Abstract
Background and Aims
Our earlier gene-expression studies with a Slovak PCBs-exposed population have revealed possible disease and disorder development in accordance with epidemiological studies. The present investigation aimed to develop an in vitro model system that can provide an indication of disrupted biological pathways associated with developing future diseases, well in advance of the clinical manifestations that may take years to appear in the actual human exposure scenario.
Methods
We used human PBMC (Primary Blood Mononuclear Cells) and exposed them to a mixture of human equivalence levels of PCBs (PCB-118,138,153,170,180) as found in the PCBs-exposed Slovak population. The microarray studies of global gene expression were conducted on the Affymetrix platform using Human Genome U133 Plus 2.0 Array along with Ingenuity Pathway Analysis (IPA) to associate the affected genes with their mechanistic pathways. High-throughput qRT-PCR Taqman Low Density Array (TLDA) was done to further validate the selected 6 differentially expressed genes of our interest, viz., ARNT, CYP2D6, LEPR, LRP12, RRAD, TP53, with a small population validation sample (n=71).
Results
Overall, we revealed a discreet gene expression profile in the experimental model that resembled the diseases and disorders observed in PCBs-exposed population studies. The disease pathways included Endocrine System disorders, Genetic disorders, Metabolic diseases, Developmental disorders, and Cancers, strongly consistent with the evidence from epidemiological studies.
Interpretation
These gene finger prints could lead to the identification of populations and subgroups at high risk for disease, and can pose as early disease biomarkers well ahead of time, before the actual disease becomes visible.
Keywords: PCBs, Human PBMC, Gene expression, Taqman Low-density array (TLDA), Pathway Analysis, Disease and Disorders, Biomarkers
1. Introduction
Even after the production of Polychlorinated biphenyls (PCBs) was banned in the 1970s, more than a billion kilograms were produced (Erickson, 1988), and they remain persistent and ubiquitous environmental contaminants that are routinely found in samples of human and animal tissues (Giera et al, 2011; Yang et al, 2010). Improper disposal of PCBs has been a major source of environmental contamination. Subsequent human exposure has been associated with toxic effects on various organs including the nervous, reproductive, and immunologic systems. The exposures to PCBs in a highly exposed Slovak population were associated with endocrine disorders (Radiková et al, 2008), diabetes (Ukropec et al, 2010), and reproductive (Plísková et al., 2005), neurological (Park et al., 2009, 2010), and hearing impairments (Trnovec et al., 2010), in addition to cancers (Pavúk et al., 2003, 2004, Bencko et al., 2009), and immunotoxicity (Horváthová et al., 2011a, b). Recent evidence suggests that exposure to some commonly encountered environmental contaminants, e.g. organochlorine compounds (OC; including several PCB congeners and chlorinated pesticides) may also contribute to Type 2 diabetes (Longnecker & Daniels, 2001, Carpenter 2008, Dirinck et al., 2011, Lee at al., 2011). There is growing evidence that perturbations of central endocrine regulatory systems by the endocrine disrupting chemicals (EDCs; e.g. Dioxins, PCBs, OC, etc.) established in early gestation may contribute to the development of obesity in later life (Alonso-Magdalena et al., 2011, Wang et al., 2008, Turyk et al., 2009a,b, Philibert et al., 2009).
The “developmental basis of disease” hypothesis posits that even seemingly minor exposures during early development can lead to functional deficits and increased disease risks later in life. However, it would be difficult to follow humans for decades to see if they develop diseases based on what they were exposed to before birth. On the other hand, researchers are now able to use new technologies to examine gene expression changes in tissues during development and link them to the pathogenetic pathways known to mediate the onset of disease later in life. Therefore, our study is based on the idea that changes in gene expression of a defined panel of genes can serve as both a robust biomarker of exposure to a group of compounds and as an indicator for future risk for specific diseases. Our earlier studies revealed that different PCB congeners (due to its structural differences) play a critical role in the mode(s) of action in vitro that changes the important cellular and signaling process and their potential to cause disease and developmental disorders (Dutta et al., 2008, Ghosh et al., 2011), including studies of gene expression in PCB-exposed children (Dutta et al., 2012, Mitra et al., 2012). The present study is designed to categorize some putative biomarkers through in vitro studies, indicating the affected molecular mechanisms and specific pathways that can be of predictive value of future risks of developing disease following an exposure event to PCBs.
2. Methods
2.1 Chemicals
PCB-118 (2,3′,4,4′,5-pentachlorobiphenyl, Product # RPC-106, CAS # 031508-00-6), PCB-138 (2,2′,3,4,4′,5′-Hexachlorobiphenyl) (Product # RPC-088, CAS # 35065-28-2), PCB-153 (2,2′,4,4′,5,5′-hexachlorobiphenyl) (Product #RPC-047, CAS # 35065-27-1), PCB-170 (2,2′, 3, 3′, 4, 4′, 5-heptachlorobiphenyl, Product # RPC-110, CAS# 035065-30-6), and PCB-180 (2,2′,3,4,4′,5,5′-heptachlorobiphenyl, Product # RPC-094, CAS # 035065-29-3) with a purity of >97.1 – 99.0 ±0.5% used herein are products of Ultra Scientific (North Kingstown, RI, USA). Dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO) was used for dissolving PCBs. A 2 ng/μl stock solution of the PCBs was prepared in DMSO to the working concentrations, in the same diluents. RPMI 1640 and Fetal Bovine Serum (FBS, Heat Inactivated, US Origin), Penicillin/Streptomycin were obtained from Invitrogen (Carlsbad, CA, USA). Phytohemagglutinin-M (PHA-M) was from Roche Diagnostics GmbH (Madison, WI, USA). Pokeweed Mitogen was from Life Technologies (Carlsbad, CA„ USA). Trizol reagent from Invitrogen Corp. (Carlsbad, CA, USA) was used for RNA extraction. For microarray, GeneChip® Human Genome HU133 Plus 2.0 was obtained from Affymetrix (Santa Clara, California, USA). The TRIzol® Plus RNA Purification System (Gaithersburg, MD, USA) was used for further clean-up and concentrate RNA samples. BD Vacutainer® CPT™ (Cell Preparation Tube, Becton Dickinson (Cat # 362753, Franklin Lakes, NJ, USA) with Sodium Heparin was used for the separation of mononuclear cells from whole blood. PBS 1× sterilized solution was procured from Quality Biological Inc. (Gaithersburg, MD, USA).
2.2 Selection of human subject participants for in vitro studies
The study was undertaken with the prior approval by the Howard University Institutional Review Board (IRB-07-GSAS-30). The informed consent was obtained from volunteers attending Howard University. The subjects were 6 young adults (age range 17–20), 3 males and 3 females, who were interviewed about themselves and about their parents, prior to collection of blood. Subjects selected for this study were free from any major reported illness. They had not undergone any major surgical procedures that required general anesthesia during the last 5 years. Those with any major environmental exposure issues in their life time were excluded.
2.3 Human subjects for small population TLDA validation
Subjects for TLDA validation study primarily belong to a well-defined cohort of mother-and-children pairs, originally enrolled in the ‘Slovak PCB Effects on Early Child Development Study’ between 2001 and 2004 (Hertz-Picciotto et al., 2003, Sonneborn et al, 2008). Details of the recruitment and characterization of this cohort have been described elsewhere (Park et al., 2010). These children live in the Michalovce area, highly contaminated by PCB from a chemical manufacturing plant. We used the subjects (n=71; Male=30 Nos., Female=41 Nos.) solely based on their PCBs concentration (with the average blood PCB 3.02±1.3 ng/mg of serum lipid; >75 percentile) from our earlier studies (Dutta et al., 2012, Mitra et al., 2012): they were included in this study only if they were free any clinical symptoms of chronic disease. Subject with no/background exposure level served as control. The Slovak Population RNAs were prepared from the whole blood and processed as previously described (Ghosh et al., 2013).
2.4 In Vitro exposure
The collected tubes of blood were brought into the lab immediately for isolation of PBMC cells. The detailed procedure can be found in our previous publications (Ghosh et al., 2011). The top five PCB congeners (PCB-118, PCB 138, PCB 153, PCB 170, and PCB 180) were the chemicals of our interest due to its maximum prevalence in the Slovak human exposed population. We chose 0.08 ng/ml of PCB 118, 0.87 ng/ml of PCB 138, 1.38 ng/ml of PCB 153, 0.52 ng/ml of PCB 170, and 1.24 ng/ml of PCB 180 according to the median concentration of PCBs (of human equivalence) in our exposed Slovak population (Hertz-Picciotto et al., 2003, Sonneborn et al., 2008). These PCBs (dissolved in DMSO) were added to each plate individually (in triplicate for each subjects), where the final concentration of DMSO was ≤0.1%. The cells remained exposed for 48 h. Control cell lines (vehicle control) were allowed to grow with DMSO only (≤0.1% of the total medium v/v) to ensure that the changes seen were not due to DMSO.
2.5 RNA preparation
RNA was extracted from the PBMC cells using a TRIzol® Plus RNA Purification System according to manufacturer’s direction. Prior to isolation, the cells from each plates were washed twice with 1× PBS. The RNAs were re-solubilized in RNase-free water. Contaminating DNA was removed with the Ambion DNA-free kit. RNA concentrations were determined spectrophotometrically on a nanodrop at 230, 260 and 280 λ. RNA quality was also verified by Agilent bioanalyzer analysis using a RNA 6000 nanochip before microarray chip hybridization. The RNA was stored at −80 °C.
2.6 cDNA Synthesis
Total RNA was reverse-transcribed to cDNA by using High-Capacity cDNA Reverse Transcription Kits (Part # 4387406; Applied Biosystems, CA, USA) according to manufacturers instruction. The reaction mixture (20 μL total volumes) was incubated at 25 °C for 10 min and then at 37 °C for 60 min followed by 95° C for 5 min. Finally, the mixture was heated at 95 °C for 5 min. The cDNAs were stored in −15 to −25° C, if not used immediately (within 24 hours), or stored in 2 to 8° C.
2.7 Microarrays
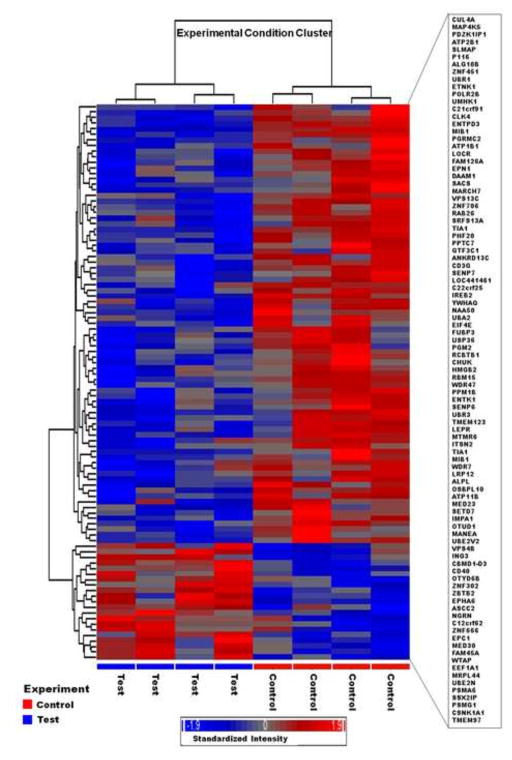
The RNAs were reversely transcribed to cDNA with an oligo-dT primer containing T7 RNA polymerase promoter. The cDNA was used as a template for in vitro transcription using the ENZO BioArray RNA transcript labeling kit (Affymetrix, CA). Biotin-labeled cRNA was purified, then fragmented randomly to approximately 200 bp prior to hybridizing to Affymetrix Human Genome Array for 16 h (in triplicate for each subjects). The microarray was washed and stained, and fluorescent images were obtained using the Affymetrix 3000 Scanner. Quality control measures included >4-fold cRNA amplification (from total RNA/cDNA), scaling factors <2 to reach a whole-chip normalization of 800, and visual observation of hybridization patterns for chip defects for quality control. The significant gene list identified with GeneSpring and dChip with Affymetrix probe set ID were imported into the dChip and clustered based on similarity in expression. Human Genome U133 Plus 2.0 Array in our microarray gene expression analysis for PCB exposure studies includes 54,675 probe sets, and was used during this study. The scan report generated by Gene Chip Operating Software (GCOS) had a scaling factor between 0.5 and 5, total percent of ‘P-calls’ between 30% and 50%, external controls cre>BioD>BioC>BioB and internal control was 1.0±0.1 (pivot table). This pivot table was then further evaluated by Hierarchical Clustering Explorer (HCE) (Seo and Shneiderman, 2002). The clustering of HCE that has been done by row by row normalization (mean ±SD) and Euclidian distance was calculated with average linkage that shows unique clustering of test and control subjects together while analyzing our data (Figure 1). After this final quality control, the data were analyzed with Partek® Genomics Suite™.
Fig. 1.
Hierarchical cluster analysis along with the heat map of the differentially expressed gene set (100) in human PBMCs in vitro study following human equivalence PCBs exposures. Red denotes up-regulation, blue down-regulation, and gray no difference; where brighter color (+/−) denotes the increasing intensities of up/down-regulations induced by PCBs exposures. The Hierarchical clustering (Dendrogram) displayed the results systematically, and showed that control and treated are grouped together and was based on average linkage with Pearson correlation.
2.8 Gene expression data analysis
The microarray data (*.cel file) were filtered and normalized by PLIER (http://www.affymetrix.com/support/technical/technotes/plier_technote.pdf) (Seo and Hoffman, 2006), and a subsequent statistical analysis was performed using the Partek® Genomics Suite™ analysis tool. Differential expression was compared using unpaired t-test statistics. A hierarchical clustering algorithm using the Pearson correlation was then used to temporally group those probe sets based on their expression pattern. Differential expression was determined by Partek’s Paired t-test and filtered with p-value <0.05. Gene’s annotations were expanded and upgraded using NCBI Entrez Gene, Unigene, and PubMed ID for all significantly different genes. “Minimum Information about a Microarray Experiment” (MIAME) compliant data has been submitted to the Gene Expression Omnibus (GEO) database. The datasets used in this paper can be accessed from the following GEO links: GSE22668.
To find common genes between the two data sets, i.e., in vitro (the current experimental) and our previous population studies (GSE22868, Dutta et al., 2012), the differentially expressed genes were compared by Partek Genomic Suites™ (version 6.6). We found 13 genes that are common in these two datasets (t-test, with FDR of p <0.05). Those are APC, ARNT, CD3G, CYP1A1, CYP2D6, ENTPD3, ITGB1, LEPR, LRP12, MYC, RRAD, TAB1, and TRAP1. We chose TP53 gene under our current investigation as a major molecule as revealed through IPA analysis (Fig. 3) which activates several cancer signaling pathways in our experimental study, resembling the human studies that provided prior suggestive evidence that PCBs are carcinogenic (ATSDR, 2000, Faroon et al., 2001, Laden et al., 2002).
Fig. 3.
Connectivity of differentially expressed genes in the important signaling pathway following mixed PCBs exposure in human PBMC in vitro depicting the connectivity between genes expressed (with ≥1.5 fold change, t-test, p <0.05). Geometric figures in red denote up-regulated genes and those are green indicate down-regulation. Genes in the top 6 networks (our experimental 100 gene sets) were allowed to grow our pathway with the direct/indirect relationship from the IPA knowledge base with the stringent filter, experimentally observed, those who were only from human study. Solid interconnecting lines show the genes that are directly connected and the dotted lines signify the indirect connection between the genes and cellular functions. Canonical functions (signaling) that are highly represented are shown within the box. Genes in uncolored notes were not identified as differentially expressed in our experiment and were integrated into computational generated networks based on evidence stored in the IPA knowledge base indicating relevance of this network.
2.9 Analysis towards the identification of cellular processes and pathways involved by Ingenuity Pathway Analysis (IPA)
Data sets containing gene identifiers and corresponding expression values (fold change) were uploaded into Ingenuity Pathway Analysis software (Ingenuity® Systems, www.ingenuity.com). Each gene identifier was mapped to its corresponding gene object in the Ingenuity Pathways Knowledge Base. We utilized the information in the Ingenuity Knowledge Base (Genes Only) as a reference set that consider both direct and indirect relationships. We included the molecules and/or the relationships only. To enrich our pathway analysis, we incorporated additional 661 gene transcripts from IPA knowledge base to our results. We used the data sources from ingenuity expert findings and use the “Core Analysis” function to interpret the data in the context of biological processes, pathways and networks. Differentially expressed gene identifiers were defined as value parameters for analysis and identified the relationship between gene expression alterations and related changes in biofunctions under the subcategories of Molecular and Cellular Functions, Physiological System Development and Function, and Disease and Disorders. Genes differentially expressed with p<0.05 were overlaid onto global molecular networks developed from information contained in the knowledge base. Networks were then algorithmically generated based on their connectivity. Networks were “named” on the most prevalent functional group(s) present. Canonical Pathway (CP) analysis identified function specific genes significantly present within the networks.
2.10 High-throughput Taqman® low density array (TLDA)
To identify altered gene expression, we used predesigned TLDA cards (Applied Biosystems®, CA) to examine the expression of 14 common genes as identified in the methods described above (including IPA knowledge base) in the experimental subjects (in vitro), and 6 genes of interest (out of 14, based on our prior investigations and epidemiological disease manifestation in PCBs-exposed population) to examine in our exposed Slovak population though a small population validation study.
For the present study, the TaqMan Low-density Array card was configured into a 14 genes set (triplicate per assay within the TLDA card, see Supplemental table-1 for detector probe information). Each set of genes contained two endogenous control genes, GAPDH and 18s RNA. The RNAs were synthesized with the cDNA (5 μL) and was mixed with 45 μL of H2O and 50 μL of 2× TaqMan Universal PCR Mix. Each sample (100 μL) was loaded into a port of the micro-fluid card, centrifuged, and run on an ABI Fast 7900HT System (ABI, CA) for 2 min at 50 °C, 10 min at 94.5 °C, followed by 40 cycles for 30 sec. at 97 °C and 1 min at 59.7 °C.
2.11 TLDA data analysis
The TLDA data were analyzed by SDS Ver. 2.4 software (ABI, CA). Threshold cycle (Ct) data for all target genes and control gene 18s RNA were used to calculate ΔCt values [ΔCt= Ct (target gene) - Ct (18s RNA)]. Then, ΔΔCt values were calculated by subtracting the calibrator (control) from the ΔCt values of each target. To visualize and further expression analysis, the data were exported in plate centric format to DataAssit V2.0 (ABI, CA) which allowed us to inspect the status of a gene(s) in the respective groups of lower and higher PCB exposures.
3. Results
3.1 Differential expression of genes with mixed PCBs exposure in vitro
Under the experimental conditions, we present here the top 100 genes that were differentially expressed (both up/down-regulated with ≥1.5 fold change, and statistically significant t-test, with FDR of p <0.05) (Fig. 1): 16% were up-regulated and 84% were down-regulated, when compared to control (Table 1).
Table 1.
List of 100 annotated gene transcription that differentially expressed in human PBMC (≥1.5 fold change, t-test, p <0.05) following exposure to human equivalence (of Slovak population) mixed PCBs.
| Probeset ID | Gene Symbol | Gene Title | Fold-Change |
|---|---|---|---|
| 1555118_at | ENTPD3 | Ectonucleoside triphosphate diphosphohydrolase 3 | −3.29083 |
| 1554726_at | ZNF655 | Zinc finger protein 655 | −1.5349 |
| 1555762_s_at | RBM15 | RNA binding motif protein 15 | −1.72036 |
| 1555837_s_at | POLR2B | Polymerase (RNA) II (DNA directed) polypeptide B, 140kDa | −1.66457 |
| 1556006_s_at | CSNK1A1 | Casein kinase 1, alpha 1 | −1.60048 |
| 1558688_at | LOC441461 | Hypothetical-LOC441461 | 1.51083 |
| 201177_s_at | UBA2 | Ubiquitin-like modifier activating enzyme 2 | −1.6631 |
| 201243_s_at | ATP1B1 | ATPase, Na+/K+ transporting, beta 1 polypeptide | −2.10046 |
| 201437_s_at | EIF4E | Eukaryotic translation initiation factor 4E | −1.77381 |
| 201449_at | TIA1 | TIA1 cytotoxic granule-associated RNA binding protein | −1.97483 |
| 201450_s_at | TIA1 | TIA1 cytotoxic granule-associated RNA binding protein | −1.74723 |
| 201523_x_at | UBE2N | Ubiquitin-conjugating enzyme E2N (UBC13 homolog, yeast) | −1.58113 |
| 202318_s_at | SENP6 | SUMO1/sentrin specific peptidase 6 | −1.83591 |
| 202320_at | GTF3C1 | General transcription factor IIIC, polypeptide 1, alpha 220kDa | 1.546298 |
| 202487_s_at | H2AFV | H2A histone family, member V | −1.55526 |
| 202653_s_at | 7-Mar | Membrane-associated ring finger (C3HC4) 7 | −1.95386 |
| 203011_at | IMPA1 | Inositol(myo)-1(or 4)-monophosphatase 1 | −2.13757 |
| 203017_s_at | SSX2IP | Synovial sarcoma, X breakpoint 2 interacting protein | −2.11359 |
| 203405_at | PSMG1 | Proteasome (prosome, macropain) assembly chaperone 1 | −1.81846 |
| 203552_at | MAP4K5 | Mitogen-activated protein kinase kinase kinase kinase 5 | −1.71411 |
| 203855_at | WDR47 | WD repeat domain 47 | −1.6205 |
| 204299_at | SFRS13A | Splicing factor, arginine/serine-rich 13A | −1.83462 |
| 206804_at | CD3G | CD3g molecule, gamma (CD3-TCR complex) | 1.65203 |
| 208805_at | PSMA6 | Proteasome (prosome, macropain) subunit, alpha type, 6 | −1.9818 |
| 208808_s_at | HMGB2 | High-mobility group box 2 | −1.84785 |
| 209096_at | UBE2V2 | Ubiquitin-conjugating enzyme E2 variant 2 | −1.51946 |
| 209422_at | PHF20 | PHD finger protein 20 | −1.50916 |
| 209666_s_at | CHUK | Conserved helix-loop-helix ubiquitous kinase | −1.50891 |
| 210285_x_at | WTAP | Wilms tumor 1 associated protein | −1.70726 |
| 211354_s_at | LEPR | Leptin receptor | −2.10571 |
| 211967_at | TMEM123 | Transmembrane protein 123 | −2.29464 |
| 212281_s_at | TMEM97 | Transmembrane protein 97 | −2.06445 |
| 212426_s_at | YWHAQ | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, theta po | −1.63874 |
| 212536_at | ATP11B | ATPase, class VI, type 11B | −1.581 |
| 212557_at | ZNF451 | Zinc finger protein 451 | −1.50106 |
| 212824_at | FUBP3 | Far upstream element (FUSE) binding protein 3 | −1.62965 |
| 212880_at | WDR7 | WD repeat domain 7 | −1.83247 |
| 213225_at | PPM1B | Protein phosphatase, Mg2+/Mn2+ dependent, 1B | −1.65843 |
| 213227_at | PGRMC2 | Progesterone receptor membrane component 2 | −1.59415 |
| 213262_at | SACS | Spastic ataxia of Charlevoix-Saguenay (sacsin) | −2.30661 |
| 214429_at | MTMR6 | Myotubularin related protein 6 | −2.26117 |
| 215684_s_at | ASCC2 | Activating signal cointegrator 1 complex subunit 2 | 2.37375 |
| 215716_s_at | ATP2B1 | ATPase, Ca++ transporting, plasma membrane 1 | −1.75211 |
| 215783_s_at | ALPL | Alkaline phosphatase, liver/bone/kidney | 2.62704 |
| 216060_s_at | DAAM1 | Dishevelled associated activator of morphogenesis 1 | −1.5949 |
| 216680_s_at | EPHB4 | EPH receptor B4 | 1.64141 |
| 216755_at | OSBPL10 | Oxysterol binding protein-like 10 | 1.52037 |
| 217203_at | GLULP4 | Glutamate-ammonia ligase (glutamine synthetase) pseudogene 4 | 1.55534 |
| 217745_s_at | NAA50 | N(alpha)-acetyltransferase 50, NatE catalytic subunit | −1.62715 |
| 217945_at | BTBD1 | BTB (POZ) domain containing 1 | −1.59839 |
| 218171_at | VPS4B | Vacuolar protein sorting 4 homolog B (S. cerevisiae) | −1.63559 |
| 218352_at | RCBTB1 | Regulator of chromosome condensation (RCC1) and BTB (POZ) domain containing prot | −1.66994 |
| 218396_at | VPS13C | Vacuolar protein sorting 13 homolog C (S. cerevisiae) | −1.66178 |
| 218846_at | MED23 | Mediator complex subunit 23 | −1.7511 |
| 219630_at | PDZK1IP1 | PDZK1 interacting protein 1 | 2.32042 |
| 219811_at | DGCR8 | DiGeorge syndrome critical region gene 8 | 1.78871 |
| 220175_s_at | CBWD1-D7 | COBW domain containing 1 -7 | −1.54632 |
| 220253_s_at | LRP12 | Low density lipoprotein receptor-related protein 12 | −2.10571 |
| 222292_at | CD40 | CD40 molecule, TNF receptor superfamily member 5 | 1.54035 |
| 222555_s_at | MRPL44 | Mitochondrial ribosomal protein L44 | −1.54162 |
| 222805_at | MANEA | mannosidase, endo-alpha | −2.24407 |
| 222825_at | OTUD6B | OTU domain containing 6B | −1.59575 |
| 222924_at | SLMAP | Sarcolemma associated protein | −1.60831 |
| 223288_at | USP38 | Ubiquitin specific peptidase 38 | −1.98296 |
| 223444_at | SENP7 | SUMO1/sentrin specific peptidase 7 | −1.82933 |
| 223875_s_at | EPC1 | Enhancer of polycomb homolog 1 (Drosophila) | −1.5408 |
| 224281_s_at | NGRN | Neugrin, neurite outgrowth associated | −1.69305 |
| 224691_at | UHMK1 | U2AF homology motif (UHM) kinase 1 | −1.9501 |
| 224720_at | MIB1 | Mindbomb homolog 1 (Drosophila) | −3.03948 |
| 224725_at | MIB1 | Mindbomb homolog 1 (Drosophila) | −2.71137 |
| 224928_at | SETD7 | SET domain containing (lysine methyltransferase) | −1.56236 |
| 225213_at | PPTC7 | PTC7 protein phosphatase homolog (S. cerevisiae) | −1.59082 |
| 225290_at | ETNK1 | Ethanolamine kinase 1 | −2.54633 |
| 225351_at | FAM45A | Family with sequence similarity 45, member A | −1.6578 |
| 225367_at | PGM2 | Phosphoglucomutase 2 | −1.80992 |
| 225772_s_at | C12orf62 | chromosome 12 open reading frame 62 | −1.63556 |
| 225892_at | IREB2 | Iron-responsive element binding protein 2 | −1.82595 |
| 226109_at | C21orf91 | Chromosome 21 open reading frame 91 | −1.94281 |
| 226140_s_at | OTUD1 | OTU domain containing 1 | −2.09994 |
| 226284_at | ZBTB2 | Zinc finger and BTB domain containing 2 | −1.58058 |
| 226432_at | ETNK1 | Ethanolamine kinase 1 | −2.68345 |
| 226520_at | LCOR | Ligand dependent nuclear receptor corepressor | −2.03599 |
| 226667_x_at | EPN1 | Epsin 1 | 1.63073 |
| 226921_at | UBR1 | Ubiquitin protein ligase E3 component n-recognin 1 | −2.25037 |
| 227003_at | RAB28 | RAB28, member RAS oncogene family | −1.94344 |
| 227239_at | FAM126A | Family with sequence similarity 126, member A | −1.6925 |
| 227375_at | ANKRD13C | Ankyrin repeat domain 13C | −1.74244 |
| 227708_at | EEF1A1 | Eukaryotic translation elongation factor 1 alpha 1 | −1.89046 |
| 227757_at | CUL4A | Cullin 4A | 2.22597 |
| 227787_s_at | MED30 | Mediator complex subunit 30 | −1.5697 |
| 228312_at | PI16 | Peptidase inhibitor 16 | 1.57939 |
| 228392_at | ZNF302 | Zinc finger protein 302 | −1.83879 |
| 228751_at | CLK4 | CDC-like kinase 4 | −2.00776 |
| 228941_at | ALG10B | Asparagine-linked glycosylation 10, alpha-1,2-glucosyltransferase homolog B | −2.07412 |
| 230029_x_at | UBR3 | Ubiquitin protein ligase E3 component n-recognin 3 (putative) | −1.94883 |
| 233184_at | EPHA6 | EPH receptor A6 | 1.87456 |
| 235396_at | C22orf25 | Chromosome 22 open reading frame 25 | 1.51629 |
| 239482_x_at | ZNF708 | Zinc finger protein 708 | −1.84726 |
| 240941_at | ITSN2 | Intersectin 2 | 1.7006 |
| 242293_at | ING3 | Inhibitor of growth family, member 3 | −1.54031 |
3.2 The effects of the differentially expressed genes
The list of biological effects caused by exposure to mixed PCBs can be found in three levels; gene function level (Table 1), network level (Table 2), and Bio-functions level (Table 3). Analysis of the genes identified above revealed six significant genetic networks (score ≥10.0), as listed in Table 2. The top-scoring networks include: Endocrine System Disorders, Genetic Disorders, Metabolic Diseases, Cancer, Auditory Diseases, Dermatological Disease and Conditions, Developmental disorders, Hematological Disease, Immunological Disease, and Neurological Disease (Figure 2; panel B). Among the genes in the Molecular and Cellular Functions network, Cellular Assembly & Organization, Carbohydrate Metabolism, Lipid Metabolism, Small Molecule Biochemistry, and Cell Cycle functions were revealed (Figure 2; panel C). In the Physiological System Development and Functions network, notable functions were Lymphoid Tissue Structure & Development, Embryonic Development, Hematological System Development & Function, Tissue Development, Cardiovascular system Development & Function, Tissue Morphology and Tumor Morphology (Figure 2, panel A).
Table 2.
Significant Top Bio-function from IPA knowledge base associated with differentially expressed genes in mixed-PCB exposure in vitro.
| Category | Important Molecule(s)/(Genes) | …log (p-value) |
|---|---|---|
| Disease & Disorders | ||
| Endocrine System Disorders | LEPR, UBR1 | 2.42E-03-5.72E-03 |
| Genetic Disorder | CD40, LEPR, DGCR8, UBR1, RBM15, ALPL | 2.42E-03-2.83E-02 |
| Metabolic Disease | LEPR, UBR1, ALPL | 2.42E-03-5.72E-03 |
| Cancer | CD3G, EPN1, CD40, ITSN2, RBM15, CHUK, EIF4E | 5.72E-03-3.84E-02 |
| Auditory Diseases | UBR1 | 5.72E-03-5.72E-03 |
| Dermatological Diseases and Conditions | UBR1 | 5.72E-03-5.72E-03 |
| Developmental Disorder | DGCR8, RCBTB1, UBR1, ALPL | 5.72E-03-1.14E-02 |
| Hematological Disease | CD3G, CD40, RBM15, CHUK | 5.72E-03-3.84E-02 |
| Immunological Disease | CD3G, CD40, CHUK | |
| Neurological Disease | UBR1, OSBPL10, EEF1A1, UBR3, NGRN, MTMR6, SLMAP, CD40, PPM1B, MIB1, C21orf91, ITSN2, SSX2IP, WDR7, HMGB2 | 5.72E-03-3.56E-02 |
| Skeletal and Muscular Disorders | C21orf91, CD40, EEF1A1, HMGB2, ITSN2, MIB1, MTMR6, NGRN, OSBPL10, PPM1B, SLMAP, SSX2IP, UBR3, WDR7 | 3.56E-02 |
| Molecular and Cellular Functions | ||
| Cellular Assembly & Organization | EEF1A1, EIF4E, UBE2N, TMEM123, SACS | 1.13E-03-2.83E-02 |
| Carbohydrate Metabolism | CD40, EEF1A1, ETNK1, IMPA1, MTMR6 | 2.13E-03-4.49E-02 |
| Lipid Metabolism | CD40, EEF1A1, ETNK1, IMPA, ATP11B, MTMR6 | 2.13E-03-4.49E-02 |
| Small Molecule Biochemistry | CD40, EEF1A1, ETNK1, IMPA, ATP11B, MTMR6 | 2.13E-03-4.49E-02 |
| Cell Cycle | CHUK, CSNK1A1, EIF4E, ZNF655, CUL4A, CD40, YWHAQ, UHMK1 | 5.72E-03-4.22E-02 |
| Physiological System Development and Function | ||
| Lymphoid Tissue Structure & Development | CD40, CHUK | 1.72E-03-4.49E-02 |
| Embryonic Development Hematological System | TMEM123, EPHB4, CUL4A, EIF4E | 5.72E-03-3.38E-02 |
| Development & Function | CD40, CUL4A, EIF4E, CHUK | 5.72E-03-4.49E-02 |
| Tissue Development | TMEM123, CD40, CHUK, EPHB4, LEPR, EIF4E | 5.72E-03-3.38E-02 |
| Cardiovascular system Development & Function | EPHB4 | 1.14E-02-1.71E-02 |
| Tissue Morphology | CD40, CUL4A, EIF4E, CHUK, LEPR | 1.62E-02-3.38E-02 |
| Tumor Morphology | CD40, CUL4A, CHUK | 1.62E-02-2.83E-02 |
Genes in Bold = Up-regulated; Italicized = Down-regulated; p-value = Fischer’s exact test was used to calculate a p-value determining the probability that each biological function and/or disease assigned to that dataset.
Table 3.
High-scoring networks (Score >10) identified by Ingenuity® Pathway Analysis in PCB mixed exposure to Human PBMC. Top 6 (Six) out of 24 networks are represented here.
| Network ID | Genes in Network | Score | Focus Molecules | Functions |
|---|---|---|---|---|
| 1. | ASCC2, ATP1B1, ATPase, Caspase, CD40, CHUK, CUL4A, EIF4E, EPC1, GTF3C1, HISTONE, ZNF451 Ikk (family), ING3, Interferon alpha, LEPR, YWHAQ, LOC100505793/SRSF10, MAP4K5, MARCH7, MED23, MED30, MIB1, IKK (complex), NFkB (complex), PI3K (complex), POLR2B, PPM1B, RNA polymerase II, TRAP/Media, UBE2N, UBE2V2, Vegf, VPS4B, WTAP | 51 | 24 | Gene Expression, Cell Death, Antigen Presentation |
| 2. | ALG10B, ATP11B, BTBD1, CALB1, CBWD1, DCAF16, ETNK1, IMPA1, KCNN4, LARP1, LCOR, MANEA, MIB1, miR-344d/miR-410, miR-520d-5p/miR-524-5p, miR-590-3p, MTMR6, MTMR9, PAPSS1, PPTC7, RAB28, RAP1GDS1, RNF182, RSL1D1, SAMSN1, SENP7, SETD7, SSX2IP, SUV39H2, UBR3, USP38, USP53, ZNF655, ZNF708, ZNRF1 | 39 | 19 | Carbohydrate Metabolism, Cell Morphology, Cellular Functions & Maintenance |
| 3. | ACTN2, ASCC3, ATP11B, C21orf91, CLK4, SIKE1, DGCR8, DROSHA, FAM40B, FUBP3, H2AFV, LRP12, MANEA, miR-194, miR-514, miR-606, MOBKL3, miR-219-2-3p/miR-219-3p, miR-297a/miR-297, UBR3, miR-548c-3p, miR-802 (human), CTTNBP2NL, NAA16, NAA50, OTUD6B, PGM2, QKI, SENP6, SLMAP, TIA1, TIAL1, TMEM123, TOB1, UBE2B | 31 | 16 | Cellular Growth & Proliferation, Lipid Metabolism, Molecular Transport |
| 4. | ANKRD13C, ATP1A2, DMXL2, DNAJB11, DROSHA, E2F1, EFNA3, EFNB2, EPHB4, FAM126A, FAM45A, HSPE1, ITSN2, MSL1, NGRN, NPLOC4, OSBPL10, PAX9, PI16, PTPN4, PTPRJ, RCBTB1, RNF144A, SACS, SRGAP2, SSX2IP, SUV420H1, TMEM123, WDR7, UBE2B, UBR1, VPS13C, miR-205, miR-27b/miR-27a, miR-20a/miR-106b/miR-17-5p (includes others) | 29 | 15 | Cardiovascular System Development & Function, Organismal Development, Cell Morphology |
| 5. | ACO2, ADRM1, C12orf11, C12orf62, CDKN1B, CKS2, COPS7A, COPS7B, DAAM1, DCAF8, EGFR, ENTPD3, EPN1, ETNK1, GRWD1, HBXIP, HNF4A, IKBKE, IREB2, miR-1264, miR-16/miR-497/miR-195 (includes others), miR-802 (human), MRPL44, NAA10, NAA20, NFKBIL1, PGRMC2, PHF20, PRPF38A, PSMA6, RBM15, SDHB, TGFB1, TIA1, UHMK1 | 23 | 13 | Cellular Growth & Proliferation, Cell Death, Cellular Function & Maintenance |
| 6. | ALPL, ANXA3, ATP2B1, BRF1 (includes EG:2972), CCL18, CD8, CD3G, CDK16, CSNK1A1, DAPK1, EFNA1, EFNA3, EFNA4, EPHA6, EPHA, FLNC, FSH, GZMA, HMGB2, IL3, IL4, Lh, Mapk, MKNK2, OSM, OTUD1, PDZK1IP1, PPP1R14A, PSMG1, RAD23A, STK25, TMEM97, TP53, ZBTB2 | 19 | 11 | Cell-To-Cell Signaling and Interaction, Lipid Metabolism, Small Molecule Biochemistry |
The genes found to be differentially regulated in our experiments and the number of such genes displayed in the “Focus Molecules” column have been highlighted in bold print that meet the criteria cutoff and/or filter criteria, and were mapped to its corresponding gene object in IPA Knowledge base (Italicized Bold= Up-regulated; Italicized = Down-regulated). The score is generated using a p-value calculation. This score indicates the likelihood that the assembly of a set of focus genes in a network could be explained by random chance alone. The data base attributed general cellular functions to each network which are determined by interrogating the Ingenuity Pathway Knowledge base for relationships between the genes in the network and the cellular functions they impact.
Fig. 2.
The key (Top) bio-functions in developing toxicities with the differentially expressed gene set following PCBs mixed-exposures in vitro as obtained through IPA analysis physiological system development and functions (A), disease and disorder development (B), and in molecular and cellular functions (C). The most statistically significant top biofunctions that were identified in the IPA Tox analysis are listed here according to their p value (−Log). The threshold line corresponds to a p value of 0.05.
3.3 The canonical pathways and GO enrichment of biological processes in vitro
In the canonical pathway analysis, we chose to build the pathways connecting the top 3 networks (Networks 1–3, Score ≥30, Table 3). The top thirteen (13) pathways identified through this approach were: Insulin Receptor Signaling, Apoptosis Signaling, Aryl Hydrocarbon Receptor Signaling, p53 Signaling, G-protein Coupled Receptor Signaling, Ovarian Cancer Signaling, Prostate Cancer Signaling, Molecular Mechanisms of Cancers, Chronic Myeloid Leukemia Signaling, Type I and Type II Diabetes Mellitus Signaling, Cardiac Hypertrophy Signaling, and Colorectal Cancer Metastasis Signaling (Fig. 3). Further in-depth analysis also identified some important pathways, viz., Leptin Signaling in Obesity, Endometrial Cancer Signaling, Cell-cycle: G2/M DNA Damage Checkpoint Regulation, NF-KB Signaling, some of which are in accord with our previous investigations (Ghosh et al., 2010, 2011, 2013, 2014).
3.4 Top biofunctions and Disease & Disorder development in in vitro
The Gene Set Enrichment Analysis (GO Enrichment Score) revealed altered expression of genes with important and shared common biological functions, chromosomal location, or regulation, e.g. Developmental process, Biological Recognition, Biological Adhesion, Reproduction, Death, Cellular Compartment Organization, Pigmentation, and Reproductive Process.. Furthermore, the IPA data analysis revealed 17 important biofunctions in the diseases and disorder categories of Cancer, Genetic Disorders, Reproductive System Disease, Skeletal and Muscular Disorder, Infection Mechanism, Renal and Urological Disease, Dermatological Disease, Connective Tissue Disorders, Endocrine System Disorders, Gastrointestinal Disease, Neurological Disease, Developmental Disorder, Hematological Disease, Immunological Disease, Cardiovascular Disease, and Inflammatory Response (Fig. 4).
Fig. 4.
Top biofunctions in disease and disorder development with the in vitro studies (PCBs mixed) generated through IPA analysis. The gene sets from the study were filtered, uploaded, and run through in the IPA comparative data Analysis module. The important disease and disorders that are represented here were at or above the threshold value (corresponds to a p value of 0.05). Fischer’s exact test was used to calculate a p-value determining the probability that each biological function and/or disease assigned to the dataset.
3.5 In vitro and Population Validation of Selected Genes through TLDA
High throughput quantitative real time PCR (TLDA in ABI platform) confirmed the PCBs-associated altered expression of our 14 common genes under the in vitro experimental condition. All 14 genes were well amplified in our experimental condition (cross-validation in mixed-PCBs in vitro exposures, n=6; Table-4, Fig. 5 & 6).
Fig. 5.
In vitro Quantitative Real-time PCR (qRT-PCR) validation of the selected 14 genes (both experimental and IPA knowledge base) by Taqman Low Density Array (TLDA) in ABI platform (7900HT Fast Real-Time PCR System) after analyzed by SDS RQ Manager Version 1.2.1 (ΔΔCt). Each panel shows the relative quantification of the selected genes up/down-regulation among the experimentally exposed condition (Subjects 1–6). The relative quantification is calculated in contrast to calibrator samples, i.e.; no-exposure in in vitro studies (control).
Fig. 6.
Quantitative Real-time PCR (qRT-PCR) validation of the selected 6 genes of interest by Taqman Low Density Array (TLDA) in ABI platform (7900HT Fast Real-Time PCR System) after analyzed by SDS RQ Manager Version 1.2.1(ΔΔCt). The panels A–F (with the respective genes) represent the relative quantification of the genes upon small population validation (the population with high PCBs in their blood; n=71) with the same gene transcript that has been used in in vitro studies. The relative quantification is calculated in contrast to calibrator samples, i.e.; the subjects with no/background PCBs exposures in the population.
Some of the signature genes, CYP2D6, LEPR, LRP12, ARNT, RRAD, and TP53, which we presume could serve as putative biomarkers under such an exposure scenario, were further cross-validated in the PCBs-exposed human population for their consistency (Fig. 6, panel A–F). The genes LEPR, LRP12, ARNT, and TP53 were well confirmed in the population samples (Figure 6, panel B, C, D, and F respectively). The CYP2D6 and RRAD genes were either up-regulated in the experiments or down-regulated in the population, or vice-versa (Fig. 6, panel A & E respectively).
4. Discussion
In the field of toxicology, most of the studied agents, including PCBs, are likely to exert their adverse effects directly or indirectly by altering “normal” signaling processes in the cell. Moreover, adverse end points resulting from toxicant exposures may be profiled and compared to normal tissue/cell samples to discern differences in gene expression between the two states (Hamadeh et al., 2002, Waring et al., 2001). Our overall hypothesis was based on several epidemiological studies showing that PCBs can cause a wide range of health effects: we posited that those health effects were initiated by receptor binding/activation, leading to altered gene and protein expression. Under the present investigation, we observed that gene expression changes resulting from the exposure may indeed reflect an investigational approach that highlights biomarkers of specific diseases and disorders, e.g. metabolic disorders (including obesity and diabetes) cardiovascular, endocrine disruption, and cancers, in accord with results observed in epidemiological settings (Langer et al, 2014; Arrebola et al., 2014; Recio-Vega et al., 2013; Lee et al., 2014; Wadzinski et al., 2014; Pereira-Fernandes et al., 2014; Sexton et al., 2013, Casas et al., 2014). Research utilizing PBMC cells in human are now being widely used to test for the efficacy of new drugs and treatments, to divulge the differential gene expression patterns induced by toxicity, and to study downstream effectors, all of which can be used as a potential source for developing disease-specific biomarkers (Reynes et al., 2014; Saidijam et al., 2014; Roccaet et al., 2014; Kong 2014). In such a model, studying the gene expression changes might provide us a valuable insights and better understanding of their mechanism(s) of action by highlighting which enzymes or proteins are targeted by these exposures.
The microarray analysis of data from this study clearly indicates that PCBs exposure caused significant differential gene expression (either up or down-regulated). We identified 14 genes for further study, viz. APC, ARNT, CD3G, CYP1A2, CYP2D6, ENTPD3, ITGB1, LEPR, LRP12, MYC, RRAD, TAB1, p53, and TRAP1, which showed notable expression changes and are known to have major impacts in mediating toxicities by altering cellular and molecular functions in developing disease and disorders under experimental condition, among which six were well validated in the PCBs-exposed Slovak population.
In the present in vitro study, RRAD was up-regulated in two of the exposed subjects in the PBMC study, and it was up-regulated in most of the subjects in the population validation study. RRAD over-expression is associated with insulin resistance in Type II (non-insulin-dependent) diabetes mellitus (Reynet and Khan, 1993). Rad (Ras associated with diabetes) GTPase is the prototypic member of a subfamily of Ras-related small G proteins, normally expressed in heart, skeletal muscle, and lung. Rad is over-expressed in skeletal muscle of some patients with type II diabetes mellitus and/or obesity. Over-expression of Rad inhibits glucose uptake in cultured muscle and fat cells (Moyers et al., 1996) and in adipocytes and muscle cells in culture, it results in diminished insulin-stimulated glucose uptake (Ilany et al., 2006). Our IPA core analysis of significant canonical pathways also highlighted the Type II diabetes mellitus signaling pathway and insulin receptor signaling.
The ARNT gene encodes the aryl hydrocarbon receptor nuclear translocator protein that forms a complex with ligand-bound aryl hydrocarbon receptor (AhR) (Reyes et al., 1992) and is required for receptor function and is involved in the induction of several enzymes that participate in xenobiotic metabolism. Induction of enzymes involved in xenobiotic metabolism occurs through binding of the ligand-bound AhR to xenobiotic responsive elements in the promoters of genes for these enzymes (Whitelaw et al., 1993). Non-ortho PCBs, also known as the coplanar PCBs, bind the AhR and are capable of producing dioxin-like effects within biological systems (Mortensen and Arukwe, 2008). In our in vitro (Fig. 5) and population validation studies (Fig. 6D), the AhR/ARNT gene was down-regulated during our TLDA validation that corroborates our 45 month gene expression studies earlier (Dutta et al., 2012). This AhR receptor signaling pathway was also important among our major canonical pathways through IPA analysis (Fig. 4).
Cytochrome P450 2D6 (CYP2D6), a member of the cytochrome P450 mixed-function oxidase system encodes a member of the cytochrome P450 superfamily of enzymes. While CYP2D6 is involved in the oxidation of a wide range of substrates, there is considerable interest in its expression induced by xenobiotic materials, e.g., PCBs (Tabb et al., 2004) which increased our interest because of the reported poor neurobehavioral development in case of PCB-exposed children (Schantz, 1996, Stewart et al., 2008). The results on CYP2D6 in our experimental condition showed that it was down-regulated in all the subjects in the PBMC experiment, and in most of those in the validation population (Fig. 6, panel A).
Leptin receptor also known as LEP-R is a protein that in humans is encoded by the LEPR gene. LEP-R functions as a receptor for the fat cell-specific hormone leptin that regulates body weight and is involved in the regulation of fat metabolism, as well as in a novel hematopoietic pathway that is required for normal lymphopoiesis (Bennett et al., 1996). In our experimental studies, the LEPR gene was down-regulated in all of the in vitro samples (Fig. 5), which is in accord with the status of this gene in a previous population study (Ghosh et al., 2013) that perfectly corroborated with the population validation analysis we report in the present paper (Fig. 6, panel B). Increasing evidence suggests that the commonly held causes of obesity, which are over-eating, inactivity and genetic pre-disposition, do not fully explain the current obesity epidemic. Interestingly, the production and use of synthetic chemicals have increased dramatically, in parallel with growing obesity and it has been suggested that EDCs may play a key role in obesity development by altering physiological control mechanisms (Tang-Péronard et al., 2011, Grün and Blumberg, 2009, Newbold et al., 2009, Elobeid et al., 2010). Among them, POPs, OC pesticides, and PCBs may be particularly interesting because low dose OC pesticides or PCBs were strongly linked to type 2 diabetes, insulin resistance, and metabolic syndrome, in all of which, obesity is believed to play a critical role (Lee et al., 2011, 2012, 2014).
Several pathways important in the molecular mechanisms of cancer were also identified in this study. In a recent study designed to evaluate the relation between PCB exposure and breast cancer risk in Mexican women, an association between heavy and potentially estrogenic PCB congeners and breast cancer risk was shown (Recio-Vega et al., 2011) corroborating our observations. Furthermore, based on epidemiological associations of PCBs and cancers at several organ sites, particularly the liver, biliary tract, intestines, and skin (melanoma), the human studies provide suggestive evidence that PCBs are carcinogenic (ATSDR, 2000, Faroon et al., 2001, Laden et al., 2002, Lauby-Secretan et al., 2013). Even after categorization of PCBs as cancer causing agents by ATSRD in 2002, the debate on the risk of cancer due to PCBs exposure in human is, however, highly controversial (Golden and Kimbrough, 2009) and has yet to be resolved.
In our experiments we observed that several important cancer-related genes were affected. p53 (TP53) is crucial in multicellular organisms, where it regulates the cell cycle and, thus, functions as a tumor suppressor that is involved in preventing cancer, whereas thec-MYC protein participates in energy-consuming processes such as cell proliferation, ribosomal biosynthesis, glycolysis, mitochondrial functions, and differentiation and its expression is often dysregulated by human cancers (Dang, 1999). Specifically, we observed that p53 was deregulated (down-regulated) in the experimental model and had a similar pattern of down-regulation in the majority of the subjects (Fig 6, panel F). Through a prior investigation, we also found that p53 was down-regulated over a short exposure period, showing loss of cell viability and apoptosis, but was up-regulated over a chronic exposure of 12 weeks period (Ghosh et al., 2007), which corroborates our present observation concerning the involvement of cell cycle and cell death pathways in the molecular and cellular functions (Fig. 2C).
LRP12 gene may also be characterized by its differential expression in cancer cells. The product of this gene is predicted to be a transmembrane protein, and was found to be lower in tumor derived cell lines compared to normal cells. LRP12 belongs to the LDLR superfamily and may play a role in signal transduction (Qing et al., 1999; Battle et al., 2003). Information on the role of LRP12 is scanty, especially while considering its involvement in some environmental toxic exposures. The work by Garnis et al., (2004) suggested that LRP12 might function as an oncogene in oral tumors. To date, we may be the first to report that this LRP12 gene may be related to PCBs toxicity.
Regarding our findings of pathways involving Type 2 diabetes, Obesity, Cardiovascular diseases, and even cancer, there is considerable evidence that the risks of these diseases may begin early in life, during pregnancy, and early childhood. There are numerous studies showing that rapid weight gain in the first few months of life is associated with obesity later in life (Boekelheide et al., 2012). Another recent study also suggests that other prenatal OC concentrations (PCBs, DDE, and DDT) were associated with being overweight at 6.5 years of age (Valvi et al., 2012). Obesity like other complex diseases is caused by myriad interactions between genetic, behavioral and environmental factors. There is an emerging hypothesis, based on data from several chemicals in animal studies, that the obesity epidemic could be due to chemical exposures during vulnerable windows of development, mainly in utero and the first few years of life (Lubrano et al., 2013; Heindel JJ, vom Saal 2009), as previously observed in our Slovak epidemiological investigations. Metabolic syndrome is also associated with the rise in obesity and may progress to type 2 diabetes. There is significant data supporting the idea that metabolic syndrome is programmed during development and that there is a role for maternal diet in its etiology (Boekelheide et al, 2012). While there are few data linking developmental exposures to environmental chemicals to actual metabolic syndrome, there are data showing effects of exposures on the development of obesity and type 2 diabetes (Uemura, 2012; Faerch et al., 2012, Ghosh et al., 2014).
During last decade, after completion of human genome project, the biomedical research community is focusing increased attention on finding biological markers (Biomarkers) so that early diagnosis and even prevention can be achieved, before the symptoms appear (Thayer et al., 2012, Sexton and Salinas 2014, Ghosh et al., 2014).
Therefore, developing a rich set of biomarkers for monitoring early health effects in the life course is becoming more important. Biomarker-based methods capable of identifying high-risk individuals with specificity and selectivity will greatly facilitate early detection directed towards reducing environmentally induced diseases, such diabetes, obesity, hearing impairments, and malignancies, as they have already started appearing in PCB-exposed population. Such efforts are also shedding new light on possible mechanisms for the genesis of disease development.
5. Conclusion
The results from the present study provide an integrated view of gene expression and potential downstream pathophysiolocal changes that might lead PCB-exposed subjects towards the development of diseases and disorders. The results thus provide a possibility to develop a screening method using these gene fingerprints that could lead to the identification of subgroups at high risk, well ahead of time, even before the actual disease becomes visible. If validated through population-based studies, such a comprehensive approach will generate new information and fill critical gaps in knowledge regarding PCB exposure-related human health effects and potentially open the door for the development of early preventive strategies.
Table 4.
Differential expression of 14 genes of interest through relative quantification (ΔΔCt) that selected for high-throughput TLDA card design and their corresponding Probe sets (pre- designed and validated, from ABI, CA) in in vitro and small set population validation study.
| Gene Name (Probe Sets) | Descriptions/Functions | In Vitro Results (n=6)* | Population Results (n=71)* |
|---|---|---|---|
| APC (Hs01568270_m1) | An antagonist of the Wnt signaling pathway | 0.33 (−) | 0.62 (−, n=53, 96%) |
| ARNT (Hs01121918_m1) | Fusin protein associated with acute myeloblastic leukemia | 0.32 (−) | 0.56 (−, n=52, 94%) |
| CD3G (Hs00173941_m1) | Gamma polypeptide (TiT3 complex), Immunodeficiency | 0.31 (−) | 0.64 (−, n=53, 96%) |
| CYP1A2 (Hs01070374_m1) | A member of Cytochrome P450 superfamily enzyme | 0 47 (+) | NV |
| CYP2D6 (Hs02576168_m1) | A member of Cytochrome P450 superfamily enzyme | 0.47 (−) | 0.45 (−, n=32, 57%) 0.22 (+, n=19, 33%) |
| ENTPD3 (Hs00928977_m1) | Ectonucleoside triphosphate diphosphoydrolase 3, Catabolism of extra cellular nucleotide | 0.72 (−) | 1.09 (−, n=50, 90%) 0.30 (+, n=4, 7.0%) |
| ITGB1 (Hs01127543_m1) | Integrin, beta 1 (fibronectin receptor, beta polypeptide) | 0.27 (−) | NV |
| LEPR (Hs00174492_m1) | Leptin receptor (Obesity) | 0.36 (−) | 0.66 (−, n=52, 94%) 0.12 (+. n=3, 5.0%) |
| LPR12 (Hs00257526_s1) | Low density lipoprotein –related protein 12; candidate tumor suppressor gene | 0.32 (−) | 0.67 (−, n=52, 94%) 0.30 (+, n=3, 5.4% |
| MYC (Hs00153408_m1) | Proto-oncogene, cell cycle progression, apoptosis and Transformation/transcription of specific target gene | 0.39 (−) | 0.73 (−, n=52, 98%) 0.10 (+, n=1, 1.8%) |
| RRAD (Hs00188163_m1) | Ras-related associated with diabetes | 0.29 (−, n=4) 0.25 (+, n=2) |
0.23 (−, n=10, 14%) 0.54 (+, n=60, 86%) |
| TAB1 (Hs00196143_m1) | TGF-beta activated kinase 1/MAP3K7 binding protein 1 | 0.27 (−) | 0.82 (−, n=54, 76%) 0.80 (+, n=16, 24%) |
| TP53 (Hs01034249_m1) | Tumor protein p53 | 0.34 (−) | 0.40 (−, n=51, 71%) 0.28 (+, n=15, 21%) |
| TRAP1 (Hs00212476_m1) | TNF receptor-associated protein 1 | 0.31 (−) | 0.64 (−, n=52, 74%) 0.14 (+, n=3, 4%) |
18s –RNA (Hs99999901_s1) Manufacturing Control;
GAPDH (Hs99999905_m1) Glyceraldehyde-3-phosphate dehydrogenase (Inter. Control)
NV- Not Validated
Total Number of Subjects in this population based pilot observation
Data represented as ΔΔCt changes (relative quantification) with Down-regulation (−)/Up-regulation (+). Number (n) in parenthesis is the total number of subjects where such changes were observed. % calculation was made only among the subjects with amplification under this validation platform.
Novelty.
Knowledge of disease pathways that were associated with human equivalence PCB exposures.
Identified discrete gene sets that were perturbed in the in vitro experiments.
Evaluated and validated the candidate genes status by comparison to the population study.
Pathways for Obesity, Type 2 diabetes, and Cancers towards potential disease development.
Explored the potential use of these gene fingerprints as disease susceptibility.
Acknowledgments
This study is supported by the 1UO1ES016127-01 from the National Institute of Environmental Health Sciences (NIEHS/NIH), the European Commission through the 7FP project OBELIX (No. 227391), Ministry of Health, Slovak Republic through projects 2007/07-SZU-03, 2012/41-SZU-5 and 2012/47-SZU-11, Slovak Research and Development Agency through projects APVV-0571-12 &APVV-0444-11, the project “Center of Excellence of Environmental Health”, ITMS No. 26240120033, based on the supporting Operational Research and Development Program financed from the European Regional Development Fund, 5G12MD007597-25 (NIMHD, PI: Southerland), and from the R200174 grant to SKD. This work also received support from U.S. National Institutes of Health grants # R01-CA96525. Thanks to Prof. Gray Harris, Dean of the Graduate School, Howard University for continuing supplemental support to this research initiative. Thanks are also due to the Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) and Dr. Annapurni Jayam Trouth, MD of Howard University for their assistance with the blood collection from healthy donors, as per approved HU IRB # IRB-07-GSAS-30.
Footnotes
The contents of this report are solely the responsibility of the authors.
Conflict of Interest
There is no conflict of interest among the authors in the present work.
Author’s Contribution
SG developed the work, design and performed the in vitro experimental work, IPA Analysis, and also wrote the manuscript. PM performed the statistical analysis of the microarray results. SGM ran the microarray experiments. TT, LM, ES were responsible for providing the epidemiological information on the human subjects, included herein. SZ provide valuable information towards this study. EH, CL and SKD provided support and direction to the manuscript. SKD held the NIEHS/UO1 and R200174 Grant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR. Toxicological profile for polychlorinated biphenyls. US Department of Health & Human Services, Public Health Service; Washington DC: 2000. [Google Scholar]
- Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7:346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- Arrebola JP, Ocaña-Riola R, Arrebola-Moreno AL, Fernández-Rodrígue M, Martin-Olmedo P, Fernández MF, Olea N. Associations of accumulated exposure to persistent organic pollutants with serum lipids and obesity in an adult cohort from Southern Spain. Environ Pollut. 2014;195C:9–15. doi: 10.1016/j.envpol.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Battle MA, Maher VM, McCormick JJ. ST7 is a novel low-density lipoprotein receptor-related protein (LRP) with a cytoplasmic tail that interacts with proteins related to signal transduction pathways. Biochemistry. 2003;42:7270–7282. doi: 10.1021/bi034081y. [DOI] [PubMed] [Google Scholar]
- Bencko V, Rames J, Ondrusova M, Plesko I, Jurickova L, Trnovec T. Human exposure to polyhalogenated hydrocarbons and incidence of selected malignancies - central European experience. Neoplasma. 2009;56:353–357. doi: 10.4149/neo_2009_04_353. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6:1170–1180. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Blumberg B, Chapin RE, Cote I, Graziano JH, Janesick A, et al. Predicting Later-Life Outcomes of Early-Life Exposures. Environ Health Perspect. 2012;120:1353–1361. doi: 10.1289/ehp.1204934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO. Health effects of persistent organic pollutants: the challenge for the Pacific Basin and for the world. Rev Environ Health. 2011;26:61–69. doi: 10.1515/reveh.2011.009. [DOI] [PubMed] [Google Scholar]
- Casas M, Nieuwenhuijsen M, Martínez D, Ballester F, Basagaña X, Basterrechea M, Chatzi L, Chevrier C, Eggesbø M, Fernandez MF, Govarts E, Guxens M, Grimalt JO, Hertz-Picciotto I, Iszatt N, Kasper-Sonnenberg M, Kiviranta H, Kogevinas M, Palkovicova L, Ranft U, Schoeters G, Patelarou E, Petersen MS, Torrent M, Trnovec T, Valvi D, Toft GV, Weihe P, Weisglas-Kuperus N, Wilhelm M, Wittsiepe J, Vrijheid M, Bonde JP. Prenatal exposure to PCB-153, p,p′-DDE and birth outcomes in 9000 mother-child pairs: Exposure-response relationship and effect modifiers. Environ Int. 2014;74C:23–31. doi: 10.1016/j.envint.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Dang CV. c-Myc target genes involved in cell growth, apoptosis and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirinck E, Jorens PG, Covaci A, Geens T, Roosens L, Neels H, Mertens I, Van Gaal L. Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity (Silver Spring) 2011;19:709–14. doi: 10.1038/oby.2010.133. [DOI] [PubMed] [Google Scholar]
- Dutta SK, Mitra PS, Ghosh S, Zang S, Sonneborn D, Hertz-Picciotto I, Trnovec T, Palkovicova L, Sovcikova E, Ghimbovschi S, Hoffman EP. Differential Gene Expression and Functional Analysis of PCB-exposed Children: Understanding Disease and Disorder Development. Environ Int. 2012;40:143–154. doi: 10.1016/j.envint.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta SK, Ghosh S, De S, Hoffman EP. CYP1A1 and MT1K are congener specific biomarker genes for liver diseases induced by PCBs Environ. Toxicol Pharmacol. 2008;25:218–221. doi: 10.1016/j.etap.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Elobeid MA, Padilla MA, Brock DW, Ruden DM, Allison DB. Endocrine disruptors and obesity: an examination of selected persistent organic pollutants in the NHANES 1999–2002 data. Int J Environ Res Public Health. 2010;7:2988–3005. doi: 10.3390/ijerph7072988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MD, Stanley JS, Turman JK, Going JE, Redford DP, Heggem DT. Determination of byproduct polychlorobiphenyls in commercial products and wastes by high-resolution gas chromatography/electron impact mass spectrometry. Environ Sci Technol. 1988;22:71–76. doi: 10.1021/es00166a007. [DOI] [PubMed] [Google Scholar]
- Faerch K, Hojlund K, Vind BF, Vaag A, Dalgard C, Nielsen F, Grandjean P. Increased Serum Concentrations of Persistent Organic Pollutants among Prediabetic Individuals: Potential Role of Altered Substrate Oxidation Patterns. J Clin Endocrinol Metab. 2012;97:E1705–1713. doi: 10.1210/jc.2012-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroon OM, Keith S, Jones D, De Rosa C. Carcinogenic effects of polychlorinated biphenyls. Toxicol Industrial Health. 2001;17:41–62. doi: 10.1191/0748233701th098oa. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas LJ. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Garnis C, Coe BP, Zhang L, Rosin MP, Lam WL. Overexpression of LRP12, a gene contained within an 8q22 amplicon identified by high-resolution array CGH analysis of oral squamous cell carcinomas. Oncogene. 2004;23:2582–2586. doi: 10.1038/sj.onc.1207367. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Murinova L, Trnovec T, Loffredo CA, Washington K, Mitra PS, Dutta SK. Biomarkers Linking PCB Exposure and Obesity. Curr Pharma Biotechnol. 2014;15:1058–1068. doi: 10.2174/1389201015666141122203509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Trnovec T, Palkovicova L, Hoffman EP, Washington K, Dutta SK. Status of LEPR Gene in PCB-exposed Population: A Quick Look. Int J Hum Genet. 2013;13:27–32. doi: 10.1080/09723757.2013.11886193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, De S, Chen Y, Sutton DC, Ayorinde FO, Dutta SK. Polychlorinated biphenyls (PCB-153) and (PCB-77) absorption in human liver (HepG2) and kidney (HK2) cells in vitro: PCB levels and cell death. Environ Int. 2010;36:893–900. doi: 10.1016/j.envint.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, De S, Dutta SK. Altered protein expressions in chronic PCB-153-induced human liver (HepG2) cells. Int J Toxicol. 2007;26:203–212. doi: 10.1080/10915810701352648. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Zang S, Mitra PS, Ghimbovschi S, Hoffman EP, Dutta SK. Global gene expression and Ingenuity biological functions analysis on PCBs 153 and 138 induced human PBMC in vitro reveals differential mode(s) of action in developing toxicities. Environ Int. 2011;37:838–857. doi: 10.1016/j.envint.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. Individual Polychlorinated Biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology. 2011;152:2909–2919. doi: 10.1210/en.2010-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden R, Kimbrough R. Weight of evidence evaluation of potential human cancer risks from exposure to polychlorinated biphenyls: an update based on studies published since 2003. Crit Rev Toxicol. 2009;39:299–331. doi: 10.1080/10408440802291521. [DOI] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Minireview: the case for obesogens. Mol Endocrinol. 2009;23:1127–1134. doi: 10.1210/me.2008-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadeh HK, Bushel PB, Jayadev S, Martin K, DiSorbo O, Seiber S, et al. Gene expression analysis reveals chemical-specific profiles. Toxicol Sci. 2002;67:219–231. doi: 10.1093/toxsci/67.2.219. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, vom Saal FS. Role of nutrition and environmental endocrine disrupting chemicals during the perinatal period on the aetiology of obesity. Mol Cell Endocrinol. 2009;304:90–96. doi: 10.1016/j.mce.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Trnovec T, Kočan A, Charles MJ, Čižnar P, Langer P, Sovčikova E, James R. PCBs and early childhood development in Slovakia: Study design and background. Fresen Environ Bull. 2003;12:208–214. [Google Scholar]
- Horvathová M, Jahnová E, Palkovičová L, Trnovec T, Hertz-Picciotto I. The kinetics of cell surface receptor expression in children perinatally exposed to polychlorinated biphenyls. J Immunotoxicol. 2011a;8:367–380. doi: 10.3109/1547691X.2011.620037. [DOI] [PubMed] [Google Scholar]
- Horvathová M, Jahnová E, Palkovičová L, Trnovec T, Hertz-Picciotto I. Dynamics of lymphocyte subsets in children living in an area polluted by polychlorinated biphenyls. J Immunotoxicol. 2011b;8:333–345. doi: 10.3109/1547691X.2011.615767. [DOI] [PubMed] [Google Scholar]
- Ilany J, Bilan PJ, Kapur S, Caldwell JS, Patti ME, Marette A, Kahn CR. Over-expression of Rad in muscle worsens diet-induced insulin resistance and glucose intolerance and lowers plasma triglyceride level. Proc Natl Acad Sci USA. 2006;103:4481–4486. doi: 10.1073/pnas.0511246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Li WJ, Huang HR, Zhong YQ, Fang JP. Differential gene expression profiles of peripheral blood mononuclear cells in childhood asthma. J Asthma. 2014:1–10. doi: 10.3390/ijerph111010146. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Laden F, Ishibe N, Hankinson SE, Wolff MS, Gertig DM, Hunter DJ, et al. Polychlorinated biphenyls, cytochrome P450 1A1, and breast cancer risk in the nurses’ health study. Cancer Epidemiol Biomarkers Prevention. 2002;11:1560–1565. [PubMed] [Google Scholar]
- Langer P, Ukropec J, Kocan A, Drobna B, Radikov Z, Huckova M, Imrich R, Gasperikova D, Klimes I, Trnovec T. Obesogenic and diabetogenic impact of high organochlorine levels (HCB, p,p′-DDE, PCBs) on inhabitants in the highly polluted Eastern Slovakia. Endocr Regul. 2014;48:17–24. doi: 10.4149/endo_2014_01_17. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K International Agency for Research on Cancer Monograph Working Group IARC Lyon France. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013;14:287–8. doi: 10.1016/S1470-2045(13)70104-9. [DOI] [PubMed] [Google Scholar]
- Lee DH, Porta M, Jacobs DR, Jr, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014;35:557–601. doi: 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lind L, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind PM. Associations of persistent organic pollutants with abdominal obesity in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Environ Int. 2012;40:170–178. doi: 10.1016/j.envint.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lind PM, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind L. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 2011;34:1778–1784. doi: 10.2337/dc10-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Daniels JL. Environmental contaminants as etiologic factors for diabetes. Environ Health Perspect. 2001;109:871–876. doi: 10.1289/ehp.01109s6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrano C, Genovesi G, Specchia P, Costantini D, Mariani S, Petrangeli E, Lenzi A, Gnessi L. Obesity and metabolic comorbidities: environmental diseases? Oxid Med Cell Longev. 2013;2013:640673. doi: 10.1155/2013/640673. Epub 2013 Mar 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuo K, Straznicky NE, Lambert GW, Katsuya T, Sugimoto K, Rakugi H, et al. Leptin-receptor polymorphisms relate to obesity through blunted leptin-mediated sympathetic nerve activation in a Caucasian male population. Hyperten Res. 2008;31:1093–1100. doi: 10.1291/hypres.31.1093. [DOI] [PubMed] [Google Scholar]
- Mitra PS, Ghosh S, Zang S, Sonneborn D, Hertz-Picciotto I, Trnovec T, Palkovicova L, Sovcikova E, Ghimbovschi S, Hoffman EP, Dutta SK. Analysis of the toxicogenomic effects of exposure to persistent organic pollutants (POPs) in Slovakian girls: correlations between gene expression and disease risk. Environ Int. 2012;39:188–199. doi: 10.1016/j.envint.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen AS, Arukwe A. Estrogenic effect of dioxin-like aryl hydrocarbon receptor (AhR) agonist (PCB congener 126) in salmon hepatocytes. Marine Environ Res. 2008;66:119–120. doi: 10.1016/j.marenvres.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Moyers JS, Bilan PJ, Reynet C, Kahn CR. Over-expression of Rad inhibits glucose uptake in cultured muscle and fat cells. J Biol Chem. 1996;271:23111–23116. doi: 10.1074/jbc.271.38.23111. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009;304:84–89. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Hertz-Picciotto I, Sovcikova E, Kocan A, Drobna B, Trnovec T. Neurodevelopmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity: a birth cohort study. Environ Health. 2010;9:51. doi: 10.1186/1476-069X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Park JS, Sovcikova E, Kocan A, Linderholm L, Bergman A, Trnovec T, Hertz-Picciotto I. Exposure to hydroxylated polychlorinated biphenyls (OH-PCBs) in the prenatal period and subsequent neurodevelopment in eastern Slovakia. Environ Health Perspect. 2009;117:1600–1606. doi: 10.1289/ehp.0900611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuk M, Cerhan JR, Lynch CF, Kocan A, Petrik J, Chovancova J. Case-control study of PCBs, other organochlorines and breast cancer in Eastern Slovakia. J Expo Anal Environ Epidemiol. 2003;13:267–275. doi: 10.1038/sj.jea.7500277. [DOI] [PubMed] [Google Scholar]
- Pavuk M, Cerhan JR, Lynch CF, Schecter A, Petrik J, Chovancova J, Kocan A. Environmental exposure to PCBs and cancer incidence in eastern Slovakia. Chemosphere. 2004;54:1509–1520. doi: 10.1016/j.chemosphere.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Pereira-Fernandes A, Vanparys C, Vergauwen L, Knapen D, Jorens PG, Blust R. Toxicogenomics in the 3T3-L1 cell line, a new approach for screening of obesogenic compounds. Toxicol Sci. 2014;140:352–363. doi: 10.1093/toxsci/kfu092. [DOI] [PubMed] [Google Scholar]
- Philibert A, Schwartz H, Mergler D. An exploratory study of diabetes in a First Nation community with respect to serum concentrations of p,p′-DDE and PCBs and fish consumption. Int J Environ Res Public Health. 2009;6:3179–3189. doi: 10.3390/ijerph6123179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plísková M, Vondrácek J, Canton RF, Nera J, Kocan A, Petrík J, et al. Impact of polychlorinated biphenyls contamination on estrogenic activity in human male serum. Environ Health Perspect. 2005;113:1277–1284. doi: 10.1289/ehp.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing J, Wei D, Maher VM, McCormick JJ. Cloning and characterization of a novel gene encoding a putative transmembrane protein with altered expression in some human transformed and tumor-derived cell lines. Oncogene. 1999;18:335–342. doi: 10.1038/sj.onc.1202290. [DOI] [PubMed] [Google Scholar]
- Rádiková Z, Tajtáková M, Kocan A, Trnovec T, Seböková E, Klimes I, Langer P. Possible effects of environmental nitrates and toxic organochlorines on human thyroid in highly polluted areas in Slovakia. Thyroid. 2008;18:353–362. doi: 10.1089/thy.2007.0182. [DOI] [PubMed] [Google Scholar]
- Recio-Vega R, Mendez-Henandez A, Gabriel AP, Jacobo-Avila A, Portales-Castanedo A, Hernandez-Gonzalez S, Gallegos-Arreola MP, Ocampo-Gomez G. Potentially estrogenic polychlorinated biphenyls congeners serum levels and its relation with lung cancer. J Appl Toxicol. 2013;33:906–914. doi: 10.1002/jat.2763. [DOI] [PubMed] [Google Scholar]
- Recio-Vega R, Velazco-Rodriguez V, Ocampo-Gómez G, Hernandez-Gonzalez S, Ruiz-Flores P, Lopez-Marquez F. Serum levels of polychlorinated biphenyls in Mexican women and breast cancer risk. J Appl Toxicol. 2011;3:270–278. doi: 10.1002/jat.1672. [DOI] [PubMed] [Google Scholar]
- Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Reynet C, Kahn CR. Rad: a member of the Ras family over-expressed in muscle of type II diabetic humans. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- Reynés B, Díaz-Rúa R, Cifre M, Oliver P, Palou A. Peripheral blood mononuclear cells as a potential source of biomarkers to test the efficacy of weight-loss strategies. Obesity (Silver Spring) 2014 Oct 8; doi: 10.1002/oby.20918. [DOI] [PubMed] [Google Scholar]
- Rocca CL, Tait S, Guerranti C, Busani L, Ciardo F, Bergamasco B, Stecca L, Perra G, Mancini FR, Marci R, Bordi G, Caserta D, Focardi S, Moscarini M, Mantovani A. Exposure to endocrine disrupters and nuclear receptor gene expression in infertile and fertile women from different Italian areas. Int J Environ Res Public Health. 2014;11:10146–10164. doi: 10.3390/ijerph111010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidijam M, Tootoonchi AS, Goodarzi MT, Hassanzadeh T, Borzuei SH, Yadegarazari R, Shabab N. Expression of interleukins 7 & 8 in peripheral blood mononuclear cells from patients with metabolic syndrome: A preliminary study. Indian J Med Res. 2014;140:238–43. [PMC free article] [PubMed] [Google Scholar]
- Schantz SL. Developmental neurotoxicity of PCBs in humans: what do we know and where do we go from here? Neurotoxicol Teratol. 1996;18:217–227. doi: 10.1016/s0892-0362(96)90001-x. [DOI] [PubMed] [Google Scholar]
- Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Hoffman EP. Probe set algorithms: is there a rational best bet? BMC Bioinformatics. 2006;7:395. doi: 10.1186/1471-2105-7-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Shneiderman B. Interactively exploring hierarchical clustering results. IEEE Computer. 2002;35:80–86. [Google Scholar]
- Sexton K, Salinas JJ, McDonald TJ, Gowen RM, Miller RP, McCormick JB, Fisher-Hoch SP. Biomarkers of maternal and fetal exposure to organochlorine pesticides measured in pregnant Hispanic women from Brownsville, Texas. Int J Environ Res Public Health. 2013;10:237–248. doi: 10.3390/ijerph10010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K, Salinas JJ. Concurrent Fetal Exposure to Multiple Environmental Chemicals along the U.S.-Mexico Border: An Exploratory Study in Brownsville, Texas. Int J Environ Res Public Health. 2014;11:10165–10181. doi: 10.3390/ijerph111010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Petrik J, Kocan A, Palkovicova L, Trnovec T, Nguyen D, Hertz-Picciotto I. Prenatal polychlorinated biphenyl exposures in eastern Slovakia modify effects of social factors on birth weight. Paediatr Perinat Epidemiol. 2008;22:202–213. doi: 10.1111/j.1365-3016.2008.00929.x. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ Health Perspect. 2008;116:1416–1422. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb MM, Kholodovych V, Grun F, Zhou C, Welsh WJ, Blumberg B. Highly Chlorinated PCBs Inhibit the Human Xenobiotic Response Mediated by the Steroid and Xenobiotic Receptor (SXR) Environ Health Perspect. 2004;112:163–167. doi: 10.1289/ehp.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Péronard JL, Andersen HR, Jensen TK, Heitmann BL. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev. 2011;12:622–636. doi: 10.1111/j.1467-789X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a national toxicology program workshop review. Environ Health Perspect. 2012;120:779–89. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnovec T, Sovcíková E, Pavlovcinová G, Jakubíková J, Jusko TA, Husták M, et al. Serum PCB concentrations and cochlear function in 12-year-old children. Environ Sci Technol. 2010;44:2884–2889. doi: 10.1021/es901918h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M, Anderson H, Knobeloch L, Imm P, Persky V. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p0-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere. 2009a;75:674–679. doi: 10.1016/j.chemosphere.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Turyk M, Anderson H, Knobeloch L, Imm P, Persky V. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ Health Perspect. 2009b;117:1076–1082. doi: 10.1289/ehp.0800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropec J, Radikova Z, Huckova M, Koska J, Kocan A, Sebokova E, et al. High prevalence of prediabetes and diabetes in a population exposed to high levels of an organochlorine cocktail. Diabetologia. 2010;53:899–906. doi: 10.1007/s00125-010-1683-2. [DOI] [PubMed] [Google Scholar]
- Uemura H. Associations of exposure to dioxins and polychlorinated biphenyls with diabetes: based on epidemiological findings. Nihon Eiseigaku Zasshi. 2012;67:363–374. doi: 10.1265/jjh.67.363. [DOI] [PubMed] [Google Scholar]
- Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, Vrijheid M. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environ Health Perspect. 2012;120:451–457. doi: 10.1289/ehp.1103862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliu O, Cameron L, Gardiner J, Deguire P, Karmaus W. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology. 2006;17:352–359. doi: 10.1097/01.ede.0000220553.84350.c5. [DOI] [PubMed] [Google Scholar]
- Wadzinski TL, Geromini K, McKinley Brewer J, Bansal R, Abdelouahab N, Langlois MF, Takser L, Zoeller RT. Endocrine Disruption in Human Placenta: Expression of the Dioxin-Inducible Enzyme, Cyp1a1, Is Correlated With That of Thyroid Hormone-Regulated Genes. J Clin Endocrinol Metab. 2014:jc20142629. doi: 10.1210/jc.2014-2629. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Tsai PC, Yang CY, Leon, Guo Y. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care. 2008;31:1574–1579. doi: 10.2337/dc07-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring JF, Ciurlionis R, Jolly RA, Heindel M, Ulrich RG. Microarray analysis of hepatotoxin in vitro reveals a correlation between gene expression profiles and mechanism of toxicity. Toxicol Lett. 2001;120:359–368. doi: 10.1016/s0378-4274(01)00267-3. [DOI] [PubMed] [Google Scholar]
- Whitelaw M, Pongratz I, Wilhelmsson A, Gustafsson JA, Poellinger L. Ligand-dependent recruitment of the Arnt coregulator determines DNA recognition by the dioxin receptor. Mol Cell Biol. 1993;13:2504–2514. doi: 10.1128/mcb.13.4.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Salmon AG, Marty MA. Development of TEFs for PCB congeners by using an alternative biomarker — thyroid hormone levels. Regul Toxicol Pharmacol. 2010;56:225–236. doi: 10.1016/j.yrtph.2009.12.008. [DOI] [PubMed] [Google Scholar]