Abstract
Stromules are stroma-containing tubules that have been observed to emanate from the main plastidic body in vivo. These structures have been shown to require cytoskeletal components for movement. Though numerous studies have shown a close association with the endoplasmic reticulum, nucleus, mitochondria, and other plastids, the mechanism of formation and their overall function remain unknown. A limiting factor in studying these structures has been the lack of a reconstituted system for in vitro stromule formation. In this study, stromule formation was induced in vitro by adding a plant extract fraction that is greater than 100 kDa to a population of isolated chloroplasts. Kinetic measurements show that stromule formation occurs within ~10 seconds after the addition of the plant extract fraction. Heat inactivation and apyrase treatment reveal that the stromule stimulating compound found in the extract fraction is a protein or protein complex 100 kDa or greater. The formation of the stromules in vitro with isolated chloroplasts and a concentrated fraction of cell extract opens an avenue for the biochemical dissection of this process that has heretofore been studied only in vivo.
Introduction
Plastids are double membrane enclosed organelles that carry out essential functions in plants. These tasks range from manufacturing and storing starch in leucoplasts to conversion of light energy into sugars as seen in chloroplasts. The adoption of fluorescent proteins by cell biologists has allowed researchers to monitor the structural changes that organelles undergo in vivo. While tubular projections emanating from plastids had been noted as long as 107 years ago [1], they did not receive much attention until they were observed using a stromal protein fused to GFP [2]. The term "stromule" was coined to describe these stroma-containing tube-like projections [3]. Stromules were observed to be 0.35 μm to 1 μm in diameter and up to ~200 μm in length [2, 4–6]. Further studies showed that actin microfilaments and myosin IX appear to be required for the movement of these structures in vivo [6–9]. Plastids without chlorophyll and those found in areas of low plastid density display a higher frequency of stromule formation [3, 10]. Plants subjected to water, temperature, or pathogenic stresses also exhibit a higher frequency of stromules [11–16], pointing to their possible role in stress signaling. Close contact with mitochondria, the endoplasmic reticulum, nuclei, and other plastids further suggests that stromules might function as conduits for the exchange of metabolites or genetic information [6, 8, 14–18], although this point remains somewhat controversial [19]. Despite many years of research activity, the mechanism of stromule formation remains unknown.
To date, most of the studies investigating stromules have been performed in vivo or on epidermal peels. In this study, we report the establishment of a robust assay for in vitro stromule formation using isolated chloroplasts. Herein we provide results showing a dependency of stromule formation on a protein or protein complex larger than 100 kDa located in an isolated plant extract fraction.
Materials and Methods
Plant growth conditions
Nicotiana benthamiana were grown in growth chambers with conditions set at 20°C with 16 hrs light cycle of 100 μmol photons/m2/sec at 60% humidity. Plants were germinated and planted following the protocol in [20].
Chloroplast isolation
N. benthamiana plants that had been transformed with NRIP1 fused to cerulean (gift from S.P. Dinesh-Kumar) were used for all experiments [12]. Plants were grown for 9–12 weeks and harvested for chloroplasts. The plastid isolation protocol is similar to the chloroplast isolation procedure for Pisum sativum [21]. Briefly, leaves of N. benthamiana were blended with a grinding buffer (50 mM Tricine- KOH, pH 8.0, 330 mM sorbitol, 1 mM MnCl2, 1 mM MgCl2, 2 mM Na2EDTA, and 0.1% BSA). The slurry was passed through 2 layers of cheesecloth and centrifuged at 3,000 x g for 5 minutes. The soluble fraction was saved as the plant extract fraction, while the pellet was resuspended and placed onto a continuous Percoll gradient and centrifuged for 10 minutes. The intact chloroplasts were removed from the Percoll gradient and washed twice with a chloroplast storage buffer (330 mM Sorbitol, 50 mM Hepes-KOH, pH 8) and stored on ice in the dark until used.
Plant extract preparation
The plant extract fraction collected after the initial centrifugation step was centrifuged again at 3,300x g for 5 minutes to remove any additional thylakoid particles. The cell extract was then concentrated 40-fold using 100 kDa mwco Amicon centrifugal filtration devices (EMD Millipore, Billerica, MA), quickly frozen in liquid nitrogen, and stored in a -80°C freezer. The 100 kDa≥ x≥10 kDa fraction was created by taking the flowthrough from the greater than 100 kDa concentration step and passing that through a 10 kDa mwco Amicon centrifugal filter device (EMD Millipore, Billerica, MA). The filtrate was also concentrated 50 fold. Heat- inactivated extract was made by placing the greater than 100 kDa plant extract fraction into a 70°C water bath for 10 minutes. The aggregates were removed by a 2 minute centrifugation at 16,000 x g in a table top centrifuge. The extract was then stored on ice for two hours before use. Fractionation of the whole-cell extract was carried out by centrifuging the concentrated extract at 16,000 x g for 10 minutes at 4°C. The supernatant was then centrifuged at 100,000 x g for 1 hour at 4°C using a TL-100 Ultracentrifuge (Beckman Coulter Inc., Brea, CA). The supernatant was collected and stored on ice before use. The pellet was resuspended in chloroplast storage buffer and stored on ice until further use. Apyrase-treated extract was prepared by incubating apyrase (Sigma-Aldrich, St. Louis, MO) with the extract at a final concentration of 125 units/ml in 200 μL for 2 hours on ice.
Microscopy and quantitation
A homemade perfusion chamber consisting of a chamber milled from a 1.8 mm x 100 mm x 1.1 mm piece of polycarbonate and a 50 x 22 mm coverslip (Fisher Scientific Houston, TX) were used for all of the imaging studies. The working perfusion chamber was assembled by mounting a poly-L-lysine (Sigma-Aldrich St. Louis, MO) coated cover slip onto the bottom of the chamber using silicone grease (Beckman Coulter Inc., Brea, CA). Isolated chloroplasts (0.5 μg Chl) were placed into each chamber and centrifuged for 15 minutes at 60 x g using a GS-6KR swinging bucket centrifuge (Beckman Coulter Inc., Brea, CA) at 10°C. The samples were then observed under the microscope. Images were taken using a Zeiss LSM 710 confocal microscope (Zeiss, Oberkochen, Germany). The Cerulean and chlorophyll were excited using the 458 nm laser. 200 μL of treatment solutions, depending on the reaction, was added to each chamber. Image compilation and analysis were performed using Fiji software. Images captured from the cerulean channel in the microscope were artificially colored with a grey LUT to enhance contrast.
Results
Plant extract stimulates stromule formation in isolated chloroplasts
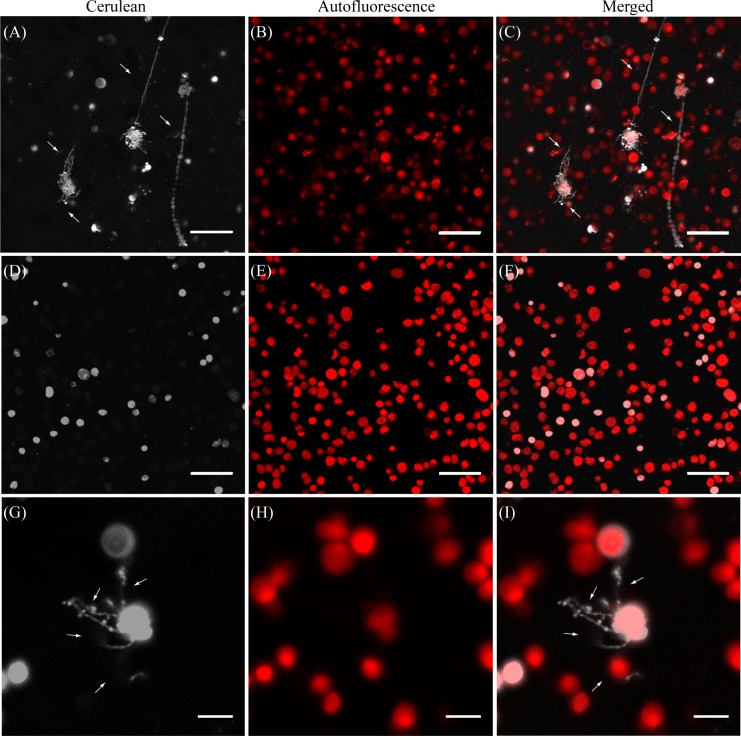
N. benthamiana plants transformed with a Cyan Fluorescent Protein (Cerulean) fused to the C- terminus of NRIP1, a chloroplast stromal protein, were placed behind its native promoter and used as a source for the isolation of intact chloroplasts [12]; the Cerulean-tagged protein serves as a stromal marker. Isolated chloroplasts were centrifuged onto a poly-L-lysine-coated coverslip in a homemade perfusion chamber, incubated for 30 minutes with various treatments, and monitored for morphological changes under a confocal microscope. Chloroplasts that were treated with a concentrated plant extract fraction prepared by using a 100 kDa molecular weight cutoff (mwco) filter displayed a significant change in their morphology (Fig 1A–1C). The images from the Cerulean channel captured several chloroplasts with protrusions containing stroma emanating from the plastid (Fig 1A). The chlorophyll autofluorescence channel showed that the thylakoids remained spherical and at the main body of the chloroplasts (Fig 1B). Chlorophyll autofluorescence was not observed in any of the protrusions, indicating that the structures contained only the plastid envelope membranes and stroma. The merged image shows that the extensions contained only stroma and that these structures were stromules (Fig 1C). The chloroplasts exhibited a variety of morphologies following the addition of the plant extract. We observed several chloroplasts that had multiple stromules forming off of a single body with lengths that varied up to ~ 40 μm. The longest projections seen in our studies was 109 μm (S1 Movie). It has been reported that stromules can reach up to 220 μm in length [10]. Other chloroplasts in our experiments showed only one large stromule, while a few did not display any stromules in our images. Higher resolution images of a stimulated chloroplast showed nodules that formed along the side or at the end of the stromules (Fig 1G–1I). The diameter of these structures were typically 1 μm with occasional nodules widening up to 2 μm. Other studies performed in Lucopersicon esculentum chromoplasts and chloroplasts [5, 6] have also reported "bead-like" sacks along the stromule which were thought to arise from the redistribution of stromal contents as a result of excessive stretching. The in vitro-formed stromules in our experiments also displayed occasional branching (S2 Movie). The plant extract stimulated stromule formation in 40.1% ± 19.9% of the intact chloroplasts (n = 15). The smallest stromule included in our quantitation was 0.8 μm in length.
Fig 1. In vitro stromule formation requires plant extract.
A-C: A concentrated cell extract fraction was added to fixed chloroplasts and observed under a microscope. D-F: Isolated chloroplasts treated with a solution of 0.5% BSA, 2 mM ATP and 200 μM GTP. G-I: A zoomed in image of a stimulated chloroplast shows multiple stromules with nodules emanating from a single plastid. Scale bars in panels A–D correspond to 30 μm; those in G–I, 10 μm. All images were organized by showing the cerulean (grey), autofluorescence (red), and merged images from left to right. White arrows indicate stromules. The total elapsed time of the recording was 10 minutes and 23 seconds.
To test whether a substance in the plant extract fraction stimulated stromule formation in our isolated chloroplasts, plastids were incubated with the storage buffer supplemented with 0.5% BSA, 2 mM ATP and 200 μM GTP (Fig 1D–1F). The isolated chloroplasts maintained a smooth spherical shape (Fig 1D) without any long tube-like projections emanating from the main body. A few chloroplasts exhibited very short and transient protrusions mostly less than 0.5 microns in length. The addition of BSA, ATP, and GTP only showed a 2.0% ± 2.3% stimulation (n = 3). These results indicate that the plant extract is responsible for the induction of stromule formation.
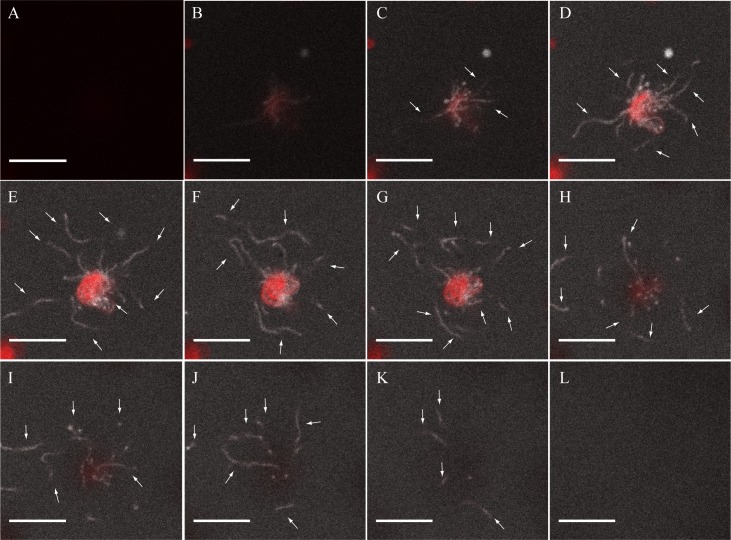
The stromules that formed in our in vitro experiments were not static distortions of the envelope membranes. This is seen in time-lapsed videos that capture the dynamic movement of these structures over the course of 10 minutes (S3 Movie). The stromules in S3 Movie initially appear to contact other chloroplasts and move around a thylakoid released from a broken plastid. Over the course of 10 minutes, the stromules can be seen to retract back to the main body of the chloroplast, and at the end appear to have been reabsorbed. Palpitations can be seen as the in vitro-stimulated stromules float in the reaction buffer, suggesting that they are under little or no tension. Not only are the stromules dynamic in the sense that they are capable of moving in two dimensions, the stimulated chloroplasts display multiple protrusions at different planes along the z—axis (Fig 2A–2L), reaching ~20 μm in the z direction as well. The dynamic structures that formed as a result of the incubation of isolated chloroplasts with plant extract prompted us to investigate the initial stages of stromule formation.
Fig 2. Stromules are dynamic structures that also move throughout the z planeA-L: An image sequence through the z axis of two chloroplasts that have been stimulated to form stromules.
The scale bar corresponds to 10 μm; the 12 panels cover 19.8 μm in the z direction. The white arrows mark stromule branches.
Stromule formation occurs rapidly after the addition of cell extract
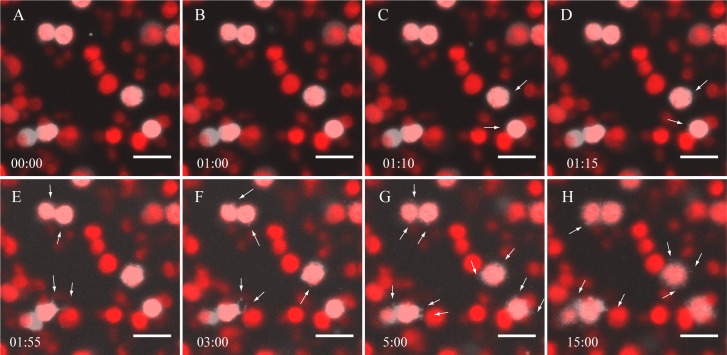
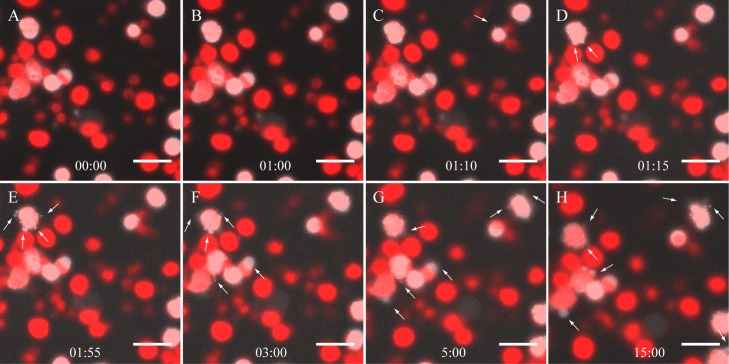
To measure the kinetics of stromule formation in vitro we monitored the changes of chloroplast morphology after cell extract addition in real time. Snapshots lasting 1.7 s were taken at 5 s intervals to prevent photobleaching. The chloroplasts imaged 60 seconds prior to the addition of the cell extract showed a smooth and spherical plastid morphology (Fig 3A and 3B). 1.8% of the total number of chloroplasts (n = 767) observed for these studies displayed short and transient protuberances as seen in the isolated chloroplasts treated with BSA, GTP, and ATP. The earliest observable transformations took place ~10 seconds after the addition of the plant extract, with a few plastids forming small beak-like structures (S4 Movie). By ~ 30 seconds tubules became evident (Fig 3C and 3D, S4 Movie). The stromules were in constant motion throughout the entire imaging process (Fig E-H). Not all the chloroplasts from which stromules eventually emerged displayed these morphological changes immediately, with some starting as long as 240 seconds after the addition of the plant extract. The observed rapid stimulation of stromules further prompted us to perform a preliminary characterization of the stromule-forming compound(s) in the concentrated cell extract.
Fig 3. Time-lapse image series of stromule formation in vitro.
Time (min) is shown in the lower left of each panel. A+B: Image of chloroplasts before the addition of the cell extract. C+D: After 1 minute, the addition of cell extract stimulates stromule formation within ~10 seconds. E+H: Stromules remain present for the remainder of the time course. White arrows mark stromules. The scale bar corresponds to 15 μm.
Stromule formation is dependent on a protein or protein complex with a molecular mass greater than 100 kDa
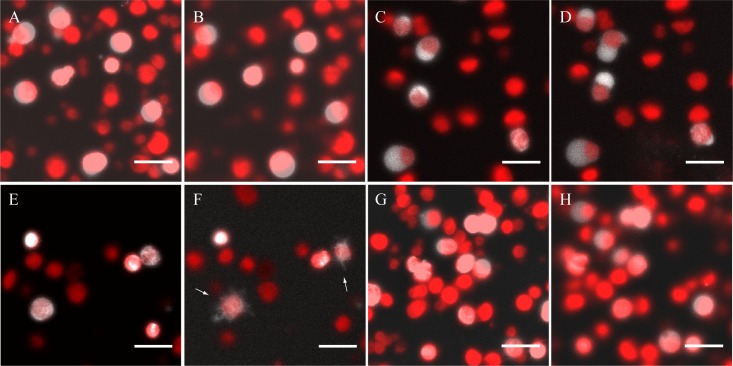
In order to determine if the stromule-stimulating component in the concentrated cell extract is a protein, we attempted to inactivate it by heat. To this end, the plant extract fraction was incubated at 70°C for 10 minutes and the protein aggregates were removed by a brief centrifugation. The remaining plant extract fraction was then transferred into a clean tube and incubated on ice for 30 minutes prior to its addition to the chloroplasts. Compared to the untreated extract, the addition of the heat-inactivated cell extract stimulated the formation of relatively few stromules (10% ± 10.4%, n = 3) (Fig 4A and 4B and S5 Movie). The chloroplasts largely maintained their spherical morphology throughout a 20 minute incubation. The paucity of stromule-forming chloroplasts treated with heat-denatured cell extract suggests that the molecules that stimulates stromule formation is protein based. Ultracentrifugational analysis of the whole-cell extract was utilized to investigate the nature of the stromule-stimulating component. The concentrated extract was centrifuged once at 16,000 x g to remove organelles. The supernatant was collected and recentrifuged at 100,000 x g for 1 hour to remove proteolipid membrane fragments. Addition of the resuspended proteolipid membrane fraction to isolated chloroplasts did not result in stromule formation (0% ± 0%, n = 5) (Fig 4C and 4D). The addition of the supernatant of the ultracentrifuged whole-cell extract to isolated chloroplasts stimulated stromule formation to levels similar to the non-fractionated cell-extract (38% ± 3.2%, n = 6) (Fig 4E and 4F). The identical stimulatory effect of the supernatant from the ultracentrifuged whole-cell extract suggests that the stromule-forming component is a soluble protein. Since the stromule-stimulating plant extract was concentrated above the 100 kDa cutoff filter, the protein(s) or complex responsible for the stimulation must be at least this large. To confirm this we also tested a fraction that passed through the 100 kDa filter and was concentrated above a 10 kDa filter; this only stimulated 5.7% ± 7.7% (n = 2) of the chloroplasts to form stromules (Fig 4G and 4H). This narrows the effort to identify the cytosolic component(s) that induce stromule formation to a protein or protein complex larger than 100 kDa.
Fig 4. Stromules are stimulated by a 100 kDa or greater sized protein or protein complex.
A-B: Images showing isolated chloroplasts before and after the addition of the heat-inactivated cell extract respectively. C-D: Pre and post images of isolated chloroplasts treated with the pellet fraction after a 100,000 x g ultracentrifugation of the whole-cell extract, respectively. E-F: Isolated chloroplasts before and after the addition of the supernatant fraction after a 100,000 x g ultracentrifugation of the whole-cell extract. G-H: Images of isolated chloroplasts before the addition and after a 15 minute incubation with a cell extract fraction containing proteins smaller than 100 kDa but greater than 10 kDa respectively. Stromules are labeled with white arrows. The scale bar corresponds to 15 μm.
Stromule formation does not require exogenous ATP in the cell extract
Stromule movement has been shown to be dependent on actin filaments and Myosin XI [7, 8, 22]. Myosin XI contains an ATPase domain at the N-terminus that drives the movement of stromules along actin filaments [23]. To determine whether formation as well as movement required ATP we tested the dependence of stromule formation on the presence of ATP by treating the plant extract with 125 units/ml of apyrase for 2 hours on ice. In conjunction with this point, the plant extract was also concentrated 40 fold with a 10 kDa mwco filter, further reducing the amount of ATP present within the plant extract. The addition of the apyrase-treated plant extract fraction to the isolated chloroplasts still stimulated the formation of stromules (51.5% ± 46.0%, n = 2), and with essentially unchanged kinetics (Fig 5A–5H and S6 Movie). Again, the earliest morphological changes took place ~10 seconds after the addition of the extract (Fig 5C and 5D), when a few chloroplasts began to form beak-like projections that eventually turned into short tubules. The number of stromules increased over the course of the 15 minute incubation (Fig 5H). Surprisingly, neither the formation nor the movement of the stromules was inhibited by the apyrase treatment. From these results we conclude that exogenously added ATP is not essential for in vitro stromule formation.
Fig 5. Stromule formation does not require exogenous ATP.
A-H: Time-lapse images showing isolated chloroplasts incubated with apyrase-treated cell extract, with duration of the reaction shown in the bottom center of each panel. The apyrase-treated cell extract was added after 1 minute. Stromules are labeled with white arrows. The scale bar corresponds to 15 μm.
Discussion
Since their rediscovery through the use of GFP and fluorescence microscopy in 1997 [2], stromules have been the subject of more than 60 publications. With but one exception (see below), all of these studies examined stromules in vivo or from epidermal peels. The obvious advantages to application of in vitro techniques to the elucidation of aspects of stromule formation prompted us to attempt to induce stromule development using isolated chloroplasts. To this end we monitored via confocal microscopy the response to various additions of isolated chloroplasts from N. benthamiana in which Cerulean is expressed in the stroma. Here we report the first, to our knowledge, observations of stromule formation in intact isolated chloroplasts that is stimulated beyond a low basal level.
In this study, we promoted stromule formation from isolated chloroplasts by the addition of a concentrated plant extract fraction generated during the chloroplast preparation (Fig 1A–1C). Very few isolated chloroplasts displayed transient protuberances (< 0.8 μm) prior to the application of any treatment. Of the total number of isolated chloroplasts observed in the study, only 1.1% of the chloroplasts spontaneously exhibited transient stromule formation, and this served as a baseline of stromule-forming activity from isolated chloroplasts. The stromules formed at the basal level tended to be short and transient in nature. Though the underlying cause of the basal level stromule formation was not further pursued, we note that ER fragments have been shown to remain adherent to the plastid membranes after isolation of intact chloroplasts through a Percoll gradient [24]. It is possible that strong membrane interactions between other proteins or organelles continue after the isolation, and that may contribute towards changes in the organelle morphology.
Stromules were induced by incubating isolated chloroplasts with an undefined cell extract fraction containing elements larger than 100 kDa (Fig 1A–1C). The in vitro-constituted stromules reached lengths up to 110 μm, with bulbous structures that appeared asymmetrically within the tubule (Fig 1G–1I). Similar structural characteristics of stromules have also been observed in epidermal peel studies and in vivo [6, 10]. We note that, as observed in numerous in vivo studies [3, 5, 10], not all isolated chloroplasts gave rise to stromules, while other plastids produced multiple stromules. Approximately 40% of the intact chloroplasts were stimulated by the plant extract to form stromules, a 40 fold increase in stromule formation compared to chloroplasts before plant extract treatment. These observations suggest that our in vitro constitution assay successfully recapitulated a number of aspects of stromule formation in live cells. Treatment of plastids with BSA and NTP's (Fig 1D–1F) only minimally increased the occurrence of stromule formation, from ~1% to ~2%. This result suggests that a specific substance within the plant extract is responsible for the formation of stromules. Interestingly, depletion of the plant extract of ATP did not inhibit its ability to induce stromule development.
The change in chloroplast morphology, including the generation of stromules, resulting from the addition of plant extract raises many questions about stromule induction. It has been suggested that stromules arise as a result of failed chloroplast photorelocation away from or towards a light stimulus [25, 26]. We do not favor this hypothesis, however, because while we did notice negative effects of illumination on stromule formation, we observed stromules emanating in all accessible directions (± x, ± y, + z) from plastids fixed in place onto coverslips through poly-lysine interactions (Fig 3, Movie 2). The movement of chloroplasts and stromules have been shown to require actin filaments and myosin [6–8, 22, 27, 28]. Yet, chloroplasts incubated with apyrase-treated cell extract formed stromules at a similar rate as those incubated with ATP present in the cell extract (Fig 4E–4L). It is noteworthy that although the cell extract was depleted of ATP by apyrase treatment, the plastids were not. Accordingly, if ATP is required for in vitro stromule formation, it must be utilized either in the chloroplast stroma, or less likely, in the inter-membrane space before it had a chance to equilibrate across the porous outer envelope membrane. This might be consistent with a mechanism suggested by Hanson and Sattarzadeh [29] in which stromules are proposed to be formed by internal pressure, albeit with an additional contribution by factors in the cytoplasm. Recent work by Caplan et al. [14] showed that the knock down of CHUP1, an outer envelope protein known to associate with actin filaments, expression caused constitutive stromule expression. This result suggests that CHUP1 is not an essential protein for stromule formation and that the association of actin with the chloroplast membrane is not crucial for generating the membrane protrusions in vitro [14].
Destruction of the plant extract component required for stromule formation by heat, and retention during concentration above a 100 mwco filter suggests that this component is a protein or protein complex with a molecular weight of 100 kDa or greater (Fig 4A–4H). This idea was further supported by the observation that the removal of membrane fragments from the whole-cell extract by ultracentrifugation did not alter the stimulatory behavior of the extract (Fig 4C–4F). These results negate the possibility that the observed chloroplast protrusions are caused by incorporating other protein-lipid fragments into the chloroplast envelope membranes. Stromules have been reported to interact with a number of different organelles, including the ER, nucleus, and mitochondria [6, 8, 17, 30]. A recent mutational analysis of RWP8.2, a protein involved in the detection of specific pathogen effectors, showed that several mutational variants localized to an area that surrounded the stromule membranes [31]. The authors suggested that this layer surrounding the stromules resulted from the rapid exchange of proteins and lipids between the ER and chloroplast, creating conduits for proteins to reach other areas in the cell. The close contact between stromules and different organelles suggests that the immediate environment surrounding the stromules may play a significant role in contributing proteins to their formation.
The generation of tubules from artificial membrane bilayers in vitro may provide some clues to understanding the formation of stromules [32–34]. These studies have raised the possibility that stromule formation is mediated by a dynamin-related protein. Plant dynamins can be categorized into 4 subfamilies, with proteins ranging from 68 kDa to 100 kDa [35, 36]. The addition of human neural dynamin to DOPC:DOPS:PI4,5-P2 lipid bilayers resulted in tubulation of the artificial bilayer after the addition of GTP [32]. The projections had diameters of ~0.5 μm and reached lengths of up to 30 μm, not unlike that observed for stromules. The rate of extension also occured on a similar time scale as that we observed for stromules in our study.
Another possible contribution to stromule formation could be the over-crowding of proteins in or along the envelope membranes. A recent study showed that proteins involved in clatharin-mediated endocytosis, epsin1 and AP180 induced vesicle tubulation through the steric pressure generated by having a high density of proteins linked to the membrane [33]. The tubulation was dependent on the carrier protein size and its concentration along the membrane. Protein crowding-induced membrane tubulation has also been reported in chloroplasts in which outer envelope membrane proteins had been overexpressed [37–39]. The stromule-like protrusions were thought to be caused by an increased concentration of membrane proteins and appeared to be independent of protein size and function. Machettira et al. [37] showed that GFP fused to the transmembrane domain of CHUP1 induced membrane protrusions, which further supports the finding that CHUP1 itself is not crucial for stromule formation. In light of this result, the proliferation of stromules following the reduction of CHUP1 expression demonstrated by Caplan et al. [14] could be explained if removal of CHUP1 made room for the accumulation of other outer envelope proteins that function to alter membrane curvature. Additionally, Caplan et al. showed that overexpression of a CHUP1 transit peptide-RFP chimera impaired the chloroplasts’ ability to form stromules. This overexpressed chimera also saturated the chloroplast import machinery, although it remains unclear whether stromule formation in vivo is linked to plastid protein import; our in vitro experiments suggest it is not. In aggregate, these studies suggest that outer envelope membrane proteins play a pivotal role in the formation of these protrusions.
While this manuscript was under revision a new study reporting stromule formation by isolated chloroplasts appeared in the literature [40]. The stromules formed in vitro in the Brunkard et al. study were often short and occurred at a low frequency, and most closely resembled structures that we classified in our study as small stromules forming at a basal level in isolated chloroplasts. We speculate they may be formed under the influence of additional proteins and membranes that remain in contact with the chloroplasts after isolation. In contrast to Brunkard et al. [40], we observed an approximately 40-fold stimulation in stromule occurrence, which appeared rapidly and often became quite long, upon addition of a concentrated cell extract containing elements, probably proteins, retained by a 100 kDa mwco filter. The reasons for the discrepancies between these two studies is currently unknown.
The development of the in vitro stromule formation assay reported here will allow for the testing of models governing stromule development, and opens a path toward identification of the protein(s) required for this process. This effort is currently ongoing in our lab. We anticipate that this new stromule assay will lead to a better understanding of the mechanism of formation and purpose of these enigmatic dynamic plastidic structures.
Supporting Information
A video showing a stromule emanating from a chloroplast body that is 109 nm in length. Scale bars correspond to 30 μm. The white arrow corresponds to a stromule.
(AVI)
A video panning out of a stromule that is branched. The white arrow indicates an area of stromule branching.
(AVI)
A video capturing the recoiling of stromules back to the main plastidic body. Scale bars correspond to 10 μm.
(AVI)
A video showing the formation of stromules in real-time. The white arrows indicate chloroplasts that are stimulated by the cell extract to form stromules. Scale bars correspond to 30 μm. The total elapsed time of the recording was 20 minutes.
(AVI)
A video showing the captured stimulation of stromules from the heat inactivated plant extract. Scale bars correspond to 30 μm. The total elapsed time of the recording was 20 minutes.
(AVI)
A video showing the formation of stromules after the addition of apyrase-treated cell extract. The white arrows correspond to chloroplasts that form stromules. Scale bars correspond to 30 μm. The total elapsed time of the recording was 20 minutes.
(AVI)
Acknowledgments
We would like to thank Dr. S.P. Dinesh-Kumar for the gift of NRP1-cerulean plants, Meenu Padmanabhan for sharing her expertise on growing N. benthamiana, Dr. Yuh-Ru Julie Lee and Jeffrey Calpan for sharing their microscopy expertise, and Dr. Lanxin Shi for sharing ideas for trouble shooting chloroplast isolations.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by MCB-1330321 from the National Science Foundation (http://www.nsf.gov/bio/mcb/about.jsp). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Senn G. Die Gestalts-und Lageveränderung der Pflanzen-Chromatophoren. Engelmann,. 1908.
- 2.Kohler RH, Cao J, Zipfel WR, Webb WW, Hanson MR. Exchange of protein molecules through connections between higher plant plastids. Science. 1997;276(5321):2039–42. 10.1126/science.276.5321.2039 . [DOI] [PubMed] [Google Scholar]
- 3.Kohler RH, Hanson MR. Plastid tubules of higher plants are tissue-specific and developmentally regulated. Journal of Cell Science. 2000;113(1):81–9. . [DOI] [PubMed] [Google Scholar]
- 4.Holzinger A, Buchner O, Lutz C, Hanson MR. Temperature-sensitive formation of chloroplast protrusions and stromules in mesophyll cells of Arabidopsis thaliana. Protoplasma. 2007;230(1–2):23–30. 10.1007/s00709-006-0222-y . [DOI] [PubMed] [Google Scholar]
- 5.Pyke KA, Howells CA. Plastid and stromule morphogenesis in tomato. Ann Bot-London. 2002;90(5):559–66. 10.1093/Aob/Mcf235 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunning BES. Plastid stromules: video microscopy of their outgrowth, retraction, tensioning, anchoring, branching, bridging, and tip-shedding. Protoplasma. 2005;225(1–2):33–42. 10.1007/s00709-004-0073-3 . [DOI] [PubMed] [Google Scholar]
- 7.Kwok EY, Hanson MR. Microfilaments and microtubules control the morphology and movement of non-green plastids and stromules in Nicotiana tabacum. Plant J. 2003;35(1):16–26. 10.1046/j.1365-313X.2003.01777.x . [DOI] [PubMed] [Google Scholar]
- 8.Kwok EY, Hanson MR. In vivo analysis of interactions between GFP-labeled microfilaments and plastid stromules. Bmc Plant Biol. 2004;4:2 10.1186/1471-2229-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray JC, Sullivan JA, Hibberd JM, Hansen MR. Stromules: Mobile protrusions and interconnections between plastids. Plant Biology. 2001;3(3):223–33. 10.1055/S-2001-15204 . [DOI] [Google Scholar]
- 10.Waters MT, Fray RG, Pyke KA. Stromule formation is dependent upon plastid size, plastid differentiation status and the density of plastids within the cell. Plant J. 2004;39(4):655–67. 10.1111/j.1365-313X.2004.02164.x . [DOI] [PubMed] [Google Scholar]
- 11.Gray JC, Hansen MR, Shaw DJ, Graham K, Dale R, Smallman P, et al. Plastid stromules are induced by stress treatments acting through abscisic acid. Plant J. 2012;69(3):387–98. 10.1111/j.1365-313X.2011.04800.x . [DOI] [PubMed] [Google Scholar]
- 12.Caplan JL, Mamillapalli P, Burch-Smith TM, Czymmek K, Dinesh-Kumar SP. Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell. 2008;132(3):449–62. 10.1016/j.cell.2007.12.031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krenz B, Jeske H, Kleinow T. The induction of stromule formation by a plant DNA-virus in epidermal leaf tissues suggests a novel intra- and intercellular macromolecular trafficking route. Front Plant Sci. 2012;3 Artn 291 10.3389/Fpls.2012.00291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan JL, Kumar AS, Park E, Padmanabhan MS, Hoban K, Modla S, et al. Chloroplast Stromules Function during Innate Immunity. Developmental Cell. 2015;34(1):45–57. 10.1016/j.devcel.2015.05.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao Y, Savchenko T, Baidoo EE, Chehab WE, Hayden DM, Tolstikov V, et al. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell. 2012;149(7):1525–35. 10.1016/j.cell.2012.04.038 . [DOI] [PubMed] [Google Scholar]
- 16.Walley J, Xiao Y, Wang JZ, Baidoo EE, Keasling JD, Shen Z, et al. Plastid-produced interorgannellar stress signal MEcPP potentiates induction of the unfolded protein response in endoplasmic reticulum. Proc Natl Acad Sci U S A. 2015;112(19):6212–7. 10.1073/pnas.1504828112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schattat M, Barton K, Baudisch B, Klosgen RB, Mathur J. Plastid Stromule Branching Coincides with Contiguous Endoplasmic Reticulum Dynamics. Plant Physiology. 2011;155(4):1667–77. 10.1104/pp.110.170480 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwok EY, Hanson MR. Plastids and stromules interact with the nucleus and cell membrane in vascular plants. Plant Cell Rep. 2004;23(4):188–95. 10.1007/s00299-004-0824-9 . [DOI] [PubMed] [Google Scholar]
- 19.Schattat MH, Barton KA, Mathur J. The myth of interconnected plastids and related phenomena. Protoplasma. 2015;252(1):359–71. 10.1007/s00709-014-0666-4 . [DOI] [PubMed] [Google Scholar]
- 20.Hayward A, Padmanabhan M, Dinesh-Kumar SP. Virus-Induced Gene Silencing in Nicotiana benthamiana and Other Plant Species. Plant Reverse Genetics: Methods and Protocols. 2011;678:55–63. 10.1007/978-1-60761-682-5_5 . [DOI] [PubMed] [Google Scholar]
- 21.Lo SM, Theg SM. Protein targeting across and into chloroplast membranes. Methods Mol Biol. 2011;684:139–57. Epub 2010/10/21. 10.1007/978-1-60761-925-3_13 . [DOI] [PubMed] [Google Scholar]
- 22.Natesan SKA, Sullivan JA, Gray JC. Myosin XI Is Required for Actin-Associated Movement of Plastid Stromules. Molecular Plant. 2009;2(6):1262–72. 10.1093/Mp/Ssp078 . [DOI] [PubMed] [Google Scholar]
- 23.Tominaga M, Kojima H, Yokota E, Orii H, Nakamori R, Katayama E, et al. Higher plant myosin XI moves processively on actin with 35 nm steps at high velocity. EMBO J. 2003;22(6):1263–72. 10.1093/emboj/cdg130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson MX, Goksor M, Sandelius AS. Optical manipulation reveals strong attracting forces at membrane contact sites between endoplasmic reticulum and chloroplasts. Journal of Biological Chemistry. 2007;282(2):1170–4. 10.1074/jbc.M608124200 . [DOI] [PubMed] [Google Scholar]
- 25.Wada M, Kagawa T, Sato Y. Chloroplast movement. Annual Review of Plant Biology. 2003;54:455–68. 10.1146/annurev.arplant.54.031902.135023 . [DOI] [PubMed] [Google Scholar]
- 26.Menzel D. An Interconnected Plastidom in Acetabularia—Implications for the Mechanism of Chloroplast Motility. Protoplasma. 1994;179(3–4):166–71. 10.1007/Bf01403955 . [DOI] [Google Scholar]
- 27.Oikawa K, Yamasato A, Kong SG, Kasahara M, Nakai M, Takahashi F, et al. Chloroplast outer envelope protein CHUP1 is essential for chloroplast anchorage to the plasma membrane and chloroplast movement. Plant Physiology. 2008;148(2):829–42. 10.1104/pp.108.123075 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Usami H, Maeda T, Fujii Y, Oikawa K, Takahashi F, Kagawa T, et al. CHUP1 mediates actin-based light-induced chloroplast avoidance movement in the moss Physcomitrella patens. Planta. 2012;236(6):1889–97. 10.1007/s00425-012-1735-6 . [DOI] [PubMed] [Google Scholar]
- 29.Hanson MR, Sattarzadeh A. Stromules: Recent Insights into a Long Neglected Feature of Plastid Morphology and Function. Plant Physiology. 2011;155(4):1486–92. 10.1104/pp.110.170852 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwok EY, Hanson MR. Plastids and stromules interact with the nucleus and cell membrane in vascular plants. Plant Cell Rep. 2004;23(4):188–95. 10.1007/s00299-004-0824-9 . [DOI] [PubMed] [Google Scholar]
- 31.Wang WM, Zhang Y, Wen YQ, Berkey R, Ma XF, Pan ZY, et al. A Comprehensive Mutational Analysis of the Arabidopsis Resistance Protein RPW8.2 Reveals Key Amino Acids for Defense Activation and Protein Targeting. Plant Cell. 2013;25(10):4242–61. 10.1105/tpc.113.117226 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pucadyil TJ, Schmid SL. Real-Time Visualization of Dynamin-Catalyzed Membrane Fission and Vesicle Release. Cell. 2008;135(7):1263–75. 10.1016/j.cell.2008.11.020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stachowiak JC, Schmid EM, Ryan CJ, Ann HS, Sasaki DY, Sherman MB, et al. Membrane bending by protein-protein crowding. Nature Cell Biology. 2012;14(9):944-+. 10.1038/Ncb2561 . [DOI] [PubMed] [Google Scholar]
- 34.Stachowiak JC, Hayden CC, Sasaki DY. Steric confinement of proteins on lipid membranes can drive curvature and tubulation. Proc Natl Acad Sci U S A. 2010;107(17):7781–6. 10.1073/pnas.0913306107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arimura S, Aida GP, Fujimoto M, Nakazono M, Tsutsumi N. Arabidopsis dynamin-like protein 2a (ADL2a), like ADL2b, is involved in plant mitochondrial division. Plant and Cell Physiology. 2004;45(2):236–42. 10.1093/Pcp/Pch024 . [DOI] [PubMed] [Google Scholar]
- 36.Praefcke GJK, McMahon HT. The dynamin superfamily: Universal membrane tubulation and fission molecules? Nat Rev Mol Cell Bio. 2004;5(2):133–47. 10.1038/Nrm1313 . [DOI] [PubMed] [Google Scholar]
- 37.Machettira AB, Gross LE, Tillmann B, Weis BL, Englich G, Sommer MS, et al. Protein-induced modulation of chloroplast membrane morphology. Front Plant Sci. 2012;2 Artn 118 10.3389/Fpls.2011.00118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breuers FKH, Brautigam A, Geimer S, Welzel UY, Stefano G, Renna L, et al. Dynamic remodeling of the plastid envelope membranes—a tool for chloroplast envelope in vivo localizations. Front Plant Sci. 2012;3 Artn 7 10.3389/Fpls.2012.00007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh ND, Li M, Lee SB, Schnell D, Daniell H. Arabidopsis Tic40 Expression in Tobacco Chloroplasts Results in Massive Proliferation of the Inner Envelope Membrane and Upregulation of Associated Proteins. Plant Cell. 2008;20(12):3405–17. 10.1105/tpc.108.063172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunkard JO, Runkel AM, Zambryski PC. Chloroplasts extend stromules independently and in response to internal redox signals. P Natl Acad Sci USA. 2015;112(32):10044–9. 10.1073/pnas.1511570112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video showing a stromule emanating from a chloroplast body that is 109 nm in length. Scale bars correspond to 30 μm. The white arrow corresponds to a stromule.
(AVI)
A video panning out of a stromule that is branched. The white arrow indicates an area of stromule branching.
(AVI)
A video capturing the recoiling of stromules back to the main plastidic body. Scale bars correspond to 10 μm.
(AVI)
A video showing the formation of stromules in real-time. The white arrows indicate chloroplasts that are stimulated by the cell extract to form stromules. Scale bars correspond to 30 μm. The total elapsed time of the recording was 20 minutes.
(AVI)
A video showing the captured stimulation of stromules from the heat inactivated plant extract. Scale bars correspond to 30 μm. The total elapsed time of the recording was 20 minutes.
(AVI)
A video showing the formation of stromules after the addition of apyrase-treated cell extract. The white arrows correspond to chloroplasts that form stromules. Scale bars correspond to 30 μm. The total elapsed time of the recording was 20 minutes.
(AVI)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.