Structured abstract
Objective
Our goal was to investigate, in a large population of women with DCIS and long follow-up, the relationship between margin width and recurrence, controlling for other characteristics.
Summary Background Data
While DCIS has minimal mortality, recurrence rates after breast-conserving surgery (BCS) are significant, and half are invasive. Positive margins are associated with increased risk of local recurrence, but there is no consensus regarding optimal negative margin width.
Methods
We retrospectively reviewed a prospective database of DCIS patients undergoing BCS from 1978–2010. Univariate and Cox proportional hazard models were used to investigate the association between margin width and recurrence.
Results
2996 cases were identified, of which 363 recurred. Median follow-up for women without recurrence was 75mo (range 0–30years); 732 were followed for ≥10yrs. Controlling for age, family history, presentation, nuclear grade, number of excisions, radiotherapy (RT), endocrine therapy, and year of surgery, margin width was significantly associated with recurrence in the entire population. Larger negative margins were associated with a lower hazard ratio compared to positive margins. An interaction between RT and margin width was significant (p<0.03); the association of recurrence with margin width was significant in those without RT (p<0.0001), but not in those with RT (p=0.95).
Conclusions
In women not receiving RT, wider margins are significantly associated with a lower rate of LR. Obtaining wider negative margins may be important in reducing the risk of recurrence in women who choose not to undergo RT, and may not be necessary in those that receive RT.
Introduction
Ductal carcinoma in situ (DCIS) now accounts for up to 21% of all breast cancers diagnosed in the United States each year.1 Management options for DCIS range from mastectomy, to breast-conserving surgery (BCS) with adjuvant radiation therapy (RT), to BCS alone. Regardless of the type of local therapy, mortality due to DCIS is uncommon. However, local recurrence rates after BCS alone are high, ranging from 25 to 35% at 13–17 years of follow-up, and approximately half of all recurrences are invasive.2–6 RT reduces the recurrence rate by about 50%, but does not reduce mortality2–6 and can be associated with increased rates of cardiovascular disease and rare malignancies.7–11 Tamoxifen also reduces recurrences among women whose DCIS expresses estrogen receptors, but like RT, does not reduce mortality, and can result in elevated risk of uterine cancer and venous thromboembolic events.3, 12–14
While no subset of patients undergoing BCS for DCIS has been identified for which adjuvant RT does not reduce recurrence risk, there is interest in identifying those at lower risk of recurrence for whom adjuvant RT would result in a small absolute benefit. Numerous risk factors for recurrence have been identified, including age,4–6, 15, 16 family history,17–19 clinical presentation,4, 5, 20 number of excisions,16 nuclear grade and necrosis,21–25 year of surgery,16, 26 and margin status.4–6, 16, 20, 24, 27–29 Three prospective studies have successfully combined multiple factors to prospectively identify women at relatively low risk for recurrence after excision alone.30–32 A nomogram that combines 10 different patient and pathological variables and adjuvant treatments to estimate risk of recurrence after BCS for DCIS allows identification of those at relatively low risk of recurrence16 and has been validated in independent populations.33–35
However, of the various risk factors for recurrence of DCIS after BCS, the only characteristic that is potentially modifiable by the clinician is width of margin. Although multiple studies have shown that positive or close margins are associated with a higher risk of recurrence after BCS for DCIS, there is no consensus as to what constitutes an optimal negative margin width. We undertook this study to evaluate the association of margin width and local recurrence in women treated with and without RT over a 30-year time period at a single institution.
Methods
After obtaining approval from the Institutional Review Board, a prospectively maintained database was used to identify all patients undergoing definitive BCS for DCIS from 1978–2010 at Memorial Sloan Kettering Cancer Center. Patients with synchronous (n=30) or metachronous (n=29) bilateral DCIS were included once for each breast.
Clinical, pathological, and treatment variables included were age at diagnosis, menopausal status (pre- or perimenopausal vs. postmenopausal), family history (at least one first or second degree family member with breast cancer), presentation (clinically palpable mass, nipple discharge or Paget’s disease vs. radiologic), nuclear grade (categorized as non-high grade [including borderline cases focally reaching or approaching low grade DCIS, low grade, and intermediate grade] or high grade), number of excisions, margin width (categorized as positive [tumor on ink], close [≤2mm], >2–10mm [includes cases with margins described as widely clear], or >10mm [includes patients with no residual disease in the re-excision specimen]), RT, endocrine therapy, and date of definitive surgery. Number of excisions was included because it is likely correlated with extent DCIS, and was previously shown to be statistically significantly associated with recurrence risk on multivariable analysis.16 Post-excision mammogram was routinely performed for cases presenting as mammographic calcifications.
The outcome of interest was any recurrence, defined as ipsilateral breast recurrence of DCIS or invasive cancer, ipsilateral axillary nodal recurrence without ipsilateral breast recurrence, or in one case, distant recurrence consistent with a breast primary carcinoma but without the presence of any ipsilateral recurrence or contralateral diagnosis of breast carcinoma. Time to event was defined as the interval between definitive surgery and date of first recurrence. 10-year Kaplan-Meier recurrence estimates were calculated by margin width for the entire cohort as well as for the subsets with and without RT, and log rank tests were used. A multivariable Cox model was created to evaluate the association of margin width with recurrence while controlling for other variables. Interaction between RT and margin width was assessed, and separate models were created for the subsets with and without RT. Proportionality of hazards was checked for all Cox models and found to be appropriate. Statistical analysis was performed using SAS 9.2 (SAS Institute, Inc., Cary, NC).
Results
From 1978 to 2010, 2996 cases were identified; the characteristics of the entire population and the cohorts without and with RT are presented in Table 1. Median age of entire population was 57 years (range 20–92). For those undergoing RT median (range) age was 55 years (27–85) and for those without RT 59 years (20–92). Recurrence occurred in 363, of which 159 were invasive (147 ipsilateral invasive breast recurrences, 2 ipsilateral axillary recurrences, 10 simultaneous breast and axillary recurrences), 192 were DCIS, 11 were unknown type of breast recurrence, and 1 was distant metastasis without locoregional recurrence. 18 developed distant disease, of which 11 have died. Sixteen had ipsilateral invasive breast recurrence and 1 had ipsilateral DCIS breast recurrence before development of distant metastases.
Table 1.
Clinical and pathologic characteristics of entire population and by receipt of radiation
| Characteristic | Entire population (N=2996)
|
No radiation (N=1374)a
|
Radiation (N=1588)a
|
||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Age | ≤50 years | 845 | 28.2% | 350 | 25.5% | 486 | 30.6% |
| >50 years | 2151 | 71.8% | 1024 | 74.5% | 1102 | 69.4% | |
|
| |||||||
| Menopausal status | Pre/peri | 1038 | 34.6% | 442 | 32.1% | 586 | 36.9% |
| Post | 1946 | 65.0% | 924 | 67.2% | 999 | 62.9% | |
| Unknown | 12 | 0.4% | 8 | 0.6% | 3 | 0.2% | |
|
| |||||||
| Family history | No | 1816 | 60.6% | 825 | 60.0% | 969 | 61.0% |
| Yes | 1136 | 37.9% | 515 | 37.5% | 610 | 38.4% | |
| Unknown | 44 | 1.5% | 34 | 2.5% | 9 | 0.6% | |
|
| |||||||
| Presentation | Clinical | 386 | 12.9% | 195 | 14.2% | 178 | 11.2% |
| Radiologic | 2606 | 87.0% | 1176 | 85.6% | 1409 | 88.7% | |
| Unknown | 4 | 0.1% | 3 | 0.2% | 1 | <0.1% | |
|
| |||||||
| Nuclear grade | Low/intermediate | 1787 | 59.6% | 975 | 71.0% | 799 | 50.3% |
| High | 994 | 33.2% | 269 | 19.6% | 716 | 45.1% | |
| Unknown | 215 | 7.2% | 130 | 9.5% | 73 | 4.6% | |
|
| |||||||
| Number of excisions | 1 | 1493 | 49.8% | 800 | 58.2% | 676 | 42.6% |
| 2 | 1282 | 42.8% | 525 | 38.2% | 745 | 46.9% | |
| ≥3 | 217 | 7.2% | 47 | 3.4% | 165 | 10.4% | |
| Unknown | 4 | 0.1% | 2 | 0.1% | 2 | 0.1% | |
|
| |||||||
| Margins | Positive | 104 | 3.5% | 43 | 3.1% | 59 | 3.7% |
| Close (≤2mm) | 449 | 15.0% | 170 | 12.4% | 271 | 17.1% | |
| >2–10mm | 888 | 29.6% | 384 | 27.9% | 498 | 31.4% | |
| >10mm | 1347 | 45.0% | 669 | 48.7% | 672 | 42.3% | |
| Unknown | 208 | 6.9% | 108 | 7.9% | 88 | 5.5% | |
|
| |||||||
| Radiation | No | 1374 | 45.9% | 1374 | 100% | 0 | 0% |
| Yes | 1588 | 53.0% | 0 | 0% | 1588 | 100% | |
| Unknown | 34 | 1.1% | 0 | 0% | 0 | 0% | |
|
| |||||||
| Endocrine therapy | No | 2321 | 77.5% | 1152 | 83.8% | 1163 | 73.2% |
| Yes | 628 | 21.0% | 210 | 15.3% | 417 | 26.3% | |
| Unknown | 47 | 1.6% | 12 | 0.9% | 8 | 0.5% | |
|
| |||||||
| Year of surgery | 1978–2000 | 1067 | 35.6% | 592 | 43.1% | 454 | 28.6% |
| 2001–2010 | 1929 | 64.4% | 782 | 56.9% | 1134 | 71.4% | |
Numbers do not sum to 2996 due to unknown receipt of radiation in 34 women.
Median follow-up for those without recurrence was 75 months (range 0–356 months). 732 women had at least 10 years of follow-up; 615 of these had complete data. Overall, 336 women died; 284 (9.5% of all women) died without having any recurrence.
Margin width
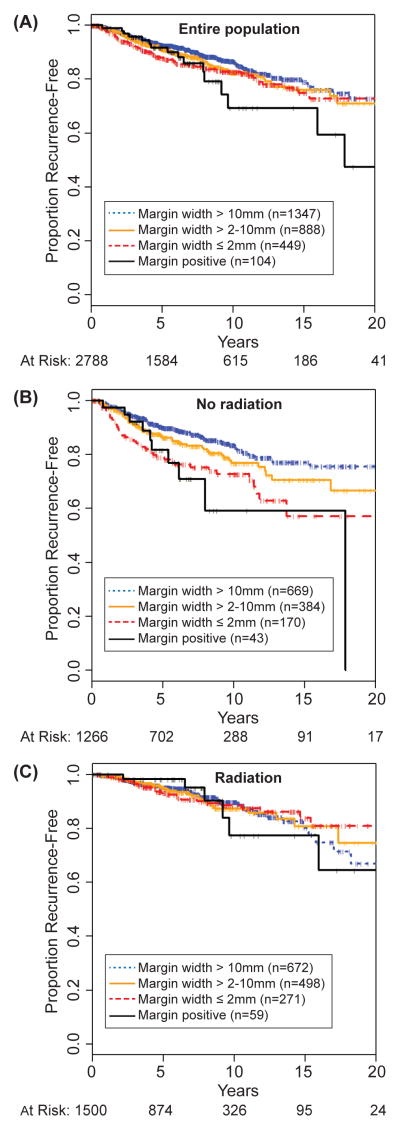
Crude recurrence rates by margin width are shown in Table 2. Figure 1A shows Kaplan Meier recurrence-free survival by margin width for entire population, and 10-year recurrence rates are shown in Table 3. A trend toward lower risk of recurrence is associated with wider margins (p=0.087). For women with positive margins, the 10-year rate of recurrence was 31%, as compared to 13% for women with >10mm margins.
Table 2.
Crude recurrences by margin width and use of radiation.
| Margin width | Entire population (N=2996)
|
No radiation (N=1374)a
|
Radiation (N=1588)a
|
|||
|---|---|---|---|---|---|---|
| Events/ N | % | Events/ N | % | Events/ N | % | |
| Positive | 16/104 | 15.4% | 10/43 | 23.3% | 6/59 | 10.2% |
|
| ||||||
| Close (≤2mm) | 69/449 | 15.4% | 42/170 | 24.7% | 27/271 | 10.0% |
|
| ||||||
| >2–10mm | 98/888 | 11.0% | 63/384 | 16.4% | 35/498 | 7.0% |
|
| ||||||
| >10mm | 145/1347 | 10.8% | 87/669 | 13.0% | 58/672 | 8.6% |
|
| ||||||
| Unknown | 35/208 | 16.8% | 21/108 | 19.4% | 14/88 | 15.9% |
Numbers do not sum to 2996 due to unknown receipt of radiation in 34 women.
Figure 1.
Proportion recurrence-free, by margin width for (A) entire population, (B) no-radiation cohort, and (C) radiation cohort.
Table 3.
Ten-year recurrence rates by margin width and by receipt of radiation.
| Margin width | Entire population with known margin width (N=2788)a | No radiation (N=1266)b | Radiation (N=1500)b | HRc for radiation | Pd for radiation | Adjusted HR for radiatione | Pf for radiation |
|---|---|---|---|---|---|---|---|
| Positive | 31% | 41% | 23% | 0.22 | 0.0026 | 0.10 | 0.0036 |
| Close (≤2mm) | 17% | 27% | 12% | 0.32 | <0.0001 | 0.29 | <0.0001 |
| >2–10mm | 18% | 23% | 13% | 0.46 | 0.0002 | 0.42 | 0.0006 |
| >10mm | 13% | 16% | 10% | 0.66 | 0.0132 | 0.54 | 0.0013 |
| Pg for margin width | P=0.087 | P=0.0003 | P=0.99 |
In entire population of 2996, 208 patients had unknown margin width
Numbers do not sum to 2788 because 22 patients with known margin width had unknown radiation status
HR, hazard ratio of radiation vs no radiation.
Log rank test for effect of radiation
Hazard ratio of radiation vs no radiation, controlling for age, family history, presentation, nuclear grade, number of excisions, endocrine therapy, year of surgery
P value for effect of radiation in Cox model, controlling for age, family history, presentation, nuclear grade, number of excisions, endocrine therapy, year of surgery
P value of log rank test for difference in recurrence by margin width
Margin width and RT
We examined the effect of margin width on recurrence, stratified by use of RT; crude recurrence rates are shown in Table 2. Figures 1B and 1C show Kaplan Meier recurrence-free survival by margin width for those not receiving and those receiving RT; 10-year recurrence rates are shown in Table 3. Among those not receiving RT, the association of wider margins and lower recurrence was highly significant (p=0.0003), while the association was not significant among those that received RT (p=0.99).
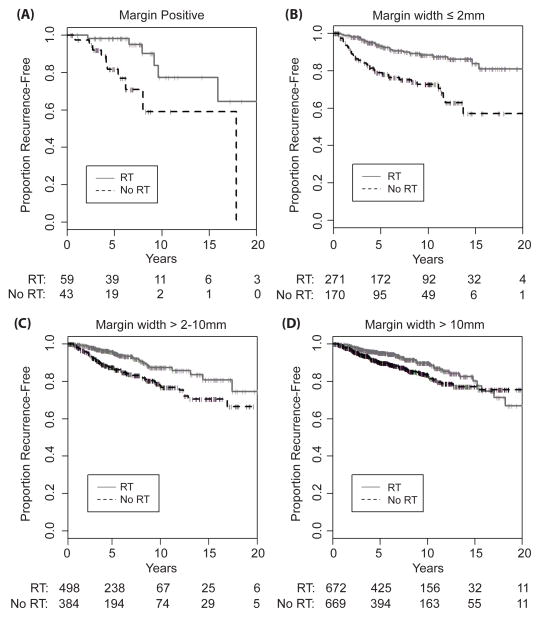
For each margin width, the use of RT was associated with a statistically significant reduction in recurrence, with greater proportional and absolute risk reduction being associated with positive or close margins (Table 3, Figure 2). This association remained significant after adjusting for 7 other variables.
Figure 2.
Proportion recurrence-free by use of radiation, for (A) positive margins, (B) margin width ≤ 2mm, (C) margin width > 2–10mm, (D) margin width > 10mm.
Multivariable analyses
Because numerous other factors are associated with recurrence, a multivariable model was built to control for factors which could affect the relationship of margin width and recurrence. Nuclear grade was not significant on either univariate (p=0.96) or multivariable analysis (p=0.2). Because its inclusion did not alter the results and because nuclear grade was unknown in 215 cases, it was not included in the final model. After controlling for age, family history, presentation, number of excisions, RT, endocrine therapy, and year of surgery (Table 4), wider margins were associated with lower risk of recurrence (p=0.0003), with progressively lower hazard ratios associated with wider margins (0.78, 0.70, and 0.44 for negative margin widths of ≤2mm, >2–10mm, and >10mm, respectively) as compared to positive margins.
Table 4.
Multivariable Cox regression analysis of the association of margin width and recurrence in 2708* women with DCIS treated with breast-conserving surgery, controlling for other factors
| Variable | N | Events | HR | P | |
|---|---|---|---|---|---|
| Age | Per year | 0.978 | <0.0001 | ||
|
| |||||
| Family history | No | 1662 | 187 | 1 | 0.03 |
| Yes | 1046 | 138 | 1.28 | ||
|
| |||||
| Presentation | Radiologic | 2394 | 264 | 1 | 0.03 |
| Clinical | 314 | 61 | 1.38 | ||
|
| |||||
| Number of excisions | 1 | 1300 | 138 | 1 | 0.006 |
| 2 | 1204 | 155 | 1.27 | ||
| ≥3 | 204 | 32 | 1.96 | ||
|
| |||||
| Radiation | No | 1225 | 201 | 1 | <0.0001 |
| Yes | 1483 | 124 | 0.44 | ||
|
| |||||
| Endocrine therapy | No | 2110 | 285 | 1 | <0.0001 |
| Yes | 598 | 40 | 0.48 | ||
|
| |||||
| Year of surgery | 1978–2000 | 826 | 188 | 1.47 | 0.002 |
| 2001–2010 | 1882 | 137 | 1 | ||
|
| |||||
| Margin width | Positive | 98 | 16 | 1 | 0.0003 |
| Close (≤2mm) | 435 | 69 | 0.78 | ||
| >2–10mm | 861 | 97 | 0.70 | ||
| >10mm | 1314 | 143 | 0.44 | ||
In entire population of 2996, 288 cases had at least one missing data point, resulting in population for multivariable analysis of 2708.
HR, hazard ratio
Because of the apparent differential effect of margin width by RT (Figures 1 & 2, Table 3), an interaction term between margin width and RT was added to the multivariable model and was found to be significant, p<0.03. To explore this relationship further, a multivariable model was fit to the subsets of patients not receiving and receiving RT (Table 5); this confirmed that there is a differential effect of margin width by RT. In those not receiving RT, the relationship between wider margins and lower rates of recurrence was even stronger (HR=0.75, 0.58, 0.31 for negative margin widths of ≤2mm, >2–10mm, >10mm, respectively, as compared to positive, p<0.0001), while for those receiving RT, there was no clear relationship (p=0.95).
Table 5.
Multivariable Cox regression analysis of recurrence, stratified by use of radiation
| Variable | No radiation (N=1225)*
|
Radiation (N=1483)*
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Events | HR | P | N | Events | HR | P | ||
| Age at surgery | Per year | 0.987 | 0.02 | 0.956 | <0.0001 | ||||
|
| |||||||||
| Family history | No | 753 | 114 | 1 | 0.05 | 909 | 73 | 1 | 0.23 |
| Yes | 472 | 87 | 1.32 | 574 | 51 | 1.25 | |||
|
| |||||||||
| Presentation | Radiologic | 1068 | 162 | 1 | 0.06 | 1326 | 102 | 1 | 0.43 |
| Clinical | 157 | 39 | 1.4 | 157 | 22 | 1.22 | |||
|
| |||||||||
| Number of excisions | 1 | 688 | 100 | 1 | 0.0003 | 612 | 38 | 1 | 0.66 |
| 2 | 492 | 85 | 1.37 | 712 | 70 | 1.18 | |||
| ≥3 | 45 | 16 | 3.18 | 159 | 16 | 1.30 | |||
|
| |||||||||
| Endocrine therapy | No | 1026 | 180 | 1 | 0.003 | 1084 | 105 | 1 | 0.002 |
| Yes | 199 | 21 | 0.50 | 399 | 19 | 0.46 | |||
|
| |||||||||
| Year of surgery | 1978–2000 | 459 | 123 | 1.60 | 0.003 | 367 | 65 | 1.18 | 0.44 |
| 2001–2010 | 766 | 78 | 1 | 1116 | 59 | 1 | |||
|
| |||||||||
| Margin width | Positive | 40 | 10 | 1 | <0.0001 | 58 | 6 | 1 | 0.95 |
| Close (≤2mm) | 167 | 42 | 0.75 | 268 | 27 | 0.95 | |||
| >2–10mm | 369 | 62 | 0.58 | 492 | 35 | 1.00 | |||
| >10mm | 649 | 87 | 0.31 | 665 | 56 | 0.88 | |||
In entire population of 2996, 288 cases had at least one missing data point, resulting in population for multivariable analysis of 2708.
HR, hazard ratio
To further explore various margin width thresholds among those receiving RT, we created multivariable models with margin width dichotomized into positive vs. tumor not on ink, ≤2mm vs. >2mm, and ≤10mm vs. >10mm, but found no significant difference (p=0.67, p=0.96, p=0.70, respectively) in risk of recurrence with any threshold.
Discussion
The overview of the four prospective randomized trials of RT for DCIS found that negative margins are associated with a lower risk of recurrence.2 However, because margin status was dichotomized as positive vs. negative4, 6, or within 1mm vs over 1mm,5, 24 the optimal negative margin width cannot be assessed in those studies. However, Pinder et al. examined a subset of 637 cases from the 1701 cases in the UK/ANZ trial for whom actual margin width was available.24 They found that the HR for risk of recurrence was halved in cases with ≥5mm margins as compared to those with <1mm margins (HR=0.46, p=0.03); they did not report the number that received RT or tamoxifen, nor did they stratify by adjuvant treatment.
Several retrospective analyses have been undertaken in an attempt to address the relationship of margin width and recurrence of DCIS. Silverstein et al. first included margin width as one of three predictors of local recurrence (along with tumor size and nuclear grade/necrosis classification) in his Van Nuys Prognostic Index.21 In a population of 333 women with a median follow-up of 79 months, larger margin widths were associated with lower risk of recurrence (p<0.04, margin widths were categorized as wide (≥10mm), intermediate (1–9mm), and close (<1mm)). Silverstein et al. later reported that among a population of 469 with a median follow-up of 81 months, 8-year recurrence rates for those not receiving RT were 3%, 20%, and 58% for those with wide, intermediate and close margins, respectively, as compared to 4%, 12%, and 30% for those receiving RT.27 They concluded that there was no significant benefit of RT in patients with wide margins.
Solin et al. reported that in a multivariable analysis of 1003 women with mammographically detected DCIS treated with BCS and RT, and median follow-up of 8.5 years, margin status and age were the only statistically significant factors associated with recurrence.36 Compared to negative margins, positive margins (tumor on ink) had a hazard ratio of 3.35 (p=0.00035) and close margins (defined as <2mm, ≤2mm, <2–3mm, or <3mm) had a hazard ratio of 1.9 (p=0.03).
In a retrospective study of 460 women treated with BCS without RT and referred to the British Columbia Cancer Agency from 1985–1999, Wai et al. reported that 10-year LR rates were lower with negative margins (9%) as compared to close (17%), positive (31%) or unknown (32%) margins (p<0.0001).37
A review of the role of margin status on recurrence in DCIS patients after BCS and RT included 7 publications for which a comparison between negative and close (variably defined as <1 to <5mm) margins could be made; the odds ratio for local recurrence with negative margins as compared to close was 0.59 (p<0.001).38 In the subset of studies for which a specific margin width could be determined, analysis showed that 2mm margins were associated with lower risk than <2mm (5.8% vs 10.4% local recurrences, OR=0.53, p<0.05), and associated with a non-significantly higher risk than 5mm margins (5.8% vs. 3.9% local recurrences, OR 1.51, p>0.05).
Wang et al. performed a network metaanalysis of the association of specific margin thresholds and recurrence for women with DCIS treated with or without RT after BCS.39 The authors used a variety of complex statistical methods, including both frequentist and Bayesian approaches, and their analyses showed that a negative margin threshold of 10mm was associated with a lower recurrence than a threshold of 2mm (p<0.001), regardless of use of RT. Because the analysis pooled many different studies, it could not adjust for the many other factors known to be associated with local recurrence.
Here, in this large cohort of well-characterized cases of DCIS treated with BCS, we have found a strong association of margin width and risk of recurrence in those not receiving RT, but not in those receiving RT. Our finding is unique because our population was large (n=2996) with substantial follow-up (615 with complete data were followed for at least 10 years), numerous patient, pathologic and treatment characteristics were known for each case, and the large size and long follow-up allowed the statistical power to control for numerous factors which are known to be associated with local recurrence. Furthermore, we were able to examine the association of margin width and local recurrence separately for those that did and did not receive RT, which revealed a differential effect.
Among women not receiving RT, the width of negative margin was strongly related to risk of recurrence, likely because a wider negative margin is associated with a lower volume of residual disease. However, among women receiving RT, there was no significant association. In an earlier report, we examined the 10-year recurrence rates of a subset (n=291) of the population in the current analysis. These women had DCIS treated with BCS from 1991–1995 and were followed for a median of 11 years.20 Most cases (93%) had pathology review by a breast pathologist. We found lower 10-year LR rates with ≥10mm margins (21%) as compared to 1–9mm margins (27%) or <1mm margins (42%) among those not receiving RT, but not among those receiving RT (13%, 12%, and 11%, respectively). The current much larger analysis confirms this finding of a differential effect of margin width depending on the use of RT.
This finding of a differential association of margin width and local recurrence, depending on the use of RT, demonstrates the complexity of understanding risk factors for recurrence for DCIS. In their most recent update of NSABP B-24, Wapnir et al. reported a differential association of margin status and local recurrence, depending on the use of tamoxifen.4 In women receiving both RT and tamoxifen, margin status (involved/uncertain vs. free) was not significantly associated with recurrence, whereas for those receiving RT alone, it was highly significant (HR 2.61, p<0.001 for invasive recurrence; HR 1.65, p=0.05 for DCIS). The interaction between use of tamoxifen and margin status was significant (p=0.04). The observation that the association of margin status or margin width with recurrence rate is affected by use of adjuvant therapy is consistent with the idea that margin status and margin width are predictors of risk of or volume of residual disease in the breast. In those that do not receive effective adjuvant treatment, margin width is highly correlated with risk of recurrence. In those that receive effective adjuvant treatment, it can eradicate the residual disease, thereby lessening the association with margin width.
A limitation of our series is that very few women had positive margins, as it is our standard practice to achieve clear margins. Most positive margins were at the dermis or the pectoralis fascia, rather than at a radial margin. Furthermore, cases with positive or close margins generally had very limited, focal disease at or near the inked margin. Together, these observations suggest that our patients with close or positive margins likely had a lower residual disease burden than some other series. This limitation may cause our reported recurrence rates for close and positive margins to underestimate recurrence rates for women with a greater volume of disease at or near the margin, as it is known that volume of disease is related to recurrence.20, 40
In contrast to the findings of Dunne et al.,38 among women receiving RT we could find no significant difference in recurrence by any categorization of margin width, including ≤2mm vs. >2mm. This is likely due to the limited amount of disease near the margin in our patients with close or positive margins. Similarly, in contrast to Wang et al.,39 we could find no significant difference in recurrence between margin widths of ≤10mm and >10mm among those receiving RT. This difference in findings may be due to our ability to control for numerous other factors in our multivariable model.
Conclusions
In a large, well-characterized population of women with DCIS, where numerous factors were controlled for, we have found that margin width is strongly associated with risk of recurrence for women undergoing BCS who do not receive RT. In contrast, we found no association among those that do receive RT, demonstrating a differential association of margin width and recurrence, depending on adjuvant treatment. These results support the conclusion that obtaining wider negative margins may be important in reducing the risk of recurrence in women who choose not to undergo RT, and may not be necessary in those that receive RT.
Acknowledgments
Sources of support: This study was funded in part by NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Correa C, McGale P, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wapnir IL, Dignam JJ, Fisher B, et al. Long-Term Outcomes of Invasive Ipsilateral Breast Tumor Recurrences After Lumpectomy in NSABP B-17 and B-24 Randomized Clinical Trials for DCIS. Journal of the National Cancer Institute. 2011;103:478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donker M, Litiere S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31:4054–4059. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 6.Warnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J Clin Oncol. 2014;32:3613–3618. doi: 10.1200/JCO.2014.56.2595. [DOI] [PubMed] [Google Scholar]
- 7.Prochazka M, Hall P, Gagliardi G, et al. Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: case-only design. J Clin Oncol. 2005;23:7467–7474. doi: 10.1200/JCO.2005.01.7335. [DOI] [PubMed] [Google Scholar]
- 8.Roychoudhuri R, Robinson D, Putcha V, et al. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer. 2007;7:9. doi: 10.1186/1471-2407-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darby SC, Ewertz M, McGale P, et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. New England Journal of Medicine. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 10.Henson KE, McGale P, Taylor C, et al. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108:179–182. doi: 10.1038/bjc.2012.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grantzau T, Mellemkjær L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: A national population based study under the Danish Breast Cancer Cooperative Group (DBCG) Radiotherapy and Oncology. 2013;106:42–49. doi: 10.1016/j.radonc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 14.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012;30:1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Zee KJ, Liberman L, Samli B, et al. Long term follow-up of women with ductal carcinoma in situ treated with breast-conserving surgery: the effect of age. Cancer. 1999;86:1757–1767. doi: 10.1002/(sici)1097-0142(19991101)86:9<1757::aid-cncr18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 2010;28:3762–3769. doi: 10.1200/JCO.2009.26.8847. [DOI] [PubMed] [Google Scholar]
- 17.McCormick B, Rosen PP, Kinne D, et al. Duct carcinoma in situ of the breast: an analysis of local control after conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 1991;21:289–292. doi: 10.1016/0360-3016(91)90773-w. [DOI] [PubMed] [Google Scholar]
- 18.Hiramatsu H, Bornstein BA, Recht A, et al. Local recurrence after conservative surgery and radiation therapy for ductal carcinoma in situ: Possible importance of family history. Cancer J Sci Am. 1995;1:55–61. [PubMed] [Google Scholar]
- 19.Szelei-Stevens KA, Kuske RR, Yantsos VA, et al. The influence of young age and positive family history of breast cancer on the prognosis of ductal carcinoma in situ treated by excision with or without radiation therapy or by mastectomy. Int J Radiat Oncol Biol Phys. 2000;48:943–949. doi: 10.1016/s0360-3016(00)00715-x. [DOI] [PubMed] [Google Scholar]
- 20.Rudloff U, Brogi E, Reiner AS, et al. The influence of margin width and volume of disease near margin on benefit of radiation therapy for women with DCIS treated with breast-conserving therapy. Ann Surg. 2010;251:583–591. doi: 10.1097/SLA.0b013e3181b5931e. [DOI] [PubMed] [Google Scholar]
- 21.Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77:2267–2274. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2267::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 22.Bijker N, Peterse JL, Duchateau L, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19:2263–2271. doi: 10.1200/JCO.2001.19.8.2263. [DOI] [PubMed] [Google Scholar]
- 23.Fisher ER, Land SR, Saad RS, et al. Pathologic variables predictive of breast events in patients with ductal carcinoma in situ. Am J Clin Pathol. 2007;128:86–91. doi: 10.1309/WH9LA543NR76Y29J. [DOI] [PubMed] [Google Scholar]
- 24.Pinder SE, Duggan C, Ellis IO, et al. A new pathological system for grading DCIS with improved prediction of local recurrence: results from the UKCCCR/ANZ DCIS trial. Br J Cancer. 2010;103:94–100. doi: 10.1038/sj.bjc.6605718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ringberg A, Nordgren H, Thorstensson S, et al. Histopathological risk factors for ipsilateral breast events after breast conserving treatment for ductal carcinoma in situ of the breast--results from the Swedish randomised trial. Eur J Cancer. 2007;43:291–298. doi: 10.1016/j.ejca.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Habel LA, Achacoso NS, Haque R, et al. Declining recurrence among ductal carcinoma in situ patients treated with breast-conserving surgery in the community setting. Breast Cancer Res. 2009;11:R85. doi: 10.1186/bcr2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverstein MJ, Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;340:1455–1461. doi: 10.1056/NEJM199905133401902. [DOI] [PubMed] [Google Scholar]
- 28.Neuschatz AC, DiPetrillo T, Safaii H, et al. Margin width as a determinant of local control with and without radiation therapy for ductal carcinoma in situ (DCIS) of the breast. International Journal of Cancer. 2001;96:97–104. doi: 10.1002/ijc.10357. [DOI] [PubMed] [Google Scholar]
- 29.Pilewskie M, Olcese C, Eaton A, et al. Perioperative breast MRI is not associated with lower locoregional recurrence rates in DCIS patients treated with or without radiation. Ann Surg Oncol. 2014;21:1552–1560. doi: 10.1245/s10434-013-3424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong JS, Chen YH, Gadd MA, et al. Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS) Breast Cancer Res Treat. 2014;143:343–350. doi: 10.1007/s10549-013-2813-6. [DOI] [PubMed] [Google Scholar]
- 32.McCormick B, Winter K, Hudis C, et al. RTOG 9804: A Prospective Randomized Trial for Good-Risk Ductal Carcinoma In Situ Comparing Radiotherapy With Observation. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.57.9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi M, Meric-Bernstam F, Kuerer HM, et al. Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol. 2012;30:600–607. doi: 10.1200/JCO.2011.36.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweldens C, Peeters S, van Limbergen E, et al. Local relapse after breast-conserving therapy for ductal carcinoma in situ: a European single-center experience and external validation of the Memorial Sloan-Kettering Cancer Center DCIS nomogram. Cancer J. 2014;20:1–7. doi: 10.1097/PPO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Li H, Tan PH, et al. Validation of a nomogram in the prediction of local recurrence risks after conserving surgery for Asian women with ductal carcinoma in situ of the breast. Clin Oncol (R Coll Radiol) 2014;26:684–691. doi: 10.1016/j.clon.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Solin LJ, Fourquet A, Vicini FA, et al. Long-term outcome after breast-conservation treatment with radiation for mammographically detected ductal carcinoma in situ of the breast. Cancer. 2005;103:1137–1146. doi: 10.1002/cncr.20886. [DOI] [PubMed] [Google Scholar]
- 37.Wai ES, Lesperance ML, Alexander CS, et al. Predictors of local recurrence in a population-based cohort of women with ductal carcinoma in situ treated with breast conserving surgery alone. Ann Surg Oncol. 2011;18:119–124. doi: 10.1245/s10434-010-1214-x. [DOI] [PubMed] [Google Scholar]
- 38.Dunne C, Burke JP, Morrow M, et al. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27:1615–1620. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 39.Wang SY, Chu H, Shamliyan T, et al. Network meta-analysis of margin threshold for women with ductal carcinoma in situ. J Natl Cancer Inst. 2012;104:507–516. doi: 10.1093/jnci/djs142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicini FA, Kestin LL, Goldstein NS, et al. Relationship between excision volume, margin status, and tumor size with the development of local recurrence in patients with ductal carcinoma-in-situ treated with breast-conserving therapy. J Surg Oncol. 2001;76:245–254. doi: 10.1002/jso.1041. [DOI] [PubMed] [Google Scholar]