Abstract
Inhalation of butter flavoring by workers in the microwave popcorn industry may result in “popcorn workers' lung.” In previous in vivo studies rats exposed for 6 h to vapor from the flavoring agents, diacetyl and 2,3-pentanedione, acquired flavoring concentration-dependent damage of the upper airway epithelium and airway hyporeactivity to inhaled methacholine. Because ion transport is essential for lung fluid balance, we hypothesized that alterations in ion transport may be an early manifestation of butter flavoring-induced toxicity. We developed a system to expose cultured human bronchial/tracheal epithelial cells (NHBEs) to flavoring vapors. NHBEs were exposed for 6 h to diacetyl or 2,3-pentanedione vapors (25 or ≥60 ppm) and the effects on short circuit current and transepithelial resistance (Rt) were measured. Immediately after exposure to 25 ppm both flavorings reduced Na+ transport, without affecting Cl− transport or Na+,K+-pump activity. Rt was unaffected. Na+ transport recovered 18 h after exposure. Concentrations (100–360 ppm) of diacetyl and 2,3-pentanedione reported earlier to give rise in vivo to epithelial damage, and 60 ppm, caused death of NHBEs 0 h post-exposure. Analysis of the basolateral medium indicated that NHBEs metabolize diacetyl and 2,3-pentanedione to acetoin and 2-hydroxy-3-pentanone, respectively. The results indicate that ion transport is inhibited transiently in airway epithelial cells by lower concentrations of the flavorings than those that result in morphological changes of the cells in vivo or in vitro.
Keywords: Diacetyl; 2,3-Pentanedione; Ion transport; Human airway epithelial cells; Vapor exposure; Dicarbonyl/l-xylulose reductase
1. Introduction
Microwave popcorn manufacturing employees who inhale butter flavoring vapor can develop “popcorn worker's lung,” an obstructive pulmonary disease which resembles clinical bronchiolitis obliterans (Kreiss et al., 2002) in which the airway epithelium is the apparent initial target of injury (Hubbs et al., 2002, 2008, 2012; Morris and Hubbs, 2009; Morgan et al., 2008, 2012; Palmer et al., 2011). In animal models of popcorn worker's lung the airway epithelium of the upper airways is a site, in morphological terms, of cytotoxicity. Inhalation of volatile α-diketone components in popcorn flavoring mixtures, such as diacetyl and 2,3-pentanedione, leads to appreciable epithelial damage in animal inhalation models and this effect is thought to be a primary contributing factor to the development of popcorn worker's lung (Akpinar-Elci et al., 2004; Day et al., 2011; Fedan et al., 2006; Hubbs et al., 2002; Kreiss et al., 2002; Lockey et al., 2009; Morgan et al., 2008, 2012; Morris and Hubbs, 2009; Morris, 2012; Palmer et al., 2011; van Rooy et al., 2007, 2009).
Regulation of the airway surface liquid (ASL) is one of the most important functions of the epithelium (Hollenhorst et al., 2011). In humans, epithelial cells throughout the respiratory tract are involved in Na+ absorption and Cl− secretion as a means of regulating the height of ASL and mucociliary clearance (Hamann et al., 2010; Hollenhorst et al., 2011). Na+ absorption occurs on the apical surface through amiloride-sensitive Na+ channels and apical Cl− channels subserve apical Cl− secretion. At the basolateral surface of the cell, Na+ is pumped outwardly by the Na+,K+-pump (Matthay et al., 2002). Ion transport disruption may result in respiratory infection and diseases including cystic fibrosis (CF) and pulmonary edema (Harris, 1996; Hollenhorst et al., 2011), as well as airway obstruction (Danahay et al., 2002; Eisenhut, 2006; Houtmeyers et al., 1999). Reduced transepithelial Na+ transport in the airways has been demonstrated to result in pulmonary edema (Egli et al., 2004). Previous findings using the guinea-pig isolated, perfused system revealed that diacetyl administered to the lumen via the perfusing physiological salt solution led to transepithelial depolarization and altered tight junction integrity (Fedan et al., 2006). In view of the importance of ion transport to normal lung function, we reasoned that understanding the effect(s) of flavorings on ion transport is needed, as they might be involved in epithelial responses to flavorings. At present the effects flavorings delivered directly to NHBE cells in vitro are unknown. In order to accomplish this characterization, a high throughput device was developed for inhalation exposure of NHBEs to diacetyl and 2,3-pentanedione.
It was heretofore not known which ion transporters might be affected by the butter flavoring agents, and we wanted to clarify a possible relationship between altered ion transport and epithelial cellular injury, and to understand the direct effects of butter flavoring agents on airway epithelial cells that are not adherent to the airway wall and not under the influence of other cell types and their mediators. This includes the question as to whether NHBEs were capable of metabolizing diacetyl and 2,3-pentanedione.
There were two primary goals of this investigation. The first was to evaluate the effects of diacetyl and 2,3-pentanedione on epithelial cell ion transport following exposure to 25 ppm of flavoring vapors in vitro, in comparison to concentrations that evoke morphological damage, as well as concentrations used in our previous vivo studies (60–360 ppm; Zaccone et al., 2013). The second was to investigate the ability of intact NHBE cells to metabolize diacetyl and 2,3-pentanedione following exposure to vapors in a novel in vitro exposure system. Diacetyl and 2,3-pentanedione are metabolized by dicarbonyl/l-xylulose reductase (DCXR; Nakagawa et al., 2002; Ebert et al., 2014). Gloede et al. (2011) and Cichocki et al. (2014) measured diacetyl levels and metabolites of diacetyl in upper and lower lung tissues as a component of a computational fluid dynamics-physiologically based pharmacokinetic (CFD-PBPK) model of diacetyl distribution and handling in the airways.
2. Materials and methods
2.1. Cell culture
Primary cultured NHBEs (Lonza; Walkersville, MA) were seeded in plastic T-75 flasks and were grown in B-ALI growth medium (Lonza) until cells were 80% confluent. The confluent monolayer was trypsinized and seeded onto rat tail collagen (BD Biosciences; San Jose, CA)-coated polyester (0.4 μm pores) transwell inserts (Corning; Corning, NY) at a density of 50,000 cells per insert. Cells were maintained at 37 °C in an air/5% CO2 mixture in an incubator. Cells were submerged for three days in B-ALI growth medium (100 μl apical; 500 μl basal chamber) before 500 μl of B-ALI differentiation medium was added to the basal chamber and the apical chamber was emptied to initiate the air-liquid interface (ALI) culture conditions. Medium was changed every 48 h. Transepithelial resistance (Rt) was measured with EVOM2 epithelial volt-ohm meter STX2 electrodes (World Precision Instruments; Sarasota, FL) to assess growth to confluence from the increase in the Rt. Cells were used after Rt reached a value of at least 700 Ω·cm2, which occurred after 7 days.
2.2. Imaging of cultured NHBEs
Differentiated, pseudostratified epithelium morphology was confirmed through a series of imaging and staining techniques. For hematoxylin and eosin (H&E) staining the transwell inserts were fixed in 10% buffered formalin, rinsed in Hank's balanced salt solution (37 °C), dehydrated in a graded series of ethanol, cleared in xylene, and infiltrated and embedded in paraffin. Sections (5 μm) were placed on microscope slides and stained with H&E. The samples were imaged on an Olympus IX70 photomicroscope (Shinjuku, Tokyo).
Mucus production by NHBEs was confirmed using alcian blue staining. Transwell inserts were stained apically with a 1% alcian blue solution (3% acetic acid, pH 2.5) for 30 s. The alcian blue solution was removed, and cells were imaged on a Zeiss Axiovert 100 microscope (Oberkochen, Germany) equipped with a Pixera Pro 150ES camera (Santa Clara, California).
For β-tubulin immunofluorescence to detect the presence of cilia transwell inserts were washed with PBS, fixed with apically applied methanol (4 °C), and stained using a monoclonal anti-tubulin-FITC antibody (F2043; Sigma-Aldrich; St. Louis, MO). Immunofluorescence was measured using the Axiovert 100 microscope equipped with a Pixera Pro 150ES camera.
The presence of cilia in the NHBEs was also confirmed using scanning electron microscopy (SEM). The samples were fixed in 4% paraformaldehyde fixative and post-fixed in osmium tetroxide. The cells were dehydrated in an ethanol series, dried using hexamethyldisilazane as the final solution, coated with gold/palladium, and imaged on a Hitachi 4800 field emission scanning electron microscope (Chiyoda, Tokyo).
Transmission electron microscopy (TEM) was employed to verify the presence of the typical 9 + 2 microtubule doublet typical of cilia. The samples were fixed in Karnovsky's fixative (2.5% glutaraldehyde, 2.5% paraformaldehyde in 0.1 M sodium cacodylic buffer), post-fixed in osmium tetroxide, mordanted in 1% tannic acid and stained en bloc in 0.5% uranyl acetate. The cells were dehydrated in an ethanol series and embedded in epon, sectioned and stained with Reynold's lead cit-rate and aqueous uranyl acetate. The sections were imaged on a JEOL 1220 transmission electron microscope (Peabody, MA).
2.3. Exposure of NHBEs to flavoring vapor
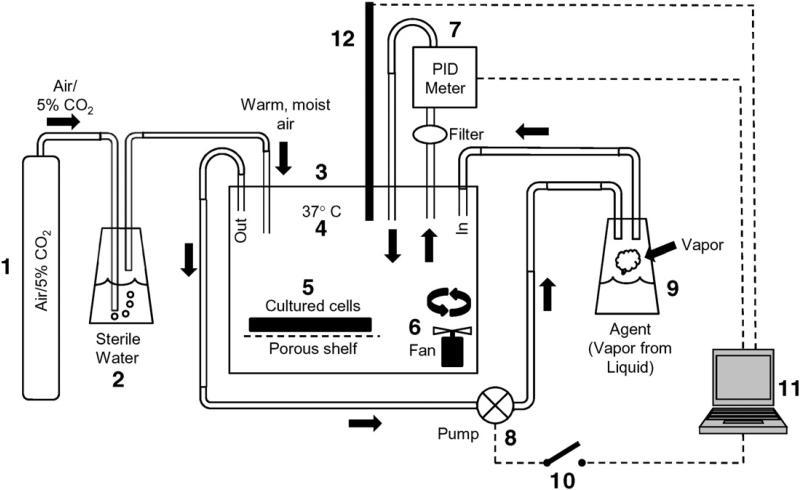
A device (Fig. 1) was designed and built for exposing NEBEs to diacetyl and 2,3-pentanedione vapor. It permitted delivery of vapors at desired concentrations that could be held constant over periods of many hours (Supplementary Fig. 1). Once the NHBEs had differentiated and reached an Rt value of at least 700 Ω·cm2, NHBEs were placed in the exposure chamber. The conditions inside the chamber were identical to those in the cell culture incubator in which the cells had been grown. Cells were exposed for 6 h to vapors from diacetyl (25, 60, 100, 200, 300, or 360 ppm) or 2,3-pentanedione (25 ppm) and were utilized 0 h or 18 h after the end of the exposure. For 18 h post-exposure experiments the medium in the basal chamber was replaced upon ending the exposure.
Fig. 1.
Schematic of the apparatus designed and used for exposing ALI NHBEs to vapors of diacetyl delivered to the apical surface. 1: Gas (5% CO2 in air) for providing oxygenation of cells and maintaining pH of the culture medium. 2: Passage of gas through sterile, distilled water (37 °C) to humidify the gas to 85% relative humidity. 3: The exposure chamber. 4: Chamber temperature was maintained at 37 °C. The exposure chamber mimicked conditions in the incubator in which the cells were grown and differentiated. 5: Cultured cell plate containing NHBEs rested on a porous stainless steel shelf. 6: Fan to ensure mixing of agent in the chamber. 7: Photoionization detector (PID) for measuring vapor level. The PID withdrew air from the chamber and returned it while measuring vapor level in the chamber. The output of the PID was sent to the proprietary computer software (11) for integration. 8: A pump was activated/deactivated by the computer in response to input from the PID. The pump delivered vapors to the chamber. 9: Vapor source from which vapor is pumped to the exposure chamber upon activation of the pump. The vapor was generated from diacetyl or 2,3-pentanedione liquid placed in the bottom of the vessel. 10: The computer software activated/ deactivated a switch to engage or disengage the pump. 11: Proprietary software was used to monitor vapor levels in the chamber and deliver vapor as needed to maintain the level within 5% of the desired level. 12: The temperature-humidity probe was monitored by computer software.
To ascertain whether incubation of NHBEs in the chamber per se had any effects on the cells, exposure chamber control (ECC) cells were placed in the exposure chamber for 6 h and were exposed only to air. The results obtained from these cells were compared to those in which NHBEs were not placed in the chamber but used immediately after removal from the incubator.
2.4. Measurement of Isc and Rt
Immediately (0 h) and 18 h after the flavoring-vapor exposure, transwell inserts were placed into Ussing chambers to measure Isc and Rt to assess alterations in epithelial ion transport due to flavoring exposure (Wu et al., 2004). Inserts were bathed in modified Krebs–Henseleit solution (MKHS) in both apical and basolateral chambers, maintained at 37 °C, and aerated with 95% O2/5% CO2. Cells were allowed to stabilize under open-circuit conditions before recording the transepithelial potential difference (Vt; −9.5 ± 1.6 mV) and applying a 0 mV voltage-clamp using an EVC 4000 automatic voltage/current amplifier (World Precision Instruments; Sarasota, FL). Square-wave voltage pulses (1 mV, 5 s) were delivered every 55 s to yield a voltage response for calculation of Rt from Ohm's law. After stabilization of baseline Isc (11.9 ± 4.7 μA/cm2), the effects of apically-applied amiloride (3.5 × 10−5 M), apically-applied 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB; 10−4 M), and serosally-applied ouabain (10−4 M) to inhibit Na+ and Cl− channels and the Na+,K+-pump, respectively, were examined.
2.5. Identification of DCXR-related metabolites of diacetyl and 2,3-pentanedione
To identify possible oxygenated compounds (i.e., aldehydes, ketones, and dicarbonyls) present in the purchased diacetyl or 2,3-pentandione or derived from flavoring metabolism by NHBE, 100 μl of O-(2,3,4,5,6-pentafluoro-benzyl)hydroxylamine hydrochloride (PFBHA) (250 mM in deionized water) was added to the sample (approximately 2 ml). This solution was heated for approximately 1 h in a 70 °C water bath to accelerate the derivatization reaction. The solution was removed from the water bath and allowed to cool and was then extracted with 3 ml of methyl tert-butyl ether (MTBE). The MTBE layer, containing the derivatized compounds (oximes) was removed (ca. 2.5 ml) and blown to dryness with clean air and reconstituted in 100 μl methanol. It should be noted that diacetyl or 2,3-pentanedione is also derivatized by PFBHA resulting in diacetyl oxime peaks in the chromatogram. Samples were analyzed using a Varian (Palo Alto, CA) 3800/Saturn 2000 gas chromatograph/mass spectrometer (GC/MS) system operated in the electron impact (EI) mode. Compound separation was achieved by a Restek (Bellefonte, PA) Rtx-5MS (0.25 mm I.D., 30-m long, 1 μm film thickness) column and the following GC oven parameters: 40 °C for 2 min, then 10 °C/min to 140 °C, then 20 °C/min to 280 °C and held for 8 min. The GC injector was initially held at 60 °C for 2 min, then ramped at 200 °C/min to 300 °C. Each sample (1 μl) was injected in the splitless mode and the injector was returned to split mode 1 min after sample injection. The Saturn 2000 ion trap mass spectrometer was tuned using perfluorotributylamine (FC-43). Full-scan EI spectra were collected from m/z 40–650. Acetonitrile was the chemical ionization reagent used for all chemical ionization spectra.
2.6. Solutions and reagents
MKHS contained: NaCl, 113 mM; KCl, 4.8 mM; CaCl2, 2.5 mM; MgCl2, 1.2 mM; KH2PO4, 1.2 mM; NaHCO3, 25 mM and glucose, 5.5 mM. All drugs and chemicals were obtained from Sigma-Aldrich (St. Louis, MO) and dissolved and diluted in saline. The purity of diacetyl (lot 03798LJ) and 2,3-pentanedione (lot 00130DJ) was 99.3% and 97%, respectively.
2.7. Statistical analysis
The results are expressed as means ± S.E.M. Electrophysiology results were expressed as Isc or as a percent change from the baseline Isc value. The results were evaluated for significant differences using one-way ANOVA. Each donor was considered an n value of 1. All Ussing chamber experiments included two donors, and there were a minimum of three replicates for each experimental condition. p < 0.05 was considered significant.
3. Results
3.1. Characteristics of NHBEs
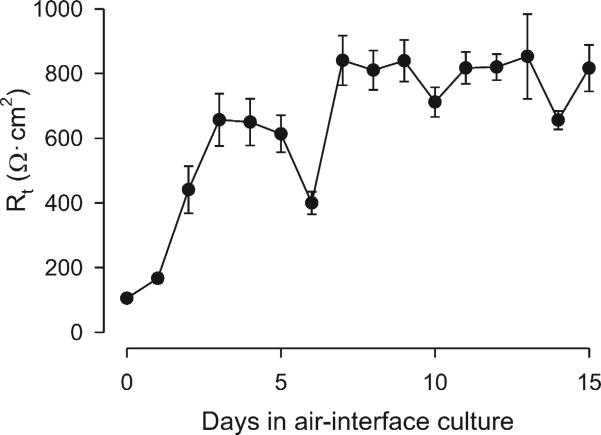
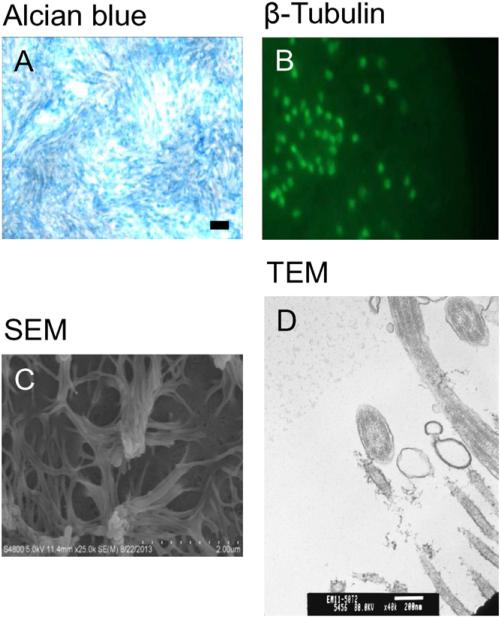
NHBEs grew to confluence and generated high Rt values after 7 days in air-interface (Fig. 2). The cells formed a differentiated layer resembling in situ cells. Alcian blue staining and β-tubulin immunofluorescence confirmed the presence of mucus and cilia, respectively (Fig. 3). SEM (Fig. 4C) and TEM imaging (Fig. 3) also confirmed the presence of cilia and the 9 + 2 microtubule configuration found in cilia.
Fig. 2.
Rt of NHBE in ALI culture. Rt values increased and stabilized over time. After raising the cultures in air interface, Rt increased from 105 ± 5 Ω·cm2 from day 1 to 816 ± 72 Ω·cm2 on day 15. The Rt was relatively stable after 1 week and thereafter.
Fig. 3.
Characteristics of cultured NHBEs in ALI. (A) Alcian blue staining for apical mucus (objective magnification = 10×). Bar = 100 μm. (B) Immunostaining for apical β-tubulin (objective magnification = 20×). (C) SEM image of apical cilia. (D) TEM image of cilia, demonstrating the 9 + 2 arrangement of ciliary microtubules.
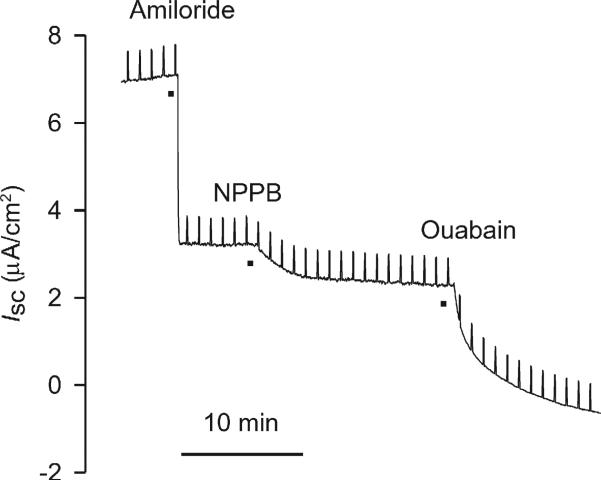
Fig. 4.
Representative bioelectric responses of naïve NHBEs to ion transport blockers amiloride (3.5 × 10−5 M), NPPB (10−4 M), and ouabain (10−4 M). Vertical deflections reflect the Isc responses to the application of 1 mV pulses across the epithelium for calculation of Rt using Ohm's law.
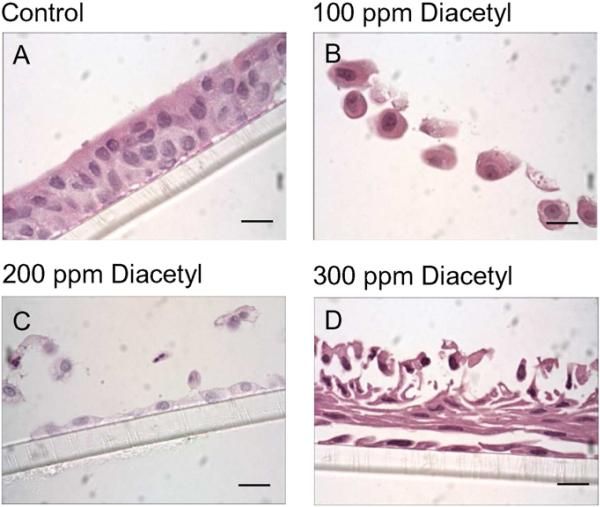
3.2. Effects of diacetyl and 2,3-pentanedione exposure on NHBE: morphology and ion transport
NHBEs were characterized with respect to ion transport and the effects of blockers. Inhibition of epithelial Na+ channels (ENaC) with apical amiloride (3.5 × 10−5 M) caused a rapid decrease in Isc in naïve cells, essentially halving the basal value. A further, smaller decrease in Isc occurred after inhibiting Cl− channels with apical NPPB (10−4 M). Upon challenge with ouabain (10−4 M) added basolaterally, Isc was inhibited completely.
Before embarking on exposures of NHBE to flavorings, we first investigated whether there were any consequences of incubation of naïve cells in the exposure chamber for 6 h. After such incubation there were no effects on cell morphology or responses of the cells to the ion channel blockers in comparison to fresh cells removed from the cell culture incubator (Supplementary Fig. 2).
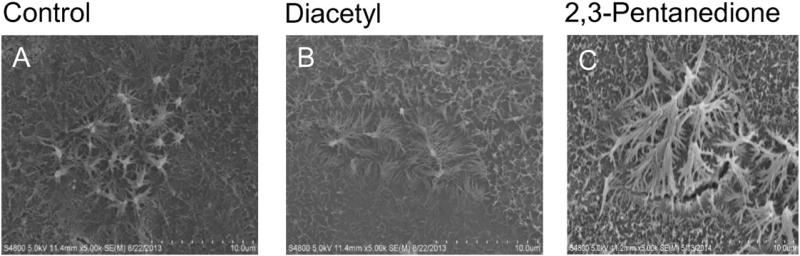
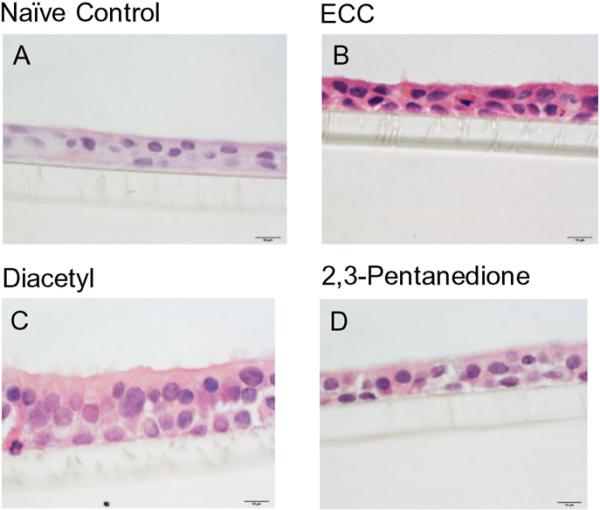
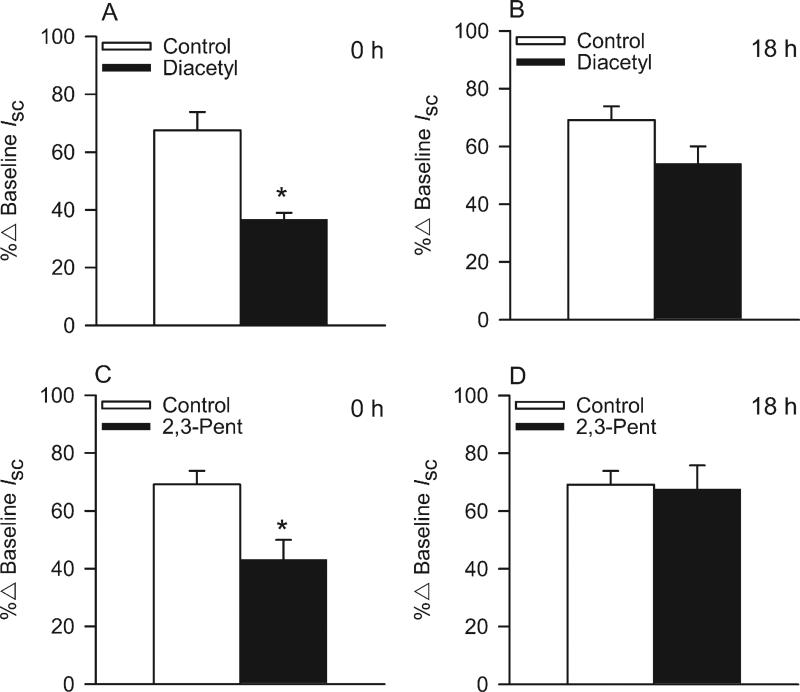
Exposure of NHBEs to 25 ppm diacetyl or 2,3-pentanedione for 6 h had no effect on cell morphology (Figs. 5 and 6). The cells remained attached to the matrix and cilia appeared normal. We, therefore, investigated changes in ion transport in these cells at two post-exposure periods (Fig. 7). At 0 h after the end of exposure, both diacetyl or 2,3-pentanedione inhibited amiloride-sensitive ion transport (Fig. 7A, C). Eighteen hours post-exposure the response to amiloride had recovered to the control levels (Fig. 7B, D). Rt values were not affected by either flavoring at either time, indicating that an effect of flavorings on active ion transport rather than on in tight junctions accounted for the decrease in the response to amiloride. Neither flavoring altered the apical Cl− conductance, demonstrated by the absence of a change in responses to NPPB responses at 0 h or 18 h post-exposure (Supplementary Fig. 3) nor Na+,K+-pump activity, as judged from the responses to ouabain (Supplementary Fig. 4) (Figs. 8 and 9).
Fig. 5.
SEM image of the apical surface of air-exposed and naïve NHBEs and cells exposed to 25 ppm diacetyl or 2,3-pentanedione. (A) SEM images of control cells, (B) 25 ppm diacetyl-exposed cells and (C) 2,3-pentanedione-exposed cells.
Fig. 6.
H&E staining (objective magnification = 100×) illustrating similar morphology between control NHBEs and cells viewed after a 6-h diacetyl (25 ppm) or 2,3-pentanedione (25 ppm) exposure. (A) Unexposed, naïve control NHBEs, (B) ECC cells, (C) cells exposed to diacetyl and (D) cells exposed to 2,3-pentanedione. Bar = 10 μm.
Fig. 7.
Effects of diacetyl and 2,3-pentanedione (25 ppm) on bioelectric responses of NHBEs to apically-applied amiloride (3.5 × 10−5 M) at 0 h and 18 h post-exposure. (A) Responses to amiloride after air or diacetyl (25 ppm) exposure at the 0 h post-exposure time point and (B) the 18 h post-exposure time point. (C) Response to amiloride after 2,3-pentanedione (2,3-Pent; 25 ppm) exposure at the 0 h time point and (D) the 18 h time point. *Significantly different from control.
Fig. 8.
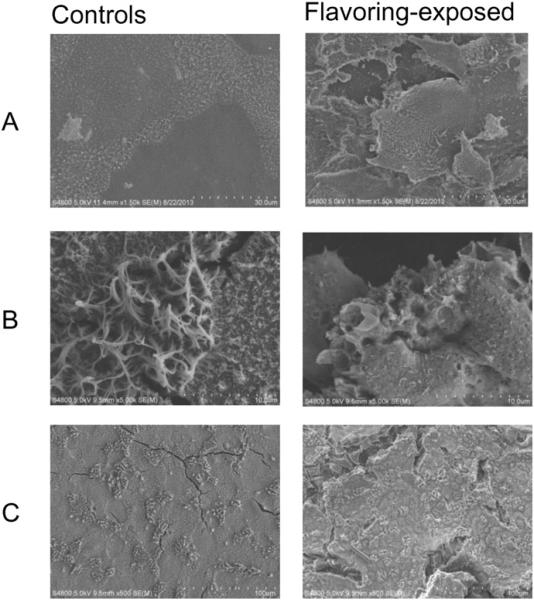
SEM images of the apical surface of NHBE control cells and cells exposed to diacetyl (60, 100, and 360 ppm). (A) SEM images of control cells (left panel) compared to 60 ppm diacetyl-exposed cells (right panel). (B) Control cells (left panel) compared to 100 pm diacetyl-exposed cells (right panel). (C) Control cells (left panel) compared to 360 ppm diacetyl-exposed cells (right panel).
Fig. 9.
H&E images (objective magnification = 100×) illustrating the effects of diacetyl (100, 200, 300 ppm) on NHBE morphology. (A) Unexposed, control epithelial cells demonstrating the presence of a confluent, pseudo-stratified epithelium. (B) Cells exposed to diacetyl at 100 ppm, (C) 200 ppm and (D) 300 ppm. Bar = 10 μM.
3.3. DCXR-related metabolism of diacetyl and 2,3-pentanedione by NHBEs
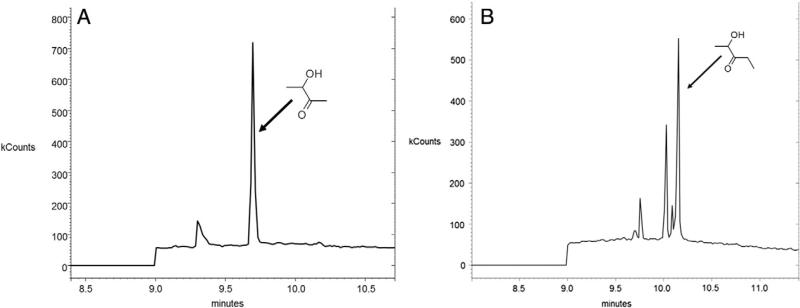
The α-diketones, diacetyl and 2,3-pentanedions, are metabolized by DCXR to give rise to acetoin and 2-hydroxy-3-pentone. Six hours after exposures of the cells to 25 ppm diacetyl or 2,3-pentanedione, acetoin and 2-hydroxy-3-pentone were detected in samples from the basolateral MKHS solution (Fig. 10). These metabolites were not observed in the basolateral media after flavoring exposures of transwell inserts devoid of cells or during air exposure, and were, therefore, products of cell metabolism.
Fig. 10.
Identification of metabolites of diacetyl (acetoin; A) and 2,3-pentanedione (2-hydroxy-3-pentanone; B) in basolateral chamber samples following exposure of NHBEs to 25 ppm diacetyl or 2,3-pentanedione. The arrows point to the peaks identified as acetoin (A) or 2-hydroxy-3-pentanone (B). The diacetyl and 2,3-pentanedione peaks are not shown. The identity of the other peaks is not known; they could be impurities or other metabolites of the flavorings.
4. Discussion
These experiments have demonstrated that the concentration ranges which cause morphological changes in NHBEs cells and those that inhibit ion transport are different, with lower concentrations capable of altering Na+ in the absence of morphological damage to the cells. Therefore, ion transport is a more sensitive measurement of acute effects of flavoring agents than morphology (i.e., changes in NHBE cell ion transport can be detected at diacetyl and 2,3-pentanedione concentrations that do not cause visible alterations in the cell).
Changes in epithelial ion transport occurring at low flavoring concentration (i.e., 25 ppm) are apparently reversible and did not lead to cell death over the 18-h time span used in our experiments. At higher concentrations, however, flavoring exposure led to cell death. Our findings do not provide any information about whether inhibition of ion transport is an initial signal leading to death at higher flavoring concentrations, however. Whether a causal relationship exists requires further investigation.
In inhalation studies using animal models, Hubbs et al. (2012) demonstrated that 2,3-pentanedione (318 ppm) inhalation did not result in severe epithelial damage immediately after exposure. Epithelial injury in the nasal cavity involved both apoptosis and necrosis, which progressed during the hours after exposure. Cell death was greater at 18 h, as opposed to immediately after exposure. Therefore, we anticipated more prominent changes in Isc or result in greater cytotoxicity at the 18 h time point than at 0 h. However, after the initial decrease in ion transport observed 0 h after exposure, the cells had recovered 18 h after exposure to 25 ppm of flavorings.
The effect of 25 ppm exposure to flavorings on ion transport appeared to be selective for amiloride-sensitive Na+ transport; Cl− channels and the Na+,K+-pump were not affected by the vapors. It is not known if these effects occurred indirectly through, for example, the effects of cytokines or other mediators derived from the epithelial cells, or as a consequence of direct interaction of α-diketones on the Na+ channel. Sun et al. (2013) demonstrated that a 30 min exposure to diacetyl (10 mM) stimulates ROS-dependent epithelial production of neutrophil chemoattractant IL-8 in cultured human airway epithelial (NCI-H292) cells. Cytokines such as IL-4 have been shown to reduce the expression of β and γ units of ENaC in association with inhibition of the amiloride-sensitive Na+ channel (Eisenhut, 2006; Galietta et al., 2004). IL-1β has also been found to reduce ENaC in human primary cultured bronchial epithelial cells without reducing ENaC expression (Gray et al., 2004). However, these authors acknowledged that this non-genomic effect could have been associated with increased expression of CFTR, because CFTR interacts with ENaC to inhibit Na+ absorption, or with other pathways that regulate ENaC. Whether inflammatory mediators are associated with reduced airway Na+ transport following flavoring exposure requires further study. Interaction of diacetyl with arginine-containing peptides and proteins is well documented (Borders and Riordan, 1975; Epperly and Dekker, 1989; Mathews et al., 2010; Mueller et al., 1995). Thus, covalent binding of these α-diketones with ENaC may be a potential mechanism of the attenuated response to amiloride.
Acute flavoring affects in the airways can now be associated with imbalances of fluid secretion due to altered Na+ transport, but probably as an initial event early in exposure and/or at low vapor concentrations. Structural epithelial damage occurring in the airways would, of course, completely abrogate ion transport by epithelium and fluid balance in the lung.
The enzyme, DCXR, has been localized in airway epithelium, as evidenced using fluorescent microscopy in rat and human airways (Hubbs et al., 2012; Ishikura et al., 2001; Nakagawa et al., 2002; Calam et al., 2013). Our investigation also has provided the first direct evidence for the metabolism of diacetyl and 2,3-pentanedione to acetoin and 2-hydroxy-3-pentanone by human airway epithelium. Both metabolites were identified in samples of basolateral MKHS medium, implying that the epithelial cells had released/secreted the metabolites in a basolateral membrane direction. However, the results indicate that epithelial DCXR is functional and, as such, buttresses the bases and conclusions of Gloede et al. (2011) and Cichocki et al. (2014) regarding flavoring metabolism in the airways.
5. Conclusions
All concentrations of diacetyl and 2,3-pentanedione above 60 ppm abolished Vt and Rt in cultured cells. The electrophysiological changes immediately after the end of the 25 ppm exposure, which did not result in cell death, help establish concentration-dependency in the toxic effects of flavorings on human airway epithelium, and discriminate functional changes from structural ones. Amiloride-sensitive Na+ transport was reduced immediately after a 6-h exposure to 25 ppm flavorings, and Rt was unaffected, but recovered by 18 h post-exposure. The results are consistent with the notion that ENaC are initially affected by diacetyl and 2,3-pentanedione vapor exposure. Thus, reductions in Na+ transport following α-diketone exposure may be a component of lung disease triggered by inhalation of popcorn flavorings.
We have demonstrated that DCXR metabolizes diacetyl and 2,3-pentanedione in the cultured NHBEs, releasing their metabolites into the submucosal medium, although the role of DCXR in altered ion transport is not clear.
Lastly, the design of the vapor exposure apparatus is such that its utility goes beyond understanding flavoring effects in the lung. That is, it is well-suited for studies of metabolism of vapors, particulates and aerosols by airway epithelial cells, including those used to deliver drugs by inhalation to patients, and should assist with the understanding of the pharmacokinetics and metabolism of inhaled drugs by epithelium. The method could potentially be extended to examine metabolism of air-borne agents by any type of cell that can be grown on permeable inserts.
Supplementary Material
Acknowledgments
This investigation was supported, in part, by an NIH Cardiovascular and Pulmonary Disease Training Grant (5T32HL090610-05) and by the National Institute for Occupational Safety and Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of brand name does not constitute product endorsement.
Abbreviations
- ASL
airway surface liquid
- CFTR
cystic fibrosis transmembrane conductance regulator
- ECC
exposure chamber control
- NHBEs
normal human bronchial/tracheal epithelial cells
- ENaC
epithelial sodium channel
- NPPB
5-nitro-2-(3-phenylpropylamino)benzoic acid
- Isc
short circuit current
- Rt
transepithelial resistance
- TEM
transmission electron microscopy
- SEM
scanning electron microscopy
- DCXR
dicarbonyl/l-xylulose reductase
- MKHS
modified Krebs–Henseleit solution
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.taap.2015.10.004.
Conflict of interest statement
The authors declare that they have no conflicts of interest in relation to this publication.
Transparency document
The Transparency document associated with this article can be found, in the online version.
References
- Akpinar-Elci M, Travis WD, Lynch DA, Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur. Respir. J. 2004;24:298–302. doi: 10.1183/09031936.04.00013903. [DOI] [PubMed] [Google Scholar]
- Borders CL, Jr., Riordan JF. An essential arginyl residue at the nucleotide binding site of creatine kinase. Biochemistry. 1975;14:4699–4704. doi: 10.1021/bi00692a021. [DOI] [PubMed] [Google Scholar]
- Calam E, Porté S, Fernández MR, Farrés J, Parés X, Biosca JA. Biocatalytic production of alpha-hydroxy ketones and vicinal diols by yeast and human aldo-keto reductases. Chem. Biol. Interact. 2013;202:195–203. doi: 10.1016/j.cbi.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Cichocki JA, Smith GJ, Morris JB. Tissue sensitivity of the rat upper and lower extrapulmonary airways to the inhaled electrophilic air pollutants diacetyl and acrolein. Toxicol. Sci. 2014;142:126–136. doi: 10.1093/toxsci/kfu165. [DOI] [PubMed] [Google Scholar]
- Danahay H, Atherton H, Jones G, Bridges RJ, Poll CT. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am. J. Physiol. Lung-C. 2002;282:L226–L236. doi: 10.1152/ajplung.00311.2001. [DOI] [PubMed] [Google Scholar]
- Day G, Lebouf R, Grote A, Pendergrass S, Cummings K, Kreiss K, Kullman G. Identification and measurement of diacetyl substitutes in dry bakery mix production. J. Occup. Environ. Hyg. 2011;8:93–103. doi: 10.1080/15459624.2011.547148. [DOI] [PubMed] [Google Scholar]
- Ebert B, Kisiela M, Maser E. Human DCXR — another “moonlighting protein” involved in sugar metabolism, carbonyl detoxification, cell adhesion and male fertility? Biol. Rev. 2014 doi: 10.1111/brv.12108. http://dx.doi.org/10.1111/brv.12108 [Epub ahead of print] April 10, 2014. [DOI] [PubMed]
- Egli M, Duplain H, Lepori M, Cook S, Nicod P, Hummler E, Sartori C, Scherrer U. Defective respiratory amiloride-sensitive sodium transport predisposes to pulmonary oedema and delays its resolution in mice. J. Physiol. 2004;560:857–865. doi: 10.1113/jphysiol.2004.066704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M. Changes in ion transport in inflammatory disease. J. Inflamm. (Lond.) 2006;3:5. doi: 10.1186/1476-9255-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperly BR, Dekker EE. Inactivation of Escherichia coli l-threonine dehydrogenase by 2,3-butanedione. Evidence for a catalytically essential arginine residue. J. Biol. Chem. 1989;264:18296–18301. [PubMed] [Google Scholar]
- Fedan JS, Dowdy JA, Fedan KB, Hubbs AF. Popcorn worker's lung: in vitro exposure to diacetyl, an ingredient in microwave popcorn butter flavoring, increases reactivity to methacholine. Toxicol. Appl. Pharmacol. 2006;215:17–22. doi: 10.1016/j.taap.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Galietta LJV, Folli C, Caci E, Pedemonte N, Taddei A, Ravazzolo R, Zegarra-Moran O. Effect of inflammatory stimuli on airway ion transport. Proc. Am. Thorac. Soc. 2004;1:62–65. doi: 10.1513/pats.2306017. [DOI] [PubMed] [Google Scholar]
- Gloede E, Cichocki JA, Baldino JB, Morris JB. A validated hybrid computational fluid dynamics-physiologically based pharmacokinetic model for respiratory tract vapor absorption in the human and rat and its application to inhalation dosimetry of diacetyl. Toxicol. Sci. 2011;123:231–246. doi: 10.1093/toxsci/kfr165. [DOI] [PubMed] [Google Scholar]
- Gray T, Coakley R, Hirsh A, Thornton D, Kirkham S, Koo JS, Burch L, Boucher R, Nettesheim P. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1β in human bronchial epithelia. Am. J. Physiol. Lung-C. 2004;286:320–330. doi: 10.1152/ajplung.00440.2002. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herrera-Perez JJ, Zeuthen T, Alvarez-Leefmans FJ. Cotransport of water by the Na+–K+–2Cl− cotransporter NKCC1 in mammalian epithelial cells. J. Physiol. 2010;588:4089–4101. doi: 10.1113/jphysiol.2010.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. Epithelial Cell Culture. Cambridge University Press; 1996. [Google Scholar]
- Hollenhorst MI, Richter K, Fronius M. Ion transport by pulmonary epithelia. J. Biomed. Biotechnol. 2011;2011:174306. doi: 10.1155/2011/174306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Regulation of mucociliary clearance in health and disease. Eur. Respir. J. 1999;13:1177–1188. doi: 10.1034/j.1399-3003.1999.13e39.x. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Battelli LA, Goldsmith WT, Porter DW, Frazer D, Friend S, Schwegler-Berry D, Mercer RR, Reynolds JS, Grote A, Castranova V, Kullman G, Fedan JS, Dowdy J, Jones WG. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol. Appl. Pharmacol. 2002;185:128–135. doi: 10.1006/taap.2002.9525. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Cumpston AM, Goldsmith WT, Battelli LA, Kashon ML, Jackson MC, Frazer DG, Fedan JS, Goravanahally MP, Castranova V, Kreiss K, Willard PA, Friend S, Schwegler-Berry D, Fluharty KL, Sriram K. Respiratory and olfac-tory cytotoxicity of inhaled 2,3-pentanedione in Sprague–Dawley rats. Am. J. Pathol. 2012;181:829–844. doi: 10.1016/j.ajpath.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbs AF, Goldsmith WT, Kashon ML, Frazer D, Mercer RR, Battelli LA, Kullman GJ, Schwegler-Berry D, Friend S, Castranova V. Respiratory toxicologic pathology of inhaled diacetyl in Sprague–Dawley rats. Toxicol. Pathol. 2008;36:330–344. doi: 10.1177/0192623307312694. [DOI] [PubMed] [Google Scholar]
- Ishikura S, Isaji T, Usami N, Kitahara K, Nakagawa J, Hara A. Molecular cloning, expression and tissue distribution of hamster diacetyl reductase. Identity with L-xylulose reductase. Chem. Biol. Interact. 2001;130-132:879–889. doi: 10.1016/s0009-2797(00)00315-x. [DOI] [PubMed] [Google Scholar]
- Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. New Engl. J. Med. 2002;347:330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- Lockey JE, Hilbert TJ, Levin LP, Ryan PH, White KL, Borton EK, Rice CH, Mckay RT, Lemasters GK. Airway obstruction related to diacetyl exposure at microwave popcorn production facilities. Eur. Respir. J. 2009;34:63–71. doi: 10.1183/09031936.00050808. [DOI] [PubMed] [Google Scholar]
- Mathews JM, Watson SL, Snyder RW, Burgess JP, Morgan DL. Reaction of the butter flavorant diacetyl (2,3-butanedione) with N-α-acetylarginine: a model for epitope formation with pulmonary proteins in the etiology of obliterative bronchiolitis. J. Agric. Food Chem. 2010;58:12761–12768. doi: 10.1021/jf103251w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol. Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Flake GP, Kirby PJ, Palmer SM. Respiratory toxicity of diacetyl in c57bl/6 mice. J. Toxicol. Sci. 2008;103:169–180. doi: 10.1093/toxsci/kfn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Jokinen MP, Price HC, Gwinn WM, Palmer SM, Flake GP. Bronchial and bronchiolar fibrosis in rats exposed to 2,3-pentanedione vapors: implications for bronchiolitis obliterans in humans. Toxicol. Pathol. 2012;40:448–465. doi: 10.1177/0192623311431946. [DOI] [PubMed] [Google Scholar]
- Morris JB. Biologically-based modeling insights in inhaled vapor absorption and dosimetry. Pharmacol. Ther. 2012;136:401–413. doi: 10.1016/j.pharmthera.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Morris JB, Hubbs AF. Inhalation dosimetry of diacetyl and butyric acid, two components of butter flavoring vapors. Toxicol. Sci. 2009;108:173–183. doi: 10.1093/toxsci/kfn222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MJ, Samuelsson B, Haeggstrom JZ. Chemical modification of leukotriene A4 hydrolase. Indications for essential tyrosyl and arginyl residues at the active site. Biochemistry. 1995;34:3536–3543. doi: 10.1021/bi00011a007. [DOI] [PubMed] [Google Scholar]
- Nakagawa J, Ishikura S, Asami J, Isaji T, Usami N, Hara A, Sakurai T, Tsuritani K, Oda K, Takahashi M, Yoshimoto M, Otsuka N, Kitamura K. Molecular characterization of mammalian dicarbonyl/L-xylulose reductase and its localization in kidney. J. Biol. Chem. 2002;277:17883–17891. doi: 10.1074/jbc.M110703200. [DOI] [PubMed] [Google Scholar]
- Palmer SM, Flake GP, Kelly FL, Zhang HL, Nugent JL, Kirby PJ, Foley JF, Gwinn WM, Morgan DL. Severe airway epithelial injury, aberrant repair and bronchiolitis obliterans develops after diacetyl instillation in rats. PLoS One. 2011;6:e17644. doi: 10.1371/journal.pone.0017644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Kelly FL, Morgan DL, Martinu T, Palmer SM. Oxidative stress in diacetyl-induced airway epithelial injury and bronchiolitis obliterans. Am. J. Respir. Crit. Care Med. 2013;187:A3369. [Google Scholar]
- van Rooy FG, Rooyackers JM, Prokop M, Houba R, Smit LA, Heederik DJ. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am. J. Resp. Crit. Care. 2007;176:498–504. doi: 10.1164/rccm.200611-1620OC. [DOI] [PubMed] [Google Scholar]
- van Rooy FG, Smit LA, Houba R, Zaat VA, Rooyackers JM, Heederik DJ. A cross-sectional study of lung function and respiratory symptoms among chemical workers producing diacetyl for food flavourings. Occup. Environ. Med. 2009;66:105–110. doi: 10.1136/oem.2008.039560. [DOI] [PubMed] [Google Scholar]
- Wu DX, Johnston RA, Rengasamy A, Van Scott MR, Fedan JS. Hyperosmolar solution effects in guinea pig airways. II. Epithelial bioelectric responses to relative changes in osmolarity. J. Pharmacol. Exp. Ther. 2004;308:19–29. doi: 10.1124/jpet.103.051615. [DOI] [PubMed] [Google Scholar]
- Zaccone EJ, Thompson JA, Ponnoth DS, Cumpston AM, Goldsmith WT, Jackson MC, Frazer DG, Hubbs AF, Shimko M, Fedan JS. Popcorn flavoring effects on reactivity of rat airways in vivo and in vitro. J. Toxic. Environ. Health A. 2013;76:669–689. doi: 10.1080/15287394.2013.796302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.