Abstract
The endocannabinoid system, including endogenous ligands ('endocannabinoids' ECs), their receptors, synthesizing and degrading enzymes, as well as transporter molecules, has been detected from the earliest stages of embryonic development and throughout pre- and postnatal development. ECs are bioactive lipids, which comprise amides, esters and ethers of long chain polyunsaturated fatty acids. Anandamide (N-arachidonoylethanolamine; AEA) and 2-arachidonoylglycerol (2-AG) are the best studied ECs, and act as agonists of cannabinoid receptors. Thus, AEA and 2-AG mimic several pharmacological effects of the exogenous cannabinoid delta9-tetrahydrocannabinol (Δ9-THC), the psychoactive principle of cannabis sativa preparations like hashish and marijuana. Recently, however, several lines of evidence have suggested that the EC system may play an important role in early neuronal development as well as a widespread role in neurodegeneration disorders. Many of the effects of cannabinoids and ECs are mediated by two G protein-coupled receptors (GPCRs), CB1 and CB2, although additional receptors may be implicated. Both CB1 and CB2 couple primarily to inhibitory G proteins and are subject to the same pharmacological influences as other GPCRs. This new system is briefly presented in this review, in order to put in a better perspective the role of the EC pathway from neurodevelopment to neurodegenerative disorders, like Alzheimer's disease, Parkinson's disease, Huntington's disease, and multiple sclerosis. In addition, the potential exploitation of antagonists of CB1 receptors, or of inhibitors of EC metabolism, as next-generation therapeutics is discussed.
Keywords: Neurodevelopment, endocannabinoids, CNS, synaptic plasticity, neurodegeneration, CB1 receptors, therapy
INTRODUCTION
The earliest anthropological evidence of Cannabis use comes from the oldest known Neolithic culture in China, where it was used in the production of hemp for ropes and textiles and also for its psychotropic effects [1]. An 1848 commentary in the British Pharmacopoeia outlined quite accurately the psychotropic effects of Cannabis and pointed out its merits as an antispasmodic and analgesic [2].
The major psychoactive constituent of Cannabis sativa is Δ9-tetrahydrocannabinol (Δ9-THC, dronabinol), (Fig. 1) which is mainly responsible for the pharmacological effects of the Cannabis plant [3, 4]. Δ9-THC was isolated, stereochemically defined, and synthesized in 1964 [5], and its psychoactive properties were recognized immediately. Currently Δ9-THC and its analogs are used for the treatment of nausea and vomiting induced by radiotherapy or chemotherapy, and wasting syndrome in AIDS patients. Although controversy exists, cannabinoids have also been suggested for the treatment of pain, spastic states, glaucoma and other disorders [6]. However, the clinical usefulness of Δ9-THC and its analogs is greatly hampered by their numerous side effects, including the potential for abuse [7, 8]. In recent years, cannabinoid research received tremendous attention from various researchers due to the breakthrough discovery of the receptors that bind Δ9-THC (Cannabinoid receptors) and their endogenous ligands, endocannabinoids (ECs), in animal tissues referred to as the endocannabinoid system. This emerging body of research has revealed multiple ways in which the EC system functions to regulate synaptic neurotransmission in various areas [9-11] of the developing as well as the adult brain. Continuing research has elucidated vital functions for EC signaling in molecular pathways that underlie both short- and long-lasting alterations in synaptic strength [12, 13]. In fact, the critical involvement of ECs in some mechanisms of synaptic neurotransmission may change the current thinking regarding the cellular models of learning and memory. These models may be pivotal in understanding and providing potential treatment for the rewarding and amnestic actions of marijuana drugs. This review is focused on our understanding of the EC system in brain function from neurodevelopment to neurodegeneration. In addition, the potential exploitation of antagonists of CB1 receptors (Fig. 2), or of inhibitors of EC metabolism, as next-generation therapeutics is discussed.
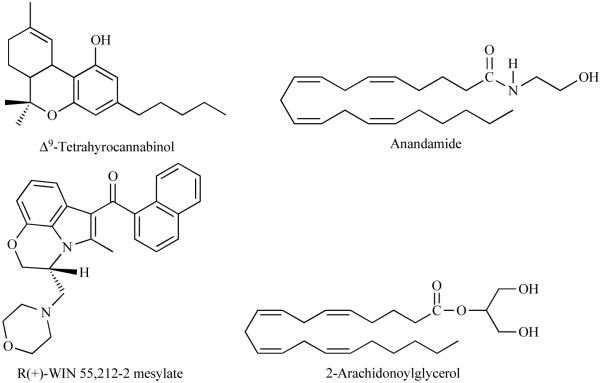
Fig. (1).
Chemical structure of CB1 receptor exogenous (THC and WIN55, 212-2) and endogenous (AEA and 2-AG) agonists.
Fig. (2).
Chemical structure of CB1 receptor antagonists.
CANNABINOID RECEPTORS
Evidence for the existence of the marijuana receptor has been available since the 1980s [14, 15]. It has now been shown that cannabinoids have two specific receptor subtypes, named CB1 and CB2, which have been cloned. Evidence for a third receptor (“CB3” or “Anandamide receptor”) in brain and in endothelial tissues has been reported in the literature [16-19], however, its cloning, expression and characterization have not yet been accomplished.
CB1 and CB2 receptors belong to the large superfamily of heptahelical G protein-coupled receptors (GPCR) and couple to Gi/o proteins (For more details see reviews [20-22]). The CB1 receptor is mainly expressed in brain and spinal cord and thus is often referred to as the “brain cannabinoid receptor”. CB1 receptors are among the most abundant GPCRs in the brain, their densities being similar to levels of γ-aminobutyric acid (GABA)- and glutamate-gated ion channels [23]. The presence of functional CB2 receptors in the CNS has provoked considerable controversy over the past few years. Formerly considered as an exclusively peripheral receptor [24, 25] and often referred as the “peripheral cannabinoid receptor”, it is now accepted that it is also present in limited amounts and distinct locations in the brain of several animal species, including humans [26, 27]. However, the functional relevance of this receptor in the CNS is emerging slowly [28].
The cDNA sequences encoding CB1- or CB2-like receptors have been reported in various species including human (For review see [21]). Human CB1 and CB2 receptors share 44% overall amino acid identity (For more details see recent review [29]). The CB2 receptor shares 81% amino acid identity between rat and mouse or human. Although significant progress has been achieved in many aspects of the biology of cannabinoid receptors and our knowledge of cannabinoid receptor genomics and proteomics is increasing, the regulation of cannabinoid receptor genes is still poorly understood.
THE SIGNAL TRANSDUCTION MECHANISM OF CB1 RECEPTORS
Activation of a cannabinoid receptor promotes its interaction with G proteins, resulting in guanosine diphosphate/guanosine triphosphate exchange and subsequent dissociation of the α and βy subunits. These subunits regulate the activity of multiple effector proteins to bring about biological functions (Fig. 3). CB1 is coupled with Gi or Go proteins. CB1 receptors differ from many other GPCR proteins in being constitutively active, as they are precoupled with G-proteins in the absence of exogenously added agonists [30]. This property of being constitutively active is similar to the majority of receptors including ionotropic and metabotropic. However, the extent of this constitutive activity varies from receptor- to receptor, species-to species, and location-to location [31]. Among its cellular actions are inhibition of adenylate cyclase activity [32-34], N-type voltage-gated channels [35-38], N-type, P/Q-type calcium channels, and D-type potassium channels [34, 39], activation of A-type and inwardly rectifying potassium channels [40], and inhibition of synaptic transmission [39, 41]. Based on these findings, it has been suggested that CB1 receptors play a role in the regulation of neurotransmitter release [39, 41].
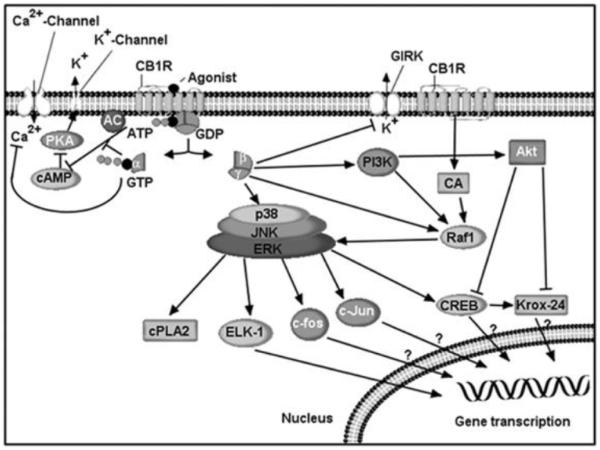
Fig. (3). CB1 receptor signaling.
CB1 receptors are G-protein-coupled transmembrane proteins located in the cell membrane. The Ca2+ channels inhibited by CB1 receptors include N-, P/Q- and L-type channels. Actions on Ca2+ channels and adenylyl cyclase (AC) are thought to be mediated by the α subunits of the G-protein, and those on GIRK, MAPK and PI3K by the βγ subunits. CB1 receptor activation stimulates phosphorylation of p130-Cas (CA), a protein associated with FAK in the hippocampus. Inhibition of AC and the subsequent decrease in cAMP decreases activation of cAMP-dependent protein kinase A (PKA), which leads to decreased phosphorylation of the K+ channels. Stimulatory effects are shown by a (→) sign and inhibitory effects by a (⟂) sign.
In addition, one of the most interesting research areas is the regulation of neuritogenesis, axonal growth and synaptogenesis by CB1 receptors (For references see recent article [42]). The molecular mechanism involved in this process is not yet clear. The CB1 receptor activates MAPK pathway [43]. In some cells, CB1 receptor-mediated activation of MAPK was mediated through the PI3 kinase pathway [43, 44]. AEA, CP,55, 940 and WIN 55,212-2 increased phosphorylation of FAK+ 6,7, a neural isoform of FAK, in hippocampal slices and in cultured neurons [45]. CB1 receptor activation stimulates phosphorylation of the Tyr-397 residue of FAK in the hippocampus, which is crucial for FAK activation [46] and increases phosphorylation of p130-Cas, a protein associated with FAK in the hippocampus. CB1 receptor-stimulated FAK-autophosphorylation was shown to be upstream of the Src family kinases [46]. These new downstream effectors of CB1 receptors are quite likely to play a role in some forms of synaptic plasticity through gene regulation, but this needs further investigation.
ENDOCANNABINOIDS
The ECs are lipid signaling molecules that bind to and activate cannabinoid receptors. These lipid compounds are formed from phospholipids precursors [47-52] within cells throughout the body, and are released from these cells on demand in a nonvesicular manner to act in a paracrine fashion [47, 49-53].
Beginning in 1992, the first endogenous cannabinoid was identified as anandamide (AEA, arachidonylethanolamide). It was named from the Sanskrit ananda, “internal bliss,’’ making reference to its chemical structure (the amide of arachidonic acid and ethanolamine) [54]. Subsequently, another endogenous cannabinoid receptor ligand, 2-arachidonylglycerol (2-AG) was discovered and characterized [55, 56]. The third ether-type EC, 2-arachidonylglycerol ether (noladin ether), was isolated from the CNS and shown to display pharmacological properties similar to AEA [57]. The fourth type of EC, virodhamine, in contrast to the previously described ECs, is a partial agonist with in vivo antagonist activity at the CB1 receptor [58]. The fifth type of EC, N-arachidonyl-dopamine (NADA), not only binds to CB1 receptor but also stimulates vanilloid receptors (VR1) [59]. It should be noted that except AEA and 2-AG, to date, there is little evidence about the physiological actions of these compounds.
AEA is believed to be synthesized by several pathways (see recent review for details [21]) (Fig. 4A). Notably, there is a strong evidence for calcium dependence in both of these synthesis steps, which may underlie the requirement for postsynaptic Ca2+ in certain forms of depolarization-induced synaptic plasticity (For details see) [13]. As a putative neuromodulator, AEA that is released into the synaptic cleft is expected to be rapidly inactivated. In general, two mechanisms are known that could remove ECs from the synaptic cleft to ensure rapid signal inactivation: re-uptake or enzymatic degradation. AEA is inactivated by reuptake [60, 61] via an uncharacterized membrane transport molecule, the ‘AEA membrane transporter’ (AMT) [52, 60, 62-66], and subsequent intracellular enzymatic degradation. AEA is metabolized to arachidonic acid and ethanolamine via the action of the fatty acid amide hydrolase (FAAH), and this activity plays a significant role in the rapid clearance of AEA from extracellular compartments [67, 68]. In addition to hydrolysis by FAAH, AEA is metabolized by COX-2, LOX and cytochrome P450 [65, 69-71]. Further research is required to elucidate the exact mechanism and enzymes involved in this pathway of AEA metabolism.
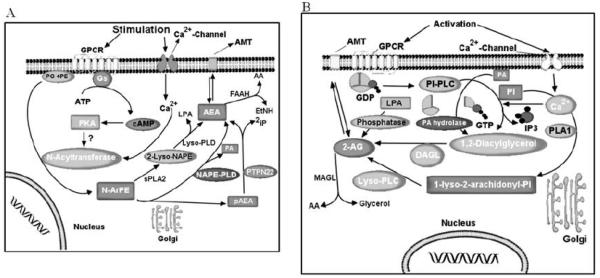
Fig. (4). The potential biosynthetic pathways of endocannabinoids.
A: The synthesis of anandamide (AEA) from membrane N-arachidonoylphosphatidylethanolamines is catalyzed by the sequential activity of N-acyltransferase and NAPE-specific phospholipase D (NAPE-PLD), which releases AEA and phosphatidic acid (PA). AEA is transported in both directions through the cell membrane by a selective AEA membrane transporter (AMT) and, once taken up, is hydrolyzed by fatty acid amide hydrolase (FAAH) to ethanolamine (EtNH2) and arachidonic acid (AA). B: 2-Arachidonoylglycerol (2-AG) is also released from membrane lipids, through the activity of diacylglycerol lipase (DAGL). Then, 2-AG can be hydrolyzed by monoacylglycerol lipase (MAGL), which both release glycerol and AA. The transport of 2-AG across the cell membrane may be mediated by AMT or a related transporter.
The second widely recognized endogenous CB1 agonist, 2-arachidonylglycerol (2-AG), was characterized soon after the discovery of AEA [55, 56]. 2-AG has been characterized as a unique molecular species of monoacylglycerol isolated from both the canine gut [55] and the rat brain [72], where it presumably functions as an endogenous cannabinoid receptor ligand. 2-AG biosynthesis occurs by two possible routes in neurons, which are illustrated in Fig. 4B and also in recent review [73]. 2-AG, like AEA, is found in a variety of tissues throughout the body and brain, and appears to be released from cells in response to certain stimuli. 2-AG activates the CB1 receptor with greater efficacy than does AEA. 2-AG is inactivated by reuptake [60, 61] via an uncharacterized membrane transport molecule, the ‘AEA membrane transporter’ (AMT) [52, 60, 62-66], and subsequent intracellular enzymatic degradation [47, 67, 74] by monoacylglycerol (MAGL) lipase, like other monoacylglycerols [75]. Furthermore, 2-AG is metabolized by enzymatic oxygenation of 2-AG by COX-2 into PGH2 glycerol esters. The biological activity and the role of oxygenated 2-AG are yet to be determined.
EC SYSTEM AND CNS DEVELOPMENT
The molecular details of EC metabolism and their receptor systems during brain development suggest that ECs may effectively regulate cellular specification programs [76]. A broad range of developmentally regulated receptors and ion channels [77-79] suggests divergent roles of EC signaling during brain development. AEA and 2-AG levels vary substantially throughout prenatal development [80, 81]. In the beginning, between days 4 and 6 of pregnancy in mice, AEA in the uterus enables embryo implantation [82]. AEA levels are low in the brain at midgestation and their levels gradually increase throughout the perinatal developmental period until adult levels are reached [80]. Like in adult brains, 2-AG concentrations (2–8 nmol/g tissue) exceed those of AEA (3–6 pmol/g tissue) throughout brain development [80, 81]. Notably, fetal 2-AG levels are similar to those in young and adult rat brains, with a remarkably distinct peak on the first day after birth [80, 81]. The cellular distribution of MAGL during development is not known, while FAAH has been detected in radial glia during late gestation and postnatal periods [83]. The distribution patterns of FAAH, together with the EC control of astrogliogenesis [83], suggest the involvement of EC signaling in neural progenitor differentiation in vivo. A fine balance between progenitor cell proliferation and programmed death guarantees the generation of adequate quantities of neural cells during brain development. It is becoming increasingly evident that ECs and related lipid mediators regulate neural progenitor commitment, survival [83-85] and synaptic connectivity in the developing brain [42, 86]. The signaling pathway responsible for their effects during development is not well characterized. The available literature suggests the participation of ERK 1 and 2 through a mechanism that involves the upstream inhibition of Rap1 and B-Raf (for a recent review, see [76]).
ECs have also been shown to regulate neuronal migration, suggesting a role in the attainment of the morphological, physiological and molecular characteristics that occur during terminal neuronal differentiation. AEA and WIN 55,212-2, in cooperation with brain-derived neurotrophic factor (BDNF), a principal pro-differentiating neurotrophin, induce migration of GABA-containing interneurons in the embryonic cortex [87]. Similarly, THC was found to increase the density of cholecystokinin-expressing interneurons in the rat hippocampus in vivo [87]. AEA [87] and WIN 55,212-2 [88] strongly inhibit neurite formation and elongation in GABA-containing interneurons. In these studies AEA was shown to abolish the morphogenic potential of BDNF. Similarly, cannabinoids, including THC, antagonize the forskolin-induced synaptogenesis of cultured hippocampal neurons [89]. In N1E-115 neuroblastoma cells, AEA and HU210 reduce the rates of neurogenic differentiation [90]. These morphological changes are mediated through the Rho family of small guanosine triphosphatases, the spatially controlled activation of which regulates cytoskeletal integrity [91]. In contrast, a synthetic cannabinoid, HU210, promotes neurite outgrowth in Neuro 2A cells through the Gao/i-mediated degradation of Rap–GAPII and the subsequent activation of Rap1 [92]. 2-AG stimulates neurite outgrowth of cerebellar neurons through a mechanism that is dependent on intrinsic DAGL activity within axonal growth cones, whereas CB1 receptor antagonists abolish N-cadherin- and Fgf8-induced neurite extension [93]. These observations suggest that EC signaling might regulate aspects of growth cone differentiation and axon guidance [94]. Further support for the potential role of ECs in the regulation of neuritogenesis derives from the similar functional effects of other lipid mediators such as lysophosphatidic acid and sphingosine-1-phosphate [90]. Further research is required to understand the precise signaling mechanism by which ECs regulate dendrite and axon development. Identification of ECs and the characterization of their metabolic enzymes together with second messenger signaling cascades will enable better understanding of the physiological role of EC signaling and will reveal the neural basis of developmental defects that are imposed by prenatal drug abuse.
The CB1 receptor has a wide expression pattern in the developing nervous system and its expression follows neuronal differentiation in the embryo from the earliest stages. Several studies have described the expression pattern of CB1 receptor mRNA and the distribution of CB1 receptors in the fetal and neonatal rat brain [80, 95-97]. The CB1 receptor mRNA levels and receptor binding could be detected from gestational day (GD) 14 in rats, coinciding with the time of phenotypic expression of most neurotransmitters (for review, see [98]). At this fetal age, CB1 receptors appear to be functional, since they are already coupled to GTP-binding proteins [95]. The developing human and rat brain contain higher levels of CB1 receptors [99, 100] than those seen in the adult brain [80]. However, the distribution of CB1 receptors is atypical in the fetal and early neonatal brain, particularly in white matter areas [97] and subventricular zones of the forebrain [80, 95], compared with the adult brain [23, 100]. This atypical location of CB1 receptors was a transient phenomenon, since the receptors progressively acquired, during the course of late postnatal development, the classic pattern of distribution observed in the adult brain [95, 97]. The existence of CB1 receptors during early brain development suggests a possible involvement of CB1 receptors during fetal and early postnatal periods in specific events of the CNS development, such as cell proliferation and migration, axonal elongation and, later, synaptogenesis and myelinogenesis (for review, see [81]). Thus, CB1 receptors contribute to generating neuronal diversity in particular brain regions during early CNS development.
Consistent with their role during early CNS development, there is evidence that perinatal exposure to cannabinoids modifies the maturation of neurotransmitter systems and their related behaviors [81, 101-104]. These effects take place through the activation of CB1 receptors, that emerge early in the developing brain [80, 81, 95, 104]. Psychoactive cannabinoids may act as epigenetic factors. The activity in the adult brain of a specific neurotransmitter is the result of a temporally ordered sequence of events that occurs during early CNS development. Perturbations of this pattern may lead to alterations in some of the functions related to this neurotransmitter. For instance, the advance or the delay in the expression of genes implicated in the synthesis of receptors at a very specific moment of development can imply alterations in some of the activities related to the physiological functions of these receptors. These physiological changes may also result from an increase or a decrease in concentration of CB1 receptors or from modifications in the activities of the CB1 receptor signaling pathways. Administration of cannabinoids, at doses similar to those found in marijuana consumption, was found to modify normal neurotransmitter development, likely producing neurobehavioral disturbances. Thus, adult animals perinatally exposed to cannabinoids exhibited, among other signs, long-term alterations in male copulatory behavior [105], open-field activity [106], learning ability [107], stress response [108], pain sensitivity [109], social interaction and sexual motivation [110], drug seeking behavior [111], neuroendocrine disturbances [112] and others (for review, see [101-103, 107]). Most of these neurobehavioral effects are likely to arise from changes in the development of several neurotransmitter systems caused by the exposure to cannabinoids, and probably through the activation of CB1 receptors during critical prenatal and early postnatal periods of brain development.
During certain periods of development, CB1 receptors may be also expressed in some subpopulations of glial cells, which play an important role in neural development. Cannabinoids induce arachidonic acid mobilization in cortical glial cells and this effect is reversed by a selective CB1 receptor antagonist, SR141716A [113], suggesting that the CB1 receptor might play a role in neural-glial signaling in the brain. In this manner, AEA released from neuronal cells may act on the astrocyte function via the activation of the CB1 receptors located in these cells. It has been observed that cannabinoids increased the rate of glucose oxidation to CO2 as well as the rate of glucose incorporation into phospholipids and glycogen. These effects of cannabinoids were prevented by forskolin, pertussis toxin, and the CB1 receptor antagonist SR 141716. Cannabinoid did not affect basal cAMP levels but partially antagonized the forskolin-induced elevation of intracellular cAMP concentration in cortical glial cells [114] and C6 glioma cells [115]. These effects were reversed by pertussis toxin or SR141716A, thus indicating the involvement of a Gi/Go protein-coupled CB1 receptor. These studies also suggest that sphingomyelin hydrolysis and mitogen-activated protein kinase stimulation are involved in this metabolic effect [114]. Cannabinoids in hippocampal glial cell cultures induce the expression of kros-24, which is reversed by SR141716A [116], suggesting the involvement of CB1 receptors.
The normal role of the EC system during early CNS development is not fully elucidated. The search for more functions is under way, and methods for finding them are improving. Still, not enough attention is focused in this direction. Modulation of this system using pharmacological and gene knockout approaches support a role for it in learning and memory, emotion and anxiety, reward, eating, nociception and motor systems, to list a few. However, none of these behavioral responses is critically dependent on the direct activity of the EC system, indicating that it serves a modulatory or facilitatory function, hence making it a highly attractive target for the development of therapeutic agents to treat CNS developmental disorders.
NEURODEVELOPMENTAL DISORDERS
An association between cannabis use and psychotic symptoms and/or schizophrenia has been evident in the literature [7]. Changes in the EC system have been reported in schizophrenia. The cannabinergic system regulates the development of dopamine systems, the differentiation of GABA interneurons, and the processes that regulate synaptogenesis and neural pruning, as well as the control of short- and long-term plasticity [13]. The activation of CB1 receptors interferes with neuronal network oscillations and impairs sensory gating function in the limbic circuitry, further supporting the connection between cannabis abuse and increased susceptibility to developing schizophrenia [117]. Early onset cannabis use may interfere with these developmental processes, constituting a neurodevelopmental insult, and account for the association between age of onset of use and an increased risk of later developing schizophrenia. (For review see [118]). A vast majority of clinical data suggests a high rate of cannabis use among people with schizophrenia and also a deteriorated course of illness [119, 120]. Recent studies identify cannabis use as a causative factor in a small proportion (~8%) of schizophrenia cases [121, 122] despite an apparent large increase in cannabis use [123]. A recent general community survey found that subclinical positive and negative symptoms of schizophrenia were more strongly associated with neurodevelopmental abuse of cannabis and this effect was independent of lifetime frequency of use [124]. In another animal study, working memory [125] and prepulse inhibition [126] were impaired in adult rats that had received a cannabinoid peri- or prepubertally, respectively. This effect was normal in rats treated for the same length of time in adulthood. These findings offer some support for cannabis-induced neurodevelopmental effects at puberty contributing to the subsequent development of schizophrenia.
It was found that regular or infrequent or heavy cannabis use at early ages (14 or 15 years) was strongly associated with other illicit drug use [127], including the development of nicotine dependence [128]. However, this association weakened with such use in 21-year olds. Administration of cannabinoids to adolescent rats induces a sensitization to morphine, cocaine and amphetamine, compared with adult rats [129]. These observations suggest long-lasting modulation of the central reward pathway in the neurodevelopmental effects of cannabinoids.
A growing body of evidence shows the association of prenatal marijuana exposure with abnormal CNS maturation as well as cognitive and attentional deficits in children (For review see [130]). Cannabis augments mid-brain dopamine release, which is known to be associated with the induction of psychosis, and, when used in higher doses, cannabis suppresses PFC dopamine utilization, resulting in cognitive dysfunction. Evidence suggests that some individuals are particularly prone to these adverse effects of cannabis due to a functional polymorphism of their COMT gene, which reduces their capacity to metabolize dopamine [131]. Despite the prevalence of marijuana use in adolescence, few studies have examined the cognitive impact of chronic marijuana use in adolescent samples. It is well known in the literature that acute cannabis impairs cognitive function in humans, but studies on the neurodevelopmental aspects of cannabis use have not been done.
EC SYSTEM AND NEURODEGENERATION
The distribution of CB1 receptors in the adult brain is highly heterogeneous, with the highest densities of receptors present in the basal ganglia, the substantia nigra pars reticulata, and the globus pallidus. In addition, very high levels of binding are present in the hippocampus, particularly within the dentate gyrus, and also in the molecular layer of the cerebellum. In contrast, there are few CB1 receptors in the brainstem [132]. There is a similar distribution of CB1 receptors in humans [99, 133]. The highest densities are found in association with limbic cortices, with much lower levels within primary sensory and motor regions, suggesting an important role in motivational (limbic) and cognitive (association) information processing. CB1 receptors have been shown to localize presynaptically on GABAergic interneurons and glutamatergic neurons [134-136]. This is consistent with the proposed role of EC compounds in modulating GABA and glutamate neurotransmission [10, 11, 137-141].
In recent years, the functions of ECs at the synaptic and network levels have been elucidated. In 2001, three groups independently revealed that ECs are released when neuronal cells (postsynaptic neurons and possibly presynaptic terminals as well) are activated. They travel in a retrograde direction and transiently (<1 min) suppress presynaptic neuro-transmitter release by activating CB1 receptor-mediated inhibition of voltage-gated Ca2+ channels [9-11]. Such a negative feedback mechanism should be effective in calming stimulated neurons after excitation. Since then, dozens of papers have been published that have confirmed the role of ECs as a retrograde messenger in various regions of the brain. It is now established that EC release can be induced by four stimulation protocols, namely, postsynaptic depolarization, activation of postsynaptic Gq-coupled receptors, combined Gq-coupled activation and depolarization, and repetitive synaptic activation (for recent review see [13]).
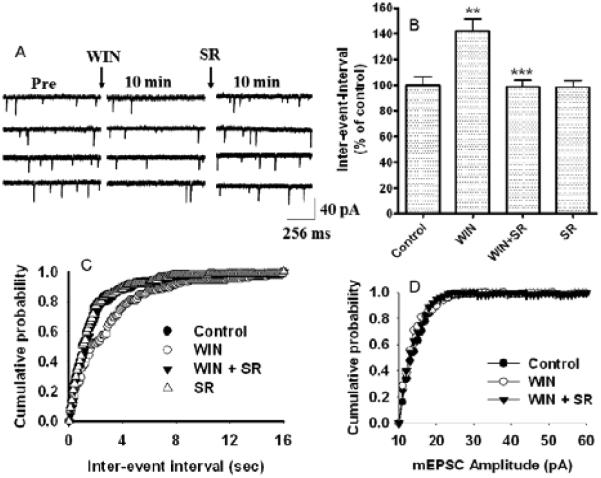
As in EC-mediated short-term plasticity, all the studies to date suggest that EC-mediated long-term plasticity takes the form of depression of neurotransmission in various brain regions. CB1 receptor agonists inhibit spontaneous excitatory postsynaptic current (mEPSCs) frequency, an effect that is reversed by CB1 receptor antagonists (Fig. 5) [142]. It was observed that long-term depression (LTD) was absent in CB1 receptor knockout mice, reduced or eliminated by CB1 receptor blockade, and enhanced by CB1 receptor activation in various brain regions, indicating the involvement of EC signaling [143]. Soon after this publication, similar EC mediated LTD was reported during both excitatory (LTDe) and inhibitory (LTDi) neurotransmission in various brain regions [144-146]. Another form of EC-mediated LTDe was described in the visual cortex [147]. All these forms of EC-mediated LTD were expressed presynaptically as persistent decreases in neurotransmitter release. In contrast, cerebellar LTD, which is well known to be expressed postsynaptically, was reported to require EC signaling [148]. It has been shown that CB1 receptor activation inhibits both LTP and LTD induction in the hippocampus [149, 150]. LTP elicited by moderate stimulations (20 or 50 pulses) was facilitated in slices treated with a CB1 antagonist, whereas LTP elicited with robust stimulations (100 or 200 pulses) was unchanged by CB1 blockade. LTP elicited with TBS was also facilitated with CB1 blockade, revealing a tonic inhibitory influence of ECs on the hippocampal LTP induction. Conversely, the inhibition of cyclooxygenase-2 (COX-2) prevented LTP elicited with TBS. Inhibition of COX-1 or other routes of EC degradation did not affect LTP. These observations suggest that COX-2 regulates the formation of ECs that negatively regulate LTP [151]. The neurophysiological consequences of the activation of CB1 receptors depend on the localization of these receptors in various brain regions and the excitatory or inhibitory pathways being stimulated. Hence, the clinical potential of cannabinoid drugs in neurological disorders is vast.
Fig. (5).
WIN 55, 212-2 –induced suppression of mEPSC frequency was antagonized by CB1 receptor antagonist SR141716A in hippocampal neurons. (A) Traces of continuously recorded mEPSCs before (control), during WIN 55,212-2 exposure, and after the addition of the CB1 receptor antagonist SR141716A (SR). (B) Combined plot showing the bath application of WIN55, 212-2 suppresses mEPSC frequency. SR141716A antagonized the WIN 55,212-2-induced depression of mEPSC frequency. SR141716A alone does not significantly affect mEPSC frequency and amplitude. (C) Average cumulative distributions of mEPSC inter-event interval (sec) showing a decrease in mEPSC frequency in WIN 55,212-2-treated cells relative to control (n = 6 neurons). (D) No change in average cumulative distributions of mEPSC amplitude was observed in WIN 55,212-2-treated cells relative to control (n = 6 neurons). (p < 0.01; Kolmogorow-Simrnov two-sample test). [The original figure was modified and reproduced from Basavarajappa et al. [142]].
Huntington’s disease (HD) is an adult-onset, dominantly inherited human neurodegenerative disorder characterized by motor deficits, cognitive impairment, and psychiatric symptoms leading to inexorable decline and death [152]. Reduced levels of ECs, CB1 receptors, and CB1 receptor mRNA have been reported in Huntington’s disease [153-157]. While the mechanism and the significance of the loss of EC function is not clear at present, these observations may indicate that the EC signaling system has a central role in the progression of neurodegeneration in Huntington’s disease, and that cannabinoid agonists could be of significant therapeutic benefit in Huntington’s disease because of their anthyperkinetic and neuroprotective effects [156]. A recent study showed a loss of CB1 receptors in progenitor cells in the adult human brain subependymal layer in Huntington's disease and suggested the possibility that these cells could be a suitable endogenous source for the replacement of cells lost due to neurodegeneration [158]. In particular, down-regulation of CB1 receptor activity and signaling seems to be a critical event within the ECS. As a consequence, neuronal functioning and GABA transmission are impaired [159]. On this basis, it can be proposed that delaying the loss of CB1 receptors, for instance by means of increasing the levels of their (endo) cannabinoid agonists, might be beneficial in the treatment of HD.
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder that selectively damages upper and lower motor neurons of the spinal cord, brainstem, and motor cortex [160, 161]. Most cases of ALS are sporadic, but about 10% are familial [160, 161]. Despite extensive research, the underlying cause of the sporadic form of ALS remains unclear, while progress has been made in understanding the mechanisms of the familial forms of the disease, and a wide range of factors have been proposed to play a role. They include glutamate excitotoxicity, mitochondrial dysfunction, oxidative stress, protein aggregation, proteosomal dysfunction, axonal transport deficits, cytoskeletal abnormalities, microglial activation, neuroinflammation and aberrant growth factor signaling [160-163]. Notably, some of these mechanisms, namely glutamate excitotoxicity, oxidative stress, neuroinflammation, and microglial activation are potentially modulated by ECs, possibly explaining the neuroprotective effects of increasing EC levels in ALS models. In line with this, it has been reported that both pharmacological agonists of CB1 and CB2 receptors and elevated levels of AEA, obtained through genetic ablation of FAAH, exerted robust anti-inflammatory and neuroprotective effects, delaying disease progression in SOD1 mice [164-166]. In addition, EC system may also provide symptomatic relief in ALS by reducing spasticity, a disabling condition which follows the lesion of the upper motor neurons [167]. These observations suggest that a hyperactive EC system and an increased EC tone underlie neurotransmission deficits in ALS. Within the EC system both CB1 receptors and FAAH are key players in the pathogenesis of ALS, with CB1 receptor activation counteracting glutamate excitotoxicity by reducing glutamate release from presynaptic terminals. In addition, enhanced EC tone in ALS may counteract the loss of mGlu5 receptor functionality [168, 169], thus representing a compensatory mechanism. It may also, at least in part, be under the control of COX-2 activity [170]. Furthermore, up-regulation of CB2 receptors in microglial cells [171], and subsequent increase of the release of pro-inflammatory cytokines [172, 173], substantially contribute to ALS neuropathology.
The main pathological feature of Parkinson’s disease (PD) is the degeneration of dopamine (DA)-containing neurons of the substantia nigra, which leads to severe DAergic denervation of the striatum. The irreversible loss of the DA-mediated control of striatal function leads to the typical motor symptoms observed in PD, i.e., bradykinesia, tremor and rigidity. Increased EC tone in the globus pallidus has been reported to be responsible for the production of Parkinsonian symptomology [174]. Several mechanisms have been considered to play a role in the selective DA neuron degeneration seen in PD, such as mitochondrial dysfunction, oxidative stress, and excitotoxicity. Interestingly, although CB1 receptors are not abundant in DA neurons of the substantia nigra, the putative involvement of the ECs in DA neuron degeneration has become apparent in the latest studies. A recent study demonstrated increased 2-AG in the globus pallidus of rats treated with reserpine, which is a rodent model of PD [175]. EC signaling was shown to be involved in the pathophysiology of parkinsonism and levodopa-induced dyskinesia (LID) in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned, non-human primate models of Parkinson's disease [79]. The deficiency in EC transmission may contribute to LID; these complications may be alleviated by the activation of CB1 receptors [176]. Increased levels of AEA have been reported in rat models of PD [175, 177]. Recently, it was discovered that CB1 receptor binding and the activation of G proteins by cannabinoid agonists were significantly increased in the postmortem basal ganglia of humans affected by PD [178]. The increase in CB1 receptors was also seen in MPTP-treated marmosets, a primate PD model [178]. A recent study found high levels of ECs in the cerebrospinal fluid of untreated PD patients [179]. Low doses of SR141716A partially attenuated the hyperkinesias shown by a rat model of PD [180]. Further studies to understand the functional interaction between dopamine and the EC system should bring new perspectives on the treatment of PD.
Several lines of evidence suggest a role for EC signaling in schizophrenia [181]. The highest densities of CB1 receptors are found in regions of the human brain implicated in schizophrenia, including the prefrontal cortex, basal ganglia, hippocampus, and the anterior cingulate cortex [181]. Increased binding of [3H]CP-55,940 to CB1 receptors in the dorsolateral prefrontal cortex of schizophrenia patients compared to controls has been demonstrated [182]. In addition, Leweke et al. [183] reported a significant twofold elevation of AEA levels in the cerebrospinal fluid (CSF) of patients with schizophrenia compared with age-matched controls. Finally, a recent study indicated that SR141716A reverses ketamine-induced impairment in prepulse inhibition of the acoustic startle reflex, an animal model of the deficient sensorimotor gating observed in schizophrenia [184]. It was recently found that CSF AEA levels are eightfold higher in antipsychotic-naive first-episode paranoid schizophrenics than in healthy controls, dementia patients or affective disorder patients. This alteration is absent in schizophrenics treated with 'typical' antipsychotics, which antagonize dopamine D2-like receptors, but not in those treated with 'atypical' antipsychotics, which preferentially antagonize 5HT(2A) receptors [185]. Recent data suggest that dysregulated striatal EC neurotransmission is associated with a hyperdopaminergic state in dopamine transporter knockout mice [186]. AEA release in the dorsal striatum is stimulated by activation of D2 dopamine receptors [176]. The amounts of AEA are significantly increased in the blood of patients with acute schizophrenia compared with healthy volunteers [187].
Alzheimer's disease (AD) is a chronic degenerative disorder of the CNS that afflicts more than 4 million people in the United States. AD also accounts for the most common form of dementia in the elderly [188]. The amyloid hypothesis, one of the operational models of AD pathogenesis, maintains that the accumulation of amyloid β peptide (Aβ; a key pathological marker of Alzheimer disease) is responsible for AD-related pathology, including Aβ deposits, neurofibrillary tangles, and eventual neuronal cell death [189]. However, a more recent variant of this model suggests that soluble Aβ oligomers disrupt glutamatergic synaptic function, which in turn leads to the characteristic cognitive deficits (for references see recent review[190]).
Several studies have demonstrated the ability of cannabinoids to provide neuroprotection against Aβ peptide toxicity [191-193]. A number of studies have established the influence of CB1 receptors on learning and memory [194-196]. Activation of CB1 receptors has been found to impair memory [197] and its blockade has been consistently found to facilitate memory [196, 198]. Stereotaxic injection of Aβ into the rat cortex caused neuronal damage in the hippocampus and increased the levels of 2-AG, but not of AEA. Further, the inhibition of EC cellular reuptake concomitantly reversed hippocampal damage in rats, and the loss of memory retention in the passive avoidance test in mice, but only when administered from the 3rd day after Aβ injection [199]. The mRNAs encoding the biosynthetic (DAGLa) enzyme of 2-AG were also found to be significantly elevated following Aß injections [199]. These observations suggest that pharmacological enhancement of brain EC levels through the inhibition of EC metabolism or uptake inhibitors may have a therapeutic value in the protection against Aβ-induced neurodegeneration [199]. Blockade of CB1 receptors by SR141716A lessens the amnesia induced by a β-amyloid fragment in mice, suggesting that the EC system may be involved in cognitive impairment in Alzheimer’s disease [200]. A recent study provides evidence that Δ9-THC inhibits the enzyme acetylcholinesterase (AchE) and prevents AchE-induced Aβ aggregation. Δ9-THC binds in the peripheral anionic site of AchE, the critical region involved in amyloid-genesis [201]. Despite the growing body of evidence that indicates the involvement of the ECs in AD pathology, the effects of cannabinoids on the clinical course of AD have been addressed only in one study. In that study, the oral administration of Dronabinol ameliorated appetite and some behavioral disturbances in a sample of patients suffering from AD [202]. Therefore, it will be of major interest to ascertain whether direct pharmacological manipulation of CB1 and/or CB2 receptors, as well as drugs that modulate EC levels, may be able to reverse cognitive impairments and slow disease progression and neuroimaging markers of brain atrophy in AD patients.
Compounds such as AEA and other NAEs present in chocolate [203] may function as "cannabinoid mimics" in the purported rewarding properties of cocoa [204], suggesting that the EC system participates in the control of food intake. Transient inhibition of food intake and reduction in fat mass were observed following treatment of mice and rats with CB1 receptor antagonist SR141716A [205]. CB1 receptor knockout mice on a high-fat diet were shown to have a lower susceptibility to obesity [206]. To date, data obtained from clinical trials (RIO North America, RIO Europe and RIO Lipid) indicate that SR141716A may have clinical benefits in relation to its anti-obesity properties and as a novel candidate for the treatment of metabolic and cardiovascular disorders associated with overweight and obesity [207, 208]. In fact, several studies have evidenced that phenotypes associated with obesity and/or alterations on insulin homeostasis (metabolic syndrome) are at increased risk for developing cognitive decline and dementia, including not only vascular dementia, but also AD (for review see[209]). Perhaps SR141716A can be beneficial against the “metabolic syndrome” observed in AD. These studies also suggested that the drug has a reasonable safety profile. Treatment with SR141716A is also associated with favorable changes in serum lipid levels and an improvement in glycemic control in type 2 diabetic patients [210]. Following 1 year of treatment, SR141716 A (rimonabant) at a dose of 20 mg/day produced significant increases, compared with a placebo, in the number cigarette smokers who quit smoking [211]. In another recent clinical study, orlistat, which specifically inhibits the critical enzymes (PLC and DAGL) involved in the biosynthetic pathway of 2-AG [212], reduced weight by 2.7 kg on average and decreased the incidence of type 2 diabetes from 9.0% to 6.2% [213]. In the same study, SR141716A significantly reduced weight by 4.6 kg (95% CI 4.3–5.0), reduced waist circumference, and improved triglyceride and HDL cholesterol profiles [213]. These observations suggest that the CB1 receptor may have a role in both control of obesity and cessation of smoking. Psychopathological disorders and depression in particular are strongly linked to eating attitude in obese patients. The identification of CB1 receptors in areas of the CNS that have been implicated in regulation of mood, food intake and ethanol-related phenomena, including tolerance, vulnerability, reinforcement, and consumption (for details, see the recent reviews [214-216]), suggests that these receptors may mediate such a behavioral link.
THERAPEUTIC OPPORTUNITY
Even though the detailed pathophysiology of the EC system is not yet fully understood, there is already overwhelming evidence indicating that a pharmacological modulation of the EC system could provide new tools for a number of disease states. In terms of drug development, the CB1 receptor antagonist has progressed furthest and a Sanofi-Aventis clinical study (surinabant) for the treatment of smoking [217, 218] is completed (ClinicalTrials.gov, Identifier: NCT00432575). An NIAAA clinical study of the efficacy of SR 141716A (rimonabant) (Fig. 2) to reduce voluntary ethanol drinking is completed recently (ClinicalTrials.gov, Identifier: NCT00075205). Pending the results of the clinical trials, CB1 receptor antagonists such as SR141716A could become an important addition to the limited arsenal of effective treatments for alcoholism. During CNS developmental deficits, neurodegenerative disease or drug abuse, including ethanol abuse, there are changes in EC levels in various regions of the brain [219-222]. Therefore, drugs or agents which regulate the level of ECs by inhibiting their metabolism (FAAH inhibitors such as URB597) or uptake (AM404) or synthesis (orlistat) could locally target sites while limiting the effects on uninvolved cognitive areas, and would thus be expected to have a higher therapeutic value [215, 223]. Recent evidence suggests that the blockade of CB1 receptors with SR 141716A might be beneficial to alleviate motor inhibition typical of PD [180]. Based on the observations from animal studies that blockade of CB1 receptors might be protective against memory loss caused by Aβ peptides[199, 200], recently, AVE 1625, a selective CB1 receptor antagonist, is being tested in a double-blind, placebo-controlled phase II clinical trial in patients with mild to moderate AD (ClinicalTrials.gov, Identifier: NCT00380302). In addition to CB1 receptor antagonist, several specific EC transport inhibitors, FAAH and MAGL inhibitors which regulate brain EC levels might have a therapeutic value in the protection against Aβ-induced neurodegeneration [199] and memory deficit in rodents [200]. Taranabant, another CB1 receptor antagonist similar to Acomplia (rimonabant), which Sanofi sells in Europe, was examined for its beneficial effects for obesity [217, 218] has been discontinued recently (Merck and Co, clinical study) based on psychiatric side effects including anxiety and depression at higher doses (theheart. org). Further research is warranted to understand the concise conceptualization of EC system function in both health and disease conditions to develop successful EC system based drugs.
CONCLUSION
The ECs, their receptors, synthesizing and degrading enzymes, as well as transporter molecules, have been detected from the earliest stages of embryonic development and throughout pre- and postnatal development. ECs such as AEA and 2-AG are bioactive lipids that mimic several pharmacological effects of Δ9-THC. Many of the effects of cannabinoids and ECs are mediated by two G protein-coupled receptors (GPCRs), CB1 and CB2, although additional receptors may be implicated. Both CB1 and CB2 couple primarily to inhibitory G proteins and are subject to the same pharmacological influences as other GPCRs. As summarized in this review, several lines of evidence have suggested that the EC system may play an important role in early neuronal development along with a widespread role in neurodegeneration disorders. The development of EC research is also very important from a clinical point of view because the EC system may provide potential targets not only for the treatment of habit-forming behaviors but also for neurological disorders.
ACKNOWLEDGMENT
The author acknowledges support in part by funding from the National Institutes of Health (NIH AA11031).
REFERENCES
- [1].Kabelik J, Krejci Z, Santavy F. Cannabis as a Medicant. Bull. Narc. 1960;12:5–23. [Google Scholar]
- [2].Christison R. Philidalphia: Lea and Blancherd. 1848:971–974. [Google Scholar]
- [3].Hollister LE. Health aspects of cannabis. Pharmacol. Rev. 1986;38:1–20. [PubMed] [Google Scholar]
- [4].Dewey WL. Cannabinoid pharmacology. Pharmacol. Rev. 1986;38:151–78. [PubMed] [Google Scholar]
- [5].Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–47. [Google Scholar]
- [6].Watson SJ, Benson JA, Jr., Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arc. Gen. Psychiatry. 2000;57:547–52. doi: 10.1001/archpsyc.57.6.547. [DOI] [PubMed] [Google Scholar]
- [7].Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611–16. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- [8].Pryce G, Baker D. Emerging properties of cannabinoid medicines in management of multiple sclerosis. Trends in Neurosci. 2005;28:272–76. doi: 10.1016/j.tins.2005.03.006. [DOI] [PubMed] [Google Scholar]
- [9].Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–27. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- [10].Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminal. Neuron. 2001;29:729–38. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- [11].Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–92. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- [12].Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog. Neurobio. 2002;68:247–86. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- [13].Basavarajappa BS, Arancio O. In: Synaptic Plasticity: New Research. Kaiser TF, Peters FJ, editors. Nova Science Publishers, Inc.; NY, USA: 2008. In Press. [Google Scholar]
- [14].Howlett AC, Johnson MR, Melvin LS, Milne GM. Nonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor model. Mol. Pharmacol. 1988;33:297–302. [PubMed] [Google Scholar]
- [15].Devane WA, Dysarz FAI, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–13. [PubMed] [Google Scholar]
- [16].Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U S A. 1999;96:14136–41. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–34. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- [18].Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J. Neurochem. 2000;75:2434–44. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- [19].Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–63. [PubMed] [Google Scholar]
- [20].Basavarajappa BS. In: New Research on Alcoholism. Baye DR, editor. Nova Science Publishers, Inc; New York: 2007. pp. 1–55. [Google Scholar]
- [21].Basavarajappa BS. Neuropharmacology of the endocannabinoid signaling system-Molecular mechanisms, biological actions and synaptic plasticity. Curr. Neuropharmacol. 2007;5:81–97. doi: 10.2174/157015907780866910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Basavarajappa BS, Yalamanchili R, Cooper TB, Hungund BL. In: Handbook of Neurochemistry and Molecular Neurobiology. Lajtha A, Sylvester EV, editors. Springer; NY: 2008. pp. 343–84. [Google Scholar]
- [23].Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Cost BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;16:8057–66. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- [25].Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. U S A. 1995;92:3376–80. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. N. Y. Acad. Sci. 2006;1074:514–36. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- [27].Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- [28].Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS ONE. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Onaivi ES, Leonard CM, Ishiguro H, Zhang PW, Lin Z, Akinshola BE, Uhl GR. Endocannabinoids and cannabinoid receptor genetics. Prog. Neurobiol. 2002;66:307–44. doi: 10.1016/s0301-0082(02)00007-2. [DOI] [PubMed] [Google Scholar]
- [30].Mukhopadhyay S, McIntosh HH, Houston DB, Howlett AC. The CB(1) cannabinoid receptor juxtamembrane C-terminal peptide confers activation to specific G proteins in brain. Mol. Pharmacol. 2000;57:162–70. [PubMed] [Google Scholar]
- [31].Kenakin T, Onaran O. The ligand paradox between affinity and efficacy: can you be there and not make a difference? Trends Pharmacol. Sci. 2002;23:275–80. doi: 10.1016/s0165-6147(02)02036-9. [DOI] [PubMed] [Google Scholar]
- [32].Childers SR, Sexton T, Roy MB. Effects of anandamide on cannabinoid receptors in rat brain membranes. Biochem. Pharmacol. 1994;47:711–15. doi: 10.1016/0006-2952(94)90134-1. [DOI] [PubMed] [Google Scholar]
- [33].Pinto JC, Potie F, Rice KC, Boring D, Johnson MR, Evans DM, Wilken GH, Cantrell CH, Howlett AC. Cannabinoid receptor binding and agonist activity of amides and esters of arachidonic acid. Mol. Pharmacol. 1994;46:516–22. [PubMed] [Google Scholar]
- [34].Howlett AC, Mukhopadhyay S. Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chem. Phys. Lipids. 2000;108:53–70. doi: 10.1016/s0009-3084(00)00187-0. [DOI] [PubMed] [Google Scholar]
- [35].Caulfield MP, Brown DA. Cannabinoid receptor agonists inhibit Ca current in NG108-15 neuroblastoma cells via a pertussis toxin-sensitive mechanism. Br. J. Pharmacol. 1992;106:231–32. doi: 10.1111/j.1476-5381.1992.tb14321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc. Natl. Acad. Sci. U S A. 1992;89:3825–29. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nogueron MI, Porgilsson B, Schneider WE, Stucky CL, Hillard CJ. Cannabinoid receptor agonists inhibit depolarization-induced calcium influx in cerebellar granule neurons. J. Neurochem. 2001;79:371–81. doi: 10.1046/j.1471-4159.2001.00567.x. [DOI] [PubMed] [Google Scholar]
- [38].Pan X, Ikeda SR, Lewis DL. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol. Pharmacol. 1996;49:707–14. [PubMed] [Google Scholar]
- [39].Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- [40].Mu J, Zhuang SY, Kirby MT, Hampson RE, Deadwyler SA. Cannabinoid receptors differentially modulate potassium A and D currents in hippocampal neurons in culture. J. Pharmacol. Exp. Ther. 1999;291:893–902. [PubMed] [Google Scholar]
- [41].Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003;83:1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- [42].Mulder J, Aguado T, Keimpema E, Barabas K, Ballester Rosado CJ, Nguyen L, Monory K, Marsicano G, Di Marzo V, Hurd YL, Guillemot F, Mackie K, Lutz B, Guzman M, Lu HC, Galve-Roperh I, Harkany T. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl. Acad. Sci. U S A. 2008;105:8760–65. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wartmann M, Campbell D, Subramanian A, Burstein SH, Davis RJ. The MAP kinase signal transduction pathway is activated by the endogenous cannabinoid anandamide. FEBS. Lett. 1995;2-3:133–36. doi: 10.1016/0014-5793(95)00027-7. [DOI] [PubMed] [Google Scholar]
- [44].Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem. J. 1995;312:637–41. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Derkinderen P, Toutant M, Burgaya F, Le Bert M, Siciliano JC, de Franciscis V, Gelman M, Girault JA. Regulation of a neuronal form of focal adhesion kinase by anandamide. Science. 1996;273:1719–22. doi: 10.1126/science.273.5282.1719. [DOI] [PubMed] [Google Scholar]
- [46].Derkinderen P, Toutant M, Kadare G, Ledent C, Parmentier M, Girault JA. Dual role of Fyn in the regulation of FAK+6,7 by cannabinoids in hippocampus. J. Biol. Chem. 2001;276:38289–96. doi: 10.1074/jbc.M105630200. [DOI] [PubMed] [Google Scholar]
- [47].Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–91. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- [48].Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J. Neurosci. 1997;17:1226–42. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur. J. Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- [50].Basavarajappa BS, Hungund BL. Chronic Ethanol Increases the Cannabinoid Receptor Agonist, Anandamide and its Precursor N-Arachidonyl phosphatidyl ethanolamine in SK-N-SH Cells. J. Neurochem. 1999;72:522–28. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- [51].Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochemica. Biophysica. Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- [52].Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur. J. Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- [53].Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat. Neurosci. 1999;2:358–63. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- [54].Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–49. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- [55].Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- [56].Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- [57].Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. U S A. 2001;98:3662–65. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, Felder CC. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002;301:1020–24. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- [59].Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U S A. 2002;99:8400–05. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Beltramo M, Piomelli D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonyl-glycerol. Neuroreport. 2000;11:1231–35. doi: 10.1097/00001756-200004270-00018. [DOI] [PubMed] [Google Scholar]
- [61].Bisogno T, MacCarrone M, De Petrocellis L, Jarrahian A, Finazzi-Agro A, Hillard C, Di Marzo V. The uptake by cells of 2-arachidonoylglycerol, an endogenous agonist of cannabinoid receptors. Eur. J. Biochem. 2001;268:1982–89. doi: 10.1046/j.1432-1327.2001.02072.x. [DOI] [PubMed] [Google Scholar]
- [62].Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J. Neurochem. 1997;69:631–38. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- [63].Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–97. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- [64].Hillard CJ, Jarrahian A. The movement of N-arachidonoyl-ethanolamine (anandamide) across cellular membranes. Chem. Phys. Lipids. 2000;108:123–34. doi: 10.1016/s0009-3084(00)00191-2. [DOI] [PubMed] [Google Scholar]
- [65].Maccarrone M, van der Stelt M, Rossi A, Veldink GA, Vliegenthart JF, Agro AF. Anandamide hydrolysis by human cells in culture and brain. J. Biol. Chem. 1998;273:32332–39. doi: 10.1074/jbc.273.48.32332. [DOI] [PubMed] [Google Scholar]
- [66].Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. J. Pharmacol. Exp. Ther. 2001;298:7–14. [PubMed] [Google Scholar]
- [67].Deutsch DG, Glaser ST, Howell JM, Kunz JS, Puffenbarger RA, Hillard CJ, Abumrad N. The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. J. Biol. Chem. 2001;276:6967–73. doi: 10.1074/jbc.M003161200. [DOI] [PubMed] [Google Scholar]
- [68].Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc. Natl. Acad. Sci. U S A. 2003;100:4269–74. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Burstein SH, Rossetti RG, Yagen B, Zurier RB. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediat. 2000;61:29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- [70].Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J. Biol. Chem. 2002;277:44877–85. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- [71].Ross RA, Craib SJ, Stevenson LA, Pertwee RG, Henderson A, Toole J, Ellington HC. Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. J. Pharmacol. Exp. Ther. 2002;301:900–07. doi: 10.1124/jpet.301.3.900. [DOI] [PubMed] [Google Scholar]
- [72].Sugiura T, Yoshinaga N, Kondo S, Waku K, Ishima Y. Generation of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, in picrotoxinin-administered rat brain. Biochem. Biophys. Res. Commun. 2000;271:654–58. doi: 10.1006/bbrc.2000.2686. [DOI] [PubMed] [Google Scholar]
- [73].Basavarajappa BS. Critical Enzymes Involved in Endocannabinoid Metabolism. Protein and Peptide let. 2007;14:237–46. doi: 10.2174/092986607780090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Day TA, Rakhshan F, Deutsch DG, Barker EL. Role of fatty acid amide hydrolase in the transport of the endogenous cannabinoid anandamide. Mol. Pharmacol. 2001;59:1369–75. doi: 10.1124/mol.59.6.1369. [DOI] [PubMed] [Google Scholar]
- [75].Konrad RJ, Major CD, Wolf BA. Diacylglycerol hydrolysis to arachidonic acid is necessary for insulin secretion from isolated pancreatic islets: sequential actions of diacylglycerol and monoacylglycerol lipases. Biochem. 1994;33:13284–94. doi: 10.1021/bi00249a015. [DOI] [PubMed] [Google Scholar]
- [76].Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [77].Matias I, Di Marzo V. Endocannabinoid synthesis and degradation, and their regulation in the framework of energy balance. J. Endocrinol. Invest. 2006;29:15–26. [PubMed] [Google Scholar]
- [78].Piomelli D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003;4:873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- [79].van der Stelt M, Fox SH, Hill M, Crossman AR, Petrosino S, Di Marzo V, Brotchie JM. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson's disease. FASEB J. 2005;19:1140–42. doi: 10.1096/fj.04-3010fje. [DOI] [PubMed] [Google Scholar]
- [80].Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–91. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [81].Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends in Neurosci. 2000;23:14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- [82].Paria BC, Song H, Wang X, Schmid PC, Krebsbach RJ, Schmid HH, Bonner TI, Zimmer A, Dey SK. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J. Biol. Chem. 2001;276:20523–28. doi: 10.1074/jbc.M100679200. [DOI] [PubMed] [Google Scholar]
- [83].Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzman M, Galve-Roperh I. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J. Neurosci. 2006;26:1551–61. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Guzman M. Cannabinoids: potential anticancer agents. Nat. Rev. Cancer. 2003;3:745–55. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- [85].Guzman M, Sanchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol. & Ther. 2002;95:175–84. doi: 10.1016/s0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- [86].Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–16. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- [87].Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratcha G, Hurd YL, Harkany T. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc. Natl. Acad. Sci. U S A. 2005;102:19115–20. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Berghuis P, Dobszay MB, Ibanez RM, Ernfors P, Harkany T. Turning the heterogeneous into homogeneous: studies on selectively isolated GABAergic interneuron subsets. Int. J. Dev. Neurosci. 2004;22:533–43. doi: 10.1016/j.ijdevneu.2004.07.012. [DOI] [PubMed] [Google Scholar]
- [89].Kim D, Thayer SA. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J. Neurosci. 2001;21:RC146. doi: 10.1523/JNEUROSCI.21-10-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Galve-Roperh I, Aguado T, Rueda D, Velasco G, Guzman M. Endocannabinoids: a new family of lipid mediators involved in the regulation of neural cell development. Curr. Pharm. Des. 2006;12:2319–25. doi: 10.2174/138161206777585139. [DOI] [PubMed] [Google Scholar]
- [91].Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- [92].Jordan JD, He JC, Eungdamrong NJ, Gomes I, Ali W, Nguyen T, Bivona TG, Philips MR, Devi LA, Iyengar R. Cannabinoid receptor-induced neurite outgrowth is mediated by Rap1 activation through G(alpha)o/i-triggered proteasomal degradation of Rap1GAPII. J. Biol. Chem. 2005;280:11413–21. doi: 10.1074/jbc.M411521200. [DOI] [PubMed] [Google Scholar]
- [93].Williams EJ, Walsh FS, Doherty P. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J. Cell Biol. 2003;160:481–86. doi: 10.1083/jcb.200210164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003;163:463–68. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Berrendero F, Garcia-Gil L, Hernandez ML, Romero J, Cebeira M, de Miguel R, Ramos JA, Fernandez-Ruiz JJ. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–88. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- [96].Buckley NE, Hansson S, Harta G, Mezey E. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neurosci. 1998;82:1131–49. doi: 10.1016/s0306-4522(97)00348-5. [DOI] [PubMed] [Google Scholar]
- [97].Romero J, Garcia-Palomero E, Berrendero F, Garcia-Gil L, Hernandez ML, Ramos JA, Fernandez-Ruiz JJ. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26:317–23. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- [98].Insel TR. In: Psychopharmacology: The Four Generation of Progress. Bloom F, EaK DJ, editors. Raven Press; New York: 1995. pp. 683–94. [Google Scholar]
- [99].Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neurosci. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- [100].Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neurosci. 1992;48:655–68. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- [101].Fernández-Ruiz JJ, Bonnin A, Cebeira M, Ramos JA, Palomo T, Archer T. Strategies for Studying Brain Disorders. Farrand Press; England: 1994. pp. 357–90. [Google Scholar]
- [102].Fernández-Ruiz JJ, Rodriguez F, Navarro M, Ramos JA. In: Neurobiology and Neurophysiology of Cannabinoids: Biochemistry and Physiology of Substance Abuse. Bartke AaM LL, editor. CRC Press; Boca Ratón, FL: 1992. pp. 119–64. [Google Scholar]
- [103].Fernández-Ruiz JJ, Romero J, García-Gil L, García-Palomero E, Ramos JA. In: Dopamine Disease States. Beninger RJ, Archer T, Palomo T, editors. CYM Press; Madrid, Spain: 1996. pp. 359–87. [Google Scholar]
- [104].Fernandez-Ruiz JJ, Berrendero F, Hernandez ML, Romero J, Ramos JA. Role of endocannabinoids in brain development. Life Sci. 1999;65:725–36. doi: 10.1016/s0024-3205(99)00295-7. [DOI] [PubMed] [Google Scholar]
- [105].Dalterio SL. Perinatal or adult exposure to cannabinoids alters male reproductive functions in mice. Pharmacol. Biochem. Behav. 1980;12:143–53. doi: 10.1016/0091-3057(80)90429-3. [DOI] [PubMed] [Google Scholar]
- [106].Navarro M, Rodriguez de Fonseca F, Hernandez ML, Ramos JA, Fernandez-Ruiz JJ. Motor behavior and nigrostriatal dopaminergic activity in adult rats perinatally exposed to cannabinoids. Pharmaco. Biochem. Behav. 1994;47:47–58. doi: 10.1016/0091-3057(94)90110-4. [DOI] [PubMed] [Google Scholar]
- [107].Dalterio SL. Cannabinoid exposure: effects on development. Neurobehav. Toxicol. Teratol. 1986;8:345–52. [PubMed] [Google Scholar]
- [108].Mokler DJ, Robinson SE, Johnson JH, Hong JS, Rosecrans JA. Neonatal administration of delta-9-tetrahydrocannabinol (THC) alters the neurochemical response to stress in the adult Fischer-344 rat. Neurotoxicol. Teratol. 1987;9:321–27. doi: 10.1016/0892-0362(87)90023-7. [DOI] [PubMed] [Google Scholar]
- [109].Vela G, Fuentes JA, Bonnin A, Fernandez-Ruiz J, Ruiz-Gayo M. Perinatal exposure to delta 9-tetrahydrocannabinol (delta 9-THC) leads to changes in opioid-related behavioral patterns in rats. Brain Res. 1995;680:142–47. doi: 10.1016/0006-8993(95)00255-o. [DOI] [PubMed] [Google Scholar]
- [110].Navarro M, de Miguel R, Rodriguez de Fonseca F, Ramos JA, Fernandez-Ruiz JJ. Perinatal cannabinoid exposure modifies the sociosexual approach behavior and the mesolimbic dopaminergic activity of adult male rats. Behav. Brain Res. 1996;75:91–98. doi: 10.1016/0166-4328(96)00176-3. [DOI] [PubMed] [Google Scholar]
- [111].Vela G, Martin S, Garcia-Gil L, Crespo JA, Ruiz-Gayo M, Fernandez-Ruiz JJ, Garcia-Lecumberri C, Pelaprat D, Fuentes JA, Ramos JA, Ambrosio E. Maternal exposure to delta9-tetrahydrocannabinol facilitates morphine self-administration behavior and changes regional binding to central mu opioid receptors in adult offspring female rats. Brain Res. 1998;807:101–09. doi: 10.1016/s0006-8993(98)00766-5. [DOI] [PubMed] [Google Scholar]
- [112].Dalterio S, Bartke A. Perinatal exposure to cannabinoids alters male reproductive function in mice. Science. 1979;205:1420–22. doi: 10.1126/science.472762. [DOI] [PubMed] [Google Scholar]
- [113].Shivachar AC, Martin BR, Ellis EF. Anandamide- and delta9-tetrahydrocannabinol-evoked arachidonic acid mobilization and blockade by SR141716A [N-(Piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboximide hydrochloride] Biochem. Pharmacol. 1996;51:669–76. doi: 10.1016/s0006-2952(95)02248-1. [DOI] [PubMed] [Google Scholar]
- [114].Sanchez C, Galve-Roperh I, Rueda D, Guzman M. Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the Delta9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Mol. Pharmacol. 1998;54:834–43. doi: 10.1124/mol.54.5.834. [DOI] [PubMed] [Google Scholar]
- [115].Sanchez C, Velasco G, Guzman M. Delta9-tetrahydrocannabinol stimulates glucose utilization in C6 glioma cells. Brain Res. 1997;767:64–71. doi: 10.1016/s0006-8993(97)00631-8. [DOI] [PubMed] [Google Scholar]
- [116].Bouaboula M, Bourrie B, Rinaldi-Carmona M, Shire D, Le Fur G, Casellas P. Stimulation of cannabinoid receptor CB1 induces krox-24 expression in human astrocytoma cells. J. Biol. Chem. 1995;270:13973–80. doi: 10.1074/jbc.270.23.13973. [DOI] [PubMed] [Google Scholar]
- [117].Hajos M, Hoffmann WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biol. Psychiatry. 2008;63:1075–83. doi: 10.1016/j.biopsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [118].Cohen M, Solowij N, Carr V. Cannabis, cannabinoids and schizophrenia: integration of the evidence. Aust. N. Z. J. Psychiatry. 2008;42:357–68. doi: 10.1080/00048670801961156. [DOI] [PubMed] [Google Scholar]
- [119].Buhler B, Hambrecht M, Loffler W, an der Heiden W, Hafner H. Precipitation and determination of the onset and course of schizophrenia by substance abuse--a retrospective and prospective study of 232 population-based first illness episodes. Schizophr, Res. 2002;54:243–51. doi: 10.1016/s0920-9964(01)00249-3. [DOI] [PubMed] [Google Scholar]
- [120].van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am. J. Epidemiol. 2002;156:319–27. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- [121].Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. Bmj. 2002;325:1212–13. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. Bmj. 2002;325:1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br. J. Psychiatry. 2004;184:110–17. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- [124].Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, Van Os J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction. 2004;99:1333–41. doi: 10.1111/j.1360-0443.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- [125].O'Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J. Psychopharmacol. 2004;18:502–08. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- [126].Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacol. 2003;28:1760–69. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- [127].Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123–35. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- [128].Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100:1518–25. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- [129].Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol. Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- [130].Gruber AJ, Pope HG., Jr. Marijuana use among adolescents. Pediatr. Clin. North. Am. 2002;49:389–413. doi: 10.1016/s0031-3955(01)00011-6. [DOI] [PubMed] [Google Scholar]
- [131].Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol. Psychiatry. 2005;57:1117–27. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- [132].Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68-69:619–31. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- [133].Biegon A, Kerman IA. Autoradiographic study of pre- and post-natal distribution of cannabinoid receptors in human brain. Neuroimage. 2001;14:1463–68. doi: 10.1006/nimg.2001.0939. [DOI] [PubMed] [Google Scholar]
- [134].Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J. Neurosci. 2001;21:9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 1999;19:4544–58. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J. Neurosci. 2006;26:5628–37. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ohno-Shosaku T, Sawada S, Kano M. Heterosynaptic expression of depolarization-induced suppression of inhibition (DSI) in rat hippocampal cultures. Neurosci. Res. 2000;36:67–71. doi: 10.1016/s0168-0102(99)00110-8. [DOI] [PubMed] [Google Scholar]
- [138].Ohno-Shosaku T, Sawada S, Yamamoto C. Properties of depolarization-induced suppression of inhibitory transmission in cultured rat hippocampal neurons. Pflugers Arch. 1998;435:273–79. doi: 10.1007/s004240050512. [DOI] [PubMed] [Google Scholar]
- [139].Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J. Neurosci. 2002;22:3864–72. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–62. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- [141].Wilson RI, Nicoll RA. Endocannabinoid Signaling in the Brain. Science. 2002;296:678–82. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- [142].Basavarajappa BS, Ninan I, Arancio O. Acute Ethanol Suppresses Glutamatergic Neurotransmission through Endocannabinoids in Hippocampal Neurons. J. Neurochem. 2008;107:1001–13. doi: 10.1111/j.1471-4159.2008.05685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002;5:446–51. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- [144].Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–72. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- [145].Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–34. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- [146].Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. U S A. 2002;99:8384–88. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–54. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- [148].Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–59. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- [149].Sullivan JM. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learning & Memory. 2000;7:132–39. doi: 10.1101/lm.7.3.132. [DOI] [PubMed] [Google Scholar]
- [150].Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–78. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- [151].Slanina KA, Roberto M, Schweitzer P. Endocannabinoids restrict hippocampal long-term potentiation via CB1. Neuropharmacol. 2005;49:660–68. doi: 10.1016/j.neuropharm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- [152].Heng MY, Detloff PJ, Albin RL. Rodent genetic models of Huntington disease. Neurobiol. Dis. 2008 doi: 10.1016/j.nbd.2008.06.005. [DOI] [PubMed] [Google Scholar]
- [153].Denovan-Wright EM, Robertson HA. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington's disease mice. Neurosci. 2000;98:705–13. doi: 10.1016/s0306-4522(00)00157-3. [DOI] [PubMed] [Google Scholar]
- [154].Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington's disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington's disease. Neurosci. 2000;97:505–19. doi: 10.1016/s0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- [155].Glass M, van Dellen A, Blakemore C, Hannan AJ, Faull RL. Delayed onset of huntington's disease in mice in an enriched environment correlates with delayed loss of cannabinoid CB1 receptors. Neurosci. 2004;123:207–12. doi: 10.1016/s0306-4522(03)00595-5. [DOI] [PubMed] [Google Scholar]
- [156].Lastres-Becker I, De Miguel R, Fernandez-Ruiz JJ. The endocannabinoid system and Huntington's disease. Curr. Drug Targets CNS Neurol. Disord. 2003;2:335–47. doi: 10.2174/1568007033482751. [DOI] [PubMed] [Google Scholar]
- [157].Lastres-Becker I, Fezza F, Cebeira M, Bisogno T, Ramos JA, Milone A, Fernandez-Ruiz J, Di Marzo V. Changes in endocannabinoid transmission in the basal ganglia in a rat model of Huntington's disease. Neuroreport. 2001;12:2125–29. doi: 10.1097/00001756-200107200-00017. [DOI] [PubMed] [Google Scholar]
- [158].Curtis MA, Faull RL, Glass M. A novel population of progenitor cells expressing cannabinoid receptors in the subependymal layer of the adult normal and Huntington's disease human brain. J. Chem. Neuroanat. 2006;31:210–15. doi: 10.1016/j.jchemneu.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [159].Lastres-Becker I, Hansen HH, Berrendero F, De Miguel R, Perez-Rosado A, Manzanares J, Ramos JA, Fernandez-Ruiz J. Alleviation of motor hyperactivity and neurochemical deficits by endocannabinoid uptake inhibition in a rat model of Huntington's disease. Synapse. 2002;44:23–35. doi: 10.1002/syn.10054. [DOI] [PubMed] [Google Scholar]
- [160].Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004;27:723–49. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- [161].Nirmalananthan N, Greensmith L. Amyotrophic lateral sclerosis: recent advances and future therapies. Curr. Opin. Neurol. 2005;18:712–19. doi: 10.1097/01.wco.0000187248.21103.c5. [DOI] [PubMed] [Google Scholar]
- [162].Bendotti C, Carri MT. Lessons from models of SOD1-linked familial ALS. Trends Mol. Med. 2004;10:393–400. doi: 10.1016/j.molmed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- [163].Rao SD, Yin HZ, Weiss JH. Disruption of glial glutamate transport by reactive oxygen species produced in motor neurons. J. Neurosci. 2003;23:2627–33. doi: 10.1523/JNEUROSCI.23-07-02627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Bilsland LG, Dick JR, Pryce G, Petrosino S, Di Marzo V, Baker D, Greensmith L. Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J. 2006;20:1003–05. doi: 10.1096/fj.05-4743fje. [DOI] [PubMed] [Google Scholar]
- [165].Raman C, McAllister SD, Rizvi G, Patel SG, Moore DH, Abood ME. Amyotrophic lateral sclerosis: delayed disease progression in mice by treatment with a cannabinoid. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:33–39. doi: 10.1080/14660820310016813. [DOI] [PubMed] [Google Scholar]
- [166].Weydt P, Hong S, Witting A, Moller T, Stella N, Kliot M. Cannabinol delays symptom onset in SOD1 (G93A) transgenic mice without affecting survival. Amyotroph. Lateral Scler Other Motor Neuron Disord. 2005;6:182–84. doi: 10.1080/14660820510030149. [DOI] [PubMed] [Google Scholar]
- [167].Young RR. Spasticity: a review. Neurology. 1994;44:S12–20. [PubMed] [Google Scholar]
- [168].Kawahara Y, Kwak S. Excitotoxicity and ALS: what is unique about the AMPA receptors expressed on spinal motor neurons? Amyotroph. Lateral Scler Other Motor Neuron Disord. 2005;6:131–44. doi: 10.1080/14660820510037872. [DOI] [PubMed] [Google Scholar]
- [169].Tortarolo M, Grignaschi G, Calvaresi N, Zennaro E, Spaltro G, Colovic M, Fracasso C, Guiso G, Elger B, Schneider H, Seilheimer B, Caccia S, Bendotti C. Glutamate AMPA receptors change in motor neurons of SOD1G93A transgenic mice and their inhibition by a noncompetitive antagonist ameliorates the progression of amytrophic lateral sclerosis-like disease. J. Neurosci. Res. 2006;83:134–46. doi: 10.1002/jnr.20715. [DOI] [PubMed] [Google Scholar]
- [170].Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res. Rev. 2006;52:201–43. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- [171].Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Klegeris A, Bissonnette CJ, McGeer PL. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br. J. Pharmacol. 2003;139:775–86. doi: 10.1038/sj.bjp.0705304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Puffenbarger RA, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- [174].Maneuf YP, Nash JE, Crossman AR, Brotchie JM. Activation of the cannabinoid receptor by delta 9-tetrahydrocannabinol reduces gamma-aminobutyric acid uptake in the globus pallidus. Eur. J. Pharmacol. 1996;308:161–64. doi: 10.1016/0014-2999(96)00326-3. [DOI] [PubMed] [Google Scholar]
- [175].Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson's disease. FASEB J. 2000;14:1432–38. doi: 10.1096/fj.14.10.1432. [DOI] [PubMed] [Google Scholar]
- [176].Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur. J. Neurosci. 2003;18:1607–14. doi: 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed] [Google Scholar]
- [177].Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agro A, Maccarrone M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J. Neurosci. 2002;22:6900–07. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Lastres-Becker I, Cebeira M, de Ceballos ML, Zeng BY, Jenner P, Ramos JA, Fernandez-Ruiz JJ. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson's syndrome and of MPTP-treated marmosets. Eur. J. Neurosci. 2001;14:1827–32. doi: 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed] [Google Scholar]
- [179].Pisani A, Fezza F, Galati S, Battista N, Napolitano S, Finazzi-Agro A, Bernardi G, Brusa L, Pierantozzi M, Stanzione P, Maccarrone M. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson's disease patients. Ann. Neurol. 2005;57:777–79. doi: 10.1002/ana.20462. [DOI] [PubMed] [Google Scholar]
- [180].Gonzalez S, Scorticati C, Garcia-Arencibia M, de Miguel R, Ramos JA, Fernandez-Ruiz J. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson's disease. Brain Res. 2006;1073-1074:209–19. doi: 10.1016/j.brainres.2005.12.014. [DOI] [PubMed] [Google Scholar]
- [181].Glass M. The role of cannabinoids in neurodegenerative diseases. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:743–765. doi: 10.1016/s0278-5846(01)00162-2. [DOI] [PubMed] [Google Scholar]
- [182].Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neurosci. 2001;103:9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- [183].Leweke FM, Schneider U, Thies M, Munte TF, Emrich HM. Effects of synthetic Delta(9)-tetrahydrocannabinol on binocular depth inversion of natural and artificial objects in man. Psychopharmacol. 1999;142:230–35. doi: 10.1007/s002130050884. [DOI] [PubMed] [Google Scholar]
- [184].Musty RE, Deyo RA, Baer JL, Darrow SM, Coleman B. Symposium on the Cannabinoids, International Cannabinoid Research Society; Burlington, Vermont. 2000. [Google Scholar]
- [185].Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, Klosterkotter J, Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacol. 2004;29:2108–14. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- [186].Tzavara ET, Li DL, Moutsimilli L, Bisogno T, Di Marzo V, Phebus LA, Nomikos GG, Giros B. Endocannabinoids activate transient receptor potential vanilloid 1 receptors to reduce hyperdopaminergia-related hyperactivity: therapeutic implications. Biol. Psychiatry. 2006;59:508–15. doi: 10.1016/j.biopsych.2005.08.019. [DOI] [PubMed] [Google Scholar]
- [187].De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:5. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Strohmeyer R, Rogers J. Molecular and cellular mediators of Alzheimer's disease inflammation. J. Alzheimers Dis. 2001;3:131–57. doi: 10.3233/jad-2001-3118. [DOI] [PubMed] [Google Scholar]
- [189].Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–55. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- [190].Venkitaramani DV, Chin J, Netzer WJ, Gouras GK, Lesne S, Malinow R, Lombroso PJ. Beta-amyloid modulation of synaptic transmission and plasticity. J Neurosci. 2007;27:11832–37. doi: 10.1523/JNEUROSCI.3478-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004;89:134–41. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- [192].Milton NG. Anandamide and noladin ether prevent neurotoxicity of the human amyloid-beta peptide. Neurosci. Lett. 2002;332:127–30. doi: 10.1016/s0304-3940(02)00936-9. [DOI] [PubMed] [Google Scholar]
- [193].Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005;25:1904–13. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J. Neurosci. 1999;19:6795–805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Nava F, Carta G, Colombo G, Gessa GL. Effects of chronic Delta(9)-tetrahydrocannabinol treatment on hippocampal extracellular acetylcholine concentration and alternation performance in the T-maze. Neuropharmacol. 2001;41:392–99. doi: 10.1016/s0028-3908(01)00075-2. [DOI] [PubMed] [Google Scholar]
- [196].Pamplona FA, Takahashi RN. WIN 55212-2 impairs contextual fear conditioning through the activation of CB1 cannabinoid receptors. Neurosci. Lett. 2006;397:88–92. doi: 10.1016/j.neulet.2005.12.026. [DOI] [PubMed] [Google Scholar]
- [197].Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J. Pharmacol. Exp. Ther. 2002;301:915–24. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- [198].Castellano C, Rossi-Arnaud C, Cestari V, Costanzi M. Cannabinoids and memory: animal studies. Curr Drug Target CNS Neurol Disord. 2003;2:389–402. doi: 10.2174/1568007033482670. [DOI] [PubMed] [Google Scholar]
- [199].van der Stelt M, Mazzola C, Esposito G, Matias I, Petrosino S, De Filippis D, Micale V, Steardo L, Drago F, Iuvone T, Di Marzo V. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol. Life Sci. 2006;63:1410–24. doi: 10.1007/s00018-006-6037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Mazzola C, Micale V, Drago F. Amnesia induced by beta-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur. J. Pharmacol. 2003;477:219–25. doi: 10.1016/j.ejphar.2003.08.026. [DOI] [PubMed] [Google Scholar]
- [201].Eubanks LM, Rogers CJ, Beuscher IV AE, Koob GF, Olson AJ, Dickerson TJ, Janda KD. A Molecular link between the active component of Marijuana and Alzheimer's Disease pathology. Mol. Pharm. 2006;3:773–77. doi: 10.1021/mp060066m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer's disease. Int. J. Geriatr Psychiatry. 1997;12:913–19. [PubMed] [Google Scholar]
- [203].di Tomaso E, Beltramo M, Piomelli D. Brain cannabinoids in chocolate. Nature. 1996;382:677–78. doi: 10.1038/382677a0. [DOI] [PubMed] [Google Scholar]
- [204].Di Marzo V, Sepe N, De Petrocellis L, Berger A, Crozier G, Fride E, Mechoulam R. Trick or treat from food endocannabinoids? Nature. 1998;396:636–37. doi: 10.1038/25267. [DOI] [PubMed] [Google Scholar]
- [205].Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am. J. Physiol. Regu.l Integr. Comp. Physiol. 2003;284:R345–53. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- [206].Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. Relat. Metab. Disord. 2004;28:640–48. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- [207].Kirkham TC, Tucci SA. Endocannabinoids in appetite control and the treatment of obesity. CNS Neurol. Disord. Drug Targets. 2006;5:272–92. doi: 10.2174/187152706777452272. [DOI] [PubMed] [Google Scholar]
- [208].Tucci SA, Halford JC, Harrold JA, Kirkham TC. Therapeutic potential of targeting the endocannabinoids: implications for the treatment of obesity, metabolic syndrome, drug abuse and smoking cessation. Curr. Med. Chem. 2006;13:2669–80. doi: 10.2174/092986706778201512. [DOI] [PubMed] [Google Scholar]
- [209].de Sa Roriz-Filho J, De-Sa-Roriz. T.M.; Rosset I, Camozzato AL, Santos AC, Chaves ML, Moriguti JC, Roriz-Cruz M. (Pre)diabetes, brain aging, and cognition. Biochim. Biophy. Acta. 2008 doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- [210].Gelfand EV, Cannon CP. Rimonabant: a selective blocker of the cannabinoid CB1 receptors for the management of obesity, smoking cessation and cardiometabolic risk factors. Expert. Opin. Investig. Drugs. 2006;15:307–15. doi: 10.1517/13543784.15.3.307. [DOI] [PubMed] [Google Scholar]
- [211].Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–72. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- [212].Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, Mendiguren A, Baer WU, Freiman I. Depolarization-induced retrograde synaptic inhibition in the mouse cerebellar cortex is mediated by 2-arachidonoylglycerol. J. Physiol. 2006;577:263–80. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- [214].Basavarajappa BS. Endocannabinoid system in the development of tolerance to alcohol. Klinik and Forschung (J. Clin. Res.) 2005;11:16–19. [Google Scholar]
- [215].Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacol. 2006;50:834–44. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- [216].Basavarajappa BS. The endocannabinoid signaling system: a potential target for next-generation therapeutics for alcoholism. Mini Rev. in Med. Chem. 2007;7:769–79. doi: 10.2174/138955707781387920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Cleland JG, Ghosh J, Freemantle N, Kaye GC, Nasir M, Clark AL, Coletta AP. Clinical trials update and cumulative meta-analyses from the American College of Cardiology: WATCH, SCD-HeFT, DINAMIT, CASINO, INSPIRE, STRATUS-US, RIO-Lipids and cardiac resynchronisation therapy in heart failure. Eur. J. Heart Fail. 2004;6:501–08. doi: 10.1016/j.ejheart.2004.04.014. [DOI] [PubMed] [Google Scholar]
- [218].Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–97. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- [219].Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno T, Di Marzo V. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15:300–02. doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- [220].Walker JM, Huang SM. Endocannabinoids in pain modulation. Prostaglandins Leukot Essent Fatty Acids. 2002;66:235–42. doi: 10.1054/plef.2001.0361. [DOI] [PubMed] [Google Scholar]
- [221].Schabitz WR, Giuffrida A, Berger C, Aschoff A, Schwaninger M, Schwab S, Piomelli D. Release of fatty acid amides in a patient with hemispheric stroke: a microdialysis study. Stroke. 2002;33:2112–14. doi: 10.1161/01.str.0000023491.63693.18. [DOI] [PubMed] [Google Scholar]
- [222].Basavarajappa BS, Hungund BL. Neuromodulatory role of the endocannabinoid signaling system in alcoholism: an overview. Prostaglandins Leukot Essent Fatty Acids. 2002;66:287–99. doi: 10.1054/plef.2001.0352. [DOI] [PubMed] [Google Scholar]
- [223].Hansson AC, Bermudez-Silva FJ, Malinen H, Hyytia P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic Impairment of Frontocortical Endocannabinoid Degradation and High Alcohol Preference. Neuropsychopharmacol. 2007;32:117–26. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]