Abstract
Ehrlichia chaffeensis is a tick-borne rickettsial pathogen and the causative agent of human monocytic ehrlichiosis. Transmitted by the Amblyomma americanum tick, E. chaffeensis also causes disease in several other vertebrate species including white-tailed deer and dogs. We have recently described the generation of an attenuated mutant strain of E. chaffeensis, with a mutation in the Ech_0660 gene, which is able to confer protection from secondary, intravenous-administered, wild-type E. chaffeensis infection in dogs. Here, we extend our previous results, demonstrating that vaccination with the Ech_0660 mutant protects dogs from physiologic, tick-transmitted, secondary challenge with wild-type E. chaffeensis; and describing, for the first time, the cellular and humoral immune responses induced by Ech_0660 mutant vaccination and wild-type E. chaffeensis infection in the canine host. Both vaccination and infection induced a rise in E. chaffeensis-specific antibody titers and a significant Th1 response in peripheral blood as measured by E. chaffeensis antigen-dependent CD4+ T cell proliferation and IFNγ production. Further, we describe for the first time significant IL-17 production by peripheral blood leukocytes from both Ech_0660 mutant vaccinated animals and control animals infected with wild-type E. chaffeensis, suggesting a previously unrecognized role for IL-17 and Th17 cells in the immune response to rickettsial pathogens. Our results are a critical first step towards defining the role of the immune system in vaccine-induced protection from E. chaffeensis infection in an incidental host; and confirm the potential of the attenuated mutant clone, Ech_0660, to be used as a vaccine candidate for protection against tick-transmitted E. chaffeensis infection.
Introduction
Ehrlichia chaffeensis is the causative agent of human monocytic ehrlichiosis (HME) [1–3]. It is an obligately intracellular Gram-negative rickettsial bacterium that is transmitted by the lone star tick, Amblyomma americanum [2]. White-tailed deer are the reservoir hosts for E. chaffeensis, but humans, dogs and other vertebrate species are common incidental hosts [2]. HME in people causes a life-threatening febrile illness and is associated with significant morbidity. About 40–60% of cases of HME require hospitalization, and fatality rates are estimated to be around 3% [4, 5]. There is currently no approved vaccine for use against E. chaffeensis infection in humans or animals.
Vaccine development and a detailed knowledge of immunity to E. chaffeensis infection have been limited due to lack of a robust experimental animal model for HME. Rodents are not a natural host for E. chaffeensis, and the pathogen is poorly infectious in mice, causing only transient infection in immunocompetent strains [1, 6–9]. However, through the use of immunodeficient animals, we and others have shown that clearance and protection from E. chaffeensis infection in mice relies primarily on antigen-specific CD4+ T cells [6, 7, 9, 10]. Infection of mice with E. muris or Ixodes ovatus Ehrlichia (IOE), strains closely related to E. chaffeensis, results in a systemic infection and has been used as a surrogate model of the human HME [8, 11–18]. Similar to our results with E. chaffeensis infection, clearance of a primary E. muris or IOE infection is associated with a strong, cellular immune response and production of IFNγ [8, 11, 17, 18]. Importantly, humoral immunity has also been shown sufficient in protecting mice from Ehrlichia infection [12, 14, 19].
Given the limitations of the mouse models of disease, our laboratory has recently turned to the use of the canine as a model for studying infection and immunity to E. chaffeensis. Dogs, like humans, are an incidental host for E. chaffeensis and are naturally infested by its tick vector, A. americanum [20]. We have recently demonstrated that E. chaffeensis infection in dogs shares similarities with infection in humans and deer, including pathogen persistence, making the canine an ideal and highly relevant model for studying host immunity [21, 22].
We recently described an approach for E. chaffeensis mutagenesis and the development of attenuated mutant strains whose growth are significantly inhibited in vivo in the vertebrate host [23]. Primary infection with one of these attenuated mutants, the Ech_0660 clone, promotes the development of protective immunity against a secondary challenge with virulent in vitro cultured E. chaffeensis in both the natural host (white-tailed deer) and an incidental host (dog) [21], suggesting our attenuated mutants are ideal candidates for vaccine development against E. chaffeensis. In this study, we tested the efficacy of vaccination with the Ech_0660 mutant against a physiologic, tick-transmitted challenge with wild-type E. chaffeensis in dogs, and for the first time conducted a detailed analysis of the humoral and cellular immune responses induced by E. chaffeensis vaccination and infection. We demonstrate that Ech_0660 mutant vaccination induces pathogen-specific antibody responses, robust CD4+ T cell immunity, and is efficacious against a tick-transmitted, secondary challenge with wild-type E. chaffeensis.
Materials and Methods
In vitro culture of E. chaffeensis
E. chaffeensis Arkansas strain (wild-type and mutant strains) and E. canis Oklahoma strain were continuously cultivated in the canine, macrophage-like DH82 cell line as described [24].
Animals and E. chaffeensis infections
Twelve female, purebred beagle dogs of 5–6 months of age were purchased from Covance Research Products (Denver, PA). Animals were housed in a climate-controlled, biosafety level-2 facility at Kansas State University. Experimental procedures were performed in strict compliance with federal and institutional guidelines and were approved by the Kansas State University Institutional Animal Care and Use Committee.
Intravenous vaccination with attenuated E. chaffeensis transposon mutant Ech_0660 in dogs was performed as previously described [21]. Animals (n = 7) were inoculated i.v. with 2x108 E. chaffeensis mutant strain, Ech_0660, organisms in 1 mL phosphate buffered saline (PBS). Ehrlichia organisms for vaccinations and challenge studies (below) were quantified by Taqman-based real-time PCR as we have described previously [25, 26].
Challenge infections were performed 31 days after Ech_0660 vaccination. Animals were either challenged by tick-transmission with wild-type E. chaffeensis (n = 3, group 2), by intravenous inoculation with ~2x108 wild-type E. chaffeensis grown in DH82 cells (n = 2, group 1), or by intravenous inoculation with ~2x108 wild-type E. canis grown in DH82 cells (n = 2, group 4). Animals that had not previously received Ech_0660 served as controls for virulent E. chaffeensis infection (n = 4, group 3). These animals were challenged via tick transmission with either wild-type E. chaffeensis or with non-attenuated Ech_0480, an isogenic mutant. We have previously demonstrated that the Ech_0480 mutant behaves similarly in culture and persists in vivo similar to the wild-type strain [27]; therefore, results from these animals were combined for antibody and T cell analyses (group 3). One unvaccinated dog was used as a wild-type E. canis infection control and was challenged via intravenous inoculation with ~2x108 E. canis grown in DH82 cells.
Animals were humanely euthanized by barbiturate overdose and necropsies performed on day 39 post challenge. Tissues were collected for histopathology and detection of E. chaffeensis in the organs.
Tick transmission
E. chaffeensis infected, A. americanum adult ticks were used for the tick-transmitted challenge. The tick infection was conducted as described in [27]. Briefly, nymphal ticks were needle-inoculated with 5 μl of concentrated bacterial culture containing ~5,000 wild-type E. chaffeensis or virulent Ech_0480 mutant. Nymphs were allowed to molt into adults at room temperature in a humidified chamber with 14 h daylight and 10 h darkness cycles [27]. The infection status of the needle-inoculated ticks was verified by nested PCR targeting to the Ech_1136 gene encoding for the p28-Omp 14 protein as previously described [27]. A small area on the back of the dog was shorn and a tick containment cell was affixed. Twenty-five pairs of adult ticks per dog were placed in the tick containment cell and permitted to feed for 6–7 days before removal.
Detection of E. chaffeensis by culture recovery and molecular methods
E. chaffeensis infection was assessed in peripheral blood using culture, semi-nested PCR, and quantitative PCR as previously described [21]. At necropsy, E. chaffeensis infection was assessed in the spleen and liver using semi-nested PCR targeting to the Ech_1136 gene for E. chaffeensis or 16s rRNA gene for E canis as previously described [22, 27].
Enzyme-linked immunosorbent assay (ELISA) for Total Ig and E. chaffeensis-specific IgG
Plasma samples collected prior to, and following infection were assessed by ELISA for the presence of E. chaffeensis–specific IgG as previously reported [22].
Preparation and culture of Peripheral blood mononuclear cells (PBMC)
PBMCs were isolated by density centrifugation from buffy coat fractions of peripheral blood collected into 2x acid citrate dextrose. Cells were washed and resuspended in complete RPMI composed of RPMI-1640 (Gibco, Carlsbad, CA) supplemented with 2 mM L-glutamine, 25 mM HEPES buffer, 1% antibiotic—antimycotic solution, 50 mg/mL gentamicin sulfate, 1% nonessential amino acids, 2% essential amino acids, 1% sodium pyruvate, 50 μM 2-mercaptoethanol, and 10% (v/v) fetal bovine serum. For lymphocyte proliferation assays, cells were labeled with 1 μM CellTrace Violet (Life Technologies Inc.) per manufacturer’s instructions. Cells were cultured for 5 days at 37°C with 4x105 cells /well in 96-well plates and were stimulated with 10 μg/mL host cell-free E. chaffeensis whole-cell lysate that was grown in ISE6 tick cells. As a positive control, cells were stimulated with 5 μg/mL Concanavalin A (Sigma-Aldrich). For proliferation and intracellular cytokine staining data, background (mock) responses were subtracted from the response to antigen and results are presented as change over mock.
Antibodies and Flow Cytometry
The following monoclonal antibodies were used in these studies: mouse anti-canine CD3-FITC (clone CA17.2112), CD4-RPE or APC (clone YKIX302.9), CD8 RPE or APC (YCATE55.9), and mouse-anti-bovine IFNγ-RPE (clone CC302) all from AbD Serotec (Raleigh, NC). The bovine IFNγ-specific clone CC302 has been previously demonstrated to cross-react with canine IFNγ [28].
For surface staining, cells were resuspended at 107 cells/mL in FACS buffer (0.1% NaN3, 10% fetal calf serum, PBS) and incubated for 20 minutes at 4°C with 10 μg/mL primary antibodies or as recommended by the manufacturer. Cells were washed and fixed in BD FACS Lysis buffer (BD Biosciences).
Intracellular cytokine staining for IFNγ was carried out using the BD Fixation and Permeabilization Solution kit (BD Biosciences). Cells were cultured with antigen for 5 days, and then Brefeldin A was added for the last 5–6 hours of incubation. Cells were surface stained and then fixed, permeabilized and stained for intracellular IFNγ (Clone CC302, 10 μg/mL) per manufacturer’s instructions.
Flow cytometry data were collected on a BD LSR Fortessa X-20 flow cytometer and analyzed using FlowJo software (Tree Star Inc., San Carlos, CA).
ELISA for canine cytokines
PBMC culture supernatants were collected after 5 days of stimulation with 10 μg/mL host-cell free E. chaffeensis lysate. IL-4, IFNγ, and IL-17A protein concentrations were determined by commercial ELISA kit (R&D Systems, Minneapolis, MN) per manufacturer’s instructions.
Statistics
Statistical analysis was performed using Prism v6.0f software (Graphpad Software, Inc.). To maximize power to detect differences, T cell and antibody responses were compared using an analysis of variance accounting for the repeated measures on animals over time, and the nesting of animals within each infection group as previously described [21–23]. ELISA results on cell culture supernatants from day 7-post infection were analyzed using a 1-way ANOVA with Bonferri post-test analysis.
Results
Attenuated mutant Ech_0660 confers protection against tick-transmitted challenge in dogs
We have demonstrated that primary infection with the attenuated Ech_0660 mutant induces protection from secondary intravenous-administered challenge with wild-type E. chaffeensis [21, 23]. To determine if the Ech_0660 mutant is also protective in a more physiologic setting of tick-transmitted challenge, we vaccinated dogs with the mutant and then performed secondary challenges on day 31-post infection. Four control dogs remained unvaccinated. Seven dogs were vaccinated i.v. with the Ech_0660 mutant organisms. Animals were monitored for the presence of Ehrlichia in the blood following Ech_0660 vaccination by PCR and culture recovery methods (Table 1). We have shown previously that the Ech_0660 mutant is highly attenuated and rapidly cleared from the canine host [23, 29]. In agreement with our prior studies, the Ech_0660 mutant was detected in only three animals on day 3 post vaccination. Thirty-one days after vaccination, dogs were divided into groups. Two Ech_0660 vaccinated dogs were challenged with wild-type E. chaffeensis via needle inoculation (group 1). Three vaccinated dogs were challenged with wild-type E. chaffeensis by tick transmission (group 2). The four unvaccinated control dogs were challenged via tick transmission with wild-type E. chaffeensis (n = 2) or a wild-type like, isogenic mutant strain Ech_0480 (n = 2) (group 3). We have previously demonstrated that the Ech_0480 mutant behaves like the wild-type strain of E. chaffeensis, displaying similar persistence in the vertebrate host (23); therefore we have combined the data for these two control groups (group 3).
Table 1. Infection status of dogs vaccinated with attenuated mutant Ech_0660.
| Days Post Vaccination | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 8 | 11 | 14 | 21 | 28 | 31 | |
| Ech_0660_1a | - | - | - | - | - | - | - | - |
| Ech_0660_2 | - | - | - | - | - | - | - | - |
| Ech_0660_3 | - | cb | - | - | - | - | - | - |
| Ech_0660_4 | - | - | - | - | - | - | - | - |
| Ech_0660_5 | - | - | - | - | - | - | - | - |
| Ech_0660_6 | - | c | - | - | - | - | - | - |
| Ech_0660_7 | - | c | - | - | - | - | - | - |
a Seven dogs were inoculated i.v. with 2x108 E. chaffeensis mutant Ech_0660 organisms.
b Dogs were tested at the indicated time points for E. chaffeensis organisms in the blood by PCR (p) and culture recovery methods (c) as described [21].
E. chaffeensis infection in dogs varies from subclinical infection to severe systemic disease. Mild clinical signs may manifest as low-grade fever or thrombocytopenia, as we and others have previously reported [21–23, 30]. In this experiment, we did not observe significant clinical disease in vaccinated or control dogs (data not shown). E. chaffeensis infection was monitored in the blood after secondary challenge using nested PCR and culture recovery methods. The results are shown in Table 2. Dogs that were vaccinated and challenged with wild-type E. chaffeensis by needle inoculation (group 1) were protected from infection, as evidenced by testing positive for infection in the blood only twice in one animal on days 8 and 11 post challenge (12.5% of the time), and testing negative for the organism in the spleen and liver at the time of necropsy. Vaccinated dogs that were challenged via tick-transmission (group 2) were also protected from secondary challenge. This group tested positive for Ehrlichia in the blood 29.1% of the time (7 out of 24 total blood samples tested). However, no blood positives were obtained after day 15 post challenge. All animals were also negative for the organism in the spleen and liver at the time of necropsy. This result suggests that while dogs may develop ehrlichemia early following infection, vaccination with the Ech_0660 mutant promotes protection from long-term pathogen persistence in the blood and organs. In contrast, unvaccinated control dogs (group 3) displayed persistent infection, testing frequently positive for the organism throughout the 31 days of assessment (about 34.3% of the time: 11 out of 32 samples tested) and testing positive for the organism in the tissues at necropsy.
Table 2. Infection status of Ech_0660 vaccinated dogs and unvaccinated control dogs following wild-type E. chaffeensis challenge.
| Days Post Challenge: WT E. chaffeensis by needle transmission | Necropsye | ||||||||||
| Group 1a | 0 | 4 | 8 | 11 | 15 | 22 | 29 | 36 | blood | spleen | liver |
| Ech_0660_1 | - | - | - | - | - | - | - | - | - | - | - |
| Ech_0660_2 | - | - | pd | c | - | - | - | - | - | - | - |
| Days Post Challenge: WT E. chaffeensis by tick transmission | |||||||||||
| Group 2b | 0 | 4 | 8 | 11 | 15 | 22 | 29 | 36 | blood | spleen | liver |
| Ech_0660_3 | - | - | c | - | c | - | - | - | - | - | - |
| Ech_0660_4 | - | - | c | - | c | - | - | - | - | - | - |
| Ech_0660_5 | - | - | p/c | p | c | - | - | - | - | - | - |
| Days Post Challenge: WT E. chaffeensis or Ech_0480 by tick transmission | |||||||||||
| Group 3c | 0 | 3 | 7 | 10 | 14 | 17 | 24 | 31 | blood | spleen | liver |
| Wild-type_1 | - | - | p | - | c | - | - | p | - | - | + |
| Wild-type_2 | - | - | p | - | c | p | - | - | - | - | - |
| Ech_0480_1 | - | - | - | - | c | - | - | - | - | - | + |
| Ech_0480_2 | - | p | - | - | c | - | p | p | - | + | - |
a Dogs from Table 1 were challenged 31 days after vaccination. Animals were challenged via i.v. inoculation with 2x108 wild-type E. chaffeensis organisms
b Dogs from Table 1 were challenged 31 days after vaccination. Animals were challenged via tick-transmission with wild-type E. chaffeensis organisms
c Unvaccinated control dogs were challenged with 2x108 wild-type E. chaffeensis organisms or 2x108 Ech_0480 mutant E. chaffeensis organisms
d Dogs were tested at the indicated time points for E. chaffeensis organisms in the blood by PCR (p) and culture recovery methods (c) as described [21]. Animals testing positive by both methods are indicated by (p/c)
e Animals were euthanized and necropsied on day 39 post challenge.
To determine if Ech_0660 mutant inoculation protects dogs against a heterologous challenge, we challenged the remaining two Ech_0660 vaccinated animals with a closely related Ehrlichia organism, E. canis, by needle inoculation (group 4). One unvaccinated control animal was also infected with wild-type E. canis by needle inoculation. Dogs in group 4 tested positive for infection in the blood 81.2% of the time (13 out of 16 samples tested), similar to the unvaccinated control animal, suggesting that the Ech_0660 mutant is not protective against heterologous E. canis infection (Table 3). Importantly, as only two animals were included in this group, additional experiments will be necessary to confirm this result and to achieve statistical significance.
Table 3. Infection status of Ech_0660 vaccinated dogs and unvaccinated control dog following wild-type E. canis challenge.
| Days Post Challenge: WT E. chaffeensis by needle transmission | Necropsyd | ||||||||||
| Group 4a | 0 | 4 | 8 | 11 | 15 | 22 | 29 | 36 | blood | spleen | liver |
| Ech_0660_6 | - | p/cc | p/c | p/c | p/c | p/c | p/c | p/c | p/c | - | + |
| Ech_0660_7 | - | p/c | - | p/c | p/c | p/c | p/c | p/c | p/c | - | - |
| Days Post Challenge: WT E. chaffeensis by tick transmission | |||||||||||
| Controlb | 0 | 3 | 7 | 10 | 14 | 17 | 24 | 31 | blood | spleen | liver |
| E_canis_1 | - | p/c | p/c | p/c | p/c | p/c | p/c | p/c | p/c | + | + |
a Dogs from Table 1 were challenged 31 days after vaccination. Animals were challenged i.v. with 2x108 wild-type E. canis organisms
b Unvaccinated control dog was challenged with ~2x108 wild-type E. canis organisms
c Dogs were tested at the indicated time points for E. canis organisms in the blood by PCR (p) and culture recovery methods (c) as described [22, 26]. Animals testing positive by both methods are indicated by (p/c)
d Animals were euthanized and necropsied on day 39 post challenge
Ech_0660 vaccination and wild-type E. chaffeensis infection induce pathogen-specific antibody production
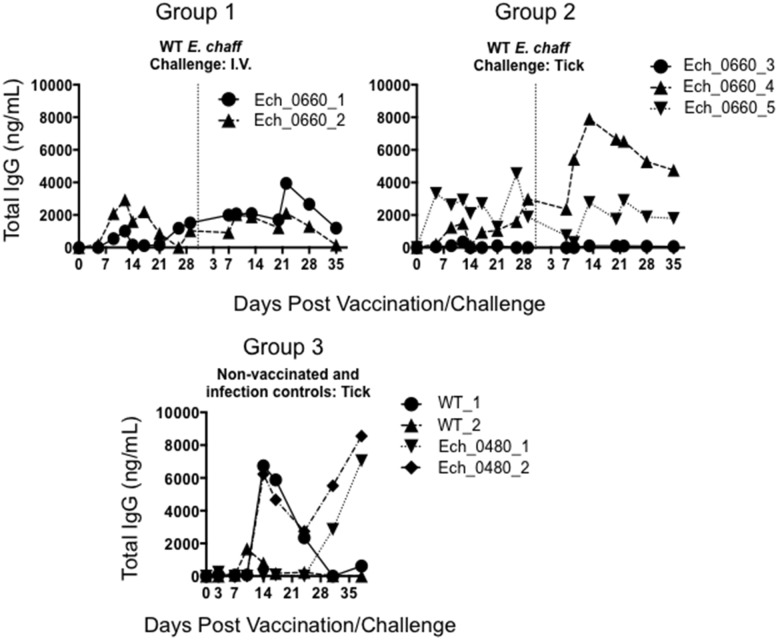
Plasma samples from vaccinated and unvaccinated control dogs were evaluated by ELISA for total E. chaffeensis-specific IgG. Vaccination resulted in an increase in E. chaffeensis specific IgG in 4 out of 5 dogs (Fig 1). We also observed an increase in pathogen-specific IgG following wild-type challenge in both vaccinated dogs and unvaccinated controls. There were no significant differences in the humoral response between vaccinated and control dogs after secondary challenge.
Fig 1. E. chaffeensis-specific IgG response following Ech_0660 vaccination and secondary challenge with wild-type E. chaffeensis.
Total E. chaffeensis-specific IgG was measured in the plasma at multiple time points by ELISA in dogs vaccinated with the Ech_0660 mutant and challenged with wild-type E. chaffeensis via needle inoculation (group 1), or vaccinated with Ech_0660 and challenged with wild-type E. chaffeensis via tick-transmission (group 2). Unvaccinated control dogs were infected with wild-type E. chaffeensis or the non-attenuated Ech_0480 mutant via tick-transmission (group 3). Each line is representative of a single animal.
Vaccination and wild-type E. chaffeensis challenge induces antigen-dependent CD4+ T cell responses
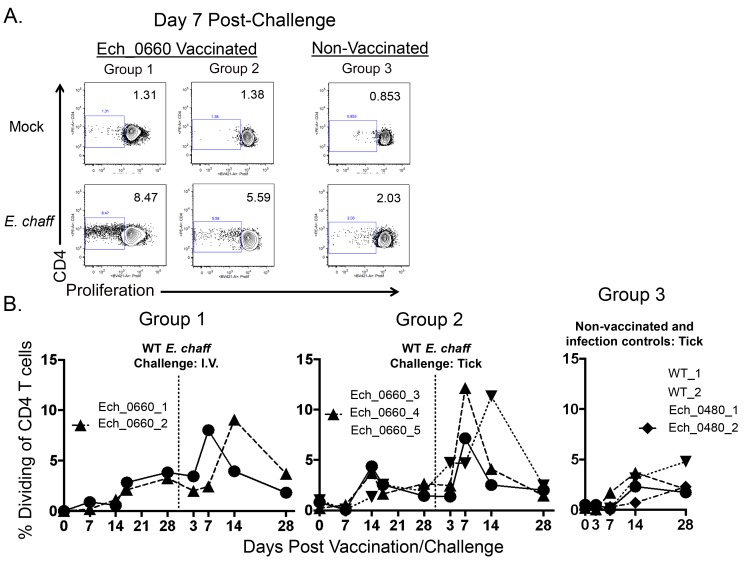
We next measured E. chaffeensis-specific CD4+ T cell recall responses in peripheral blood from vaccinated and control dogs. PBMC were labeled with Cell Trace Violet, stimulated with host cell-free E. chaffeensis whole cell lysate, and then analyzed by flow cytometry. Antigen-dependent CD4+ T cells were identified based upon proliferation in response to E. chaffeensis antigen as determined by dilution of the Cell Trace Violet dye. Fig 2A shows representative dilution profiles of mock and antigen-stimulated CD4+ T cells from one animal per group on day 7 post secondary challenge. The numbers depicted in Fig 2A represent the percent of proliferating CD3+CD4+ cells contained within each gate. Fig 2B shows the percentage of CD4+ T cells dividing in response to antigen that was measured in all animals over the course of the experiment. Background levels of proliferation were subtracted from these values, and results represent change in proliferation over mock stimulated cultures. We observed an increase in the percentage of CD4+ T cells that divided in response to E. chaffeensis antigen in PBMC collected on day 14–17 post inoculation with the Ech_0660 mutant. This percentage was further increased following wild-type E. chaffeensis challenge. Vaccinated animals displayed significantly higher percentages of proliferating E. chaffeensis antigen-dependent CD4+ T cells compared to unvaccinated dogs (Fig 2B, p = 0.0081).
Fig 2. CD4+ T cells from Ech_0660 mutant vaccinated and wild-type E. chaffeensis infected animals proliferate in response to E. chaffeensis antigen.
PBMC from dogs vaccinated with Ech_0660 and challenged with wild-type E. chaffeensis via needle inoculation (group 1, left panels), vaccinated with Ech_0660 and challenged with wild-type E. chaffeensis via tick inoculation (group 2, middle panels), or unvaccinated and infected with wild-type E. chaffeensis or Ech_0480 via tick inoculation (group 3, right panels) were labeled with Cell Trace Violet, then cultured for 5 days at 4x106 cells/mL in the presence or absence of 10 ug/mL E. chaffeensis host-cell free lysate grown in the tick ISE6 cell line. On day 5, CD4+ T cells were analyzed by flow cytometry for Cell Trace Violet dilution as a measure of proliferation. (A) Representative Cell Trace Violet dilution profiles, gated on total live cells and total CD3+CD4+ T cells. (B) The percentage of CD4+ T cells that have proliferated in response to E. chaffeensis antigens as measured over the course of the experiment. The background (mock stimulated) proliferation was subtracted, and results represent change over mock. Each line is representative of a single animal.
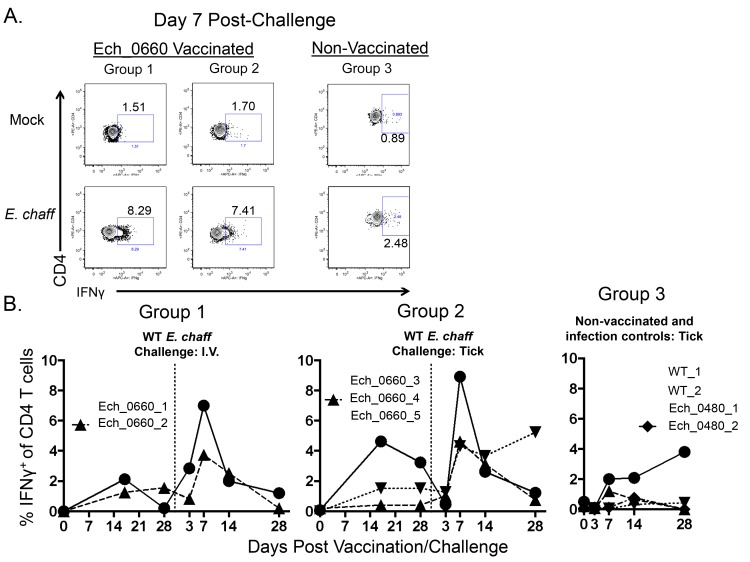
We also measured antigen-dependent IFNγ production by CD4+ T cells in the blood using intracellular cytokine staining. Fig 3A shows representative flow plots of mock and antigen-stimulated CD4+ T cells gated on IFNγ+ cells. Flow plots are from one animal per group on day 7 post secondary challenge. Fig 3B shows the combined results from all animals. We observed significantly increased percentages of CD4+ T cells producing IFNγ in response to E. chaffeensis antigen in samples from vaccinated animals, compared to unvaccinated controls (Fig 3B, p = 0.0025).
Fig 3. CD4+ T cells from Ech_0660 mutant vaccinated and wild-type E. chaffeensis infected animals produce IFNγ in response to E. chaffeensis antigen.
PBMC from dogs vaccinated with the Ech_0660 mutant and challenged with wild-type E. chaffeensis (groups 1–3, as in Fig 2) were cultured for 5 days at 4x106 cells/mL in the presence or absence of 10 ug/mL E. chaffeensis host-cell free lysate grown in the tick ISE6 cell line. On day 5, brefeldin A was added for the last 6 hours of culture. CD4+ T cells were stained for intracellular expression of IFNγ and analyzed by flow cytometry. (A) Representative flow plots from animals in groups 1, 2 and 3, gated on total live cells and total CD3+CD4+ T cells. (B) The percentage of IFNγ+ cells of total CD4+ T cells in the blood measured over the course of the experiment. Background (mock stimulated) IFNγ production was subtracted, and results represent change over mock.
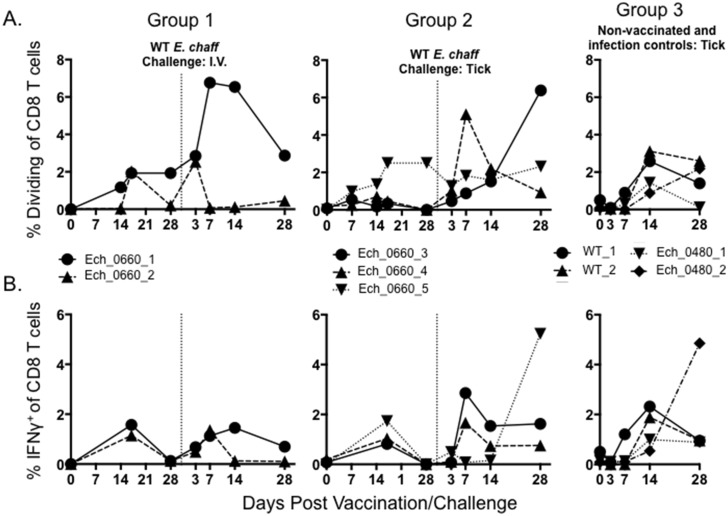
E. chaffeensis-specific CD8+ T cell proliferation and IFNγ production was also measured by flow cytometry (Fig 4). Neither vaccination nor infection with wild-type E. chaffeensis induced a significant CD8+ T cell response as measured by proliferation assay (Fig 4A) or by intracellular cytokine staining for IFNγ (Fig 4B). While we observed a trend towards an increase in the CD8+ T cell response from vaccinated dogs following secondary challenge, this response was not significant over that observed in control dogs (group 3).
Fig 4. CD8+ T cells from Ech_0660 vaccinated and wild-type E. chaffeensis infected animals proliferate and produce IFNγ in response to E. chaffeensis antigen.
CD8+ T cell proliferation and IFNγ production were measured using similar approaches as in Figs 2 and 3. PBMC from dogs in groups 1–3 were cultured for 5 days at 4x106 cells/mL in the presence or absence of 10 ug/mL E. chaffeensis host-cell free lysate. On day 5 of culture, CD8+ T cells were analyzed by flow cytometry for (A) proliferation as measured by Cell Trace Violet dilution; and (B) intracellular production of IFNγ. The frequencies of responding CD8+ T cells were measured over the course of the experiment. Results were gated on total live cells and total CD3+CD8+ T cells. Background (mock stimulated) proliferation or IFNγ production was subtracted and results represent change over mock.
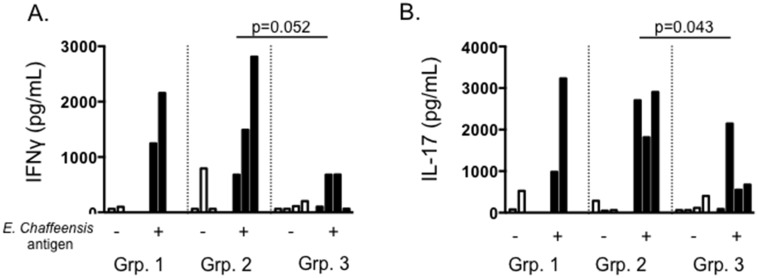
ELISAs were used to measure Th1, Th2 and Th17 cytokines secreted by PBMC in recall responses to E. chaffeensis antigen. PBMC from Ech_0660 vaccinated animals secreted IFNγ (Th1) in response to E. chaffeensis antigen (Fig 5A); and this response was significantly increased over the response from unvaccinated control dogs. We did not observe appreciable IL-4 (Th2) production by PBMC from vaccinated or control dogs; however, all three groups mounted a vigorous IL-17 response to E. chaffeensis antigen (Fig 5B). IL-17 production by cells from Ech_0660 vaccinated dogs was significantly increased over unvaccinated controls.
Fig 5. PBMC from Ech_0660 vaccinated and wild-type E. chaffeensis infected animals secrete IFNγ and IL-17 in response to E. chaffeensis antigen.
PBMC from dogs vaccinated with Ech_0660 and challenged with wild-type E. chaffeensis (groups 1–3, as in Fig 2) were collected on day 7 post-secondary challenge with wild-type E. chaffeensis. PBMC were cultured for 5 days at 4x106 cells/mL in the presence or absence of 10 ug/mL E. chaffeensis host-cell free lysate grown in the tick ISE6 cell line. On day 5, cell culture supernatants were collected and later analyzed by ELISA for secretion of (A) IFNγ, (B) IL-17, and IL-4 (not shown). Each bar is representative of a single animal.
Discussion
We recently reported the ability of a clonally purified transposon mutant of E. chaffeensis, Ech_0660, to induce protection from secondary, intravenous challenge with wild-type E. chaffeensis in the incidental canine host [21]. In the current study, we demonstrate that Ech_0660 mutant vaccinated animals are also protected from a physiologically relevant, tick-transmitted infection challenge. In this study, we also report the induction of robust E. chaffeensis-specific antibody and CD4+ T cell responses in animals receiving Ech_0660 mutant vaccination and secondary challenge.
In nature, E. chaffeensis is transmitted to a host when an infected A. americanum tick takes a blood meal [2]. Previous studies have shown that the route of inoculation of vector-borne pathogens such as Ehrlichia can have a profound effect on vaccine-induced protection and ultimately disease outcome. For example, a recent study by Pretorius et al. showed that an experimental prime/boost DNA vaccine regimen afforded 100% protection to sheep challenged via needle-transmitted Ehrlichia ruminantium challenge; while the same regimen provided less than 20% protection from a physiologic tick-transmitted E. ruminantium challenge [31]. While a number of factors were at play in this study, the authors attribute the vaccine failure to fundamental differences between tick-transmitted and needle-transmitted challenge infections. In our study, we analyzed the immune response to dogs challenged with wild-type E. chaffeensis via needle-inoculation or via a more physiologic route of tick-transmitted challenge. We did not observe obvious differences in the immunologic parameters we measured in this study between the two routes of inoculation, including the magnitude of the humoral or cell-mediated immune response. However, we used only 2 animals in the group receiving E. chaffeensis via needle-inoculation (group 2), because this route of inoculation was not the primary focus of this study. Further, we previously reported that Ech_0660 vaccination promoted protection against intravenous inoculation challenge in our recent report [21]. It is possible that critical differences may exist between the immune response induced by intravenous infection compared to tick-transmitted challenge. We have previously demonstrated in studies using the murine model of E. chaffeensis infection that the route of inoculation, and the source of inoculum (e.g. organisms grown in tick cells vs. those grown in canine macrophage cells) can have a significant effect on the specificity and nature of the immune response [25]. We hypothesize this effect is due to the differential expression of Ehrlichia outer membrane proteins expressed during growth in tick cells vs. macrophage cells. In support of this theory, we have demonstrated that approximately 50% of E. chaffeensis proteins are expressed in a host-cell specific manner [32]. In addition, ticks employ a number of well-described immunomodulatory factors that play a critical role in allowing vector-borne pathogens to establish infection (reviewed in [33]). Amongst these strategies: inhibition of host inflammatory cytokine production such as IL-1, IL-12, TNF-α and IFNγ inhibition of lymphocyte proliferative responses; and downregulation of macrophage nitric oxide production.
We demonstrate here that E. chaffeensis vaccination and challenge in dogs induces robust antigen-dependent CD4+ T cell responses, but not a significant antigen-dependent CD8+ T cell response. This result may be due to large animal-to-animal variability, or it may be that CD8+ T cell responses are not a major component of the Ehrlichia-specific immune response in dogs. In support of our observations, studies from mice agree that CD4+ T cell immunity is critical for the response to Ehrlichia, while the role of CD8+ T cells is less clear [6, 11, 13, 14, 17]. We observe only minor CD8+ T cell responses following primary E. chaffeensis infection in C57BL/6 mice [6], and a similar result is observed during IOE infection of C57BL/6 mice [11]. In contrast, cytotoxic T cells appear critical during E. muris infection of C3H/HeN mice, as infection of animals lacking MHC class I results in over 80% lethality [14]. In the future, it will be important to more clearly define the contribution of CD8+ T cells in the response to E. chaffeensis vaccination and protection in the natural host.
E. canis is genetically related to E. chaffeensis, and the primary etiologic agent of canine monocytic ehrlichiosis [34]. In this study, we tested the ability of our E. chaffeensis mutant vaccine strain to promote protection from heterologous E. canis challenge. We did not observe protection from E. canis, as two dogs that received the Ech_0660 mutant tested positive for ehrlichemia for the duration of the study. While this result is disappointing, similar examples of poor protection from heterologous challenge have been previously reported for the rickettsials, including lack of protection between strains of E. ruminantum [35], of Anaplasma phagocytophilum [36, 37] and of E. canis [38, 39].
To the best of our knowledge, ours is the first report of IL-17 production in the context of Ehrlichia infection. IL-17 is a pro-inflammatory cytokine that is primarily produced by activated CD4+ and γδ T cells [40]. It induces expression of a number of chemotactic factors, particularly IL-8, and is critical for the recruitment and activation of neutrophils. A balanced IL-17 response seems favorable for control of a number of intracellular bacterial infections, but excessive IL-17 contributes to damaging immunopathology. For example, in a mouse model of Mycobacterium bovis infection, IL-17 is essential for pathogen control and for appropriate maturation of granulomas [41]; while excessive IL-17 promotes exacerbated inflammation and increased mortality [42]. Similarly, in a mouse model of Chlamydia muridarum infection, IL-17 contributes to disease pathology, but is also essential for protection from secondary infection [43, 44]. Our results suggest that IL-17 production may correlate with protection from wild-type E. chaffeensis infection in a canine host. However, as observed during other intracellular infections, it is probable that balance is critical for host defense, and excessive IL-17 production may be detrimental to the host. In support of this, a recent report in humans showed that elevated levels of IL-8 are associated with fatal HME [45]. While this study did not examine expression of IL-17, it is a mechanism that should be considered in light of our recent findings. Future studies will be required to determine the role of IL-17 and Th17 immunity in protection or possibly immunopathology during E. chaffeensis infection in a natural host.
In conclusion, we demonstrate that vaccination with the live, attenuated mutant Ech_0660 induces pathogen-specific humoral and cellular immunity, and protection from tick-transmitted E. chaffeensis infection in a physiologic host. This report represents the first detailed analysis of the immune responses induced by vaccination and infection in a natural host for E. chaffeensis, and a critical first step in developing our understanding of immunity to this important, emerging pathogen.
Acknowledgments
The authors wish to thank Dr. S. K. Chapes for critical review of the manuscript, Dr. Sally Olson and the animal care staff for their attentive care of the animals, and Courtney Sobba for her excellent technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by PHS grant number AI070908 from the National Institute of Allergy and Infectious Diseases to RRG, and KSU-CVM start-up funds to JLM. Research reported in this publication was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20 GM103638 to JLM. This manuscript is contribution number 16-102-J from the Kansas Agricultural Experiment Station. Publication of this article was funded in part by the Kansas State University Open Access Publishing Fund. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
References
- 1.McBride JW, Walker DH. Progress and obstacles in vaccine development for the ehrlichioses. Expert Rev Vaccines 2010;9(9):1071–82. 10.1586/erv.10.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Rev 2003;16(1):37–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker DH, Dumler JS. Emergence of the ehrlichioses as human health problems. Emerg infect Dis 1996;2(1):18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng TR, Harkess JR, Fishbein DB, Dawson JE, Greene CN, Redus MA, et al. Epidemiologic, clinical, and laboratory findings of human ehrlichiosis in the United States, 1988. Jama. 1990;264(17):2251–8. . [PubMed] [Google Scholar]
- 5.Fishbein DB, Dawson JE, Robinson LE. Human ehrlichiosis in the United States, 1985 to 1990. Annal Intern Med 1994;120(9):736–43. . [DOI] [PubMed] [Google Scholar]
- 6.Ganta RR, Cheng C, Wilkerson MJ, Chapes SK. Delayed clearance of Ehrlichia chaffeensis infection in CD4+ T-cell knockout mice. Infect Immun 2004;72(1):159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganta RR, Wilkerson MJ, Cheng C, Rokey AM, Chapes SK. Persistent Ehrlichia chaffeensis infection occurs in the absence of functional major histocompatibility complex class II genes. Infect Immun 2002;70(1):380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winslow GM, Bitsaktsis C, Yager E. Susceptibility and resistance to monocytic ehrlichiosis in the mouse. Ann N Y Acad Sci 2005;1063:395–402. 10.1196/annals.1355.071 . [DOI] [PubMed] [Google Scholar]
- 9.Winslow GM, Yager E, Shilo K, Collins DN, Chu FK. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect Immun 1998;66(8):3892–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winslow GM, Yager E, Shilo K, Volk E, Reilly A, Chu FK. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect Immun 2000;68(4):2187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitsaktsis C, Huntington J, Winslow G. Production of IFN-gamma by CD4 T cells is essential for resolving ehrlichia infection. J Immunol 2004;172(11):6894–901. . [DOI] [PubMed] [Google Scholar]
- 12.Bitsaktsis C, Nandi B, Racine R, MacNamara KC, Winslow G. T-Cell-independent humoral immunity is sufficient for protection against fatal intracellular ehrlichia infection. Infect Immun 2007;75(10):4933–41. 10.1128/IAI.00705-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bitsaktsis C, Winslow G. Fatal recall responses mediated by CD8 T cells during intracellular bacterial challenge infection. J Immunol 2006;177(7):4644–51. . [DOI] [PubMed] [Google Scholar]
- 14.Feng HM, Walker DH. Mechanisms of immunity to Ehrlichia muris: a model of monocytotropic ehrlichiosis. Infect Immun 2004;72(2):966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawahara M, Suto C, Shibata S, Futohashi M, Rikihisa Y. Impaired antigen specific responses and enhanced polyclonal stimulation in mice infected with Ehrlichia muris. Microbiol Immunol 1996;40(8):575–81. . [DOI] [PubMed] [Google Scholar]
- 16.Sotomayor EA, Popov VL, Feng HM, Walker DH, Olano JP. Animal model of fatal human monocytotropic ehrlichiosis. Am J Pathol 2001;158(2):757–69. 10.1016/S0002-9440(10)64018-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismail N, Soong L, McBride JW, Valbuena G, Olano JP, Feng HM, et al. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J Immunol 2004;172(3):1786–800. . [DOI] [PubMed] [Google Scholar]
- 18.Stevenson HL, Jordan JM, Peerwani Z, Wang HQ, Walker DH, Ismail N. An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect Immun 2006;74(8):4856–64. 10.1128/IAI.00246-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yager E, Bitsaktsis C, Nandi B, McBride JW, Winslow G. Essential role for humoral immunity during Ehrlichia infection in immunocompetent mice. Infect Immun 2005;73(12):8009–16. 10.1128/IAI.73.12.8009-8016.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yabsley MJ. Natural history of Ehrlichia chaffeensis: vertebrate hosts and tick vectors from the United States and evidence for endemic transmission in other countries. Vet Parasitol 2010;167(2–4):136–48. . [DOI] [PubMed] [Google Scholar]
- 21.Nair AD, Cheng C, Jaworski DC, Ganta S, Sanderson MW, Ganta RR. Attenuated Mutants of Ehrlichia chaffeensis in Inducing Protection against Wild-type Infection Challenge in the Reservoir Host and in an Incidental Host. Infect Immun 2015. 10.1128/IAI.00487-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair AD, Cheng C, Jaworski DC, Willard LH, Sanderson MW, Ganta RR. Ehrlichia chaffeensis infection in the reservoir host (white-tailed deer) and in an incidental host (dog) is impacted by its prior growth in macrophage and tick cell environments. PloS One 2014;9(10):e109056 10.1371/journal.pone.0109056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng C, Nair AD, Indukuri VV, Gong S, Felsheim RF, Jaworski D, et al. Targeted and random mutagenesis of Ehrlichia chaffeensis for the identification of genes required for in vivo infection. PLoS Pathog 2013;9(2):e1003171 10.1371/journal.ppat.1003171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng C, Ganta RR. Laboratory maintenance of Ehrlichia chaffeensis and Ehrlichia canis and recovery of organisms for molecular biology and proteomics studies. Curr Protoc Microbiol 2008;Chapter 3:Unit 3A 1. 10.1002/9780471729259.mc03a01s9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganta RR, Cheng C, Miller EC, McGuire BL, Peddireddi L, Sirigireddy KR, et al. Differential clearance and immune responses to tick cell-derived versus macrophage culture-derived Ehrlichia chaffeensis in mice. Infect Immun 2007;75(1):135–45. 10.1128/IAI.01127-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sirigireddy KR, Ganta RR. Multiplex detection of Ehrlichia and Anaplasma species pathogens in peripheral blood by real-time reverse transcriptase-polymerase chain reaction. J Molec Diag 2005;7(2):308–16. 10.1016/S1525-1578(10)60559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng C, Nair AD, Jaworski DC, Ganta RR. Mutations in Ehrlichia chaffeensis Causing Polar Effects in Gene Expression and Differential Host Specificities. PloS One 2015;10(7):e0132657 10.1371/journal.pone.0132657 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen LG, Castelruiz Y, Jacobsen S, Aasted B. Identification of monoclonal antibodies that cross-react with cytokines from different animal species. Vet Immunol Immunopathol 2002;88(3–4):111–22. . [DOI] [PubMed] [Google Scholar]
- 29.Nair AD, Cheng C, Jaworski DC, Ganta S, Sanderson MW, Ganta RR. Attenuated Mutants of Ehrlichia chaffeensis Induce Protection against Wild-Type Infection Challenge in the Reservoir Host and in an Incidental Host. Infect Immun 2015;83(7):2827–35. 10.1128/IAI.00487-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XF, Zhang JZ, Long SW, Ruble RP, Yu XJ. Experimental Ehrlichia chaffeensis infection in beagles. J Med Microbiol 2003;52(Pt 11):1021–6. . [DOI] [PubMed] [Google Scholar]
- 31.Pretorius A, van Kleef M, Collins NE, Tshikudo N, Louw E, Faber FE, et al. A heterologous prime/boost immunisation strategy protects against virulent E. ruminantium Welgevonden needle challenge but not against tick challenge. Vaccine 2008;26(34):4363–71. 10.1016/j.vaccine.2008.06.006 . [DOI] [PubMed] [Google Scholar]
- 32.Singu V, Liu H, Cheng C, Ganta RR. Ehrlichia chaffeensis expresses macrophage- and tick cell-specific 28-kilodalton outer membrane proteins. Infect Immun 2005;73(1):79–87. 10.1128/IAI.73.1.79-87.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wikel S. Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front Microbiol 2013;4:337 10.3389/fmicb.2013.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu XJ, McBride JW, Walker DH. Restriction and expansion of Ehrlichia strain diversity. Vet Parasitol 2007;143(3–4):337–46. . [DOI] [PubMed] [Google Scholar]
- 35.Shkap V, de Vos AJ, Zweygarth E, Jongejan F. Attenuated vaccines for tropical theileriosis, babesiosis and heartwater: the continuing necessity. Trends parasitol 2007;23(9):420–6. 10.1016/j.pt.2007.07.003 . [DOI] [PubMed] [Google Scholar]
- 36.Levin ML, Coble DJ, Ross DE. Reinfection with Anaplasma phagocytophilum in BALB/c mice and cross-protection between two sympatric isolates. Infect Immun 2004;72(8):4723–30. 10.1128/IAI.72.8.4723-4730.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuen S, Bergstrom K, Petrovec M, Van de Pol I, Schouls LM. Differences in clinical manifestations and hematological and serological responses after experimental infection with genetic variants of Anaplasma phagocytophilum in sheep. Clin Diag Lab Immunol 2003;10(4):692–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breitschwerdt EB, Hegarty BC, Hancock SI. Doxycycline hyclate treatment of experimental canine ehrlichiosis followed by challenge inoculation with two Ehrlichia canis strains. Antimicrob Agents Chemother. 1998;42(2):362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buhles WC Jr., Huxsoll DL, Ristic M. Tropical canine pancytopenia: Clinical, hematologic, and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation. J Infect Dis 1974;130(4):357–67. . [DOI] [PubMed] [Google Scholar]
- 40.Khader SA, Gopal R. IL-17 in protective immunity to intracellular pathogens. Virulence. 2010;1(5):423–7. 10.4161/viru.1.5.12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamoto Yoshida Y, Umemura M, Yahagi A, O'Brien RL, Ikuta K, Kishihara K, et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol 2010;184(8):4414–22. 10.4049/jimmunol.0903332 . [DOI] [PubMed] [Google Scholar]
- 42.Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med 2010;207(8):1609–16. 10.1084/jem.20100265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrew DW, Cochrane M, Schripsema JH, Ramsey KH, Dando SJ, O'Meara CP, et al. The duration of Chlamydia muridarum genital tract infection and associated chronic pathological changes are reduced in IL-17 knockout mice but protection is not increased further by immunization. PloS One 2013;8(9):e76664 10.1371/journal.pone.0076664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Meara CP, Armitage CW, Harvie MC, Andrew DW, Timms P, Lycke NY, et al. Immunity against a Chlamydia infection and disease may be determined by a balance of IL-17 signaling. Immunol Cell Biol 2014;92(3):287–97. 10.1038/icb.2013.92 . [DOI] [PubMed] [Google Scholar]
- 45.Ismail N, Walker DH, Ghose P, Tang YW. Immune mediators of protective and pathogenic immune responses in patients with mild and fatal human monocytotropic ehrlichiosis. BMC immunol. 2012;13:26 10.1186/1471-2172-13-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.