Abstract
Recent studies suggest that exposure to perfluorinated alkylate substances (PFASs) may induce immunosuppression in humans and animal models. In this exploratory study, twelve healthy adult volunteers were recruited. With each subject, serum-PFAS concentrations were measured and their antibody responses prospectively followed for 30 days after a booster vaccination with diphtheria and tetanus. The results indicated that serum-PFAS concentrations were positively correlated, and positively associated, with age and male sex. The specific antibody concentrations in serum were increased from Day 4 to Day 10 post-booster, after which a constant concentration was reached. Serum PFAS concentrations showed significant negative associations with the rate of increase in the antibody responses. Interestingly, this effect was particularly strong for the longer-chain PFASs. All significant associations remained significant after adjustment for sex and age. Although the study involved a small number of subjects, these findings of a PFAS-associated reduction of the early humoral immune response to booster vaccination in healthy adults supported previous findings of PFAS immunosuppression in larger cohorts. Furthermore, the results suggested to us that cellular mechanisms right after antigen exposure should be investigated more closely to identify possible mechanisms of immunosuppression from PFAS.
Keywords: Antibodies, immunotoxicity, perfluorinated alkylate substances, prospective study, vaccinations
Introduction
Perfluorinated alkylate substances (PFASs) are widespread environmental chemicals that are immunosuppressive in experimental animal models (DeWitt et al., 2012). In humans, exposure to the most common PFASs is associated with decreased responses to childhood immunizations (Grandjean et al., 2012) and to increased incidence of common infections (Granum et al., 2013). In vitro studies using human leukocytes support the causality of those associations (Corsini et al., 2012).
The occurrence of diminished responses to T-cell-dependent vaccinations in children, along with an inverse association between antibody concentration at 5-years-of-age and prenatal PFAS exposure (Grandjean et al., 2012), suggested to us that the effect may be due to reprogramming of early immune system development. However, prenatal exposure to PFAS in mice does not seem to cause more serious effects than in adult mice (Keil et al., 2008). Furthermore, in children examined after a booster vaccination at 5-years-of-age, the current exposure level was inversely associated with the estimated peak of their antibody responses (Grandjean et al., 2012). In addition, vaccination against Influenza Type B of adults in Ohio and West Virginia exposed to perfluorooctanoic acid (PFOA) in their drinking water resulted in hosts with significantly lower levels of specific antibodies associated with the highest levels of exposures (Looker et al., 2014). Thus, while developmental immunotoxic effects from exposure are a key concern - as they may have long-term consequences (Dietert, 2009) - toxicity associated with PFAS may also occur as a result of current/ongoing exposures, independent of host age.
Therefore, to assess the possible influence of current exposure to PFASs on vaccine responses, an exploratory vaccine intervention study was carried out wherein adults were boosted with tetanus and diphtheria toxoids, and their antibody responses were followed closely during a subsequent one-month period.
Materials and Methods
Subjects
Twelve self-reported healthy volunteers who did not have a history of tetanus-diphtheria booster vaccination in the past 5 years were recruited from among the staff at Copenhagen University Hospital Rigshospitalet. Written informed consent was obtained from all participants. The Ethics Review Committee serving Copenhagen, Denmark approved this protocol (#H-4-2012-049).
Exposure measurements
Past exposures to PFASs were assessed based on analyses of serum obtained from each participant 10 days post-vaccination. Analyses of blinded samples for PFAS concentrations were carried out by on-line solid-phase extraction and analysis using high-pressure liquid chromatography with tandem mass spectrometry (Haug et al., 2009).
Antibody measurements
Vaccination was performed using DiTeBooster (Statens Serum Institut, Copenhagen, Denmark). A pre-vaccination blood sample was collected at the time of vaccination; post-vaccination samples were collected 2, 4, 7, 10, 14 and 30 days later. Serum was prepared from each sample and stored at −80°C until analyzed. Serum concentrations of antibodies against the tetanus toxoid were measured using a Statens Serum Institut enzyme-linked immunosorbent assay (Copenhagen). In contrast, antibodies against diphtheria toxoid were measured using a standard Vero cell-based neutralization assay (Heilmann et al., 2010), using 2-fold dilutions of each serum sample.
Statistical analyses
Concentrations of both antibodies and PFASs were log-transformed to avoid right-skewed distributions in the statistical calculations. The development of antibody concentrations after the vaccination was illustrated with a smooth curve using natural cubic splines. To estimate how PFAS exposures may affect the trajectories, the present study used a model assuming a constant concentration level until Day 4 followed by a linear increase between Days 4 and 10 (on the log-scale), after which the concentration was again assumed to be constant. The PFAS concentration was allowed to affect the intercept of this curve and, most importantly, the linear slope from Day 4 to Day 10. If one lets Yij denote the antibody concentration in subject i at the j’th measurement, the model is given by the regression equation:
where tj is the number of days since vaccination and g(t) = t - 4 if 4 ≤ t ≤ 10. If t < 4 then g(t) = 0 and if t > 10 then g(t) = 6. Thus, the model assumes that between Day 4 and 10, the antibody concentration level was increased by a certain factor every day. These analyses estimated how much this factor is changed (in percent) when the PFAS concentration is doubled.
To adjust for effects of sex and age, these variables were included in the same way as PFAS in a second model. Thus, sex and age were allowed to have a linear effect on the intercept and an effect on the slope from Day 4 to Day 10. Error terms εij in the same subject were assumed to have an autoregressive covariance structure, thereby allowing measurement in the same subject to be correlated such that the correlation was increasing the closer in time the measurements were. Confidence intervals and p-values were based on the robust sandwich variance estimator that was correct even if the covariance of the residuals had been misspecified. All analyses were performed using SAS 9.3 software (SAS Institute Inc., Cary, NC).
Results
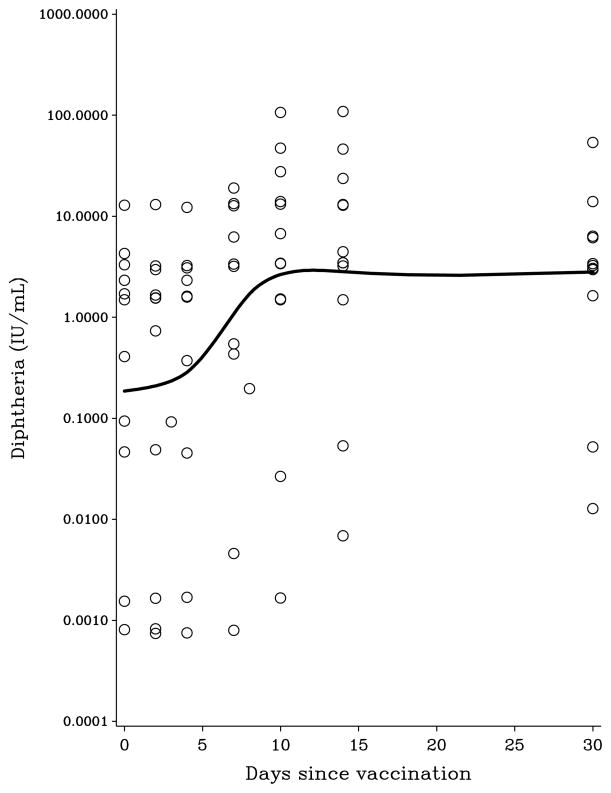
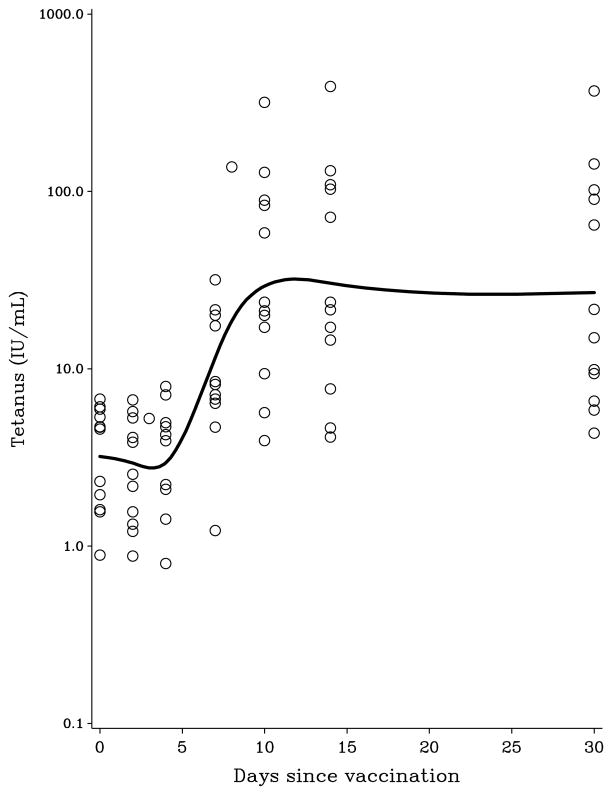
The characteristics of participating study subjects are presented in Table 1. Log-transformed PFAS concentrations showed positive correlations with Pearson coefficients from 0.07 (PFHxS and PFDoDA) to 0.98 (PFUnDA and PFDoDA). Age was significantly associated with serum concentrations of PFAS, although not for the two compounds with the longest carbon chains. Men showed higher serum concentrations of PFHxS, PFOA, PFOS, and PFNA. All subjects had been appropriately vaccinated with diphtheria and tetanus toxoids during childhood, but not boosted within the last 5 years. The serum antibody concentrations remained at a constant concentration the first few days and began to increase from Day 4. The log-transformed antibody responses suggest a linear increase from Day 4 to Day 10 post-vaccination, where a constant concentration was reached (Figure 1). Sex and age did not show a clear pattern of associations with antibody concentrations.
Table 1.
Characteristics of healthy study participants (n = 12).
| Characteristics | Result |
|---|---|
| Age in years, median (range) | 37.9 (23.2–65.8) |
| Men, N (%) | 6 (50) |
| PFAS concentration, median (IQR), ng/ml | |
| PFHxS | 0.37 (0.27–0.70) |
| PFHpA | 0.12 (0.094–0.14) |
| PFOS | 9.52 (5.38–14.3) |
| PFOA | 1.69 (1.30–2.79) |
| PFNA | 0.66 (0.46–0.80) |
| PFDA | 0.30 (0.20–0.32) |
| PFUnDA | 0.21 (0.18–0.27) |
| PFDoDA | 0.039 (0.035–0.048) |
| Antibody concentration, median (IQR), IU/ml | |
| Diphtheria, pre-vaccination | 1.00 (0.038–2.53) |
| Tetanus, pre-vaccination | 4.03 (1.95–5.63) |
Figure 1.
Time-dependent change in serum antibody concentrations against (A) diphtheria and (B) tetanus after vaccination, as modeled by a cubic spline function.
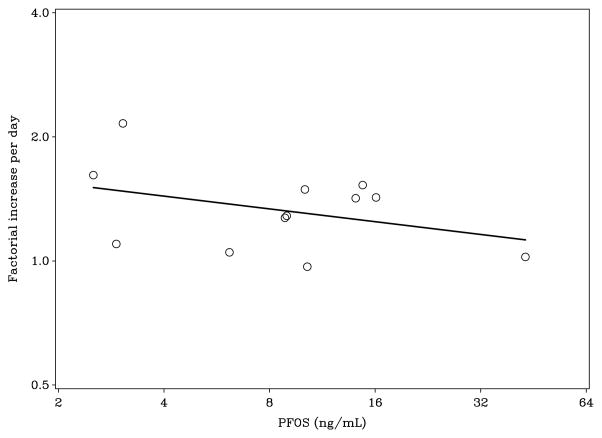
Table 2 shows the associations between serum concentrations of major PFASs and the steepness of the two antibody slopes between Days 4 and 10. The majority of PFASs negatively affected the increase in antibody responses towards vaccination; several of the associations were statistically significant, despite the small number of participants. Thus, at a doubling of PFOS exposure, the relative increase in the diphtheria antibody concentration was decreased by an average of ≈ 12%, an outcome that was statistically significant (Figure 2). Interestingly, this immunotoxic effect was especially significant for the longer-chain PFASs. Adjustment for age and sex tended to increase the apparent impact of PFAS exposure, and all significant associations remained significant.
Table 2.
Percentage change and 95% confidence interval (95% CI) in exponential change of antibody concentrations between Days 4 and 10 post-vaccination associated with a doubling in serum concentrations of different PFASs.
| Antibody/Exposure | Change (%) | 95% CI | p-value |
|---|---|---|---|
| Diphtheria | |||
| PFHxS | −13.31 | 0.29 to −25.07 | 0.055 |
| PFHpA | 6.52 | 57.7 to −28.04 | 0.750 |
| PFOS | −11.90 | −0.33 to −21.92 | 0.044 |
| PFOA | −8.22 | 6.44 to −20.85 | 0.250 |
| PFNA | −17.90 | −6.39 to −27.99 | 0.004 |
| PFDA | −18.18 | −5.00 to −29.52 | 0.009 |
| PFUnDA | −12.11 | −0.90 to −22.06 | 0.036 |
| PFDoDA | −15.64 | −0.98 to −28.14 | 0.038 |
| Tetanus | |||
| PFHxS | −4.35 | 6.04 to −13.72 | 0.390 |
| PFHpA | 15.40 | 49,9 to −11.16 | 0.280 |
| PFOS | −3.59 | 5.51 to −11.91 | 0.420 |
| PFOA | 0.23 | 12.1 to −10.40 | 0.970 |
| PFNA | −5.96 | 4.55 to −15.41 | 0.250 |
| PFDA | −8.31 | 2.66 to −18.10 | 0.130 |
| PFUnDA | −7.88 | −0.42 to −14.79 | 0.039 |
| PFDoDA | −10.78 | −0.64 to −19.90 | 0.038 |
Results are unadjusted, but a model adjusted for sex and age showed similar results.
Figure 2.
Relative change in diphtheria antibody concentration between Days 4 and 10 after booster vaccination, as a function of the PFOS concentration (p = 0.044).
Discussion
As tetanus and diphtheria vaccines have been available for many decades, few recent studies report details on the antibody response to booster vaccinations. However, the responses noted here between Days 4 and 10 after the booster vaccination were in accordance with the expected antibody production lag phase. A wide variability in individual responses to vaccinations is also well known. However, apart from immunodeficiency syndromes and effects due to chemotherapy, little is known about the reasons for this variability in the healthy background population. Although in a limited number of subjects, the present study suggested that exposure to environmental chemicals such as PFAS could cause an immunodepression that may explain some of this variability. These results are consistent with data obtained in larger cohorts (Grandjean et al., 2012, Looker et al., 2014).
The exposures documented in the present study were quite similar to those occurring in other Western countries (Kato et al., 2011). The associations between PFAS exposure and a decrease in the early humoral immune response suggested to us that cellular mechanisms early after vaccination should be investigated more closely to extend the current knowledge of the immunosuppression associated with PFAS. In the current study, the strongest effects were seen in regard to the diphtheria response. This finding was in accordance with our previous prospective study in children (Grandjean et al., 2012). Tetanus toxoid is generally a more immunogenic antigen and may be less sensitive to slight decreases in T- or B-lymphocyte functions.
In regard to the PFASs, our previous work focused on major substances, especially PFHxS, PFOA, and PFOS. Due to inter-correlations between these three PFASs, it was not possible to ascribe any of the immunotoxicity observed to any specific PFAS. Although the present study showed a significant effect from PFOS, the longer-chain PFASs were also associated with significant decreases in the host immune response, and they seemed also to affect tetanus vaccination-related endpoints. While the present study included only 12 subjects and low p-values were obtained due to inclusion of all observations from each subject in the calculations, important outcomes were nevertheless noted. However, the small size of the study group suggests that these results - at this point - should be considered preliminary only.
Although PFASs came into production more than 60 years ago (Grandjean and Clapp, 2014), there is still very little toxicological information available on these substances, and virtually none on the long-chain PFASs. The present study suggests that the immunotoxicity of these environmental chemicals deserves more attention, and that cellular mechanisms operating immediately after antigen exposure should be explored to identify possible novel PFAS modes of action.
Acknowledgments
This study was supported by the National Institute of Environmental Health Sciences, National Institutes of Health (ES012199).
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content of this manuscript.
References
- Corsini E, Sangiovanni E, Avogadro A, et al. In vitro characterization of the immuno-toxic potential of several perfluorinated compounds (PFCs) Toxicol Appl Pharmacol. 2012;258:248–255. doi: 10.1016/j.taap.2011.11.004. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol Pathol. 2012;40:300–311. doi: 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- Dietert RR. Developmental immunotoxicology: Focus on health risk. Chem Res Toxicol. 2009;22:17–23. doi: 10.1021/tx800198m. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz-Jorgensen E, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–397. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Clapp R. Changing interpretation of human health risks from perfluorinated compounds. Public Health Rep. 2014;129:482–485. doi: 10.1177/003335491412900605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug LS, Namork E, et al. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol. 2013;10:373–379. doi: 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr. 2009;1216:385–393. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, et al. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol. 2011;45:8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Keil DE, Mehlmann T, Butterworth L, Peden-Adams MM. Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol Sci. 2008;103:77–85. doi: 10.1093/toxsci/kfn015. [DOI] [PubMed] [Google Scholar]
- Looker C, Luster MI, Calafat AM, et al. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol Sci. 2014;138:76–88. doi: 10.1093/toxsci/kft269. [DOI] [PMC free article] [PubMed] [Google Scholar]