the target language (Bates and MacWhinney, 1982, Bates et al., 1984). Mastery of these cues only occurs gradually over time during language development (Bates et al., 1984, Chan et al., 2009, Dittmar et al., 2008). For the German language, previous behavioral studies have demonstrated that German-speaking children show primacy in the acquisition of animacy cues, followed by word order, and do not rely on case marking over other cues until the age of seven or later (Chan et al., 2009, Dittmar et al., 2008, Lindner, 2003). The late acquisition of syntactic case marking may be attributed to its low validity in German particularly due to lower availability of unambiguous case marking (Mahlstedt, 2007). Other studies have also suggested that semantic information directly influences syntactic analysis in children's sentence processing (Deutsch et al., 1999, Friederici, 1983). Hence, semantic cues seem to play a more important role for children compared to adults during sentence comprehension (MacWhinney et al., 1984).
Apparently, sentence comprehension is achieved by integrating the syntactic and semantic information provided. Evidence from functional neuroimaging studies with adults has shown that while syntactic and semantic processing both involve a left-lateralized fronto-temporal network, each function seems to be supported by segregated regions in the brain (Friederici et al., 2000, Newman et al., 2003, Newman et al., 2010, Ni et al., 2000). While the pars opercularis of the left inferior frontal gyrus (IFG), that is, Brodmann Area (BA) 44, and the left posterior superior temporal gyrus/sulcus (posterior STG/STS) have been reported to show increased activation for processing more complex syntactic sentences compared to less complex sentences (Constable et al., 2004, Friederici, 2011, Friederici et al., 2009, Grewe et al., 2007, Kinno et al., 2008, Mack et al., 2013, Makuuchi et al., 2009, Meltzer et al., 2010, Santi and Grodzinsky, 2010), sentential semantic processing has been shown to be subserved by pars triangularis (BA 45) and pars orbitalis (BA 47) of the left IFG as well as the left posterior STS (Binder et al., 2009, Bruffaerts et al., 2013, Grewe et al., 2007, Mestres-Missé et al., 2008, Newman et al., 2010, Rodd et al., 2005). These neuroimaging findings from adults provide a valuable neurocognitive model of language against which the development of semantic and syntactic processing in children can be discussed.
Yet the neural network underlying sentence processing in young children has only been sparsely investigated. Existing studies suggest that functional segregation in the fronto-temporal regions in children is not as distinct as in adults. Using transitive sentences containing either syntactic or semantic violations, Brauer and Friederici (2007) demonstrated that while adults showed function-specific activation in the left STG and the frontal operculum for syntactic as compared with semantic processing, five- to six-year-old children recruited largely overlapped activation in the left STG and bilateral IFG. Skeide et al. (2014) used correct sentences in a sentence–picture matching task with manipulations of syntactic complexity and semantic plausibility, and demonstrated that three- to four-year-old children showed no main effect, but only interaction effects between syntax and semantics in the mid and posterior portion of STG. In addition to interaction effects, six- to seven-year-old children also started to show main effects of syntax and semantics in the mid to posterior STG/STS, but children at the ages of nine to ten had a segregated main effect of syntax in the left IFG and a main effect of semantics in the anterior STG/STS. This study provides strong neural evidence that children do not process syntax independently from semantics in sentence interpretation until the age of ten.
Moreover, there are a number of studies that report cortical activation for syntactic processing to be associated with children's behavioral performance. In children aged between seven and fifteen years, it was found that the activation in the left IFG in response to syntactic processing increased with above average proficiency of syntactic skills independent of age (Nuñez et al., 2011). In another study with children between four and six years of age, it was shown that even in these young children, a subgroup with better syntactic knowledge already showed enhanced activation in the left BA 44 for non-canonical object-first sentences compared to canonical subject-first sentences (Knoll et al., 2012). These studies indicate that the neural representation underlying language processing is dependent on the development and maturation of the brain, which in turn is correlated with children's linguistic skills. A direct correlation between the brain's functional development of four language-related regions as well as the structural maturation between these and the syntactic processing skills between the ages of three to ten years has recently been demonstrated by Skeide et al. (2015).
The current study used functional magnetic resonance imaging (fMRI) to specify the neural correlates underlying processing of syntactic canonicity and of semantic animacy, as well as the interaction between these, during auditory sentence comprehension in the developing and mature brain. In a sentence listening paradigm, we manipulated case marking as the syntactic cue and animacy hierarchy as the semantic cue. Five-year-old children were selected to compare with adults as children at this age are already sensitive to the animacy hierarchy but have only started to learn case marking cues. This allows us to examine whether children with different levels of syntactic knowledge, independent of age, may show different patterns of neural activation for syntactic processing and how this interacts with animacy information. Adults were chosen as the control group as their neural responses would serve as a reference model for sentence comprehension under the task manipulation. Moreover, in the current study, analyses tested for whole brain effects as well as for anatomically defined a priori regions-of-interest (ROIs) in the perisylvian areas that have been identified relevant for processing sentence comprehension in previous studies as described above, namely the pars opercularis and pars triangularis in the left IFG, the left posterior STS, and the left posterior STG (Bahlmann et al., 2007, Ben-Shachar et al., 2003, Binder et al., 2009, Bornkessel et al., 2005, Bruffaerts et al., 2013, Constable et al., 2004, Friederici, 2011, Friederici et al., 2009, Grewe et al., 2007, Kinno et al., 2008, Makuuchi et al., 2009, Mestres-Missé et al., 2008, Moro et al., 2001, Musso et al., 2003, Newman et al., 2010, Obleser et al., 2007, Rodd et al., 2005, Röder et al., 2002, Saur et al., 2008, Tyler et al., 2005).
Materials and methods
Participants
Fifty-six children at the age range of 5;1 to 5;11 were initially recruited. A number of children had to be excluded from the study for the following reasons: two children showed incidental findings; eleven did not finish the fMRI task; three were ambidextrous or left-handed (scores ≤ 20 in the modified version of Edinburgh Handedness Inventory (Oldfield, 1971)); three had large movement during fMRI scanning exceeding 3 mm at any translation axis and/or 3° at any rotation. Consequently, data from thirty-seven children were preprocessed and analyzed. A group of sixteen adults served as a control group. After individual-level analyses, activation maps contrasting all individual sentence conditions versus the silence condition (rest) were examined as a basic activation check, which were expected to show activation in the bilateral auditory cortices as the sentences were auditorily presented. Seven children and one adult were excluded from group-level analyses as they did not show any activation in the auditory cortices for this baseline contrast even at the threshold of p < 0.05 (uncorrected). As a result, the final group-level analyses consisted of thirty children (ten boys, age range 5;1–5;11, M = 5;6, SD = 0;3.45; handedness scores range 40–100, M = 78.1, SD = 17.8) and fifteen adults (eight males; age range 21–32, M = 25.5, SD = 3.27; handedness scores range 36.8–100, M = 83.0, SD = 16.9). All participants were native German speakers, and had no history of medical, psychiatric or neurological disorders. Written informed consent was obtained from all adult participants and the parents of the children. Children gave verbal assent prior to participation. The study was approved by the Institutional Review Board of the University of Leipzig.
Task design and materials
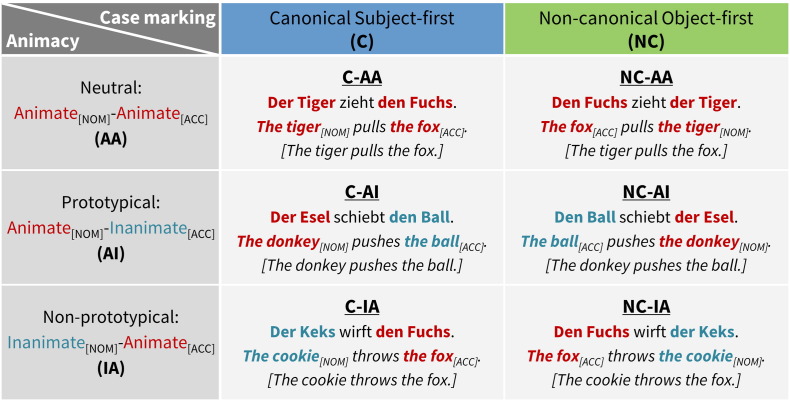
In the German language, case marking in the article of the noun phrase indicates the subject (nominative case) and the object (here: accusative case). While canonical sentences are subject-first, German as a free word order language also possesses non-canonical object-first sentences. Stimulus materials consisted of 150 five-word German sentences composed of two noun phrases (NPs) and one verb (V) following a NP–V–NP structure. Only grammatically masculine nouns were used, for which case marking variation of the nominative and accusative forms is unambiguous. The sentences varied in two factors: syntactic case marking and semantic animacy hierarchy. Case marking variation was used to introduce canonical subject-first and non-canonical object-first sentences that differed by syntactic structure but not by semantic content. Animacy hierarchy of the nominatives and the accusatives in the NPs was defined with three levels as neutral hierarchy (animate agent and animate patient, AA), prototypical hierarchy (animate agent and inanimate patient, AI), and non-prototypical hierarchy (inanimate agent and animate patient, IA). The manipulation of case marking and animacy hierarchy factors resulted in a 2 × 3 within-subjects designed experiment composed of six conditions: C-AA, C-AI, C-IA, NC-AA, NC-AI, and NC-IA (see Fig. 1 for examples of sentences). Each condition consisted of 25 sentences, and each sentence lasted for 3.29 s on average (SD = 0.02 s). For both children and adults, the fMRI task contained 150 sentence trials and additionally 15 null events modeled as the control baseline. Trials were presented every 6 s, with the trial onsets randomly jittered at 0, 500, 1000, or 1500 ms after the beginning of the first scan, which allowed for the sampling of several different time points along the hemodynamic response curve.
Fig. 1.
The fMRI task conditions.
The task consisted of two levels of case marking (canonical subject-first, non-canonical object-first) and three levels of animacy hierarchy (neutral, prototypical, non-prototypical), resulting in six conditions: C-AA, C-AI, C-IA, NC-AA, NC-AI, and NC-IA. NOM: nominative; ACC: accusative. The animate arguments are highlighted in red and inanimate in aqua.
The nouns and verbs were selected from the German corpora in the Child Language Data Exchange System (CHILDES; http://childes.psy.cmu.edu/) and the SETK-2 test (Grimm et al., 2000). Several criteria were applied for the selection of verbs: (1) transitive verbs; (2) verbs that indicate physical contact or intention of physical contact and do not require a third object for the action; (3) verbs that are semantically accessible in their bare forms, that is, without particles. The word length was controlled at no more than two syllables. We first generated 9,044 sentences from the selected words and conducted a pilot rating study with 57 adults in order to select the sentences with low semantic animacy acceptability for the IA condition and high semantic animacy acceptability for the other two conditions. The sentences were recorded by a trained female native German speaker in a child-directed manner. The recorded sentences were digitized (44.1 kHz, 32-bit sampling rate, mono) and normalized to the root mean squared amplitude.
Task procedure
To familiarize the children with experimental settings and the magnetic resonance (MR) environment, they underwent a practice session in a mock MR scanner prior to the actual scanning session. During the mock-up session, children were instructed to practice lying still in the scanner while listening to sentences. Verbal feedback regarding movement was given to the children via headphones during the simulated scanning. Those children who passed the mock-up session were invited to participate in the fMRI experiment within a week. Prior to scanning, they performed a picture naming task, in which they were introduced to pictures of the animate and inanimate nouns included in the sentence stimuli and were asked to name the pictures. This task was to assure that children were familiar with the nouns. If they failed to name a noun, correct answers would be given and repeated twice to them by the experimenter.
For the auditory sentence comprehension task, children were instructed to listen to the sentences carefully during the scanning, in particular to pay attention to “who is doing what to whom”, and they were reminded that there would be a post-scanning test about the sentences they were about to listen to. Stimuli were presented using the software Presentation® (Neurobehavioral Systems, Inc., Albany, CA, USA) via headphones. During the fMRI scanning session and while listening to the sentences, participants viewed a screensaver that did not involve any kind of human or animal action via goggles. The entire scanning session including preparation, functional and anatomical scans lasted for approximately 40 min. No immediate responses were required during the fMRI run. Instead, children performed a post-scanning behavioral sentence comprehension task outside the scanner. Experimental stimuli from the fMRI run were used and auditorily presented via a computer. With each sentence, two pictures were shown on the screen side by side. In the case of inanimate actors, pictures were represented with the minimal features that were required for a specific action, such as a hand in a throwing action or a foot in a kicking action. No other human-like features, such as faces or other body parts, were added to the inanimate actors. By doing so, we ensured that the action would be possible to be depicted and at the same time the inanimate actor would not receive overall animate features. Foils contained the same lexical items as the correct pictures but with reversed thematic roles. The correct pictures appeared in 50% of the trials on the left or the right sides of the screen, and actions were performed in 50% of the trials from the left-to-right or right-to-left directions between the two nouns. The children had to indicate whether the left or the right picture matched with the sentence they heard, and their responses were recorded. This task was specialized to test the behavioral performance on the sentences used in the fMRI experiment.
The choice to measure behavioral performance during a post-scanning task instead of during the scan was based on careful methodological considerations. First, requiring young children at this age to respond to a task by a button press often incurs additional movement, which could very likely translate to undesirable motion artifacts during fMRI scans. Second, it is always a challenging trade-off for developmental neuroimaging studies to reduce the scanning time as much as possible while still preserving enough power of task effects. The longer the scanning time, the more difficult it is to ensure that children keep their attention and stay still in the scanner. Hence, we chose to remove in-scanner responses as it allowed us to shorten the scanning time and minimize the likelihood of increasing head motion artifacts.
Adults underwent a similar experimental procedure as children, but they did not have a simulated scanning session in a mock MR scanner. They also completed the picture naming task prior to entering the scanner and were instructed to listen to the sentences carefully during the experiment. The same auditory sentence comprehension task as used for children was applied. After scanning, they were not administered the post-scanning behavioral sentence comprehension task. Rather, in order to test their attention during scanning, they completed a questionnaire about the task stimuli including a number of probes of the task as well as questions, such as which verbs/nouns they remembered.
Standardized behavioral measures
Standardized behavioral measures were acquired from both groups, which covered a variety of cognitive functions, such as language (TSVK—Test zum Satzverstehen von Kindern (Siegmüller et al., 2011)), working memory (forward and backward digit span, Mottier test (Mottier, 1951)), and non-verbal intelligence (K-ABC—Kaufman Assessment Battery for Children (Kaufman et al., 2003)). Children who performed at T < 40 in TSVK or K-ABC were excluded from the fMRI experiment. Among these measures, the TSVK test is the most relevant to the current study. It is a standardized German language test of sentence comprehension for children at different age groups. It consists of 36 sentences varying along several syntactic dimensions of sentence complexity including word order (subject-/object-first structures), tense (present/past tense), mode (active/passive constructions), clause complexity (coordinate/subordinate constructions), number (singular/plural of nouns and verbs), pronoun type (definite/indefinite), and verb type (reflexive/non-reflexive verbs). The TSVK is a picture matching test, in which a sentence is presented to the child auditorily and the child has to choose the picture that matches with the sentence from a set of three pictures. The incorrect pictures vary from the correct picture on several of the syntactic dimensions mentioned above. Therefore, the TSVK serves as a measure of children's general syntactic abilities for sentence comprehension. Crucially, as the syntactic dimension of interest in the current study was subject-/object-first structures indicated by case marking, we specifically extracted a subset of 12 items which test the understanding of case marking cues, and used the number of correct items out of 12 (TSVK-subtest) for the relevant analysis.
Image acquisition
Imaging was performed in a 3 Tesla Magnetom Tim Trio scanner (Siemens, Erlangen, Germany) with a 12-channel head coil. An echo planar imaging sequence was used to acquire functional images with the following parameters: repetition time (TR) = 2 s, echo time (TE) = 30 ms, flip angle (FA) = 90°, field of view (FOV) = 192 mm, matrix size 64 × 64, in-plane resolution 3 × 3 mm2, slice thickness 3 mm, and 28 axial slices acquired bottom-up sequentially with 0.99-mm gaps between slices. For anatomical reference, a high-resolution 3D MP2RAGE (Magnetization Prepared 2 Rapid Acquisition Gradient Echoes; Marques et al., 2010) sequence (TR 5 s, TE 2.82 ms, FA 0°, matrix size 168 × 192, resolution 1.3 × 1.3 × 1.3 mm3) and a MPRAGE sequence (TR 1480 ms, TE 3.46 ms, FA 10°, matrix size 240 × 256, resolution 1 × 1 × 1.5 mm3) were used to obtain T1-weighted images covering the whole-brain. The MP2RAGE images were used for preprocessing for most of the participants, whereas for four children whose MP2RAGE images were not available, the MPRAGE images were used instead.
Data preprocessing and analysis
Functional images were preprocessed and analyzed using the Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm/). In order to ensure that the anatomical and functional images were aligned in the same space, the children's images were initially coregistered to a standardized 4.5- to 8.5-year-old template (Fonov et al., 2011) and the adults' data were reoriented, after which the anterior commissure is aligned to the origin and the y-plane is aligned to the anterior commissure–posterior commissure line. All functional images were slice-timing corrected with the middle slice in the acquisition order as the reference slice. All volumes were realigned to the first volume to correct for movement with the inhomogeneity of the static magnetic field taken into account. The T1 anatomical image was coregistered to the mean functional image and then segmented using the standardized 4.5- to 8.5-year-old tissue probability maps for the children (Fonov et al., 2011) and the ICBM Tissue Probabilistic Atlases for the adults (provided by the International Consortium for Brain Mapping, John C. Mazziotta and Arthur W. Toga, http://www.loni.ucla.edu/ICBM/ICBM-TissueProb.html). The segmentation parameters were carried over to normalize the T1 and the functional images to the Montreal Neurological Institute (MNI) space. The functional images were smoothed with a Gaussian kernel of 6 mm full-width at half-maximum.
The same procedure of data analysis was performed in both children and adults groups. Condition-specific effects were analyzed using a general linear model (GLM). The blood-oxygen-level dependent (BOLD) responses were convolved with a canonical hemodynamic response function with time derivatives. Motion parameters generated from realignment were included into the design matrix as regressors of non-interest to control for the variances contributed by head movement. For each participant, six contrasts were generated to access the effect of each sentence condition contrasted to the silence condition.
Subsequently, the six condition-specific contrast images were used for random-effects group-level analyses. One-sample t-tests were performed to show the group activation maps for each condition in each age group (see results in Supplementary Material 1). A 2 × 3 flexible factorial analysis with two levels of the case marking factor (C, NC) and three levels of the animacy hierarchy factor (AA, AI, IA) was conducted to examine the main effects of case marking and animacy hierarchy as well as the interaction effect. The probability of false detection was determined by a Monte Carlo simulation using AlphaSim in AFNI (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim) at a voxel-wise threshold (p < 0.005) combined with a cluster size threshold (k ≥ 27 voxels), which led to an equivalent threshold of α < 0.05 corrected for multiple comparisons. The resulting activation maps were masked with the average gray matter mask among each age group. Moreover, to demonstrate the between-condition differences that have driven the main effects revealed by the flexible factorial analyses, we first identified the peak activation coordinates within the activation clusters that fell within the predefined regions of interest, and then created 6-mm sphere masks centered at the peak coordinates. The percent signal change of BOLD responses from these masks was extracted using the MarsBaR Toolbox in SPM8 (v0.43; Brett et al., 2002) for the examination of main effects.
In addition to the two-way flexible factorial analysis performed in each group, we also conducted a three-way flexible factorial analysis adding groups as a between-subjects factor to directly examine group differences for the effects of case marking and animacy hierarchy. The analysis procedure and results are provided in Supplementary Material 2.
Region-of-interest analysis
To obtain the ROI masks, the NIH 4.5- to 8.5-year-old T1 template was segmented and parcellated using the software package FreeSurfer (available at https://surfer.nmr.mgh.harvard.edu/). A priori ROIs, including pars opercularis and pars triangularis of the left IFG, the left posterior STS, and the left posterior STG, were identified according to the automated anatomical labeling system by Desikan et al. (2006), and binary ROI masks were created using the utility, fslmaths, in FSL (available at http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/, Smith et al., 2004). To obtain a mask of the left posterior STG, we split the left STG by the middle point along the y-axis and retained the posterior mask for subsequent ROI analyses. This pSTG mask is located adjacently posterior to the Heschl's gyrus.
The relationship between TSVK-subtest performance and BOLD responses to the case marking effect in the a priori ROIs was examined for the five-year-old children. Each participant's percent signal change of BOLD responses for all sentence conditions was extracted from the a priori ROIs using MarsBaR. We first computed the differences in percent signal change between all non-canonical conditions compared to all canonical conditions (NC > C). Hierarchical multiple regression analyses were performed to examine whether TSVK-subtest performance (independent variable) explained a significant amount of variances in the NC > C differences (dependent variable) in each ROI with other variables controlled as covariates of non-interest. For each hierarchical multiple regression model, variables were entered at two steps. The covariates of non-interest were entered at step 1 and then the TSVK-subtest performance at step 2. Comparing the R2 in the models at step 1 and step 2, we would know how much predictive power was increased by adding the variable of TSVK-subtest performance at step 2. In other words, when the covariates of non-interest were controlled, we could examine the amount of variances in the NC > C differences that could be accounted for by the TSVK-subtest performance and if the amount was significant. Several factors, including gender (dummy coding), age, verbal working memory measures (i.e., forward and backward digit span, Mottier test), were considered as potential covariates of non-interest. In addition, a whole-brain multiple regression analysis was performed to examine the overall relationship between brain responses to the case marking effect (NC > C) and children's syntactic ability (TSVK-subtest performance). The results of the whole-brain analysis are reported in Supplementary Material 3.
Results
Behavioral results
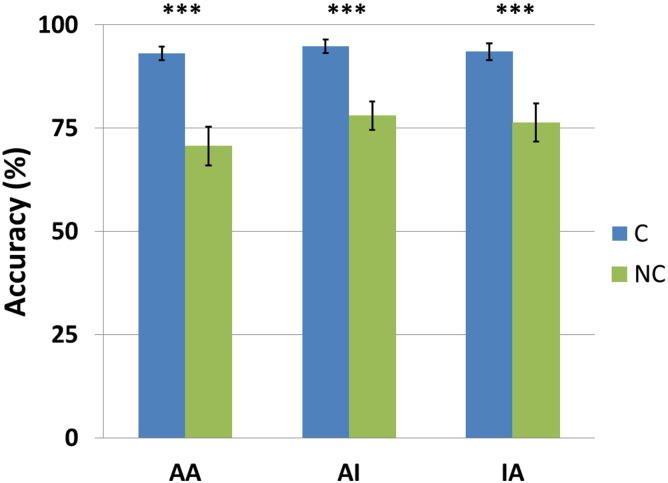
The sample size for the analysis of the post-scanning behavioral sentence comprehension task was 29 as one child's performance could not be obtained. The results are summarized in Fig. 2. The accuracy rates for all six conditions (C-AA: M = 93.1%, SD = 9.20%; NC-AA: M = 70.7%, SD = 25.5%; C-AI: M = 94.8%, SD = 9.16%; NC-AI: M = 78.0%, SD = 18.8%; C-IA: M = 93.5%, SD = 10.9%; NC-IA: M = 76.3%, SD = 24.9%) were above the chance level (all p < 0.001). A repeated measures GLM with case marking (C/NC) and animacy hierarchy (AA/AI/IA) factors showed a significant main effect of case marking driven by better performance for C (M = 93.8%, SD = 9.70%) than for NC (M = 75%, SD = 23.2%), F(1, 28) = 32.8, p < 0.001, and a marginally significant main effect of animacy hierarchy, F(2, 56) = 3.09, p = 0.053. Post-hoc analyses revealed that the averaged performance for AI (M = 86.4%, SD = 16.9%) was higher than for AA (M = 81.9%, SD = 22.1%), p = 0.06. No gender differences were found. The total accuracy (M = 84.4%, SD = 12.7%) was found to be positively correlated with performance in the standardized syntactic TSVK-subtest (M = 6.7, SD = 2.0), r = 0.43, p = 0.019.
Fig. 2.
Performance of the post-scanning behavioral sentence comprehension task. Children's performance for canonical sentences was better than for non-canonical sentences in all animacy conditions (***p < 0.001). Error bars represent standard errors. C: canonical; NC: non-canonical; AA: animate agent and animate patient; AI: animate agent and inanimate patient; IA: inanimate agent and animate patient.
Functional MRI results
Flexible factorial analysis
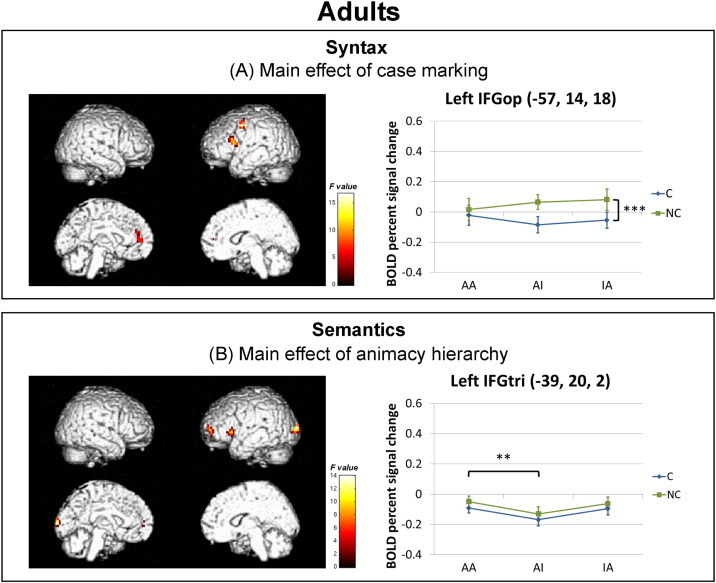
For adults (see Fig. 3 and Table 1), the significant main effect of case marking was identified in the pars opercularis (BA 44), the precentral gyrus, and the superior medial fontal gyrus in the left hemisphere. Within the activation cluster in the left pars opercularis, the percent signal change of a 6-mm sphere centered at the peak activation coordinate (− 57, 14, 18) was greater for the NC compared to the C conditions (p < 0.001). The significant main effect of animacy hierarchy involved a cluster including the left insula, pars triangularis and pars opercularis as well as the left middle frontal gyrus (MFG) and the left middle occipital gyrus. The main effect of animacy hierarchy in the left pars triangularis, centered at (− 39, 20, 2), was mainly contributed by greater activation for the neutral AA conditions compared to the prototypical AI conditions (p = 0.004) as well as a marginally significant difference between non-prototypical IA and prototypical AI conditions (p = 0.067). No significant interaction effects were found.
Fig. 3.
Whole-brain activation of (A) the main effect of case marking and (B) the main effect of animacy hierarchy for the adults overlaid onto the ICBM rendered template (p < 0.005 uncorrected, cluster ≥ 27 voxels, masked by the group-specific average gray matter mask). The graphs below illustrate the BOLD percent signal change within a 6-mm sphere mask centered at the peak activation coordinate in the a priori ROI for each main effect, that is, the left pars opercularis (IFGop) for the main effect of case marking and the left pars triangularis (IFGtri) for the main effect of animacy hierarchy. Significance level for the post-hoc comparisons of the main effects: ***p < 0.001, **p < 0.01. Error bars represent standard errors.
Table 1.
Activation clusters of the main effects of case marking and animacy hierarchy for adults and five-year-old children.
| Hemisphere | Region | X | Y | Z | Cluster size | z score |
|---|---|---|---|---|---|---|
| Adults | ||||||
| (A) Main effect of case marking | ||||||
| Left | Inferior frontal gyrus (pars opercularis) | − 57 | 14 | 18 | 39 | 3.69 |
| Left | Precentral gyrus | − 39 | − 1 | 46 | 30 | 3.30 |
| Left | Superior medial frontal gyrus | − 9 | 44 | 22 | 27 | 3.27 |
| (B) Main effect of animacy hierarchy | ||||||
| Left | Middle frontal gyrus | − 27 | 56 | 2 | 39 | 4.33 |
| Left | Middle occipital gyrus | − 18 | − 94 | 6 | 64 | 3.93 |
| Left | Calcarine gyrus | − 3 | − 100 | 6 | 3.50 | |
| Left | Insula lobe | − 36 | 20 | 2 | 37 | 3.65 |
| Five-year-old children | ||||||
| (A) Main effect of case marking | ||||||
| Left | Middle frontal gyrus | − 24 | 26 | 46 | 38 | 3.39 |
| Right | Caudate nucleus | 9 | 14 | − 2 | 72 | 3.35 |
| Left | Caudate nucleus | − 12 | 17 | − 2 | 3.19 | |
| (B) Main effect of animacy hierarchy | ||||||
| Right | Insula lobe | 39 | − 13 | − 6 | 359 | 4.01 |
| Right | Superior temporal gyrus | 51 | − 1 | − 14 | 3.84 | |
| Left | Hippocampus | − 21 | − 22 | − 18 | 86 | 3.52 |
| Left | Superior temporal gyrus | − 51 | − 1 | − 10 | 164 | 3.77 |
| Left | Middle temporal gyrus | − 57 | − 16 | − 2 | 3.61 | |
| Left | Supplementary motor area | − 12 | − 1 | 46 | 44 | 3.68 |
| Right | Superior occipital gyrus | 21 | − 88 | 30 | 41 | 3.61 |
| Right | Middle occipital gyrus | 30 | − 70 | 22 | 3.00 | |
| Left | Insula lobe | − 30 | 23 | − 2 | 93 | 3.31 |
| Left | Inferior frontal gyrus (pars triangularis) | − 39 | 38 | 6 | 3.23 | |
| Left | Inferior frontal gyrus (pars orbitalis) | − 42 | 20 | − 10 | 3.10 | |
| Right | Precentral gyrus | 39 | − 19 | 54 | 38 | 3.18 |
Coordinates are in the MNI space.
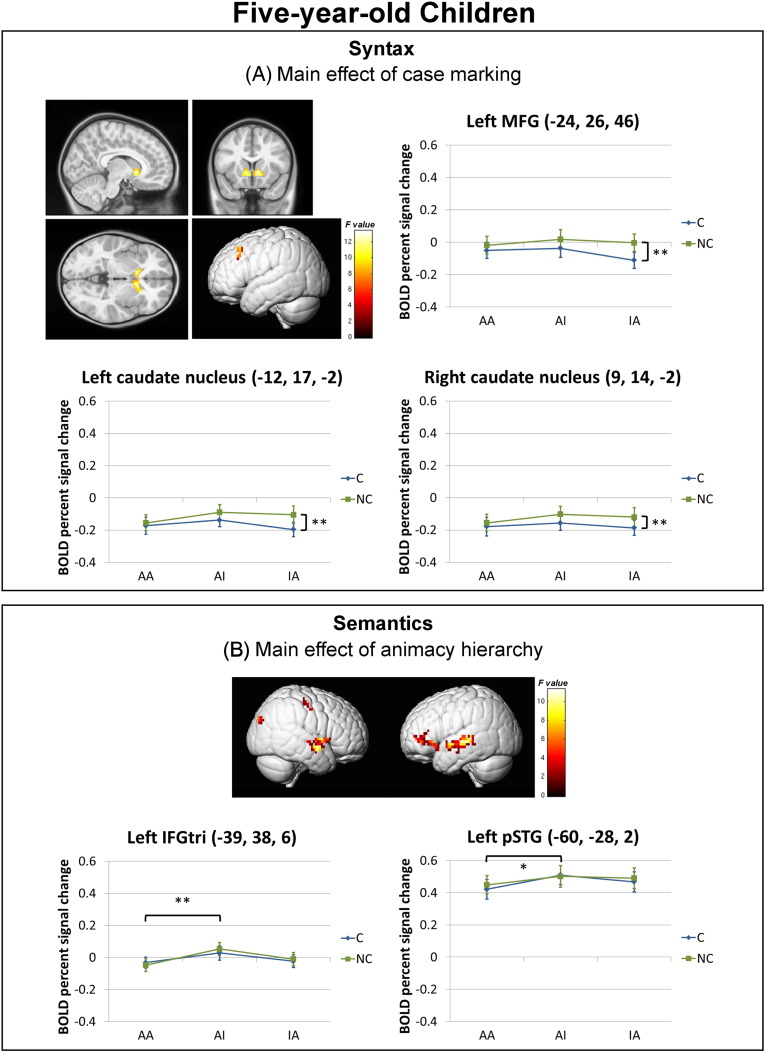
Five-year-old children (see Fig. 4 and Table 1) did not show a significant main effect of case marking in the left inferior frontal gyrus but rather in the left MFG and the left and right caudate nuclei, the activation of which was greater for the NC compared to the C conditions (p = 0.008, 0.003, and 0.002, respectively). An extended network of significant activation was found for the main effect of animacy hierarchy in the left middle and superior temporal gyri, the left pars triangularis and pars orbitalis, and the insula bilaterally. Additional significant activation was found in the right superior and middle occipital gyri and the precentral gyrus. Within the activation clusters located in the regions of interest, the BOLD percent signal change was greater for the prototypical AI conditions compared to the neutral AA conditions in the left pars triangularis cluster centered at (− 39, 38, 6) (p = 0.005) and the left pSTG cluster centered at (− 60, − 28, 2) (p = 0.021). Activation maps of specific contrasts for all individual conditions are shown in Supplementary Material 1.
Fig. 4.
Whole-brain activation of (A) the main effect of case marking and (B) the main effect of animacy hierarchy for the five-year-old children overlaid onto the 4.5- to 8.5-year-old rendered template (Fonov et al., 2011) (p < 0.005 uncorrected, cluster ≥ 27 voxels, masked by the group-specific average gray matter mask). The graphs below illustrate the BOLD percent signal change within a 6-mm sphere mask centered at the peak activation coordinate in each of the activation clusters for the main effect of case marking, including the left middle frontal gyrus (MFG), and the left and right caudate nuclei, as well as in the a priori ROIs for the main effect of animacy hierarchy, that is, the left pars triangularis (IFGtri) and the left posterior superior temporal gyrus (pSTG). Significance level for the post-hoc comparisons of the main effects: **p < 0.01, *p < 0.05. Error bars represent standard errors.
Region-of-interest analysis
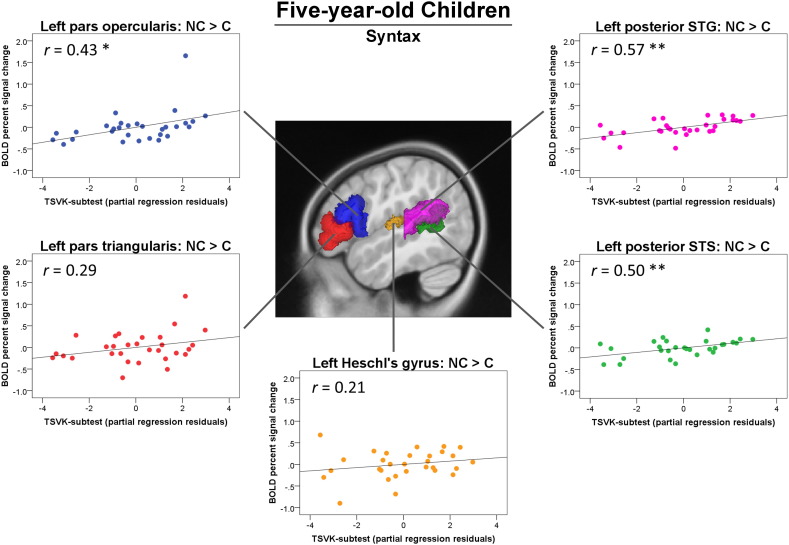
In the whole-brain analysis, the five-year-old children did not show any significant main effects of case marking in the hypothesized areas in the left frontal and temporal cortices. However, since we had strong anatomical hypotheses about the network of brain areas involved in syntactic processing from the adult model, we examined if brain responses to the case marking effect in predefined anatomical ROIs could be predicted by children's syntactic abilities using hierarchical multiple regression analyses. We included gender and age as covariates of non-interest at step 1 and TSVK-subtest performance at step 2. Gender was controlled due to unequal sample sizes for the gender groups (10 boys and 20 girls); age was included as it showed significant effects on the whole-brain activation as revealed by the whole-brain multiple regression analysis (see Supplementary Material 3). The hierarchical multiple regression results revealed that with gender and age controlled, children's TSVK-subtest performance was positively correlated with the percent signal change of the case marking effect (all NC > all C conditions) in the left pars opercularis (partial correlation r = 0.43, p = 0.023; R2 change = 0.17, p = 0.023), the left posterior STS (partial correlation r = 0.50, p = 0.007; R2 change = 0.23, p = 0.007), and the left posterior STG (partial correlation r = 0.57, p = 0.002; R2 change = 0.28, p = 0.002) (Fig. 5). No significant correlation with TSVK-subtest performance was observed in the left pars triangularis (partial correlation r = 0.29, p = 0.13; R2 change = 0.08, p = 0.13). These significant correlations were independent of working memory measures (i.e., forward and backward digit span, Mottier test). In addition, to confirm that the correlations with children's TSVK-subtest performance were constrained in brain regions relevant to syntactic processing but not in non-language regions, we examined the relationship between TSVK-subtest performance and the BOLD percent signal change in a control region, the primary auditory cortex (left Heschl's gyrus), and found no significant correlation (partial correlation r = 0.26, p = 0.18; R2 change = 0.041, p = 0.29).
Fig. 5.
Correlations between the percent signal change for the effect of case marking (all non-canonical > all canonical conditions) and the partial residuals of TSVK-subtest scores for the five-year-old children, with gender and age controlled as covariates of non-interest. Positive correlations were found in the left pars opercularis (p = 0.023), the left posterior superior temporal sulcus (STS, p = 0.007), and the left posterior superior temporal gyrus (STG, p = 0.002). No significant correlation was found in the left pars triangularis (p = 0.13) and the Heschl's gyrus (p = 0.18). **p < 0.01, *p < 0.05.
Discussion
The current study investigated the neural correlates of syntactic and semantic processing during sentence comprehension with the manipulation of case marking and animacy hierarchy cues, in children and adults respectively. We found interesting developmental differences in the reliance on semantic and syntactic cues. In adults, a functional dissociation of Broca's area in the left IFG was observed, with the main effect of syntactic case marking in the left pars opercularis and the main effect of semantic animacy hierarchy in the left pars triangularis. In five-year-old children who already possess knowledge of animacy hierarchy, but not yet have reached a full mastery in the use of case marking, a main effect of animacy hierarchy was evident in the left pars triangularis, pars orbitalis, and posterior STG, whereas no main effect of case marking was found in the left fronto-temporal language network in the whole brain analysis. However, ROI analyses revealed that children's brain responses to case marking in the frontal and temporal regions were associated with their behavioral performance. Those children with better syntactic capabilities showed greater activation to non-canonical object-first sentences compared to canonical subject-first sentences, most prominently in the left posterior superior temporal cortex.
In the adult brain, a functional segregation between syntactic and semantic processing was observed. The main effect of case marking was shown in the left pars opercularis, with greater activation for the non-canonical object-first sentences compared to canonical subject-first sentences. This main effect of syntactic complexity in the left pars opercularis supports its role for syntactic processing and is consistent with previous results (Friederici et al., 2006, Grewe et al., 2007, Kinno et al., 2008). However, the main effect of animacy hierarchy was found in the left pars triangularis, which was mainly contributed by greater activation for sentences with neutral (animate agent and animate patient) and non-prototypical (inanimate agent and animate patient) compared to prototypical (animate agent and inanimate patient) animacy hierarchy. This is in line with previous results that attributed activation in the left pars triangularis to semantic processes at the sentential level (Binder et al., 2009, Bruffaerts et al., 2013, Newman et al., 2010). In the most prototypical AI conditions where the subject noun of the sentence is animate and the object noun is inanimate, case marking and animacy hierarchy cues are in coalition. However, additional semantic analysis is required for the other conditions, that is, when the animacy hierarchy of both nouns is in competition for the neutral AA condition, or when case marking cues are in conflict with animacy hierarchy cues for the non-prototypical IA condition. Therefore, greater activation in the left pars triangularis is recruited for these conditions. These findings reflect that adults process both case marking and animacy hierarchy cues during sentence comprehension, and they do so independent of each other with specialized brain regions being sensitive to each type of sentential cue. Consistent with previous findings (Friederici et al., 2000, Goucha and Friederici, 2015, Newman et al., 2003), the functional dissociation in the left IFG provides further evidence for segregated roles of the left pars opercularis and the left pars triangularis for syntactic and semantic processing respectively.
At the behavioral level, we found that performance in the post-scanning sentence comprehension task for the five-year-old children revealed a significant main effect of case marking (C better than NC) and a marginally significant main effect of animacy hierarchy (AI better than AA), with no interaction. These results suggest that not all children have mastered the use of case marking cues for sentence interpretation by the age of five and that there is wide variability. If children used case marking cues—which were the most reliable cues in the task—they would have performed comparatively well in all conditions. By contrast, if they only relied on animacy hierarchy cues and not on case marking at all, they would have shown the best performance in the AI conditions, at chance level in the AA conditions, and below chance level in the IA conditions, regardless of case marking. However, the behavioral results did not show such a pattern across the animacy conditions. Instead, the average performance of all conditions was significantly above chance level, and there was a marginally significant difference between the AI and AA conditions. The latter indicates that five-year-old children made use of animacy cues, which helped them with sentence comprehension—especially in the prototypical animacy conditions (i.e., AI). Moreover, the above-chance performance suggests that five-year-old children might also use case marking cues, which ensured their correct responses even when animacy cues conflicted with case marking cues (i.e., IA) or when animacy hierarchy was not available (i.e., AA). Our behavioral results implicate early reliance on animacy cues and later acquisition of case marking. This finding is consistent with those in previous studies, which have shown that sentence comprehension for children before the age of five depends primarily on the use of animacy and word order cues, whereas syntactic cues such as case marking only become relevant later (Chan et al., 2009, Dittmar et al., 2008, Lindner, 2003).
At the neural level, five-year-old children showed the main effect of animacy hierarchy in the left fronto-temporal cortex, including the left pars triangularis, pars orbitalis, and posterior STG, which was mainly driven by greater activation for sentences with the prototypical (animate agent and inanimate patient) compared to neutral (animate agent and animate patient) animacy hierarchy. Compared to the adults whose results serve as a model of mature sentence processing and show greater activation for sentences with neutral and non-prototypical animacy hierarchy, children demonstrate enhanced activation for the prototypical animacy hierarchy conditions. The difference between AI and AA conditions at the neural level corresponds to the difference at the behavioral level, with greater activation for the conditions in which children could make better use of animacy cues. While adults only showed the main effect of animacy in the left pars triangularis, five-year-old children recruited both frontal and temporal regions for semantic animacy processing. This difference indicates that children at that age are more reliant on semantic animacy cues for sentence interpretation and, moreover, suggests a gradual shift from a widespread fronto-temporal network for sentential semantic processing in children to a more specialized involvement of the left IFG (Broca's area) in adults.
Unlike adults, five-year-old children did not show a main effect of case marking in the left pars opercularis, but instead extended into the left MFG and the bilateral caudate nuclei, with greater activation for the non-canonical object-first sentences compared to canonical subject-first sentences. These regions may not be directly involved in syntactic processing, but are associated with cognitive control processes. The enhanced activation in the left MFG has been implicated in coping with cognitive demands (Fu et al., 2006) and inhibitory control (Minamoto et al., 2010). The bilateral caudate nuclei have been suggested to be involved in cognitive control, with increased activation for processing complex sentences (Jeon et al., 2014) and grammatically ambiguous sentences (Mestres-Missé et al., 2012). An anterior–posterior organization for cognitive hierarchy in the caudate nuclei has also been demonstrated, in which the rostral to caudal parts of the caudate nuclei support higher to lower levels of cognitive hierarchy. We found greater activation for non-canonical object-first sentences in the bilateral anterior-ventral caudate nuclei, which may be responsible for recruiting controlled processes as processing non-canonical sentences has not yet become automatic for five-year-old children during language development (Friederici, 2006).
As apparent from the behavioral performance, some of the five-year-old children may have started to gain proficiency in using case marking cues, which helped them to correctly identify the agent and the patient in sentences even when animacy cues were in conflict with case marking cues. Therefore, we used individual syntactic performance measured by a standardized test for children's sentence comprehension ability for the ROI analyses in those predefined anatomical regions known to be involved in syntactic processes during sentence comprehension. Regression analyses revealed that brain activation in the left pars opercularis, posterior STS, and posterior STG in response to increasing syntactic complexity (NC greater than C) was positively correlated with syntactic abilities. In other words, those children with better syntactic proficiency showed greater activation in the left fronto-temporal language network for non-canonical object-first sentences compared to canonical subject-first sentences. The correlation was most prominent in the left posterior STS and STG. This finding suggests that the neural basis for processing syntactic cues may emerge in the posterior superior temporal cortex and gradually shift to the left IFG, in particular the pars opercularis, with increasing age and better syntactic proficiency. The shift from the left posterior superior temporal cortex to the inferior frontal cortex for syntactic processing is in line with the data of Skeide et al.'s (2014) study, in which children at the ages of three to seven recruited the posterior superior temporal cortex for syntactic processing, and it was only until the ages of nine to ten that children showed a syntax-specific involvement in the left IFG.
Neuroanatomical maturation of the underlying cortical and connecting white matter structures may be a neurobiological basis for the delayed reliance on syntactic information during sentence comprehension (Friederici et al., 2012, Skeide et al., 2015). It has been suggested that comprehension of syntactically complex sentences is supported by the left posterior STS/STG as well as pars opercularis in Broca's area, which are connected by a dorsal pathway via the arcuate fasciculus/superior longitudinal fasciculus (AF/SLF). By contrast, semantic processing involves a ventral pathway connecting the left posterior temporal cortex and BAs 45/47 in the IFG via the inferior fronto-occipital fasciculus (IFOF) (Friederici and Gierhan, 2013). Diffusion MRI studies have shown that the maturation of the AF/SLF is slower and prolonged compared to other fiber tracts (Lebel et al., 2012, Zhang et al., 2007). In particular, while the IFOF is already in place at birth, the myelination of the AF/SLF that supports complex syntactic processing is not fully developed by the age of seven or later (Brauer et al., 2011, Brauer et al., 2013, Skeide et al., 2015). Before full maturation of the dorsal pathway, children seem to recruit supplementary processing areas in the pars triangularis as part of Broca's area (Brauer et al., 2011, Brauer et al., 2013, Skeide et al., 2015) and may make use of the ventral pathway via the IFOF. The primary development of the ventral pathway and delayed maturation of the dorsal pathway may provide a neurobiological explanation for preschoolers' dependence on semantic information before their brains become ready for processing complex syntax.
Furthermore, the difference between more diffuse activation in children versus more focal activation in adults during sentence comprehension is in agreement with the pattern of functional neurocognitive development reported in the literature. Cross-sectional and longitudinal studies on different cognitive domains have demonstrated a shift from diffuse to more focal activity in the task-relevant cortical regions along with learning and cognitive development (Brown et al., 2005, Casey et al., 2005). Compared to adults who had focal and specialized recruitment in the left IFG for syntactic and semantic processing, five-year-old children showed diffuse activation in the frontal and temporal cortices. Along with previous studies (Brauer and Friederici, 2007, Skeide et al., 2014), this pattern suggests that the language networks for semantics and syntax are not yet specialized in younger children and that segregated and focal recruitment emerges gradually through development.
By varying semantic and syntactic complexities in a sentence listening task, the current study adds both behavioral and neural evidence to our understanding of the sentence-processing network for preschool children. Nevertheless, limitations to the present study have to be considered. To minimize motion artifacts and to shorten scanning time, we applied a passive listening task instead of a task taxing participants' behavioral responses during scanning. By instructing children to listen to the sentences carefully during the scan, it was ensured that children were paying attention to the task without direct measurement. This challenge is prominent in neuroimaging studies with preschoolers. However, it is important to note that passive listening tasks have been successfully applied in previous fMRI experiments with young children, revealing activation patterns similar to those by active tasks (Karunanayaka et al., 2007, Knoll et al., 2012, Schmithorst et al., 2007, Vannest et al., 2009).
Conclusions
Sentence comprehension requires the integration of syntactic and semantic information carried by words, the processing of which is subserved by segregated regions in the Broca's area in the mature brain. In the current study, we demonstrated that children at the age of five were most sensitive to animacy hierarchy cues, suggesting that semantic information is used already at early stages in language development. Syntactic information signaling the grammatical relationship in sentences seems to become relevant only at later developmental stages. Here we show that in five-year-old children the increased capability to process syntactic case marking cues is associated with an increase of brain responses in the language network. Our findings provide neural evidence for a gradual acquisition of sentential cues in the developing brain.
Acknowledgments
This work was supported by a grant from the European Research Council (ERC-2010-AdG 20100407, NEUROSYNTAX) awarded to AF.
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.10.036.
Appendix A. Supplementary data
Supplementary material.
References
- Bahlmann J., Rodriguez-Fornells A., Rotte M., Münte T.F. An fMRI study of canonical and noncanonical word order in German. Hum. Brain Mapp. 2007;28(10):940–949. doi: 10.1002/hbm.20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E., MacWhinney B. Functionalist approaches to grammar. In: Wanner E., Gleitman L., editors. Language Acquisition: The State of the Art. Cambridge University Press; New York: 1982. [Google Scholar]
- Bates E., MacWhinney B., Caselli C., Devescovi A., Natale F., Venza V. A cross-linguistic study of the development of sentence interpretation strategies. Child Dev. 1984;55(2):341–354. [PubMed] [Google Scholar]
- Ben-Shachar M., Hendler T., Kahn I., Ben-Bashat D., Grodzinsky Y. The neural reality of syntactic transformations: evidence from functional magnetic resonance imaging. Psychol. Sci. 2003;14(5):433–440. doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where Is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkessel I., Zysset S., Friederici A.D., von Cramon D.Y., Schlesewsky M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. NeuroImage. 2005;26(1):221–233. doi: 10.1016/j.neuroimage.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Brauer J., Friederici A.D. Functional neural networks of semantic and syntactic processes in the developing brain. J. Cogn. Neurosci. 2007;19(10):1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Brauer J., Anwander A., Friederici A.D. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb. Cortex. 2011;21(2):459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Brauer J., Anwander A., Perani D., Friederici A.D. Dorsal and ventral pathways in language development. Brain Lang. 2013;127(2):289–295. doi: 10.1016/j.bandl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.-L., Valabregue R., Poline J.-B. Paper presented at the 8th International Conference of Functional Mapping of the Human Brain, Sendai, Japan. 2002. Region of interest analysis using an SPM toolbox. [Google Scholar]
- Brown T.T., Lugar H.M., Coalson R.S., Miezin F.M., Petersen S.E., Schlaggar B.L. Developmental changes in human cerebral functional organization for word generation. Cereb. Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bruffaerts R., Dupont P., Peeters R., De Deyne S., Storms G., Vandenberghe R. Similarity of fMRI activity patterns in left perirhinal cortex reflects semantic similarity between words. J. Neurosci. 2013;33(47):18597–18607. doi: 10.1523/JNEUROSCI.1548-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chan A., Lieven E., Tomasello M. Children's understanding of the agent–patient relations in the transitive construction: cross-linguistic comparisons between Cantonese, German, and English. Cogn. Linguist. 2009;20(2):267–300. [Google Scholar]
- Constable R.T., Pugh K.R., Berroya E., Mencl W.E., Westerveld M., Ni W., Shankweiler D. Sentence complexity and input modality effects in sentence comprehension: an fMRI study. NeuroImage. 2004;22(1):11–21. doi: 10.1016/j.neuroimage.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D.…Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Deutsch A., Bentin S., Katz L. Semantic influence on processing gender agreement: evidence from Hebrew. J. Psycholinguist. Res. 1999;28(5):515–535. doi: 10.1023/a:1023220527357. [DOI] [PubMed] [Google Scholar]
- Dittmar M., Abbot-Smith K., Lieven E., Tomasello M. German children's comprehension of word order and case marking in causative sentences. Child Dev. 2008;79(4):1152–1167. doi: 10.1111/j.1467-8624.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D. Children's sensitivity to function words during sentence comprehension. Linguistics. 1983;21(5):717–739. [Google Scholar]
- Friederici A.D. What's in control of language? Nat. Neurosci. 2006;9(8):991–992. doi: 10.1038/nn0806-991. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Gierhan S.M.E. The language network. Curr. Opin. Neurobiol. 2013;23(2):250–254. doi: 10.1016/j.conb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Opitz B., von Cramon D.Y. Segregating semantic and syntactic aspects of processing in the human brain: an fMRI investigation of different word types. Cereb. Cortex. 2000;10(7):698–705. doi: 10.1093/cercor/10.7.698. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Fiebach C.J., Schlesewsky M., Bornkessel I.D., von Cramon D.Y. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb. Cortex. 2006;16(12):1709–1717. doi: 10.1093/cercor/bhj106. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Makuuchi M., Bahlmann J. The role of the posterior superior temporal cortex in sentence comprehension. Neuroreport. 2009;20(6):563–568. doi: 10.1097/WNR.0b013e3283297dee. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Oberecker R., Brauer J. Neurophysiological preconditions of syntax acquisition. Psychological Research. 2012;76(2):204–211. doi: 10.1007/s00426-011-0357-0. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., McIntosh A.R., Kim J., Chau W., Bullmore E.T., Williams S.C.R.…McGuire P.K. Modulation of effective connectivity by cognitive demand in phonological verbal fluency. NeuroImage. 2006;30(1):266–271. doi: 10.1016/j.neuroimage.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Goucha T., Friederici A.D. The language skeleton after dissecting meaning: a functional segregation within Broca's Area. NeuroImage. 2015;114:294–302. doi: 10.1016/j.neuroimage.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Grewe T., Bornkessel-Schlesewsky I., Zysset S., Wiese R., von Cramon D.Y., Schlesewsky M. The role of the posterior superior temporal sulcus in the processing of unmarked transitivity. NeuroImage. 2007;35(1):343–352. doi: 10.1016/j.neuroimage.2006.11.045. [DOI] [PubMed] [Google Scholar]
- Grimm H., Aktas M., Frevert S. Göttingen: Hogrefe; 2000. Sprachentwicklungstest für zweijährige Kinder (SETK-2) [Google Scholar]
- Jeon H.-A., Anwander A., Friederici A.D. Functional network mirrored in the prefrontal cortex, caudate nucleus, and thalamus: high-resolution functional imaging and structural connectivity. J. Neurosci. 2014;34(28):9202–9212. doi: 10.1523/JNEUROSCI.0228-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanayaka P.R., Holland S.K., Schmithorst V.J., Solodkin A., Chen E.E., Szaflarski J.P., Plante E. Age-related connectivity changes in fMRI data from children listening to stories. NeuroImage. 2007;34(1):349–360. doi: 10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L., Melchers P. Frankfurt am Main. Swets & Zeitlinger; Germany: 2003. K-ABC — Kaufman assessment battery for children. [Google Scholar]
- Kinno R., Kawamura M., Shioda S., Sakai K.L. Neural correlates of noncanonical syntactic processing revealed by a picture–sentence matching task. Hum. Brain Mapp. 2008;29(9):1015–1027. doi: 10.1002/hbm.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll L.J., Obleser J., Schipke C.S., Friederici A.D., Brauer J. Left prefrontal cortex activation during sentence comprehension covaries with grammatical knowledge in children. NeuroImage. 2012;62(1):207–216. doi: 10.1016/j.neuroimage.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lindner K. The development of sentence-interpretation strategies in monolingual German-learning children with and without specific language impairment. Linguistics. 2003;41(2):213–254. [Google Scholar]
- Mack J., Meltzer-Asscher A., Barbieri E., Thompson C. Neural correlates of processing passive sentences. Brain Sci. 2013;3(3):1198–1214. doi: 10.3390/brainsci3031198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacWhinney B., Bates E., Kliegl R. Cue validity and sentence interpretation in English, German, and Italian. J. Verbal Learn. Verbal Behav. 1984;23(2):127–150. [Google Scholar]
- Mahlstedt A. Vol. 99. Max Planck Institute for Human Cognitive and Brain Sciences; Leipzig, Germany: 2007. (The acquisition of case marking information as a cue to argument interpretation in German: an electrophysiological investigation with pre-school children). [Google Scholar]
- Makuuchi M., Bahlmann J., Anwander A., Friederici A.D. Segregating the core computational faculty of human language from working memory. Proc. Natl. Acad. Sci. 2009;106(20):8362–8367. doi: 10.1073/pnas.0810928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques J.P., Kober T., Krueger G., van der Zwaag W., Van de Moortele P.-F., Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage. 2010;49(2):1271–1281. doi: 10.1016/j.neuroimage.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Meltzer J.A., McArdle J.J., Schafer R.J., Braun A.R. Neural aspects of sentence comprehension: syntactic complexity, reversibility, and reanalysis. Cereb. Cortex. 2010;20(8):1853–1864. doi: 10.1093/cercor/bhp249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestres-Missé A., Càmara E., Rodriguez-Fornells A., Rotte M., Münte T.F. Functional neuroanatomy of meaning acquisition from context. J. Cogn. Neurosci. 2008;20(12):2153–2166. doi: 10.1162/jocn.2008.20150. [DOI] [PubMed] [Google Scholar]
- Mestres-Missé A., Turner R., Friederici A.D. An anterior–posterior gradient of cognitive control within the dorsomedial striatum. NeuroImage. 2012;62(1):41–47. doi: 10.1016/j.neuroimage.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Minamoto T., Osaka M., Osaka N. Individual differences in working memory capacity and distractor processing: possible contribution of top-down inhibitory control. Brain Res. 2010;1335:63–73. doi: 10.1016/j.brainres.2010.03.088. [DOI] [PubMed] [Google Scholar]
- Moro A., Tettamanti M., Perani D., Donati C., Cappa S.F., Fazio F. Syntax and the brain: disentangling grammar by selective anomalies. NeuroImage. 2001;13(1):110–118. doi: 10.1006/nimg.2000.0668. [DOI] [PubMed] [Google Scholar]
- Mottier G. Der Mottier-Test. Über Untersuchungen zur Sprache lesegestörter Kinder. Folia Phoniatr. 1951;3:170–177. [PubMed] [Google Scholar]
- Musso M., Moro A., Glauche V., Rijntjes M., Reichenbach J., Buchel C., Weiller C. Broca's area and the language instinct. Nat. Neurosci. 2003;6(7):774–781. doi: 10.1038/nn1077. [DOI] [PubMed] [Google Scholar]
- Newman S.D., Just M.A., Keller T.A., Roth J., Carpenter P.A. Differential effects of syntactic and semantic processing on the subregions of Broca's area. Cogn. Brain Res. 2003;16(2):297–307. doi: 10.1016/s0926-6410(02)00285-9. [DOI] [PubMed] [Google Scholar]
- Newman S.D., Ikuta T., Burns T., Jr. The effect of semantic relatedness on syntactic analysis: an fMRI study. Brain Lang. 2010;113(2):51–58. doi: 10.1016/j.bandl.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W., Constable R.T., Mencl W.E., Pugh K.R., Fulbright R.K., Shaywitz S.E.…Shankweiler D. An event-related neuroimaging study distinguishing form and content in sentence processing. J. Cogn. Neurosci. 2000;12(1):120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Nuñez S.C., Dapretto M., Katzir T., Starr A., Bramen J., Kan E.…Sowell E.R. fMRI of syntactic processing in typically developing children: structural correlates in the inferior frontal gyrus. Dev. Cogn. Neurosci. 2011;1(3):313–323. doi: 10.1016/j.dcn.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J., Wise R.J., Dresner M.A., Scott S.K. Functional integration across brain regions improves speech perception under adverse listening conditions. J. Neurosci. 2007;27(9):2283–2289. doi: 10.1523/JNEUROSCI.4663-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rodd J.M., Davis M.H., Johnsrude I.S. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb. Cortex. 2005;15(8):1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Röder B., Stock O., Neville H., Bien S., Rösler F. Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: a functional magnetic resonance imaging study. NeuroImage. 2002;15(4):1003–1014. doi: 10.1006/nimg.2001.1026. [DOI] [PubMed] [Google Scholar]
- Santi A., Grodzinsky Y. fMRI adaptation dissociates syntactic complexity dimensions. NeuroImage. 2010;51(4):1285–1293. doi: 10.1016/j.neuroimage.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D., Kreher B.W., Schnell S., Kümmerer D., Kellmeyer P., Vry M.-S.…Weiller C. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K., Plante E. Development of effective connectivity for narrative comprehension in children. Neuroreport. 2007;18(14):1411–1415. doi: 10.1097/WNR.0b013e3282e9a4ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmüller J., Kauschke C., van Minnen S., Bittner D. Urban & Fischer Verlag, Elsevier GmbH; Munich, Germany: 2011. Test zum Satzverstehen von Kindern (TSVK) — Eine profilorientierte diagnostik der Syntax. [Google Scholar]
- Skeide M.A., Brauer J., Friederici A.D. Syntax gradually segregates from semantics in the developing brain. NeuroImage. 2014;100:106–111. doi: 10.1016/j.neuroimage.2014.05.080. [DOI] [PubMed] [Google Scholar]
- Skeide M.A., Brauer J., Friederici A.D. Brain functional and structural predictors of language performance. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv042. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Matthews P.M.… Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1(0)):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tyler L.K., Stamatakis E.A., Post B., Randall B., Marslen-Wilson W. Temporal and frontal systems in speech comprehension: an fMRI study of past tense processing. Neuropsychologia. 2005;43(13):1963–1974. doi: 10.1016/j.neuropsychologia.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Vannest J.J., Karunanayaka P.R., Altaye M., Schmithorst V.J., Plante E.M., Eaton K.J.…Holland S.K. Comparison of fMRI data from passive listening and active-response story processing tasks in children. J. Magn. Reson. Imaging. 2009;29(4):971–976. doi: 10.1002/jmri.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Evans A., Hermoye L., Lee S.-K., Wakana S., Zhang W.…Mori S. Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. NeuroImage. 2007;38(2):239–247. doi: 10.1016/j.neuroimage.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.