Abstract
BACKGROUND
The standard therapy for women with unexplained infertility is gonadotropin or clomiphene citrate. Ovarian stimulation with letrozole has been proposed to reduce multiple gestations while maintaining live birth rates.
METHODS
We enrolled couples with unexplained infertility in a multicenter, randomized trial. Ovulatory women 18 to 40 years of age with at least one patent fallopian tube were randomly assigned to ovarian stimulation (up to four cycles) with gonadotropin (301 women), clomiphene (300), or letrozole (299). The primary outcome was the rate of multiple gestations among women with clinical pregnancies.
RESULTS
After treatment with gonadotropin, clomiphene, or letrozole, clinical pregnancies occurred in 35.5%, 28.3%, and 22.4% of cycles, and live birth in 32.2%, 23.3%, and 18.7%, respectively; pregnancy rates with letrozole were significantly lower than the rates with standard therapy (gonadotropin or clomiphene) (P = 0.003) or gonadotropin alone (P<0.001) but not with clomiphene alone (P = 0.10). Among ongoing pregnancies with fetal heart activity, the multiple gestation rate with letrozole (9 of 67 pregnancies, 13%) did not differ significantly from the rate with gonadotropin or clomiphene (42 of 192, 22%; P = 0.15) or clomiphene alone (8 of 85, 9%; P = 0.44) but was lower than the rate with gonadotropin alone (34 of 107, 32%; P = 0.006). All multiple gestations in the clomiphene and letrozole groups were twins, whereas gonadotropin treatment resulted in 24 twin and 10 triplet gestations. There were no significant differences among groups in the frequencies of congenital anomalies or major fetal and neonatal complications.
CONCLUSIONS
In women with unexplained infertility, ovarian stimulation with letrozole resulted in a significantly lower frequency of multiple gestation but also a lower frequency of live birth, as compared with gonadotropin but not as compared with clomiphene. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT01044862.)
Therapeutic options for couples with unexplained infertility include assisted reproductive technologies, such as in vitro fertilization (IVF) and embryo transfer, and empirical ovarian stimulation combined with intrauterine insemination. The high cost and limited insurance coverage of IVF in all but a few locales in the United States make it an unattainable option for most infertile couples.1 Empirical ovarian stimulation has been thought to promote childbearing by increasing the number of ova ovulated, as well as possibly by enhancing implantation, placentation, or both through hormonal effects on the endometrium.2–4 However, empirical ovarian stimulation (with clomiphene or particularly with gonadotropin) is frequently complicated by the ovarian hyperstimulation syndrome and by multiple gestations, with an increased risk of preterm birth and associated neonatal morbidity and costs.5–10
Aromatase inhibitors have been used successfully to induce ovulation in women with the polycystic ovary syndrome.11 In addition, multiple reports suggest that aromatase inhibitors may be effective alternative agents for ovarian stimulation in couples with unexplained infertility.12–19 Their administration is reported to be associated with monofollicular development in most cases,3,17 which may result in enhanced fertility and a reduced risk of ovarian hyperstimulation and multiple births,12,13,20 as compared with current standard therapies such as gonadotropin and clomiphene. Use of an aromatase inhibitor to promote conception has not been associated with a significantly increased risk of congenital anomalies.11,21 We designed the present randomized trial to assess whether ovarian stimulation with letrozole, an aromatase inhibitor, as compared with clomiphene or gonadotropin, would result in a lower rate of multiple gestation without lowering the likelihood of pregnancy.
METHODS
STUDY DESIGN
The Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) clinical trial was conducted by the National Institute of Child Health and Human Development (NICHD) Cooperative Reproductive Medicine Network; the Collaborative Center for Statistics and Science at Yale University served as the data coordinating center. The trial was conducted at 12 clinical sites throughout the United States.
The trial design has been published previously.22 Briefly, this was a multicenter, randomized clinical trial involving 900 couples with unexplained infertility. Women were between 18 and 40 years of age with regular menses (nine or more cycles per year), had a normal uterine cavity with at least one patent fallopian tube, and had a male partner with a semen specimen of at least 5 million sperm per milliliter. This threshold for the sperm count was used because the protocol incorporated a sperm wash with intrauterine insemination. (World Health Organization [WHO] thresholds for normal sperm counts, which are higher, are based on intercourse rather than insemination.) In addition, a sperm wash was performed to concentrate motile sperm; this approach has been shown to improve pregnancy rates with sperm counts below the WHO threshold.23
Approval for the study was obtained from the institutional review board at each site. Written informed consent was provided by all female and male participants. The first and last authors assume responsibility for the accuracy and completeness of the data reporting and for the fidelity of the report to the study protocol, which is available with the full text of this article at NEJM.org.
STUDY TREATMENT
Women received gonadotropin (Menopur, Ferring Pharmaceuticals), administered by subcutaneous injection, or clomiphene or letrozole, administered orally by means of coated tablets. Gonadotropin was purchased from Ferring; clomiphene and letrozole were acquired and coated by a third-party company; none of these companies had any involvement in the conduct of the trial. Letrozole and clomiphene were assigned in a double-blind fashion; letrozole was administered for ovarian stimulation under Investigational New Drug application 107705 with the Food and Drug Administration. Medications were initiated on day 3, 4, or 5 of the menstrual cycle, with randomization stratified according to study site and age group (18 to 34 years or 35 to 40 years) and performed on the basis of a varying block design, with ovarian stimulation for up to four treatment cycles and intrauterine insemination. Women who conceived were followed through pregnancy and delivery.
OUTCOMES
The primary outcome was the rate of multiple gestation among women with pregnancies in which fetal heart motion was confirmed on ultrasonography performed at approximately 4 to 6 weeks of gestation; if ultrasonography was performed more than once, we used the highest number of fetal heartbeats identified. The primary outcome was compared first between the letrozole group and the overall standard-therapy group (the clomiphene and gonadotropin groups combined) and then among the three groups, with the use of a superiority design. Secondary outcomes included rates of live birth, multiple gestation with live birth, and pregnancy loss; length of gestation; and maternal, fetal, and neonatal complications. Congenital anomalies were identified at the time of birth or at the time of an examination by a pediatric dysmorphologist, usually within 6 months after birth. Examiners were neither routinely informed nor explicitly kept unaware of the treatment group. We prespecified a 25% reduction in the rate of live births in the letrozole group versus the other two groups combined as the margin in a noninferiority design.
STATISTICAL ANALYSIS
The power calculations and data analysis plan have been published previously.22 Briefly, we calculated that a sample of 900 couples in this three-group study would provide more than 80% power, at a one-sided alpha level of 0.05 and assuming rates for the primary outcome of 25%, 12.5%, and 6.25% in the gonadotropin, clomiphene, and letrozole groups, respectively. Intention-to-treat analyses were performed primarily to compare the letrozole group with the combined gonadotropin and clomiphene groups (since the latter two groups were receiving current standard ovarian-stimulation agents) and secondarily to compare the letrozole group individually with each of the groups receiving standard treatment. Categorical data are reported as frequencies and percentages; differences in these measures between treatment groups were assessed by means of a chi-square analysis, with Fisher’s exact test used for expected frequencies of less than 5. Continuous data are expressed as means ±SD, with a Wilcoxon rank-sum test used for testing differences between two groups, and a Kruskal–Wallis test used for testing differences among the three groups. Analyses were performed with SAS software, version 9.2 (SAS Institute). Statistical significance was defined as a two-sided P value of less than 0.05.
RESULTS
BASELINE CHARACTERISTICS
We prescreened 3730 couples. Of the 1220 couples with unexplained infertility who provided written informed consent and completed the screening, 900 were randomly assigned to a treatment group (see Fig. S1 in the Supplementary Appendix, available at NEJM.org), and 746 of those couples completed the study. There were no significant differences in the frequency of dropouts among study groups (gonadotropin group, 17.3%; clomiphene group, 16.3%; and letrozole group, 17.7%) (Fig. S1 and S2 in the Supplementary Appendix). Baseline characteristics have been reported previously24 and are presented in Table 1; baseline characteristics in the per-protocol analysis are provided in Table S1 in the Supplementary Appendix. Treatment-cycle cancellation rates were 6.9% for gonadotropin, 3.3% for clomiphene, and 3.7% for letrozole (P<0.001 for differences among the three groups) (see Table S2 in the Supplementary Appendix for reasons for cycle cancellation).
Table 1.
Baseline Characteristics of the Women Enrolled in the Study.*
| Characteristic | Gonadotropin (N = 301) |
Clomiphene (N = 300) |
Letrozole (N = 299) |
|---|---|---|---|
| Biometric features | |||
| Age — yr | 32.3±4.1 | 32.0±4.6 | 32.2±4.3 |
| Body-mass index† | 26.8±6.7 | 26.7±6.4 | 27.3±6.5 |
| Race or ethnic group — no. (%)‡ | |||
| White | 238 (79.1) | 241 (80.3) | 243 (81.3) |
| Black | 23 (7.6) | 31 (10.3) | 30 (10.0) |
| Asian | 28 (9.3) | 17 (5.7) | 14 (4.7) |
| Mixed race | 9 (3.0) | 7 (2.3) | 9 (3.0) |
| Hispanic or Latino | 29 (9.6) | 30 (10.0) | 35 (11.7) |
| Fertility history | |||
| Length of time attempting conception — mo | 34.8±26.2 | 34.2±24.1 | 35.2±26.8 |
| Previous live birth — no. (%) | 64 (21.3) | 67 (22.3) | 52 (17.4) |
| Ultrasonographic findings | |||
| Antral follicle count, both ovaries | 20.6±12.6 | 20.3±11.3 | 21.2±11.2 |
| Endometrial thickness, sagittal plane — mm | 6.4±3.1 | 7.0±3.3 | 6.7±3.1 |
| Fasting serum biochemical values | |||
| Estradiol — pg/ml | 33.7±40.4 | 32.4±21.5 | 32.7±28.0 |
| Antimüllerian hormone — ng/ml | 2.6±2.1 | 2.6±2.1 | 2.6±1.9 |
| Follicle-stimulating hormone — mIU/ml | 6.9±2.4 | 7.2±2.8 | 7.0±2.9 |
Data are from Diamond et al.24 Plus–minus values are means ±SD. There were no significant differences (P<0.05) among the three groups in any of the baseline characteristics.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Race or ethnic group was reported by the patients. Some patients chose more than one category, including Hispanic or Latino.
PREGNANCY OUTCOMES
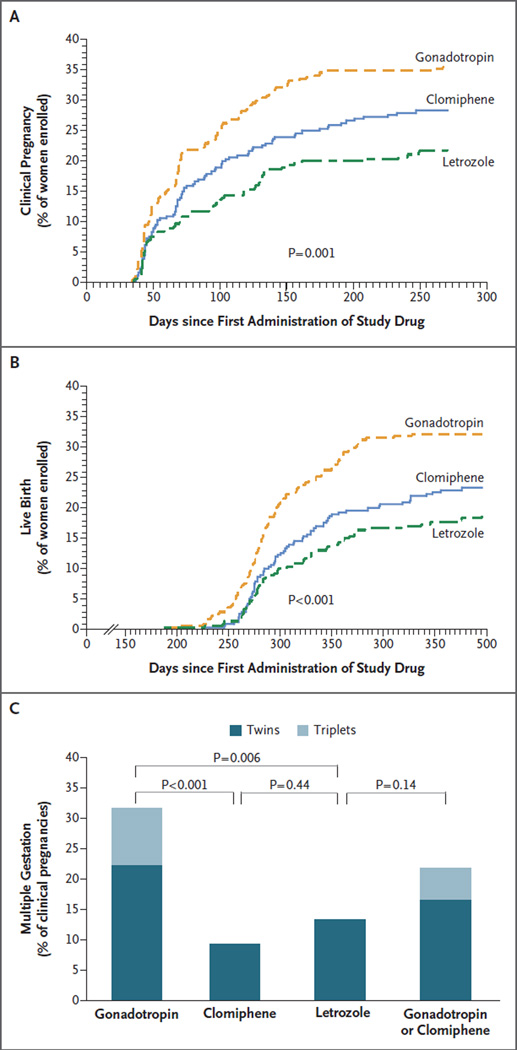
Rates of conception, clinical pregnancy (as evidenced by fetal heart activity), and live birth in the letrozole group (Table S3 and Fig. S2 and S3 in the Supplementary Appendix) were within the prespecified noninferiority margin (i.e., were statistically noninferior) but were statistically significantly lower than those in the standard-therapy group (the combined gonadotropin and clomiphene groups) (Table 2). Rates of live birth were 32.2% after gonadotropin administration, 23.3% after clomiphene administration (P = 0.02 for the comparison with gonadotropin), and 18.7% after letrozole administration (P<0.001 for the comparison with gonadotropin) (Fig. 1A and 1B). The rates of live birth, ongoing clinical pregnancy, and conception per treatment cycle are presented in Table S4 and Fig. S4 in the Supplementary Appendix. There was no significant difference in the time to conception among the three groups (Table S3 in the Supplementary Appendix). Among the women who conceived, there was no significant difference among groups in the incidence of clinical pregnancy (Table S3 in the Supplementary Appendix). The presence of multiple fetal hearts among women who conceived did not differ significantly between the women who received gonadotropin or clomiphene (combined group) and those who received letrozole (P = 0.15) (Table S3 in the Supplementary Appendix). Similarly, among women who had a clinical pregnancy, the frequency of ongoing multiple gestation did not differ significantly between the gonadotropin and clomiphene groups combined and the letrozole group (P = 0.14) (Table 2). When individual groups were compared, the incidence of multiple clinical pregnancy was significantly higher with gonadotropin than with clomiphene or letrozole (Table 2 and Fig. 1C). All the multiple gestations in the clomiphene and letrozole groups were twins; there were 24 twins and 10 triplets in the gonadotropin group (Table S3 in the Supplementary Appendix). Two patients with triplet clinical pregnancies underwent selective embryo reduction, resulting in 2 sets of twin live births.
Table 2.
Rates of Clinical Pregnancy, Live Birth, and Pregnancy Loss.*
| Variable | Gonadotropin (N=301) |
Clomiphene (N = 300) |
Letrozole (N = 299) |
Gonadotropin or Clomiphene (N=601) |
Absolute Difference |
|||
|---|---|---|---|---|---|---|---|---|
| Clomiphene vs. Gonadotropin |
Letrozole vs. Gonadotropin |
Clomiphene vs. Letrozole |
Gonadotropin or Clomiphene vs. Letrozole |
|||||
| number/total number (percent) | percent (95% confidence interval) | |||||||
| Clinical pregnancy among all women enrolled |
107/301 (35.5) | 85/300 (28.3) | 67/299 (22.4) | 192/601 (31.9) | −7.2 (−14.7 to 0.2) |
−13.1 (−20.3 to −6.0)¶ |
5.9 (−1.0 to 12.9) |
9.5 (3.5 to 15.6)§ |
| Multiple gestations among clinical pregnancies |
34/107 (31.8) | 8/85 (9.4) | 9/67 (13.4) | 42/192 (21.9) | −22.4 (−33.2 to −11.6)¶ |
−18.3 (−30.4 to −6.3)§ |
−4.0 (−14.3 to 6.2) |
8.4 (−1.6 to 18.5) |
| Live birth among all women enrolled† |
97/301 (32.2) | 70/300 (23.3) | 56/299 (18.7) | 167/601 (27.8) | −8.9 (−16.0 to −1.8) |
−13.5 (−20.4 to −6.6)¶ |
4.6 (−1.9 to 11.1) |
9.1 (3.4 to 14.8)§ |
| Singleton live birth among all women enrolled |
66/301 (21.9) | 66/300 (22.0) | 48/299(16.1) | 132/601 (22.0) | 0.1 (−6.5 to 6.7) |
−5.9 (−12.1 to 0.4) |
6.0 (−0.3 to 12.2) |
5.9 (0.6 to 11.2) |
| Twin live birth among all women enrolled |
25/301 (8.3) | 4/300 (1.3) | 8/299 (2.7) | 29/601 (4.8) | −7.0 (−10.4 to −3.6)¶ |
−5.6 (−9.2 to −2.0)§ |
−1.3 (−3.6 to 0.9) |
2.2 (−0.4 to 4.7) |
| Triplet live birth among all women enrolled |
6/301 (2.0) | 0 | 0 | 6/601 (1.0) | −2.0 (−3.6 to −0.4) |
−2.0 (−3.6 to −0.4) |
0 | 1.0 (−0.1 to 2.0) |
| Multiple gestations among live births‡ |
31/97(32.0) | 4/70 (5.7) | 8/56 (14.3) | 35/167 (21.0) | −26.2 (−37.0 to −15.5)¶ |
−17.7 (−30.7 to −4.6) |
−8.6 (−19.2 to 2.1) |
6.7 (−4.4 to 17.7) |
| Gestations with ≥1 loss | 51/140 (36.4) | 31/106(29.3) | 26/85 (30.6) | 82/246 (33.3) | −7.2 (−19.9 to 4.6) |
−5.8 (−18.5 to 6.8) |
−1.3 (−14.4 to 11.7) |
2.8 (−8.7 to 14.2) |
| Loss in first trimester | 48/140 (34.3) | 28/106 (26.4) | 25/85 (29.4) | 76/246 (30.9) | −7.9 (−19.4 to 3.6) |
−4.9 (−17.4 to 7.6) |
−3.0 (−15.8 to 9.8) |
1.5 (−9.8 to 12.8) |
| Loss in second or third trimester | 3/140 (2.1) | 3/106 (2.8) | 1/85 (1.2) | 6/246 (2.4) | 0.7 (−3.3 to 4.7) |
−1.0 (−4.3 to 2.4) |
1.7 (−2.3 to 5.6) |
1.3 (−1.7 to 4.3) |
Clinical pregnancy was defined as an intrauterine pregnancy with fetal heart motion, as determined by transvaginal ultrasonography. Live birth was defined as the delivery of a viable infant.
Ten patients with clinical pregnancy (one in the gonadotropin group, six in the clomiphene group, and three in the letrozole group) were excluded because they were lost to follow-up before delivery.
Multiple gestations were calculated as the number of deliveries with multiple babies divided by the number of women with live births.
P<0.01.
P=0.001.
Figure 1. Pregnancy Rates in the Gonadotropin, Clomiphene, and Letrozole Groups.
Shown are rates of clinical pregnancy among all women enrolled in the study (Panel A), rates of live birth among all women enrolled (Panel B), and rates of multiple gestation among all clinical pregnancies (Panel C), according to the study treatment. In Panels A and B, the P value is for the comparison among the three treatment groups.
The rate of pregnancy loss among established pregnancies did not differ significantly according to treatment (36.4% with gonadotropin, 29.2% with clomiphene, and 30.6% with letro-zole) (Table S3 in the Supplementary Appendix). Most losses occurred in the first trimester (94.1%, 90.3%, and 96.2% of pregnancy losses, respectively); pregnancies with no identified fetal heart motion accounted for approximately half of the first-trimester losses in each treatment group. There were 21 documented ectopic pregnancies, and 3 losses of pregnancies of unknown location; frequencies of pregnancy loss were similar with the three treatments for loss after identification of fetal heart activity in the first trimester and for loss in the second or third trimester.
The pregnancy outcomes based on a perprotocol analysis are shown in Table S5 in the Supplementary Appendix. These results were consistent with the results of the intention-to-treat analysis.
NEONATAL OUTCOMES
A total of 272 infants were live-born. There was a significant difference among groups in the mean pregnancy duration overall (P = 0.02) but not for singleton gestations specifically (Table 3). As anticipated, the mean gestational age at delivery decreased with an increasing number of fetuses (38.6 weeks for singletons, 35.3 weeks for twins, and 34.2 weeks for triplets [P<0.001]). There were 9 infants (from 8 pregnancies) in whom congenital anomalies were identified (4, 3, and 2 in the gonadotropin, clomiphene, and letrozole groups, respectively) (Table 3); no treatment-specific patterns were identified (Table S6 in the Supplementary Appendix). There was 1 neonatal death, which was in the letrozole group and was due to preterm labor and delivery, at approximately 23 weeks of gestation.
Table 3.
Neonatal Outcomes According to Treatment Group and Number of Fetuses.*
| Outcome | Gonadotropin | Clomiphene | Letrozole | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Singleton | Twin | Triplet | Total | Singleton | Twin | Total | Singleton | Twin | Total | |
| Live delivery by mother — no. |
66† | 25‡ | 6‡ | 97 | 66 | 4 | 70 | 48 | 8 | 56 |
| Estimated gestational age at delivery — wk |
38.6±1.5 | 35.0±3.2 | 34.2±1.6 | 37.4±2.7§ | 38.6±1.7 | 37.3±1.5 | 38.5±1.7 | 38.5±3.3 | 35.0±1.6 | 37.7±4.0 |
| Birth weight — g¶ | 3211±540 | 2271±602 | 1847±245 | 2891±726§ | 3215±529 | 2693±605 | 3184±543 | 3267±805 | 2256±439 | 3138±837 |
| Male infants, including twins and triplets — no./total no. (%) |
32/66 (48) | 23/50 (46) | 10/18 (56) | 65/134 (49) | 30/66 (46) | 4/8 (50) | 34/74 (46) | 25/48 (52) | 8/16 (50) | 33/64 (52) |
| NICU admission — no./total no. (%) |
5/66 (8) | 10/25 (40)║ | 6/6 (100)║ | 21/97 (22) | 7/66 (11) | 0/4§ | 7/70 (10) | 5/48 (10) | 6/8 (75) | 11/56 (20) |
| Length of neonatal hospi- talization — days** |
8.0±3.6 | 39.4±23.0 | 17.6±4.8 | 24.3±19.9 | 17.5±3.9 | NA | 17.5±3.9 | 23.5±15.5 | 17.8±8.1 | 21.1±12.3 |
| Neonatal complications — no./total no. (%) |
15/66 (23) | 11/25 (44) | 4/6 (67) | 30/97 (31) | 12/66 (18) | 1/4 (25) | 13/70 (19) | 2/48 (25) | 3/8 (38) | 15/56 (27) |
| Congenital malformations — no./total no. (%) |
1/66 (2) | 2/25 (8)†† | 0/6 | 3/97 (3) | 3/66 (5) | 0/4 | 3/70 (4) | 2/48 (4) | 0/8 | 2/56 (4) |
Plus-minus values are means ±SD. Differences in outcomes were compared among the three treatment groups. In the clomiphene group, estimated gestational age was missing for one singleton, and birth weight was missing for two singletons; in the gonadotropin and letrozole groups, birth weights were missing for one set of twins. NA denotes not applicable, and NICU neonatal intensive care unit.
P<0.001 for the comparison among the three treatment groups.
P<0.05 for the comparison among the three treatment groups.
P<0.05.
For multiple births, the mean birth weight of each of the twins or triplets was calculated first; this average was used in the calculation of the means ±SD for that treatment group.
Shown are the number of twin or triplet deliveries for which the twins (except one in the letrozole group) or triplets were admitted to the NICU.
Data shown are only for infants hospitalized for more than 3 days. For twins and triplets, the length of hospitalization was calculated as the average number of days that the twins or triplets were in the hospital.
One set of twins had congenital malformations; in the other set, only one neonate had a congenital malformation.
Mean birth weights are presented in Table 3; for singleton gestations, the mean birth weight did not differ significantly among the three treatment groups. There was also no significant difference among groups in the distribution of the infants according to sex. Among singleton infants, rates of neonatal complications and admission to the neonatal intensive care unit did not differ significantly according to the treatment group. The most common neonatal complications in all groups were jaundice, the respiratory distress syndrome, and intrauterine growth restriction (Table S7 in the Supplementary Appendix).
SAFETY
The frequency of individual serious adverse maternal events (before conception or during or after pregnancy) and serious adverse fetal or neonatal events did not differ significantly among the three treatment groups, although cumulative serious adverse events were more common in the gonadotropin group than in the other two groups (P = 0.009) (Table 4). Overall rates of non-serious adverse events were similar among the groups, but the frequencies of specific adverse events differed according to the group. The frequency of abdominal bloating was higher with gonadotropin administration than with the other two treatments. One woman, who was in the gonadotropin group, received a diagnosis of the ovarian hyperstimulation syndrome; also, the frequency of treatment-cycle cancellation owing to concern about possible development of the ovarian hyperstimulation syndrome was higher in the gonadotropin group than in the other two groups (see Table S2 in the Supplementary Appendix).
Table 4.
Adverse Events and Serious Adverse Events According to Treatment Group.*
| Event | Gonadotropin | Clomiphene | Letrozole | P Value† |
|---|---|---|---|---|
| no./total no. (%) | ||||
| Overall adverse events | ||||
| ≥1 Serious adverse event | 21/297 (7.1) | 8/298 (2.7) | 8/291 (2.7) | 0.009 |
| ≥1 Adverse event | 208/297 (70.0) | 212/298 (71.1) | 214/291 (73.5) | 0.63 |
| Adverse events before conception | ||||
| Serious adverse events | ||||
| Presumed pyelonephritis | 1/297 (0.3) | 0/298 | 0/291 | 0.66 |
| Pyosalpinx after intrauterine insemination | 1/297 (0.3) | 0/298 | 0/291 | 0.66 |
| Other adverse events | ||||
| Abdominal bloating | 81/297 (27.3) | 50/298 (16.8) | 54/291 (18.6) | 0.003 |
| Breast pain | 65/297 (21.9) | 19/298 (6.4) | 21/291 (7.2) | <0.001 |
| Constipation | 6/297 (2.0) | 28/298 (9.4) | 8/291 (2.7) | <0.001 |
| Headache | 89/297 (30.0) | 104/298 (34.9) | 122/291 (41.9) | 0.01 |
| Hot flashes | 25/297 (8.4) | 92/298 (30.9) | 49/291 (16.8) | <0.001 |
| Injection-site reaction | 32/297 (10.8) | 6/298 (2.0) | 9/291 (3.1) | <0.001 |
| Joint or limb pain | 5/297 (1.7) | 8/298 (2.7) | 17/291 (5.8) | 0.02 |
| Adverse events after conception | ||||
| Serious adverse events | ||||
| First trimester | ||||
| Cholecystitis | 1/139 (0.7) | 0/107 | 0/83 | 1.00 |
| Ectopic pregnancy | 11/139 (7.9) | 5/107 (4.7) | 5/83 (6.0) | 0.58 |
| Ovarian hyperstimulation syndrome | 1/139 (0.7) | 0/107 | 0/83 | 1.00 |
| Pain due to ovarian enlargement | 1/139 (0.7) | 0/107 | 0/83 | 1.00 |
| Pregnancy of unknown location | 1/139 (0.7) | 2/107 (1.9) | 0/83 | 0.48 |
| After first trimester | ||||
| Hemorrhagic hematoma | 0/139 | 0/107 | 1/83 (1.2) | 0.25 |
| Hospitalization | 1/139 (0.7)‡ | 0/107 | 1/83 (1.2)§ | 0.72 |
| Hyperemesis | 2/139 (1.4) | 0/107 | 0 | 0.51 |
| Hypertension | 0/139 | 1/107 (0.9) | 1/83 (1.2) | 0.33 |
| Severe preeclampsia | 0/139 | 1/107 (0.9) | 0/83 | 0.58 |
| Emergency cesarean section | 1/139 (0.7) | 0/107 | 0/83 | 1.00 |
| Other adverse events | ||||
| Breast pain, first trimester | 0/139 | 0/107 | 5/83 (6.0) | 0.001 |
| Serious adverse events after 20 wk of gestation | ||||
| Congenital anomaly¶ | 3/96 (3.1) | 3/70 (4.3) | 2/56 (3.6) | 0.90 |
| Neonatal death | 0/96 | 0/70 | 1/56 (1.8) | 0.25 |
The event summary for mothers includes all women with at least one adverse event or serious adverse event. Specific events listed in this table include all the serious adverse events and all the adverse events that were observed in at least 5% of women in any of the treatment groups and that differed significantly among the three groups.
P values are for the comparison among the three treatment groups. The chi-square test or Fisher’s exact test was used.
The patient was admitted to the hospital at 34 weeks of gestation for lower abdominal, pelvic, and vaginal pain, with a second admission for management of severe groin pain.
One week after an uncomplicated delivery by cesarean section, the patient presented to the emergency department with shortness of breath. She was admitted to the hospital and evaluated for pulmonary edema.
For details, see Table S6 in the Supplementary Appendix.
A list of maternal serious adverse events (all) and nonserious adverse events (those that occurred in >2% of women in any group) before conception, as well as all other adverse events that occurred after conception, is presented in Table S8 in the Supplementary Appendix. The frequency of placental abnormalities did not differ significantly among the groups (Table S9 in the Supplementary Appendix).
DISCUSSION
In this study of couples with unexplained infertility, the rate of multiple gestation was not significantly reduced among women treated with letrozole for ovarian stimulation, as compared with a combined group of women receiving current standard therapy (gonadotropin or clomiphene). The multiple-gestation rate in the letrozole group was significantly lower than the rate in the gonadotropin group alone but was similar to the rate in the clomiphene group; as compared with gonadotropin, clomiphene also resulted in a significantly lower rate of multiple gestation. The only higher-order multiple gestations (all triplets) occurred after gonadotropin administration.
Administration of letrozole resulted in rates of conception, clinical pregnancy, and live birth that were statistically noninferior to the rates with clomiphene, according to our prespecified noninferiority margin of 25%. However, clomiphene and letrozole each resulted in significantly lower rates of conception, clinical pregnancy, and live birth, as compared with gonadotropin. In contrast, many reports in the literature have suggested a similar or improved pregnancy rate with an aromatase inhibitor, as compared with standard therapy.12–14,16–18,25 Possible explanations for these conflicting results may be the randomized design of our study, our standardized criteria for the timing of gonadotropin administration, the standardized timing of insemination in all treatment groups, or some combination of these factors.
The live birth rate among women receiving clomiphene in this study was slightly higher than the rate observed after clomiphene administration in the Reproductive Medicine Network’s Pregnancy in Polycystic Ovarian Syndrome II (PPCOS II) study,11 which involved women with infertility due to the polycystic ovary syndrome, rather than unexplained infertility. However, in the AMIGOS trial, the rate of live birth was reduced after letrozole administration, whereas in the PPCOS II study, letrozole treatment was associated with a significant increase in live births, as compared with clomiphene treatment. The explanation for this difference is uncertain. It is possible that letrozole has a greater antiestrogenic effect on endometrial development in women with unexplained infertility than in women with the polycystic ovary syndrome, resulting in a suboptimal endometrium or altered hormonal milieu that is detrimental to implantation and placentation. Another possibility is that aromatase inhibition, which led to lower androgen levels in women with unexplained infertility and to higher androgen levels in those with the polycystic ovary syndrome, resulted in differential elevations of tissue androgen levels in the hypothalamus, pituitary, ovary, or endometrium.
In this large study, letrozole was not associated with increased risks of serious adverse maternal, fetal, or neonatal outcomes, as compared with the risks in the gonadotropin and clomiphene groups combined. Longer-term follow-up of these infants is ongoing. Although data about the risk of congenital anomalies after the use of an aromatase inhibitor have been limited,21 the frequency of congenital anomalies in our trial after treatment with letrozole (3.6%) did not differ significantly from the frequency after treatment with gonadotropin (3.1%) or clomiphene (4.3%).
A limitation of our study is the lack of blinding for the gonadotropin group. In addition, the trial was powered for a comparison of the letrozole group with the gonadotropin and clomiphene groups combined, not for individualized group comparisons. This prespecified primary comparison of letrozole with standard therapy is limited by the fact that the two standard agents had different effects. We did not include a placebo control group because we considered it inappropriate to do so in a study involving infertile couples who had been trying to achieve pregnancy for more than 1 year (mean, almost 3 years). A previous study, which included a control group of women who underwent timed intracervical insemination without ovarian stimulation, showed significantly lower pregnancy and delivery rates in the control group than in the gonadotropin group or the clomiphene group.9
In conclusion, in our study involving couples with unexplained infertility, the use of letrozole for ovarian stimulation resulted in significantly reduced rates of ongoing clinical pregnancy and live birth, but not of multiple gestations, as compared with a combined group receiving standard therapy (gonadotropin or clomiphene), but the two comparators had different effects on these outcomes. As compared with gonadotropin use, the use of letrozole resulted in lower rates of live birth and multiple gestation, whereas the rates of these outcomes did not differ significantly between letrozole and clomiphene.
Supplementary Material
Acknowledgments
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Supported by grants from the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10 HD39005, to Dr. Diamond; U10 HD38992, to Dr. Legro; U10 HD27049, to Dr. Coutifaris; U10 HD38998, to Dr. Alvero; U10 HD055942, to Dr. Robinson; U10 HD055944, to Dr. Casson; U10 HD055936, to Dr. Christman; U10HD055925, to Dr. Zhang; and U10 U54-HD29834, to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core of the Specialized Cooperative Centers Program in Reproduction and Infertility Research), the National Center for Research Resources, and the National Center for Advancing Translational Sciences (UL1 TR000127, to Pennsylvania State University) and by the American Recovery and Reinvestment Act.
APPENDIX
The authors’ full names and academic degrees are as follows: Michael P. Diamond, M.D., Richard S. Legro, M.D., Christos Coutifaris, M.D., Ph.D, Ruben Alvero, M.D., Randal D. Robinson, M.D., Peter Casson, M.D., Gregory M. Christman, M.D., Joel Ager, Ph.D., Hao Huang, M.D., M.P.H., Karl R. Hansen, M.D., Ph.D., Valerie Baker, M.D., Rebecca Usadi, M.D., Aimee Seungdamrong, M.D., G. Wright Bates, M.D., R. Mitchell Rosen, M.D., Daniel Haisenleder, Ph.D., Stephen A. Krawetz, Ph.D., Kurt Barnhart, M.D., J.C. Trussell, M.D., Dana Ohl, M.D., Yufeng Jin, M.S., Nanette Santoro, M.D., Esther Eisenberg, M.D., M.P.H., and Heping Zhang, Ph.D., for the NICHD Reproductive Medicine Network
The authors’ affiliations are as follows: the Department of Obstetrics and Gynecology, Georgia Regents University, Augusta (M.P.D.); Department of Obstetrics and Gynecology, Wayne State University, Detroit (M.P.D., J.A., S.A.K.); Department of Obstetrics and Gynecology, Pennsylvania State University, Hershey (R.S.L.); Department of Obstetrics and Gynecology, University of Pennsylvania School of Medicine, Philadelphia (C.C., K.B.); Department of Obstetrics and Gynecology, University of Colorado, Denver (R.A., N.S.); Department of Obstetrics and Gynecology, University of Texas Health Science Center at San Antonio, San Antonio (R.D.R.); Department of Obstetrics and Gynecology, University of Vermont, Burlington (P.C.); Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor (G.M.C., D.O.); Department of Biostatistics, Yale University School of Public Health, New Haven, CT (H.H., Y.J., H.Z.); Department of Obstetrics and Gynecology, University of Oklahoma College of Medicine, Oklahoma City (K.R.H.); Stanford University Medical Center, Stanford, CA (V.B.); Carolinas Medical Center, Charlotte, NC (R.U.); University of Medicine and Dentistry of New Jersey, Newark (A.S.); University of Alabama at Birmingham, Birmingham (G.W.B.); Department of Reproductive Endocrinology and Infertility, University of California, San Francisco, San Francisco (R.M.R.); Ligand Core Laboratory, University of Virginia Center for Research in Reproduction, Charlottesville (D.H.); Upstate University Hospital, Syracuse, NY (J.C.T.); and Fertility and Infertility Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, MD (E.E.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.McClamrock HD, Jones HW, Jr, Adashi EY. Ovarian stimulation and intrauterine insemination at the quarter centennial: implications for the multiple births epidemic. Fertil Steril. 2012;97:802–809. doi: 10.1016/j.fertnstert.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Ghesquiere SL, Castelain EG, Spies-sens C, Meuleman CL, D’Hooghe TM. Relationship between follicle number and (multiple) live birth rate after controlled ovarian hyperstimulation and intrauter-ine insemination. Am J Obstet Gynecol. 2007;197(4):589.e1–589.e5. doi: 10.1016/j.ajog.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Samani FG, Farzadi L, Nezami N, Tar-zamni MK, Soleimani F. Endometrial and follicular development following letro-zole intervention in unexplained infertile patients failed to get pregnant with clo-miphene citrate. Arch Gynecol Obstet. 2009;280:201–205. doi: 10.1007/s00404-008-0888-9. [DOI] [PubMed] [Google Scholar]
- 4.Fisch P, Casper RF, Brown SE, et al. Unexplained infertility: evaluation of treatment with clomiphene citrate and human chorionic gonadotropin. Fertil Steril. 1989;51:828–833. doi: 10.1016/s0015-0282(16)60674-x. [DOI] [PubMed] [Google Scholar]
- 5.Practice Committee of American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion. Fertil Steril. 2012;97:825–834. doi: 10.1016/j.fertnstert.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds MA, Schieve LA, Martin JA, Jeng G, Macaluso M. Trends in multiple births conceived using assisted reproductive technology, United States, 1997–2000. Pediatrics. 2003;111:1159–1162. [PubMed] [Google Scholar]
- 7.Schieve LA, Devine O, Boyle CA, Pe-trini JR, Warner L. Estimation of the contribution of non-assisted reproductive technology ovulation stimulation fertility treatments to US singleton and multiple births. Am J Epidemiol. 2009;170:1396–1407. doi: 10.1093/aje/kwp281. [DOI] [PubMed] [Google Scholar]
- 8.Cook JL, Geran L, Rotermann M. Multiple births associated with assisted human reproduction in Canada. J Obstet Gynaecol Can. 2011;33:609–616. doi: 10.1016/S1701-2163(16)34909-X. [DOI] [PubMed] [Google Scholar]
- 9.Guzick DS, Carson SA, Coutifaris C, et al. Efficacy of superovulation and intra-uterine insemination in the treatment of infertility. N Engl J Med. 1999;340:177–183. doi: 10.1056/NEJM199901213400302. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni AD, Jamieson DJ, Jones HW, Jr, et al. Fertility treatments and multiple births in the United States. N Engl J Med. 2013;369:2218–2225. doi: 10.1056/NEJMoa1301467. [DOI] [PubMed] [Google Scholar]
- 11.Legro RS, Brzyski RG, Diamond MP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitwally MF, Casper RF. Aromatase inhibition reduces gonadotrophin dose required for controlled ovarian stimulation in women with unexplained infertility. Hum Reprod. 2003;18:1588–1597. doi: 10.1093/humrep/deg311. [DOI] [PubMed] [Google Scholar]
- 13.Al-Fozan H, Al-Khadouri M, Tan SL, Tulandi T. A randomized trial of letrozole versus clomiphene citrate in women un- dergoing superovulation. Fertil Steril. 2004;82:1561–1563. doi: 10.1016/j.fertnstert.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 14.Bayar U, Tanriverdi HA, Barut A, Ayoğlu F, Ozcan O, Kaya E. Letrozole vs. clomiphene citrate in patients with ovulatory infertility. Fertil Steril. 2006;85:1045–1048. doi: 10.1016/j.fertnstert.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 15.Al-Fadhli R, Sylvestre C, Buckett W, Tan SL, Tulandi T. A randomized trial of superovulation with two different doses of letrozole. Fertil Steril. 2006;85:161–164. doi: 10.1016/j.fertnstert.2005.07.1283. [DOI] [PubMed] [Google Scholar]
- 16.Gregoriou O, Vlahos NF, Konidaris S, Papadias K, Botsis D, Creatsas GK. Randomized controlled trial comparing superovulation with letrozole versus re-combinant follicle-stimulating hormone combined with intrauterine insemination for couples with unexplained infertility who had failed clomiphene citrate stimulation and intrauterine insemination. Fer-til Steril. 2008;90:678–683. doi: 10.1016/j.fertnstert.2007.06.099. [DOI] [PubMed] [Google Scholar]
- 17.Badawy A, Elnashar A, Totongy M. Clomiphene citrate or aromatase inhibitors for superovulation in women with unexplained infertility undergoing intra- uterine insemination: a prospective randomized trial. Fertil Steril. 2009;92:1355–1359. doi: 10.1016/j.fertnstert.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Fouda UM, Sayed AM. Extended letro-zole regimen versus clomiphene citrate for superovulation in patients with unexplained infertility undergoing intrauter-ine insemination: a randomized controlled trial. Reprod Biol Endocrinol. 2011;9:84. doi: 10.1186/1477-7827-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavone ME, Bulun SE. The use of aro-matase inhibitors for ovulation induction and superovulation. J Clin Endocrinol Metab. 2013;98:1838–1844. doi: 10.1210/jc.2013-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintero RB, Urban R, Lathi RB, Westphal LM, Dahan MH. A comparison of letrozole to gonadotropins for ovula-tion induction, in subjects who failed to conceive with clomiphene citrate. Fertil Steril. 2007;88:879–885. doi: 10.1016/j.fertnstert.2006.11.166. [DOI] [PubMed] [Google Scholar]
- 21.Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006;85:1761–1765. doi: 10.1016/j.fertnstert.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Diamond MP, Mitwally M, Casper R, et al. Estimating rates of multiple gestation pregnancies: sample size calculation from the Assessment of Multiple Intra-uterine Gestations from Ovarian Stimulation (AMIGOS) trial. Contemp Clin Trials. 2011;32:902–908. doi: 10.1016/j.cct.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickey RP, Pyrzak R, Lu PY, Taylor SN, Rye PH. Comparison of the sperm quality necessary for successful intrauterine insemination with World Health Organization threshold values for normal sperm. Fertil Steril. 1999;71:684–689. doi: 10.1016/s0015-0282(98)00519-6. [DOI] [PubMed] [Google Scholar]
- 24.Diamond MP, Legro RS, Coutifaris C, et al. Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) trial: baseline characteristics. Fertil Steril. 2015;103:962–973. e4. doi: 10.1016/j.fertnstert.2014.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polyzos NP, Tzioras S, Badawy AM, Valachis A, Dritsas C, Mauri D. Aromatase inhibitors for female infertility: a systematic review of the literature. Re-prod Biomed Online. 2009;19:456–471. doi: 10.1016/j.rbmo.2009.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.