Abstract
The addictive power of drugs of abuse such as cocaine comes from their ability to hijack natural reward and plasticity mechanisms mediated by dopamine signaling in the brain. Reward learning involves burst firing of midbrain dopamine neurons in response to rewards and cues predictive of reward. The resulting release of dopamine in terminal regions is thought to act as a teaching signaling to areas such as the prefrontal cortex and striatum. In this review, we posit that a pool of extrasynaptic dopaminergic D1-like receptors activated in response to dopamine neuron burst firing serve to enable synaptic plasticity in the prefrontal cortex in response to rewards and their cues. We propose that disruptions in these mechanisms following chronic cocaine use contribute to addiction pathology, in part due to the unique architecture of the mesocortical pathway. By blocking dopamine reuptake in the cortex, cocaine elevates dopamine signaling at these extra-synaptic receptors, prolonging D1-receptor activation and the subsequent activation of intracellular signaling cascades, and thus inducing long-lasting maladaptive plasticity. These cellular adaptations may account for many of the changes in cortical function observed in drug addicts, including an enduring vulnerability to relapse. Therefore, understanding and targeting these neuroadaptations may provide cognitive benefits and help prevent relapse in human drug addicts.
Keywords: Dopamine, Prefrontal cortex, Addiction, Cocaine, Learning, Electrophysiology
1. Introduction
A better understanding of the brain will facilitate the development of successful treatments for neuropsychiatric diseases. One neuropsychiatric disease especially resistant to treatment is drug addiction, which has a major negative impact on society. Addiction results from chronic use of substances like cocaine that hijack reward pathways in the brain, causing cellular adaptations that contribute to the disease. As a result, the behavior of a drug addict changes. They become more impulsive, less reflexive, demonstrate general cognitive impairment, and despite a desire to end their drug-use, remain vulnerable to relapse even after extensive periods of abstinence (Goldstein and Volkow, 2011, 2002; Kalivas and McFarland, 2003; Kalivas and O’Brien, 2007). Thus, a better understanding of the neurobiology that contributes to relapse vulnerability will aid in the development of successful addiction therapies.
Prefrontal cortex (PFC) disruption is a hallmark of addiction (Goldstein and Volkow, 2011; Kalivas et al., 2005; Kalivas and Volkow, 2005). The current dogma, based largely upon clinical imaging data with support from pre-clinical studies, is that activity in the PFC is reduced during abstinence from chronic cocaine, but elevated during periods of intoxication or craving (for reviews see Goldstein and Volkow (2011, 2002)). However, the majority of studies that examine PFC neuronal excitability in reduced preparations (acute slices or dissociated neurons) find that the intrinsic excitability of cortical neurons is enhanced following chronic cocaine exposure. In this review, we posit that the following chronic cocaine experience, the loss of intrinsic potassium (K+) channel mediated inhibition underlies the enhanced PFC responsiveness to cues seen in drug addicts. Additional cellular adaptations beyond the scope of this review may account for the hypofrontality observed during resting conditions. However a recent study has attributed PFC hypofrontality to a reduction in PFC intrinsic excitability (Chen et al., 2013), and in Section 5 we provide a plausible, albeit untested, scenario that may explain these conflicting observations, as well as additional possibilities that could contribute to this discrepancy.
Addiction-induced changes in cortical function are thought to be the result of drug-induced plasticity in dopaminergic signaling, producing enduring cellular adaptations that contribute to relapse vulnerability (Volkow et al., 1991, 1993, 2002, 2008, 2009). Dopaminergic signaling in the cortex has been extensively investigated at multiple levels, ranging from investigations of dopamine-induced changes in intracellular signaling to dopamine’s role in complex behaviors. In healthy animals, dopamine’s actions facilitate the learning of cues that predict rewards (Schultz, 1998). Drugs of abuse such as cocaine ‘hijack’ this dopaminergic signal (Schultz, 2011), increasing dopamine beyond natural levels to enhance the encoding of cues predictive of drug rewards (Heien et al., 2005; Phillips et al., 2003), thus establishing powerful and enduring memories that promote drug use. Thereafter, learned drug-paired cues initiate relapse-events by activating the PFC, which via its glutamatergic projections to the nucleus accumbens, induce drug craving and initiate drug seeking (Gipson et al., 2013; Kalivas and McFarland, 2003; Stefanik et al., 2012).
Understanding how dopamine encodes rewards in the PFC will reveal the mechanisms by which drug-paired cues come to exert such powerful control over the behavior of those suffering from drug addiction. Therefore, in this literature review we explore a cellular mechanism by which cortical dopamine signaling could facilitate reward learning, we discuss this mechanism’s vulnerability to cocaine-induced adaptations, and we develop a conceptual framework for future explorations.
2. Anatomy of the mesocortical dopamine system
Midbrain dopamine neurons fire bursts of action potentials in response to rewards or cues predictive of reward, releasing dopamine in their terminal regions (Garris and Wightman, 1994; Owesson-White et al., 2008; Paladini and Roeper, 2014; Robinson et al., 2003; Schultz, 1998, 2002; Schultz and Hollerman, 1998). A variety of brain regions receive input from midbrain dopamine neurons, including the PFC and nucleus accumbens (Garris and Wightman, 1994; Lammel et al., 2008; Yetnikoff et al., 2014). Dopamine released from these terminals provides a teaching signaling that is thought to encode information about rewards and their associated cues (Schultz, 2002, 2006; Schultz and Hollerman, 1998). Drugs of abuse such as cocaine can commandeer this pathway, and cause adaptations in mesocortical dopamine signaling that lead to addiction-induced changes in PFC function (Volkow et al., 2002, 2009) and the loss of control over drug-seeking behavior (Goldstein and Volkow, 2011).
The mesocortical system refers to the dopaminergic projection from the ventral midbrain, including the ventral tegmental area (VTA) and substantia nigra, to the PFC. This pathway is distinct from the mesolimbic system involving dopaminergic projections to striatal regions (Garris and Wightman, 1994; Lammel et al., 2008; Yetnikoff et al., 2014). In comparison to dopaminergic terminals in the striatum, mesocortical terminals are slow to mature, and not fully developed until early adulthood (Benes et al., 1996; Kalsbeek et al., 1988; Naneix et al., 2012; Tarazi et al., 1998, 1999). The dopamine cells that project to the PFC are also markedly different from the mesolimbic dopamine neurons innervating the accumbens in neurotransmitter release and reuptake mechanisms, as well as plasticity and associated behaviors (Ford and Williams, 2008; Garris et al., 1993; Garris and Wightman, 1994; Hoffmann et al., 1988; Lammel et al., 2011, 2012). While dopamine transmission in the mesolimbic system is better understood and findings from the mesolimbic system are often generalized to dopamine signaling in the cortex, several unique architectural features of the mesocortical system may render it especially vulnerable to drugs of abuse like cocaine.
In rodents and nonhuman primates, dopaminergic fibers appear densest in deeper cortical layers (Benes et al., 1996, 2000; Martin and Spühler, 2013). These terminals contact both pyramidal cells and GABA interneurons, with at least one synapse per dendrite (Krimer et al., 1997). Tyrosine hydroxylase (TH; the enzyme necessary for dopamine synthesis) is expressed at similar locations. TH+ terminals make symmetrical (and presumably inhibitory) contacts onto pyramidal cells, mostly at locations adjacent to spines receiving excitatory input, suggesting dopamine regulates excitation and could facilitate plasticity at synaptic spines (Goldman-Rakic et al., 1989; Sesack et al., 2003). However the majority of dopaminergic axons do not appear to form synapses, consistent with a role for non-synaptic dopaminergic transmission (Rice and Cragg, 2008; Spühler and Hauri, 2013).
Indeed, dopamine fibers in the PFC display an anatomical architecture that enables nonsynaptic (or volumetric) signaling. The PFC exhibits sparse immunoreactivity for dopamine transporters (DAT) (Sesack et al., 1998) that are further away from sites of synaptic release compared with the location of DATs in the striatum (Sesack et al., 2003). The relative scarcity of DATs and lack of autoreceptors mean that in the PFC dopamine likely spreads substantially from release sites and persists at higher concentrations for prolonged periods of time as compared to striatal regions (Garris et al., 1993; Garris and Wightman, 1994; Hoffmann et al., 1988). Furthermore, unlike in the striatum, the concentration of dopamine in the cortex is likely not uniform but graded, with ‘hotspots’ of dopamine concentrations greatest near VTA-terminal release sites (Spühler and Hauri, 2013). Measurements of dopamine levels suggest basal dopamine concentrations fluctuate in the low (0.3–15) nanomolar range during tonic dopamine neuron firing (Devoto et al., 2001; Garris et al., 1993; Garris and Wightman, 1994; Hernandez and Hoebel, 1995; Hildebrand et al., 1998; Ihalainen et al., 1999; Izaki et al., 1998; Seamans and Yang, 2004), while dopamine neuron bursting could elicit transients in the cortex that approach ~1 micro-molar near release sites (Garris and Wightman, 1994; Spühler and Hauri, 2013).
Dopamine exerts its action by binding to dopamine receptors (D1–5), all of which are metabotropic G-protein coupled receptors (GPCRs) often broadly grouped into subgroups: D1-like (D1, D5) and D2-like (D2–4). Both D1- and D2-like receptors have similar affinities for dopamine that depends upon their conformation state. In their high-affinity state, both groups of receptors bind dopamine in the tens of nanomolar range, while in the low affinity state they bind with dopamine only at higher (1–5 micromolar) concentrations (Rice and Cragg, 2008; Richfield et al., 1989; Spühler and Hauri, 2013). Agonist binding to receptors in the high affinity state results in the activation of downstream signaling cascades, and switches receptor conformation to the low-affinity state. While D1 receptors account for a larger portion of dopamine receptors (~78% in most brain regions), only ~30% of D1 receptors exist in the high affinity state compared to ~80% for D2 receptors (Richfield et al., 1989). Of the two families, D1-like receptors in the cortex are found almost exclusively in perisynaptic or extrasynaptic locales, while D2 receptors may be located more synaptically (Seamans and Yang, 2004). Modeling studies of the primate cortex suggest even without dopamine neuron burst firing, cortical dopamine levels should be at high enough concentrations to effectively activate dopamine receptors in their high affinity state even at locations distant from dopamine release sites through diffusion (Spühler and Hauri, 2013). This suggests that in the healthy cortex, both D1 and D2 dopamine receptors should be sensitive to graded changes in tonic dopamine neuron firing, and that dopamine release during VTA neuron burst firing likely teaches the cortex by changing dopamine concentrations relative to baseline levels, transiently increasing the likelihood of dopamine binding to extrasynaptic D1 receptors via diffusion.
Dopamine D1 receptors exist on spines and dendritic shafts, but are most commonly found proximal to large asymmetric (presumably excitatory) synapses that do not receive contacts from TH+ terminals (Bergson et al., 1995; Goldman-Rakic et al., 1989; Smiley et al., 1994), suggesting these receptors may be exclusively activated by dopamine diffusion. Furthermore, many shafts contain extrasynaptic D5 receptors that form signaling microdomains by coupling with IP3 and intracellular calcium signaling (Bergson et al., 1995; Paspalas and Goldman-Rakic, 2004), further suggestive of a specific role for the D1-like receptor subtype in nonsynaptic (or volumetric) dopamine signaling. This unique architecture suggests that dopamine released in the PFC during VTA neuron burst firing may induce plasticity via dopaminergic spillover to extrasynaptic D1-like receptors at synapses distant from dopamine release sites. Perhaps the degree to which these extrasynaptic receptors are recruited during reward learning depends on the degree of dopamine neuron burst firing, with greater dopamine spillover activating more extra-synaptic dopamine D1/5 receptors.
How does the activation of extrasynaptic D1-like dopamine receptors promote synaptic plasticity and reward-learning? As described in Section 3, existing studies suggest that dopamine may function as a metaplasticity signal in the cortex, priming plasticity at strongly activated glutamatergic synapses by regulating the intrinsic excitability of pyramidal neurons.
3. Plasticity in intrinsic excitability as a cellular mechanism for learning
It is now well established that both dopamine and learning increase neuronal excitability via nonsynaptic (intrinsic) mechanisms (Daoudal and Debanne, 2003; Mozzachiodi, 2010; Oh and Disterhoft, 2014; Saar and Barkai, 2009; Zhang and Linden, 2003). Non-synaptic changes in intrinsic excitability are one of the many mechanisms of plasticity in neurons and are thought to serve as a form of metaplasticity, regulating the likelihood of future plasticity (Abraham and Bear, 1996; Abraham and Tate, 1997, but see Sehgal et al., 2013). These changes reflect adaptations in ion channels and intracellular signaling cascades that alter the integration and processing of electrical signals such as those mediated by glutamate and GABA. Intrinsic excitability mechanisms also regulate the synchronization between networks of neurons throughout the brain, including the rhythmic activity of neuronal oscillations important for cognitive function (Buschman et al., 2012; Gutkin et al., 2001; Hu et al., 2002; McCormick and Sanchez-Vives, 2000; Mueller et al., 2014; Paz et al., 2008; Uhlhaas, 2009; Waldhauser et al., 2014; Ward, 2003).
One of the most widely observed learning-induced changes in intrinsic excitability involves changes in after-hyperpolarization potentials that regulate repetitive action potential firing. In particular, as reviewed below, the slow after-hyperpolarization (sAHP) that mediates spike-frequency adaptation (the slowing of action potential firing in response to sustained depolarization) seems to be especially sensitive to learning and neuromodulation (Andrade et al., 2012; Oh and Disterhoft, 2014; Sehgal et al., 2013), such that learning often reduces the sAHP, reducing spike-frequency adaptation, and enhancing neuronal excitability. Despite their clear importance for learning, these mechanisms are poorly understood compared to their synaptic counterparts.
Unlike the fast synaptic excitation mediated by glutamatergic activation of NMDA and AMPA receptors (Kessels and Malinow, 2009), the sAHP appears to reflect a “generalized potassium channel gating mechanism” that results from the activation of complex intracellular signaling cascades downstream of intra-cellular calcium (see Andrade et al. (2012) for details).
Cytoplasmic rises in calcium either via calcium influx through voltage-gated calcium channels or calcium release from intra-cellular stores binds with proteins of the neuronal calcium sensor (NCS) family, such as hippocalcin and neurocalcin δ (Markova et al., 2008; Tzingounis et al., 2007; Villalobos and Andrade, 2010). Upon binding to calcium, these calcium sensors translocate to the plasma membrane, where they are thought to localize to the K+ channels underlying the sAHP (Andrade et al., 2012; Markova et al., 2008). However, the molecular identity of the K+ channel(s) mediating the sAHP remain undefined (Andrade et al., 2012). Leading candidates are big conductance calcium activated K+ channels (BK), small conductance calcium activated K+ channels (sK) and Kv7/KCNQ channels (N. Gu et al., 2007; Soh and Tzingounis, 2010; Tzingounis et al., 2010; Tzingounis and Nicoll, 2008; Villalobos, 2004; Vogalis et al., 2003). The complexity of this response is likely in part responsible for its remarkable utility in many forms of associative learning (Oh and Disterhoft, 2014; Sehgal et al., 2013).
3.1. Plasticity in the sAHP and spike accommodation during learning
Learning induced increases in intrinsic plasticity occur in simple organisms including aplysia (Brembs et al., 2002; Lorenzetti et al., 2005, 2008; Mozzachiodi et al., 2008) as well as in complex mammalian circuits such as the piriform cortex (Saar et al., 1998), hippocampus (Coulter et al., 1989; Matthews et al., 2009; McKay et al., 2009; Moyer et al., 2000; Oh et al., 2003; L. Zhang et al., 2013), and amygdala (Motanis et al., 2014; Sehgal et al., 2014). The medial PFC also displays these mechanisms with subregion specificity (dorsal prelimbic neurons and ventral infralimbic neurons often show site specific learning-induced changes in intrinsic excitability) (Hayton et al., 2011a; Santini et al., 2008; Sepulveda-Orengo et al., 2013). In these systems, transient reductions in the sAHP and spike accommodation are learning-specific and behaviorally appropriate. Animals that fail to learn tasks lack these excitability changes (Kaczorowski and Disterhoft, 2009; Moyer et al., 1996). In those that do learn, the excitability reverses with extinction training (McKay et al., 2009; Santini et al., 2008). Pharmacological manipulation of K+ channels (BK (Matthews and Disterhoft, 2009), sK (McKay et al., 2012), or Kv7/KCNQ (Santini and Porter, 2010)—all of which play roles in regulating excitability and the AHP), can either enhance or prevent learning.
3.2. Dopamine regulation of the slow after-hyperpolarization (sAHP)
Could dopamine neuron burst firing teach the cortex about rewards by reducing the sAHP to enhance neuronal excitability? If so, dopamine must inhibit the sAHP by binding to the extra-synaptic D1-like receptors. Indeed, in PFC brain slices, bath application of dopamine enhances cortical neuron excitability via modulation of multiple ion channels (Dong et al., 2004; Dong and White, 2003; Kisilevsky et al., 2008; Maurice et al., 2001; Penit-Soria et al., 1987; Seong and Carter, 2012; Witkowski et al., 2008; Yang and Seamans, 1996, but see Seamans and Yang (2004) for review), including by inhibiting the sAHP that mediates late spike-frequency adaptation (accommodation) (Thurley et al., 2008; Yi et al., 2013). In vivo stimulation of the VTA to mimic burst firing also produces similar increases in PFC neuron excitability that last for extended periods of time (>45 min) (Lavin et al., 2005), further supporting the idea that VTA neuron burst firing teaches the cortex about rewards by enhancing neuronal excitability in the cortex.
The effects of dopamine on the sAHP appear to be mediated by an increase in cAMP production and PKA activity, but not via closure of voltage-gated calcium channels (Malenka and Nicoll, 1986; Pedarzani et al., 1998; Pedarzani and Storm, 1993, 1995). However evidence also suggests a role for intracellular calcium signaling, as dopamine D1 receptor stimulation in PFC slices has been shown to produce a long-lasting enhancement of intrinsic excitability via calcium dependent intracellular signaling (Chen et al., 2007). PFC dopamine receptors can couple to Gαq and stimulate protein lipase-C (PLC) activation to mediate calcium release from intracellular stores (Lee et al., 2004; Liu et al., 2009; So et al., 2009). Furthermore, anatomical studies demonstrate that extrasynaptic D5 receptors form microdomains with IP3-gated intracellular calcium stores (Paspalas and Goldman-Rakic, 2004). Dopamine receptors also regulate calcium release from stores via Gβγ signaling independent of cAMP/PKA (Zeng et al., 2003), as well as indirectly via PKA signaling (Dai et al., 2008). For a detailed account of these dopamine signaling cascades, see Beaulieu and Gainetdinov (2011), Neve et al. (2004), and Tritsch and Sabatini (2012). These dopamine-regulated signaling cascades that alter calcium release from intracellular stores may be important for the inhibition or “gating” of the sAHP by dopamine.
It is worth noting that many neurotransmitters with various intracellular signaling cascades increase neuronal excitability by reducing the sAHP. This includes receptors for dopamine as well as glutamate (Burke and Hablitz, 1996; Young et al., 2008), acetylcholine (Knöpfel et al., 1990; Pedarzani and Storm, 1993; Power and Sah, 2008), norepinephrine (Knöpfel et al., 1990; Mueller et al., 2008; Otis and Mueller, 2011; Power and Sah, 2008), serotonin (Pedarzani and Storm, 1993; Satake et al., 2008), and various neuropeptides like CRF (Haug and Storm, 2000) and kisspeptin (C. Zhang et al., 2013). Therefore, while the mechanisms are not fully understood and may vary by cell type, the K+ channel inhibition provided by the sAHP appears to be under complex regulation by multiple neurotransmitters and intracellular signaling cascades, including cAMP/PKA signaling, PLC/PKC/ Ptdlns(4,5)P2 signaling, and calcium release from intracellular stores as well as diffusible intracellular calcium sensor proteins of the NCS family (Andrade et al., 2012; Delmas and Brown, 2005; Haug and Storm, 2000; Sah, 1996; Villalobos and Andrade, 2010). Neuromodulation of any of these signaling molecules may reduce the sAHP.
In regards to dopamine and reward learning, it appears that burst firing of VTA neurons during rewards or cues predictive of rewards would lead to dopamine release in the cortex and activation of extrasynaptic D1-like receptors. This would activate multiple intracellular signaling cascades important for plasticity, including cAMP, PKA, and intracellular calcium signaling. While this likely has many consequences for cellular function, the dopamine teaching signal may involve a reduction in the sAHP and a subsequent increase in intrinsic excitability. This would facilitate the establishment of neuronal ensembles, as enhancing neuronal excitability in neuronal subsets preferentially recruits the storage of memory traces in these neurons (Sehgal et al., 2013, 2014; Yiu et al., 2014).
3.3. Implications for synaptic plasticity and memory consolidation
Dopaminergic modulation of intrinsic excitability gates the induction of synaptic plasticity, which is required for the establishment of neuronal ensembles (O’Donnell, 2003; Rogerson et al., 2014; Yuan et al., 2011). Dopamine does this via interplay between multiple mechanisms that occur beyond the insertion of glutamatergic receptors (Durstewitz and Seamans, 2006; Malinow and Malenka, 2002; Seamans and Yang, 2004). Increased postsynaptic excitability enhances the likelihood of excitatory inputs eliciting action potentials, thus facilitating the induction of long-term potentiation (LTP) at active synapses via Hebbian learning mechanisms (Sehgal et al., 2013). Indeed, dopaminergic activation of D1-like receptors increases the insertion of glutametergic AMPA receptors (specifically GluR1 containing subunits via a PKA dependent mechanism) into the cellular membrane, however, only at extrasynaptic sites (Sun et al., 2005). These newly inserted receptors remain extrasynaptic and are only incorporated into synapses when the activation of D1 receptors is followed by strong NMDA receptor activation.
Dopamine inhibition of the sAHP potentiates NMDA receptor signaling, as NMDA mediated excitatory potentials and after-depolarizations are inhibited by the sAHP (Fernández deSevilla et al., 2007; Lancaster et al., 2001; Wu et al., 2004). Therefore, inhibition of the sAHP by dopamine and learning would promote NMDA receptor mediated depolarizations, allowing for AMPA receptor translocation to active synapses. In addition to being necessary for the synaptic insertion of AMPA receptors, dopamine-induced potentiation of NMDA receptor conductance is also thought to facilitate cortical upstates (sustained depolarizations important for learning and cognitive function) (Lewis and O’Donnell, 2000; O’Donnell, 2003). These upstates contribute to maintained PFC neuron firing observed during behavioral states such as working memory, where PFC activity persists for a period of time beyond cue presentation (Arnsten et al., 2012; Durstewitz et al., 2000a, 2000b; Sawaguchi and Goldman-Rakic, 1991; Wang, 2001; Williams and Goldman-Rakic, 1995).
Activation of dopamine D1 receptors also facilitates changes in NMDA receptor expression. D1-like receptors and NMDA receptors appear to interact directly, forming perisynaptic clusters of receptor reservoirs near glutamatergic synapses (Kruse et al., 2009; Ladepeche et al., 2013). When D1 receptors are activated, they dissociate from NMDA receptors, allowing the NMDA receptors to translocate into synapses and facilitate LTP (Ladepeche et al., 2013). The co-localization of D1 and NMDA receptors (specifically GluN1 subunits) also promotes D1 receptor induced potentiation of NMDA mediated increases in cytosolic calcium, effects blocked by PKA inhibition (Kruse et al., 2009). Furthermore, activation of D1 receptors appears to increase the surface expression of NMDA receptors (specifically GluN1 and GluN2B subunits, but not GluN2A) via mechanisms that instead of involving PKA may involve Fyn and/or tyrosine kinase activity (Gao and Wolf, 2008; Hu et al., 2010). These findings are consistent with electrophysiological studies demonstrating that activation of D1 receptor signaling potentiates NMDA receptor signaling to promote LTP at excitatory synapses in the PFC (Chen et al., 2004; Gurden et al., 2000; Seamans et al., 2001).
These changes in glutamate receptor signaling appear secondary to dopamine-induced changes in intrinsic excitability that gate the induction of synaptic plasticity. Inhibition of the sAHP facilitates the expression of LTP by enhancing NMDA conductance and reducing the threshold for LTP expression (Cohen et al., 1999; Kramár et al., 2004; Sah and Bekkers, 1996). Furthermore, inhibition of the sAHP gates the induction of spike-timing dependent plasticity (STDP) (Zaitsev and Anwyl, 2012), facilitating LTP by extending the temporal window for its induction (Fuenzalida et al., 2007; Ruan et al., 2014; Xu and Yao, 2010; Zhang et al., 2009), ultimately promoting the consolidation of memories (Cohen-Matsliah et al., 2010; Thompson et al., 1996).
Therefore, it appears that increasing the intrinsic excitability of neurons by reducing the inhibition provided by the sAHP underlies the ability of neurotransmitters like dopamine to gate synaptic plasticity in cortical–striatal circuits (Izhikevich, 2007; Pawlak, 2010; Pawlak and Kerr, 2008; Sheynikhovich et al., 2011, 2013; Vogt and Hofmann, 2012) (see Fig. 1). Together, these results suggest that behaviorally relevant neuronal ensembles in cortical–striatal circuits are established by dopamine-induced enhancement of cortical intrinsic excitability in response to cues and rewards.
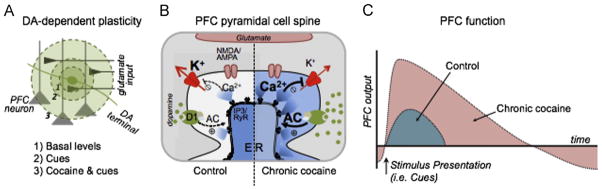
Fig. 1.
Chronic cocaine disrupts mesocortical learning mechanisms. (A, 1) VTA terminals that provide dopaminergic tone to the PFC regulate executive function. (A, 2) In response to salient stimuli such as rewards and cues predictive of rewards, VTA neurons fire bursts of action potentials. This facilitates learning and appropriate behavioral responses by increasing dopamine (DA) levels to transiently activate extrasynaptic D1 receptors. (A, 3) Drugs of abuse such as cocaine block dopamine reuptake and dysregulate this process. With reuptake blocked, the spread of dopamine becomes disproportional to the original stimulus and endures for an excessively long period of time. This change in the spatial-temporal signature of dopamine signaling imparts a strong salience to drug-paired cues. (B, left) In a control (drug naïve) setting, dopamine binding to D1-receptors stimulates adenylyl cyclase (AC) leading to the generation of cAMP, activation of PKA, and potentiation of calcium release from intracellular stores. Transient activation of these signaling cascades increases action potential firing in response to excitatory input by reducing inhibition provided by potassium (K+) channels and the slow after-hyperpolarization (sAHP). This in turn facilitates the induction of synaptic plasticity at excitatory synapses. (B, right) Chronic cocaine exposure enhances basal levels of dopamine D1-receptor signaling, disrupting the inhibition mediated by K+ channels. This renders pyramidal cells more responsive to incoming excitatory inputs, such as those activated by drug-paired cues. (C) In control (drug naïve) individuals, PFC output is proportional to the stimulus presentation (i.e., cue). In cocaine-experienced individuals, however, cellular adaptations cause persistent cortical dysfunction. That is, in human cocaine addicts, PFC activity is reduced during low-arousal states, but hyperactive in response to cocaine-associated cues. We posit that the hyper-responsiveness to cues reflects a loss of intrinsic inhibition mediated by K+ channels and the sAHP. Multiple other mechanisms may contribute to the hypoactivity in the PFC under basal conditions.
Although both dopamine and learning regulate intrinsic excitability by modulating the sAHP and spike accommodation, these mechanisms are often overlooked when considering pathological neuroadaptations associated with addiction. As plasticity in the sAHP and spike accommodation appear fundamental to learning processes, they likely undergo adaptations following chronic cocaine that contributes to PFC dysfunction and addiction pathology. Indeed, as discussed in Section 4, most if not all of the intracellular cascades that regulate the sAHP undergo adaptations following chronic in vivo exposure to drugs of abuse.
4. Adaptations in mesocortical function following chronic cocaine
Chronic cocaine use induces adaptations in dopaminergic signaling within the PFC that disrupt the intricate signaling cascades regulating intrinsic excitability and synaptic plasticity. An understanding of these cellular adaptations and their contribution to PFC dysfunction and addiction pathology will aid the development of treatments for relapse prevention. Therefore, below we highlight known adaptations caused by chronic cocaine and their potential impact on the signaling cascades regulating spike-frequency adaptation and the sAHP, as well as cortical function in general.
4.1. Reduced basal function
Human cocaine addicts demonstrate reduced levels of basal metabolic activity in the PFC after prolonged abstinence from cocaine (Volkow et al., 1993, 2001). The cocaine-induced reduction of PFC activity observed in humans has also been observed in animal models of cocaine self-administration. Under resting state conditions after weeks of cocaine exposure, measurements of cerebral blood volume indicate reduced metabolic activity in the medial PFC and accumbens (Gozzi et al., 2011), and electrophysiology and fMRI studies denote decreased communication between the PFC, accumbens core, and orbital frontal cortex (Lu et al., 2014; McCracken and Grace, 2013). Rats that self-administer sucrose do not develop such adaptations (Lu et al., 2012), suggesting that these adaptations in cortical function are a response to cocaine-induced elevations in dopaminergic signaling.
4.2. Heightened reactivity to cues
The PFC of drug addicts becomes hyper-active in response to cocaine-associated cues or tasks requiring sustained attention (Garavan et al., 2000; Goldstein and Volkow, 2002; Howell and Wilcox, 2002; Kosten et al., 2006; Maas et al., 1998; Tomasi et al., 2007a, 2007b). Similar findings have also been observed in animal models of cocaine addiction, where after weeks of cocaine exposure cortical neurons demonstrate reduced baseline firing rates but resumption of cocaine self-administration increases bursting and the firing rates within bursts (Sun and Rebec, 2006). Furthermore, re-exposure to cocaine predictive cues potentiates PFC prelimbic neuron firing during drug seeking (Rebec and Sun, 2005; West et al., 2014). While it is unknown which brain regions drive the PFC to respond to drug-cues, basolateral amygdala (BLA)-induced control over the PFC appears increased, while dopaminergic regulation of BLA-evoked responses degraded in psychostimulant-experienced animals (McCracken and Grace, 2013; Tse et al., 2011), suggesting the amygdala could be driving cue-induced responses in the PFC.
4.3. Unique vulnerability of the mesocortical system
Chronic exposure to cocaine increases the amount of dopamine released from mesocortical terminals in response to subsequent challenges of cocaine or re-exposure to drug-paired cues (Ben-Shahar et al., 2012; Ikegami et al., 2007; Nakagawa et al., 2011; Parsegian and See, 2013; Sorg et al., 1997). Following repeated cocaine self-administration, PFC levels of DAT increase (Grimm et al., 2002; McIntosh et al., 2013), possibly to compensate for the enhanced release of dopamine. Although sucrose self-administration triggers dopamine release, it does not enhance DAT expression in the PFC, suggesting the magnitude of dopamine release (rather than frequency) causes these adaptations. As the amygdala, nucleus accumbens, or orbitofrontal cortex display no such adaptations, the PFC displays a unique sensitivity to cocaine (Grimm et al., 2002).
The unique features of the mesocortical system highlighted in Section 2 may render it especially vulnerable to cocaine-induced adaptations. Drugs of abuse like cocaine prevent the reuptake of dopamine by blocking DATs and norepinephrine transporters (Han and Gu, 2006), resulting in enhanced dopamine signaling in the PFC that, according to modeling studies, quickly reaches micromolar concentrations even in the absence of dopamine neuron burst firing (Spühler and Hauri, 2013). As observed in Febo (2011), larger numbers of cortical neurons are recruited in reward learning under these conditions. With chronic drug use this could sensitize extrasynaptic dopamine D1/5 receptors, perhaps by increasing the proportion of D1-like receptors in high-affinity states as observed after methamphetamine sensitization (Shuto et al. 2008). This could render the PFC insensitive to any subsequent burst firing from dopamine neurons. If the D1/5 receptors are occupied or in an active state prior to dopamine neuron burst firing, there will be no receptors available to respond to additional dopamine release, thus preventing (via occlusion) learning in response to changes in dopamine. This could underlie the reduced ability of addicts to adapt to changes in reward contingency (Jentsch et al., 2002; LeBlanc et al., 2013; Stalnaker et al., 2007) and make extinguishing or unlearning drug-paired associations extremely difficult.
Thus, acute use of cocaine likely facilitates plasticity in the PFC by recruiting greater pools of extrasynaptic dopamine D1-receptors and synapses. As discussed further in Section 4.5, basal levels of dopamine D1-receptor signaling are enhanced by chronic cocaine use, which renders cortical neurons hyper-excitable. This hyperexcitability may be responsible for both the establishment of extremely strong drug-associated memories in addicted individuals, as well as their heightened reactivity to drug-associated cues.
4.4. Loss of dopamine neuromodulation
Cocaine experience interferes with dopamine modulation of cortical plasticity. For example, under control conditions, D1 receptor stimulation reduces an mGluR5 mediated after-depolarization that follows action potential firing; however, after cocaine experience D1 receptor activation no longer has any effect on this after-depolarization (Sidiropoulou et al., 2009). The ability for dopamine (or glutamate) to enhance neuronal firing in the PFC is also reduced after methamphetamine experience (Peterson et al., 2000). Furthermore, chronic (but not acute) cocaine experience impairs long-term depression (LTD) in the PFC due to elevated D1 and cAMP signaling (Huang et al., 2007). Also, using similar regiments of cocaine injections, it was found that LTP induction was facilitated in the PFC after withdrawal from cocaine experience (Lu et al., 2010). These findings are what would be expected if the K+ channel gate on synaptic plasticity provided by the sAHP were removed by chronic cocaine experience. Under control conditions, dopamine tightly regulates this plasticity; however, chronic cocaine experience appears to lock PFC synapses in a potentiated state by enhancing basal levels of dopamine D1 receptor signaling even in the absence of dopamine.
4.5. Cyclase superactivation
This loss of dopamine modulation after cocaine experience likely occurs due to an increase in intracellular D1-receptor signaling (cyclic adenosine monophosphate (cAMP) accumulation; defined as adenylyl cyclase superactivation) resulting in enhancement of basal PKA activity. In support of this, Dong et al. (2005) found that chronic cocaine treatment elevated cortical excitability, an adaptation that could be reversed by inhibiting PKA activity. This adaptation was enduring, as it could be observed even after 3 weeks of withdrawal and appears to result from a PKA dependent enhancement of L-type calcium channel activity (Ford et al., 2009; Nasif, 2005). Furthermore, dopamine neuromodulation lost after methamphetamine exposure is restored by inhibition of PKA (Peterson et al., 2006). The enhanced PKA activity observed in these studies likely results from the chronic elevations in dopamine release that occurred during cocaine exposure. Cocaine exposure also degrades D2 receptor signaling in the PFC, which normally acts to reduce AC/PKA activity. Indeed, AGS-3, which reduces the ability of all Gαi coupled GPCRs (like D2 receptors and mGluR2/3 receptors) to inhibit adenylyl cyclase, is increased in the PFC following cocaine experience, resulting in tonically elevated PKA weeks after cocaine (Bowers et al., 2004; Ford et al., 2009). This may be a widespread adaptation in addiction, as upregulated AGS-3 during cocaine or opiate withdrawal also leads to super-activation of adenylyl cyclase in the striatum (Fan et al., 2009). The emergence of elevated PFC PKA activity must occur early during withdrawal from cocaine and contribute to drug-seeking behavior, because blocking PKA activity immediately following withdrawal from cocaine self-administration prevents reinstatement of drug-seeking weeks later (Sun et al., 2014).
4.6. Increased calcium load
Cyclase superactivation following chronic cocaine experience likely disrupts other intracellular signaling cascades in the PFC as well. For instance, cyclase superactivation in the striatum during morphine withdrawal increases PLC and PKC signaling, suggesting disruption of Gq receptor signaling (Fan et al., 2009). Similar adaptations could occur in the PFC after withdrawal from chronic cocaine. Similar to the actions of acute dopamine at D1-like receptors increasing intracellular calcium signaling, superactivation of cyclase activity would be expected to increase the calcium load within pyramidal cells, likely disrupting the compartmentalization of calcium signaling. Perhaps chronic cocaine renders ryanodine receptors that gate calcium release from intracellular stores leaky due to enhanced phosphorylation by PKA, as was observed after chronic stress (Liu et al., 2012). Because of the ubiquitous importance of calcium signaling in cellular functions, including transcription and translational regulation, spine dynamics (altered following chronic cocaine (Crombag et al., 2005; Muñoz-Cuevas et al., 2013; Rasakham et al., 2014), apoptosis, and synaptic plasticity, altered handling of intracellular calcium would have major consequences for pyramidal cell function and PFC dependent behaviors. For a complete synopsis of the consequences of aberrant calcium signaling in disease, see Verkhratsky (2005).
5. Can these adaptations account for cortical dysfunction in addiction?
As discussed in Section 4, addiction in humans has been described as a disease of hypofrontalility with symptoms that include reduced cognitive function, increased impulsivity, and poor decision-making. Despite considerable variability in findings as to how the PFC responds to acute exposure to cocaine (Breiter et al., 1997; Herning et al., 1994; Howell et al., 2010; Howell and Wilcox, 2002; London, 1990; Lu et al., 2012), it is generally acknowledged that PFC activity is reduced during abstinence from cocaine, while intoxication and craving activate the PFC (Goldstein and Volkow, 2011, 2002). Thus, chronic cocaine self-administration reduces PFC responsiveness to everyday behaviors, but heightens responses during drug seeking behaviors. Can the cellular adaptations reviewed above account for the reduction in basal activity as well as the increased responses to drug-cues?
Our hypothesis is that by hijacking dopamine D1-receptor signaling, chronic cocaine experience results in an enduring enhancement of calcium release from intracellular stores that disrupts K+ channel inhibition provided by the sAHP (see Fig. 1). With elevated cytosolic calcium, the opening of calcium activated K+ channels such as BK and sK channels may be enhanced. Furthermore, some K+ channels, such as KCNQ (Kv7) channels, increase in expression in response to global increases in calcium signaling (Zhang and Shapiro, 2012). Thus it may be that under some conditions, the activity of these inhibitory K+ channels dominates, and PFC neurons are hypo-excitable as observed in Chen et al. (2013). However, Kv7/KCNQ channels, as well as other K+ channels that control neuronal excitability, can be inhibited by high levels of intracellular calcium (Delmas and Brown, 2005; Kirkwood et al., 1991). Furthermore, as noted in Section 3.2, these same K+ channels (and those underlying the sAHP) also are subject to inhibition by a number of neuromodulators, including dopamine and acetylcholine, which are released in response to salient cues (Heien et al., 2005; Paolone et al., 2013; Phillips et al., 2003; Robinson et al., 2003).
Perhaps then under low arousal conditions, in the absence of salient drug-cues, PFC neurons appear hypo-active after chronic cocaine due to elevations in intracellular calcium and enhanced inhibition from calcium-activated K+ channels, such as BK and sK. However, K+ channels such as KCNQ channels and others that participate in the sAHP may not tolerate extreme elevations in intracellular calcium and therefore inactivate, rendering cortical cells hyper-responsive to incoming excitatory inputs. Drug-paired stimuli would inhibit additional opening of K+ channels via further release of neuromodulators into the cortex. This would further dis-inhibit cortical neurons and facilitate PFC responses to excitatory input.
The interplay of these cellular mechanisms could explain apparently controversial results, where some have reported reduced intrinsic excitability (Chen et al., 2013), while others report enhanced intrinsic excitability (Dong et al., 2005; Ford et al., 2009; Hearing et al., 2012, 2013) in the PFC after cocaine experience. Other mechanisms may account for these differences as well, including different behavioral models as suggested in Jasinska et al. (2014), because training to withhold responding for a reward as used by Chen et al. (2013) has been shown to decrease neuronal excitability in the dorsal PFC (Hayton et al., 2011b). Other factors, such as differences between PFC subregions (dorsal prelimbic versus ventral infralimbic regions), neuronal layer (layer 5 versus 2/3), or the projection targets of neurons studied could contribute to this discrepancy as well. For example, in one study using i.p. cocaine injections, only layer 5/6 prelimbic neurons (including cells projecting to the ventral midbrain and nucleus accumbens) were found to be hyperexcitable after cocaine treatment (Hearing et al., 2013). Nevertheless, it appears that after prolonged abstinence from chronic cocaine, PFC activity is reduced during everyday behaviors, but can become hyperactive in response to drug-associated cues that promote drug seeking and relapse.
6. Summary
Rewards and their cues induce dopamine neuron burst firing that facilitates plasticity in cortical–striatal circuits. The unique architecture of the mesocortical system allows burst firing of dopamine neurons to activate extrasynaptic D1-like receptors and facilitate synaptic plasticity and circuit remodeling. Dopamine D1-like receptors facilitate this plasticity via the dis-inhibition of cortical neurons, removing the K+ channel inhibition provided by the sAHP and unlocking the gate for synaptic plasticity to occur.
The disruption of dopamine modulation of the PFC by cocaine experience likely occurs as a direct result of the unique architecture of the mesocortical system and consequential adaptations in dopaminergic signaling. Cocaine experience appears to enhance dopamine release from terminals, as well as elevate basal levels of intracellular D1-receptor signaling, resulting in adenylyl cyclase superactivation, elevated PKA activity, and increased calcium load. These adaptations appear to dramatically alter PFC physiology, increasing responsiveness to strong excitatory input, while reducing the ability of cortical neurons to respond to changes in VTA neuron firing.
This scenario is supported by both preclinical models of drug addiction and human drug addicts displaying cognitive dysfunction in dopamine-dependent processes, as well as deficits in response inhibition, attention, impulsivity, working memory, and cognitive flexibility, including a reduced ability to adapt to changes in reward contingency (Briand et al., 2008; George et al., 2008; Groman and Jentsch, 2011; Jentsch et al., 2002; Olausson et al., 2007; Porter et al., 2011; Sofuoglu, 2010; Spronk et al., 2013; van Holst and Schilt, 2011). Drug-paired cues display strong control of PFC neuron firing only following cocaine experience. PFC activity in response to cues correlates with drug craving in human addicts (Garavan et al., 2000; Goldstein and Volkow, 2002; Volkow et al., 2008) and is thought to initiate drug seeking by releasing glutamate into the nucleus accumbens. This pattern of PFC activity in response to drug-cues likely reflects the activation of neuronal ensembles encoding powerful memories of drug-experience, that retain their strength due to elevated dopamine/ D1/AC/PKA signaling in cortical neurons following cocaine abuse.
The hypothesis put forth in this review that cocaine experience disrupts K+ channel inhibition in the PFC by enhancing calcium release from intracellular stores remains to be fully vetted. To do so, studies should investigate rodent models of cocaine addiction and examine changes in neuronal excitability in the PFC with a focus on the sAHP and intracellular calcium signaling. Any observed changes should be validated as important for addiction by assessing their relation to cue-induced cocaine seeking. Comparisons between natural rewards and drug rewards will be vital, as learning alone can produce changes in neuronal excitability. These important future studies would address unresolved questions as to how cortical function changes following chronic cocaine use, and would further our understanding of the resulting cortical neuroadaptations, the targeting of which may provide cognitive benefits and help prevent relapse in human drug addicts.
Acknowledgments
Research reported in this publication was supported by the National Institute On Drug Abuse of the National Institutes of Health under Award Number F31DA036989 to W. B., RO1-DA033342 to A.C.R., and P50-DA015369 (PI: A.C.R., PD: P. Kalivas). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. http://dx.doi.org/10.1016/S0166-2236(96)80018-X. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Andrade R, Foehring RC, Tzingounis AV. The calcium-activated slow AHP: cutting through the Gordian knot. Front Cell Neurosci. 2012;6:47. doi: 10.3389/fncel.2012.00047. http://dx.doi.org/10.3389/fncel.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. http://dx.doi.org/10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. http://dx.doi.org/10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar OM, Szumlinski KK, Lominac KD, Cohen A, Gordon E, Ploense KL, DeMartini J, Bernstein N, Rudy NM, Nabhan AN, Sacramento A, Pagano K, Carosso GA, Woodward N. Extended access to cocaine self-administration results in reduced glutamate function within the medial prefrontal cortex. Addict Biol. 2012;17:746–757. doi: 10.1111/j.1369-1600.2011.00428.x. http://dx.doi.org/10.1111/j.1369-1600.2011.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. http://dx.doi.org/10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Molloy R, Khan Y. Increased interaction of dopamine-immunoreactive varicosities with GABA neurons of rat medial prefrontal cortex occurs during the postweanling period. Synapse. 1996;23:237–245. doi: 10.1002/(SICI)1098-2396(199608)23:4<237::AID-SYN1>3.0.CO;2-8. http://dx.doi.org/10.1002/(SICI)1098-2396(199608)23:4<237::AID-SYN1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew R, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. http://dx.doi.org/10.1016/S0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brembs B, Lorenzetti FD, Reyes FD, Baxter DA, Byrne JH. Operant reward learning in Aplysia: neuronal correlates and mechanisms. Sci Signal. 2002;296:1706. doi: 10.1126/science.1069434. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Ann Med Vet. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. http://dx.doi.org/10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JP, Hablitz JJ. G-protein activation by metabotropic glutamate receptors reduces spike frequency adaptation in neocortical neurons. Neuroscience. 1996;75:123–131. doi: 10.1016/0306-4522(96)00244-8. http://dx.doi.org/10.1016/0306-4522(96)00244-8. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Denovellis EL, Diogo C, Bullock D, Miller EK. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron. 2012;76:838–846. doi: 10.1016/j.neuron.2012.09.029. http://dx.doi.org/10.1016/j.neuron.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. http://dx.doi.org/10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Chen G, Greengard P, Yan Z. Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex. Proc Natl Acad Sci USA. 2004;101:2596–2600. doi: 10.1073/pnas.0308618100. http://dx.doi.org/10.1073/pnas.0308618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bohanick JD, Nishihara M, Seamans JK, Yang CR. Dopamine D1/5 receptor-mediated long-term potentiation of intrinsic excitability in rat prefrontal cortical neurons: Ca2+-dependent intracellular signaling. J Neurophysiol. 2007;97:2448–2464. doi: 10.1152/jn.00317.2006. http://dx.doi.org/10.1152/jn.00317.2006. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Coussens CM, Raymond CR, Abraham WC. Long-lasting increase in cellular excitability associated with the priming of LTP induction in rat hippocampus. J Neurophysiol. 1999;82:3139–3148. doi: 10.1152/jn.1999.82.6.3139. [DOI] [PubMed] [Google Scholar]
- Cohen-Matsliah SI, Motanis H, Rosenblum K, Barkai E. A novel role for protein synthesis in long-term neuronal plasticity: maintaining reduced postburst after hyperpolarization. J Neurosi. 2010;30:4338–4342. doi: 10.1523/JNEUROSCI.5005-09.2010. http://dx.doi.org/10.1523/JNEUROSCI.5005-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Lo Turco JJ, Kubota M, Disterhoft JF, Moore JW, Alkon DL. Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J Neurophysiol. 1989;61:971–981. doi: 10.1152/jn.1989.61.5.971. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. http://dx.doi.org/10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- Dai R, Ali MK, Lezcano N, Bergson C. A crucial role for cAMP and protein kinase A in D1 dopamine receptor regulated intracellular calcium transients. Neurosignals. 2008;16:112–123. doi: 10.1159/000111557. http://dx.doi.org/10.1159/000111557. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem. 2003;10:456–465. doi: 10.1101/lm.64103. http://dx.doi.org/10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. http://dx.doi.org/10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Pani L, Gessa GL. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry. 2001;6:657–664. doi: 10.1038/sj.mp.4000904. http://dx.doi.org/10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- Dong Y, Cooper D, Nasif F, Hu XT, White FJ. Dopamine modulates inwardly rectifying potassium currents in medial prefrontal cortex pyramidal neurons. J Neurosci. 2004;24:3077–3085. doi: 10.1523/JNEUROSCI.4715-03.2004. http://dx.doi.org/10.1523/JNEUROSCI.4715-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, Malenka RC, White FJ. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci. 2005;25:936–940. doi: 10.1523/JNEUROSCI.4715-04.2005. http://dx.doi.org/10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, White FJ. Dopamine D1-class receptors selectively modulate a slowly inactivating potassium current in rat medial prefrontal cortex pyramidal neurons. J Neurosci. 2003;23:2686–2695. doi: 10.1523/JNEUROSCI.23-07-02686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. Beyond bistability: biophysics and temporal dynamics of working memory. NSC. 2006;139:119–133. doi: 10.1016/j.neuroscience.2005.06.094. http://dx.doi.org/10.1016/j.neuroscience.2005.06.094. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000a;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat Neurosci. 2000b;3:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Fan P, Jiang Z, Diamond I, Yao L. Up-regulation of AGS3 during morphine withdrawal promotes cAMP superactivation via adenylyl cyclase 5 and 7 in rat nucleus accumbens/striatal neurons. Mol Pharmacol. 2009;76:526–533. doi: 10.1124/mol.109.057802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M. Prefrontal cell firing in male rats during approach towards sexually receptive female: interactions with cocaine. Synapse. 2011;65:271–277. doi: 10.1002/syn.20843. http://dx.doi.org/10.1002/syn.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández de Sevilla D, Fuenzalida M, Porto Pazos AB, Buño W. Selective shunting of the NMDA EPSP component by the slow afterhyperpolarization in rat CA1 pyramidal neurons. J Neurophysiol. 2007;97:3242–3255. doi: 10.1152/jn.00422.2006. http://dx.doi.org/10.1152/jn.00422.2006. [DOI] [PubMed] [Google Scholar]
- Ford CP, Williams JT. Mesoprefrontal dopamine neurons distinguish themselves. Neuron. 2008;57:631–632. doi: 10.1016/j.neuron.2008.02.027. http://dx.doi.org/10.1016/j.neuron.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Ford KA, Wolf ME, Hu XT. Plasticity of L-type Ca 2+channels after cocaine withdrawal. Synapse. 2009;63:690–697. doi: 10.1002/syn.20651. http://dx.doi.org/10.1002/syn.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuenzalida M, de Sevilla DF, Buño W. Changes of the EPSP waveform regulate the temporal window for spike-timing-dependent plasticity. J Neurosci. 2007;27:11940–11948. doi: 10.1523/JNEUROSCI.0900-07.2007. http://dx.doi.org/10.1523/JNEUROSCI.0900-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Wolf ME. Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons. J Neurochem. 2008;106:2489–2501. doi: 10.1111/j.1471-4159.2008.05597.x. http://dx.doi.org/10.1111/j.1471-4159.2008.05597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. http://dx.doi.org/10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garris PA, Collins LB, Jones SR, Wightman RM. Evoked extracellular dopamine in vivo in the medial prefrontal cortex. J Neurochem. 1993;61:637–647. doi: 10.1111/j.1471-4159.1993.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Ann Med Vet. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. http://dx.doi.org/10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. http://dx.doi.org/10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci USA. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. http://dx.doi.org/10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Tessari M, Dacome L, Agosta F, Lepore S, Lanzoni A, Cristofori P, Pich EM, Corsi M, Bifone A. Neuroimaging evidence of altered fronto-cortical and striatal function after prolonged cocaine self-administration in the rat. Ann Med Vet. 2011;36:2431–2440. doi: 10.1038/npp.2011.129. http://dx.doi.org/10.1038/npp.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol. 2002;13:379–388. doi: 10.1097/00008877-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Jentsch JD. Cognitive control and the dopamine D2-like receptor: a dimensional understanding of addiction. Depress Anxiety. 2011;29:295–306. doi: 10.1002/da.20897. http://dx.doi.org/10.1002/da.20897. [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. http://dx.doi.org/10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkin BS, Laing CR, Colby CL, Chow CC, Ermentrout GB. Turning on and off with excitation: the role of spike-timing asynchrony and synchrony in sustained neural activity. J Comput Neurosci. 2001;11:121–134. doi: 10.1023/a:1012837415096. [DOI] [PubMed] [Google Scholar]
- Han D, Gu H. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. http://dx.doi.org/10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug T, Storm JF. Protein kinase A mediates the modulation of the slow Ca(2+)-dependent K(+) current, I (sAHP), by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J Neurophysiol. 2000;83:2071–2079. doi: 10.1152/jn.2000.83.4.2071. [DOI] [PubMed] [Google Scholar]
- Hayton SJ, Olmstead MC, Dumont ÉC. Shift in the intrinsic excitability of medial prefrontal cortex neurons following training in impulse control and cued-responding tasks. PLoS One. 2011a;6:e23885. doi: 10.1371/journal.pone.0023885. http://dx.doi.org/10.1371/journal.pone.0023885.t002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayton SJ, Olmstead MC, Dumont ÉC. Shift in the intrinsic excitability of medial prefrontal cortex neurons following training in impulse control and cued-responding tasks. CORD Conf Proc. 2011b;6:e23885. doi: 10.1371/journal.pone.0023885. http://dx.doi.org/10.1371/journal.pone.0023885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Luján R, Wickman K. Repeated cocaine weakens GABAB-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron. 2013;80:159–170. doi: 10.1016/j.neuron.2013.07.019. http://dx.doi.org/10.1016/j.neuron.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Zink AN, Wickman K. Cocaine-induced adaptations in metabotropic inhibitory signaling in the mesocorticolimbic system. Rev Neurosci. 2012;23:325–351. doi: 10.1515/revneuro-2012-0045. http://dx.doi.org/10.1515/revneuro-2012-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci USA. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. http://dx.doi.org/10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Chronic clozapine selectively decreases prefrontal cortex dopamine as shown by simultaneous cortical, accumbens, and striatal microdialysis in freely moving rats. Pharmacol Biochem Behav. 1995;52:581–589. doi: 10.1016/0091-3057(95)00144-l. [DOI] [PubMed] [Google Scholar]
- Herning RI, Glover BJ, Koeppl B, Phillips RL. cocaine-induced increases in eeg alpha and beta activity: evidence for reduced cortical processing. Neuropsychopharmacology. 1994;11:1–9. doi: 10.1038/npp.1994.30. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilström B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779:214–225. doi: 10.1016/s0006-8993(97)01135-9. http://dx.doi.org/10.1016/S0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann IS, Talmaciu RK, Ferro CP, Cubeddu LX. Sustained high release at rapid stimulation rates and reduced functional autoreceptors characterize prefrontal cortex dopamine terminals. J Pharmacol Exp Ther. 1988;245:761–772. [PubMed] [Google Scholar]
- Howell LL, Votaw JR, Goodman MM, Lindsey KP. Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology. 2010;208:191–199. doi: 10.1007/s00213-009-1720-3. http://dx.doi.org/10.1007/s00213-009-1720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. Functional imaging and neurochemical correlates of stimulant self-administration in primates. Psychopharmacology. 2002;163:352–361. doi: 10.1007/s00213-002-1207-y. http://dx.doi.org/10.1007/s00213-002-1207-y. [DOI] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol. 2002;545:783–805. doi: 10.1113/jphysiol.2002.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JL, Liu G, Li YC, Gao WJ, Huang YQ. Dopamine D1 receptor-mediated NMDA receptor insertion depends on Fyn but not Src kinase pathway in prefrontal cortical neurons. Mol Brain. 2010;3:20. doi: 10.1186/1756-6606-3-20. http://dx.doi.org/10.1186/1756-6606-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Yang PC, Lin HJ, Hsu KS. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J Neurosci. 2007;27:2958–2968. doi: 10.1523/JNEUROSCI.4247-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.4247-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihalainen JA, Riekkinen P, Feenstra MG. Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using microdialysis. Neurosci Lett. 1999;277:71–74. doi: 10.1016/s0304-3940(99)00840-x. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Olsen CM, D’Souza MS, Duvauchelle CL. Experience-dependent effects of cocaine self-administration/ conditioning on prefrontal and accumbens dopamine responses. Behav Neurosci. 2007;121:389–400. doi: 10.1037/0735-7044.121.2.389. http://dx.doi.org/10.1037/0735-7044.121.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki Y, Hori K, Nomura M. Dopamine and acetylcholine elevation on lever-press acquisition in rat prefrontal cortex. Neurosci Lett. 1998;258:33–36. doi: 10.1016/s0304-3940(98)00841-6. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM. Solving the distal reward problem through linkage of STDP and dopamine signaling. Cereb Cortex. 2007;17:2443–2452. doi: 10.1093/cercor/bhl152. http://dx.doi.org/10.1093/cercor/bhl152. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Chen BT, Bonci A, Stein EA. Dorsal medial prefrontal cortex (MPFC) circuitry in rodent models of cocaine use: implications for drug addiction therapies. Addict Biol. 2014;20:215–226. doi: 10.1111/adb.12132. http://dx.doi.org/10.1111/adb.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. http://dx.doi.org/10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft JF. Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learn Mem. 2009;16:362–366. doi: 10.1101/lm.1365609. http://dx.doi.org/10.1101/lm.1365609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. http://dx.doi.org/10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2007;33:166–180. doi: 10.1038/sj.npp.1301564. http://dx.doi.org/10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. http://dx.doi.org/10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. http://dx.doi.org/10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. http://dx.doi.org/10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Simmons MA, Mather RJ, Lisman J. Muscarinic suppression of the M-current is mediated by a rise in internal Ca 2+ concentration. Neuron. 1991;6:1009–1014. doi: 10.1016/0896-6273(91)90240-z. [DOI] [PubMed] [Google Scholar]
- Kisilevsky AE, Mulligan SJ, Altier C, Iftinca MC, Varela D, Tai C, Chen L, Hameed S, Hamid J, Macvicar BA, Zamponi GW. D1 receptors physically interact with N-type calcium channels to regulate channel distribution and dendritic calcium entry. Neuron. 2008;58:14. doi: 10.1016/j.neuron.2008.03.002. http://dx.doi.org/10.1016/j.neuron.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Knöpfel T, Vranesic I, Gähwiler BH, Brown DA. Muscarinic and beta-adrenergic depression of the slow Ca2 (+)-activated potassium conductance in hippocampal CA3 pyramidal cells is not mediated by a reduction of depolarization-induced cytosolic Ca2+ transients. Proc Natl Acad Sci USA. 1990;87:4083–4087. doi: 10.1073/pnas.87.11.4083. http://dx.doi.org/10.2307/2354893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. http://dx.doi.org/10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Lin Bin, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. http://dx.doi.org/10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimer LS, Jakab RL, Goldman-Rakic PS. Quantitative three-dimensional analysis of the catecholaminergic innervation of identified neurons in the macaque prefrontal cortex. J Neurosci. 1997;17:7450–7461. doi: 10.1523/JNEUROSCI.17-19-07450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MS, Prémont J, Krebs MO, Jay TM. Interaction of dopamine D1 with NMDA NR1 receptors in rat prefrontal cortex. Eur Neuropsychopharmacol. 2009;19:296–304. doi: 10.1016/j.euroneuro.2008.12.006. http://dx.doi.org/10.1016/j.euroneuro.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Ladepeche L, Dupuis JP, Bouchet D, Doudnikoff É, Yang L, Campagne Y, Bezard E, Hosy E, Groc L. Single-molecule imaging of the functional crosstalk between surface NMDA and dopamine D1 receptors. Proc Natl Acad Sci. 2013;110:18005–18010. doi: 10.1073/pnas.1310145110. http://dx.doi.org/10.1073/pnas.1310145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. http://dx.doi.org/10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. http://dx.doi.org/10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. http://dx.doi.org/10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Hu H, Ramakers GM, Storm JF. Interaction between synaptic excitation and slow afterhyperpolarization current in rat hippocampal pyramidal cells. J Physiol. 2001;536:809–823. doi: 10.1111/j.1469-7793.2001.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PEM, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci. 2005;25:5013–5023. doi: 10.1523/JNEUROSCI.0557-05.2005. http://dx.doi.org/10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc KH, Maidment NT, Ostlund SB. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One. 2013;8:e61355. doi: 10.1371/journal.pone.0061355. http://dx.doi.org/10.1371/journal.pone.0061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O’Dowd BF, George SR. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. http://dx.doi.org/10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Lewis BL, O’Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential “up”states in pyramidal neurons via D1 dopamine receptors. Cereb Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. http://dx.doi.org/10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang F, Huang C, Long LH, Wu WN, Cai F, Wang JH, Ma LQ, Chen JG. Activation of phosphatidylinositol-linked novel D1 dopamine receptor contributes to the calcium mobilization in cultured rat prefrontal cortical astrocytes. CORD Conf Proc. 2009;29:317–328. doi: 10.1007/s10571-008-9323-9. http://dx.doi.org/10.1007/s10571-008-9323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Betzenhauser MJ, Reiken S, Meli AC, Xie W, Chen BX, Arancio O, Marks AR. Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell. 2012;150:1055–1067. doi: 10.1016/j.cell.2012.06.052. http://dx.doi.org/10.1016/j.cell.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED. Cocaine-induced redoppuction of glucose utilization in human brain. Arch Gen Psychiatry. 1990;47:567–574. doi: 10.1001/archpsyc.1990.01810180067010. http://dx.doi.org/10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- Lorenzetti FD, Baxter DA, Byrne JH. Molecular mechanisms underlying a cellular analog of operant reward learning. Neuron. 2008;59:815–828. doi: 10.1016/j.neuron.2008.07.019. http://dx.doi.org/10.1016/j.neuron.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti FD, Mozzachiodi R, Baxter DA, Byrne JH. Classical and operant conditioning differentially modify the intrinsic properties of an identified neuron. Nat Neurosci. 2005;9:17–19. doi: 10.1038/nn1593. [DOI] [PubMed] [Google Scholar]
- Lu H, Chefer S, Kurup PK, Guillem K, Vaupel DB, Ross TJ, Moore A, Yang Y, Peoples LL, Stein EA. fMRI response in the medial prefrontal cortex predicts cocaine but not sucrose self-administration history. NeuroImage. 2012;62:1857–1866. doi: 10.1016/j.neuroimage.2012.05.076. http://dx.doi.org/10.1016/j.neuroimage.2012.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Chefer S, Ross TJ, Vaupel DB, Guillem K, Rea W, Peoples LL, Stein EA. Abstinence from cocaine and sucrose self-administration reveals altered mesocorticolimbic circuit connectivity. Brain Connect. 2014;4:499–510. doi: 10.1089/brain.2014.0264. http://dx.doi.org/10.1089/brain.2014.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67:13. doi: 10.1016/j.neuron.2010.08.012. http://dx.doi.org/10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Dopamine decreases the calcium-activated afterhyperpolarization in hippocampal CA1 pyramidal cells. Brain Res. 1986;379:210–215. doi: 10.1016/0006-8993(86)90773-0. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. http://dx.doi.org/10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Markova O, Fitzgerald D, Stepanyuk A, Dovgan A, Cherkas V, Tepikin A, Burgoyne RD, Belan P. Hippocalcin signaling via site-specific translocation in hippocampal neurons. Neurosci Lett. 2008;442:152–157. doi: 10.1016/j.neulet.2008.06.089. http://dx.doi.org/10.1016/j.neulet.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KAC, Spühler IA. The fine structure of the dopaminergic innervation of area 10 of macaque prefrontal cortex. Eur J Neurosci. 2013;37:1061–1071. doi: 10.1111/ejn.12124. http://dx.doi.org/10.1111/ejn.12124. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Disterhoft JF. Blocking the BK channel impedes acquisition of trace eyeblink conditioning. Learn Mem. 2009;16:106–109. doi: 10.1101/lm.1289809. http://dx.doi.org/10.1101/lm.1289809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Linardakis JM, Disterhoft JF. The fast and slow afterhyperpolarizations are differentially modulated in hippocampal neurons by aging and learning. J Neurosci. 2009;29:4750–4755. doi: 10.1523/JNEUROSCI.0384-09.2009. http://dx.doi.org/10.1523/JNEUROSCI.0384-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Sanchez-Vives MV. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. http://dx.doi.org/10.1038/79848. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. Persistent cocaine-induced reversal learning deficits are associated with altered limbic cortico-striatal local field potential synchronization. J Neurosci. 2013;33:17469–17482. doi: 10.1523/JNEUROSCI.1440-13.2013. http://dx.doi.org/10.1523/JNEUROSCI.1440-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh S, Howell L, Hemby SE. Dopaminergic dysregulation in prefrontal cortex of rhesus monkeys following cocaine self-administration. Front Psychiatry. 2013;4:88. doi: 10.3389/fpsyt.2013.00088. http://dx.doi.org/10.3389/fpsyt.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Matthews EA, Oliveira FA, Disterhoft JF. Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J Neurophysiol. 2009;102:2763–2770. doi: 10.1152/jn.00347.2009. http://dx.doi.org/10.1152/jn.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Oh MM, Galvez R, Burgdorf J, Kroes RA, Weiss C, Adelman JP, Moskal JR, Disterhoft JF. Increasing SK2 channel activity impairs associative learning. J Neurophysiol. 2012;108:863–870. doi: 10.1152/jn.00025.2012. http://dx.doi.org/10.1152/jn.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motanis H, Maroun M, Barkai E. Learning-induced bidirectional plasticity of intrinsic neuronal excitability reflects the valence of the outcome. Cereb Cortex. 2014;24:1075–1087. doi: 10.1093/cercor/bhs394. http://dx.doi.org/10.1093/cercor/bhs394. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci. 2000;20:5476–5482. doi: 10.1523/JNEUROSCI.20-14-05476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R. More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends Neurosci. 2010;33:17–26. doi: 10.1016/j.tins.2009.10.001. http://dx.doi.org/10.1016/j.tins.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R, Lorenzetti FD, Baxter DA, Byrne JH. Changes in neuronal excitability serve as a mechanism of long-term memory for operant conditioning. Nat Neurosci. 2008;11:1146–1148. doi: 10.1038/nn.2184. http://dx.doi.org/10.1038/nn.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. http://dx.doi.org/10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller EM, Panitz C, Hermann C, Pizzagalli DA. Prefrontal oscillations during recall of conditioned and extinguished fear in humans. J Neurosci. 2014;34:7059–7066. doi: 10.1523/JNEUROSCI.3427-13.2014. http://dx.doi.org/10.1523/JNEUROSCI.3427-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Cuevas FJ, Athilingam J, Piscopo D, Wilbrecht L. Cocaine-Induced Structural Plasticity in Frontal Cortex Correlates with Conditioned Place Preference. Vol. 16. Nature Publishing Group; 2013. pp. 1367–1369. http://dx.doi.org/10.1038/nn.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Suzuki Y, Nagayasu K, Kitaichi M, Shirakawa H, Kaneko S. Repeated exposure to methamphetamine, cocaine or morphine induces augmentation of dopamine release in rat mesocorticolimbic slice co-cultures. PLoS One. 2011;6:e24865. doi: 10.1371/journal.pone.0024865. http://dx.doi.org/10.1371/journal.pone.0024865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J Neurosci. 2012;32:16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. http://dx.doi.org/10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ. Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci. 2005;25:3674–3679. doi: 10.1523/JNEUROSCI.0010-05.2005. http://dx.doi.org/10.1523/JNEUROSCI.0010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. http://dx.doi.org/10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- Oh MM, Disterhoft JF. Increased excitability of both principal neurons and interneurons during associative learning. Neuroscientist. 2014 doi: 10.1177/1073858414537382. http://dxdoi.org/10.1177/1073858414537382. [DOI] [PMC free article] [PubMed]
- Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF. Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol. 2003;90:2171–2179. doi: 10.1152/jn.01177.2002. http://dx.doi.org/10.1152/jn.01177.2002. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Krueger DD, Tronson NC, Nairn AC, Taylor JR. Orbitofrontal cortex and cognitive-motivational impairments in psychostimulant addiction: evidence from experiments in the non-human primate. Ann N Y Acad Sci. 2007;1121:610–638. doi: 10.1196/annals.1401.016. http://dx.doi.org/10.1196/annals.1401.016. [DOI] [PubMed] [Google Scholar]
- Otis JM, Mueller D. Inhibition of β-adrenergic receptors induces a persistent deficit in retrieval of a cocaine-associated memory providing protection against reinstatement. Neuropsychopharmacology. 2011;36:1912–1920. doi: 10.1038/npp.2011.77. http://dx.doi.org/10.1038/npp.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci USA. 2008;105:11957–11962. doi: 10.1073/pnas.0803896105. http://dx.doi.org/10.1073/pnas.0803896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini C, Roeper J. Generating bursts (and pauses) in dopamine midbrain neurons. Neuroscience. 2014;282:109–121. doi: 10.1016/j.neuroscience.2014.07.032. http://dx.doi.org/10.1016/j.neuroscience.2014.07.032. [DOI] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33:8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. http://dx.doi.org/10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology. 2013;39:811–822. doi: 10.1038/npp.2013.231. http://dx.doi.org/10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J Neurosci. 2004;24:5292–5300. doi: 10.1523/JNEUROSCI.0195-04.2004. http://dx.doi.org/10.1523/JNEUROSCI.0195-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V. Timing is not everything: neuromodulation opens the STDP gate. Front Synaptic Neurosci. 2010;2:146. doi: 10.3389/fnsyn.2010.00146. http://dx.doi.org/10.3389/fnsyn.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, Kerr JND. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. http://dx.doi.org/10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Paré D. Theta synchronizes the activity of medial prefrontal neurons during learning. Learn Mem. 2008;15:524–531. doi: 10.1101/lm.932408. http://dx.doi.org/10.1101/lm.932408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, Krause M, Haug T, Storm JF, Stühmer W. Modulation of the Ca2+-activated K+ current sIAHP by a phosphatase-kinase balance under basal conditions in rat CA1 pyramidal neurons. J Neurophysiol. 1998;79:3252–3256. doi: 10.1152/jn.1998.79.6.3252. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron. 1993;11:1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. Dopamine modulates the slow Ca(2+)-activated K+ current IAHP via cyclic AMP-dependent protein kinase in hippocampal neurons. J Neurophysiol. 1995;74:2749–2753. doi: 10.1152/jn.1995.74.6.2749. [DOI] [PubMed] [Google Scholar]
- Penit-Soria J, Audinat E, Crepel F. Excitation of rat prefrontal cortical neurons by dopamine: an in vitro electrophysiological study. Brain Res. 1987;425:263–274. doi: 10.1016/0006-8993(87)90509-9. http://dx.doi.org/10.1016/0006-8993(87)90509-9. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Repeated amphetamine administration decreases D1 dopamine receptor-mediated inhibition of voltage-gated sodium currents in the prefrontal cortex. J Neurosci. 2006;26:3164–3168. doi: 10.1523/JNEUROSCI.2375-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.2375-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Altered responsiveness of medial prefrontal cortex neurons to glutamate and dopamine after withdrawal from repeated amphetamine treatment. Synapse. 2000;36:342–344. doi: 10.1002/(SICI)1098-2396(20000615)36:4<342::AID-SYN11>3.0.CO;2-9. http://dx.doi.org/10.1002/(SICI)1098-2396(20000615)36:4<342::AID-SYN11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in Rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. http://dx.doi.org/10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Sah P. Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. J Neurosci. 2008;28:3209–3220. doi: 10.1523/JNEUROSCI.4310-07.2008. http://dx.doi.org/10.1523/JNEUROSCI.4310-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasakham K, Schmidt HD, Kay K, Huizenga MN, Calcagno N, Pierce RC, Spires-Jones TL, Sadri-Vakili G. Synapse density and dendritic complexity are reduced in the prefrontal cortex following seven days of forced abstinence from cocaine self-administration. PLoS One. 2014;9:e102524. doi: 10.1371/journal.pone.0102524. http://dx.doi.org/10.1371/journal.pone.0102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Sun W. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J Exp Anal Behav. 2005;84:653–666. doi: 10.1901/jeab.2005.105-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. http://dx.doi.org/10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. NSC. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien MLAV, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. http://dx.doi.org/10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Rogerson T, Cai DJ, Frank A, Sano Y, Shobe J, Lopez-Aranda MF, Silva AJ. Synaptic tagging during memory allocation. Nat Rev Neurosci. 2014;15:157–169. doi: 10.1038/nrn3667. http://dx.doi.org/10.1038/nrn3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Saur T, Yao WD. Dopamine-enabled anti-Hebbian timing-dependent plasticity in prefrontal circuitry. Front Neural Circuits. 2014;8:38. doi: 10.3389/fncir.2014.00038. http://dx.doi.org/10.3389/fncir.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long-lasting maintenance of learning-induced enhanced neuronal excitability: mechanisms and functional significance. Mol Neurobiol. 2009;39:171–177. doi: 10.1007/s12035-009-8060-5. http://dx.doi.org/10.1007/s12035-009-8060-5. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci. 1998;10:1518–1523. doi: 10.1046/j.1460-9568.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sah P, Bekkers JM. Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long-term potentiation. J Neurosci. 1996;16:4537–4542. doi: 10.1523/JNEUROSCI.16-15-04537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Porter JT. M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J Neurosci. 2010;30:12379–12386. doi: 10.1523/JNEUROSCI.1295-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.1295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. http://dx.doi.org/10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake T, Mitani H, Nakagome K, Kaneko K. Individual and additive effects of neuromodulators on the slow components of afterhyperpolarization currents in layer V pyramidal cells of the rat medial prefrontal cortex. Brain Res. 2008;1229:47–60. doi: 10.1016/j.brainres.2008.06.098. http://dx.doi.org/10.1016/j.brainres.2008.06.098. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:23. doi: 10.1016/s0896-6273(02)00967-4. http://dx.doi.org/10.1016/S0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. http://dx.doi.org/10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W. Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron. 2011;69:603–617. doi: 10.1016/j.neuron.2011.02.014. http://dx.doi.org/10.1016/j.neuron.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Schultz W, Hollerman JR. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. http://dx.doi.org/10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci. 2001;98:301–306. doi: 10.1073/pnas.011518798. http://dx.doi.org/10.1073/pnas.98.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:58. doi: 10.1016/j.pneurobio.2004.05.006. http://dx.doi.org/10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sehgal M, Ehlers VL, Moyer JR. Learning enhances intrinsic excitability in a subset of lateral amygdala neurons. Learn Mem. 2014;21:161–170. doi: 10.1101/lm.032730.113. http://dx.doi.org/10.1101/lm.032730.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M, Song C, Ehlers VL, Moyer JR., Jr Learning to learn – intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiol Learn Mem. 2013;105:186–199. doi: 10.1016/j.nlm.2013.07.008. http://dx.doi.org/10.1016/j.nlm.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong HJ, Carter AG. D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. J Neurosci. 2012;32:10516–10521. doi: 10.1523/JNEUROSCI.1367-12.2012. http://dx.doi.org/10.1523/JNEUROSCI.1367-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda-Orengo MT, Lopez AV, Soler-Cedeño O, Porter JT. Fear extinction induces mGluR5-mediated synaptic and intrinsic plasticity in infralimbic neurons. J Neurosci. 2013;33:7184–7193. doi: 10.1523/JNEUROSCI.5198-12.2013. http://dx.doi.org/10.1523/JNEUROSCI.5198-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, CARR DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. http://dx.doi.org/10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynikhovich D, Otani S, Arleo A. The role of tonic and phasic dopamine for long-term synaptic plasticity in the prefrontal cortex: a computational model. J Physiol Paris. 2011;105:45–52. doi: 10.1016/j.jphysparis.2011.08.001. http://dx.doi.org/10.1016/j.jphysparis.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Sheynikhovich D, Otani S, Arleo A. Dopaminergic control of long-term depression/long-term potentiation threshold in prefrontal cortex. J Neurosci. 2013;33:13914–13926. doi: 10.1523/JNEUROSCI.0466-13.2013. http://dx.doi.org/10.1523/JNEUROSCI.0466-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto T, Seeman P, Kuroiwa M, Nishi A. Repeated administration of a dopamine D1 receptor agonist reverses the increased proportions of striatal dopamine D1High and D2High receptors in methamphetamine-sensitized rats. Eur J Neurosci. 2008;27:2551–2557. doi: 10.1111/j.1460-9568.2008.06221.x. http://dx.doi.org/10.1111/j.1460-9568.2008.06221.x. [DOI] [PubMed] [Google Scholar]
- Sidiropoulou K, Lu FM, Fowler MA, Xiao R, Phillips C, Ozkan ED, Zhu MX, White FJ, Cooper DC. Dopamine modulates an mGluR5-mediated depolarization underlying prefrontal persistent activity. Nat Neurosci. 2009;12:190–199. doi: 10.1038/nn.2245. http://dx.doi.org/10.1038/nn.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So CH, Verma V, Alijaniaram M, Cheng R, Rashid AJ, O’Dowd BF, George SR. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1–D2 receptor hetero-Oligomers. Mol Pharmacol. 2009;75:843–854. doi: 10.1124/mol.108.051805. http://dx.doi.org/10.1124/mol.108.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. http://dx.doi.org/10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh H, Tzingounis AV. The specific slow afterhyperpolarization inhibitor UCL2077 is a subtype-selective blocker of the epilepsy associated KCNQ channels. Mol Pharmacol. 2010;78:1088–1095. doi: 10.1124/mol.110.066100. http://dx.doi.org/10.1124/mol.110.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Davidson DL, Kalivas PW, Prasad BM. Repeated daily cocaine alters subsequent cocaine-induced increase of extracellular dopamine in the medial prefrontal cortex. J Pharmacol Exp Ther. 1997;281:54–61. [PubMed] [Google Scholar]
- Spronk DB, van Wel JHP, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: a comprehensive review. Neurosci Biobehav Rev. 2013;37:1838–1859. doi: 10.1016/j.neubiorev.2013.07.003. http://dx.doi.org/10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Spühler IA, Hauri A. Decoding the dopamine signal in macaque prefrontal cortex: a simulation study using the Cx3Dp simulator. PLoS One. 2013;8:e71615. doi: 10.1371/journal.pone.0071615. http://dx.doi.org/10.1371/journal.pone.0071615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Roesch MR, Calu DJ, Burke KA, Singh T, Schoenbaum G. Neural correlates of inflexible behavior in the orbitofrontal amygdalar circuit after cocaine exposure. Ann N Y Acad Sci. 2007;1121:598–609. doi: 10.1196/annals.1401.014. http://dx.doi.org/10.1196/annals.1401.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2012;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. http://dx.doi.org/10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WL, Coleman NT, Zelek Molik A, Barry SM, Whitfield TW, McGinty JF. Relapse to cocaine-seeking after abstinence is regulated by cAMP-dependent protein kinase A in the prefrontal cortex. Addict Biol. 2014;19:77–86. doi: 10.1111/adb.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. http://dx.doi.org/10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Brain Res Dev Brain Res. 1998;110:227–233. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: an autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Disterhoft JF. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol. 1996;76:1836–1849. doi: 10.1152/jn.1996.76.3.1836. [DOI] [PubMed] [Google Scholar]
- Thurley K, Senn W, Lüscher HR. Dopamine increases the gain of the input–output response of rat prefrontal pyramidal neurons. J Neurophysiol. 2008;99:2985–2997. doi: 10.1152/jn.01098.2007. http://dx.doi.org/10.1152/jn.01098.2007. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007a;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. http://dx.doi.org/10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007b;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. http://dx.doi.org/10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. http://dx.doi.org/10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse MTL, Cantor A, Floresco SB. Repeated amphetamine exposure disrupts dopaminergic modulation of amygdala-prefrontal circuitry and cognitive/emotional functioning. J Neurosci. 2011;31:11282–11294. doi: 10.1523/JNEUROSCI.1810-11.2011. http://dx.doi.org/10.1523/JNEUROSCI.1810-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Heidenreich M, Kharkovets T, Spitzmaul G, Jensen HS, Nicoll RA, Jentsch TJ. The KCNQ5 potassium channel mediates a component of the afterhyperpolarization current in mouse hippocampus. Proc Natl Acad Sci USA. 2010;107:1032–10237. doi: 10.1073/pnas.1004644107. http://dx.doi.org/10.1073/pnas.1004644107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Kobayashi M, Takamatsu K, Nicoll RA. Hippocalcin gates the calcium activation of the slow afterhyperpolarization in hippocampal pyramidal cells. Neuron. 2007;53:487–493. doi: 10.1016/j.neuron.2007.01.011. http://dx.doi.org/10.1016/j.neuron.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Nicoll RA. Contribution of KCNQ2 and KCNQ3 to the medium and slow afterhyperpolarization currents. Proc Natl Acad Sci USA. 2008;105:19974–19979. doi: 10.1073/pnas.0810535105. http://dx.doi.org/10.1073/pnas.0810535105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009;3 doi: 10.3389/neuro.07.017.2009. http://dx.doi.org/10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr Drug Abuse Rev. 2011;4:42–56. doi: 10.2174/1874473711104010042. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. http://dx.doi.org/10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Villalobos C. SKCa channels mediate the medium but not the slow calcium-activated afterhyperpolarization in cortical neurons. J Neurosci. 2004;24:3537–3542. doi: 10.1523/JNEUROSCI.0380-04.2004. http://dx.doi.org/10.1523/JNEUROSCI.0380-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Andrade R. Visinin-like neuronal calcium sensor proteins regulate the slow calcium-activated afterhyperpolarizing current in the rat cerebral cortex. J Neurosci. 2010;30:14361–14365. doi: 10.1523/JNEUROSCI.3440-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.3440-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F, Storm JF, Lancaster B. SK channels and the varieties of slow after-hyperpolarizations in neurons. Eur J Neurosci. 2003;18:3155–3166. doi: 10.1111/j.1460-9568.2003.03040.x. http://dx.doi.org/10.1111/j.1460-9568.2003.03040.x. [DOI] [PubMed] [Google Scholar]
- Vogt SM, Hofmann UG. Neuromodulation of STDP through short-term changes in firing causality. Cogn Neurodyn. 2012;6:353–366. doi: 10.1007/s11571-012-9202-4. http://dx.doi.org/10.1007/s11571-012-9202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. http://dx.doi.org/10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):S3–S8. doi: 10.1016/j.neuropharm.2008.05.022. http://dx.doi.org/10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. http://dx.doi.org/10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. NeuroImage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. http://dx.doi.org/10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhauser GT, Bäuml K-HT, Hanslmayr S. Brain oscillations mediate successful suppression of unwanted memories. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu138. http://dxdoi.org/10.1093/cercor/bhu138. [DOI] [PubMed]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cognit Sci. 2003;7:553–559. doi: 10.1016/j.tics.2003.10.012. http://dx.doi.org/10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- West EA, Saddoris MP, Kerfoot EC, Carelli RM. Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine-seeking before and following abstinence. Eur J Neurosci. 2014;39:1891–1902. doi: 10.1111/ejn.12578. http://dx.doi.org/10.1111/ejn.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. http://dx.doi.org/10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Witkowski GG, Szulczyk BB, Rola RR, Szulczyk PP. D”1 dopaminergic control of G protein-dependent inward rectifier K\widehat+ (GIRK)-like channel current in pyramidal neurons of the medial prefrontal cortex. NSC. 2008;155:11. doi: 10.1016/j.neuroscience.2008.05.021. http://dx.doi.org/10.1016/j.neuroscience.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Wu WW, Chan CS, Disterhoft JF. Slow afterhyperpolarization governs the development of NMDA receptor-dependent afterdepolarization in CA1 pyramidal neurons during synaptic stimulation. J Neurophysiol. 2004;92:2346–2356. doi: 10.1152/jn.00977.2003. http://dx.doi.org/10.1152/jn.00977.2003. [DOI] [PubMed] [Google Scholar]
- Xu TX, Yao WD. D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proc Natl Acad Sci USA. 2010;107:16366–16371. doi: 10.1073/pnas.1004108107. http://dx.doi.org/10.1073/pnas.1004108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V-VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS. An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience. 2014;282C:23–48. doi: 10.1016/j.neuroscience.2014.04.010. http://dx.doi.org/10.1016/j.neuroscience.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Zhang XH, Yang CR, Li BM. Contribution of dopamine d1/5 receptor modulation of post-spike/burst afterhyperpolarization to enhance neuronal excitability of layer v pyramidal neurons in prepubertal rat prefrontal cortex. PLoS One. 2013;8:e71880. doi: 10.1371/journal.pone.0071880. http://dx.doi.org/10.1371/journal.pone.0071880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HLL, Pressey J, Mahadevan V, Tran MM, Kushner SA, Woodin MA, Frankland PW, Josselyn SA. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. http://dx.doi.org/10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Young SR, Bianchi R, Wong RKS. Signaling mechanisms underlying group I mGluR-induced persistent AHP suppression in CA3 hippocampal neurons. J Neurophysiol. 2008;99:1105–1118. doi: 10.1152/jn.00435.2007. http://dx.doi.org/10.1152/jn.00435.2007. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Isaacson JS, Scanziani M. Linking neuronal ensembles by associative synaptic plasticity. PLoS One. 2011;6:e20486. doi: 10.1371/journal.pone.0020486. http://dx.doi.org/10.1371/journal.pone.0020486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Anwyl R. Inhibition of the slow afterhyperpolarization restores the classical spike timing-dependent plasticity rule obeyed in layer 2/3 pyramidal cells of the prefrontal cortex. J Neurophysiol. 2012;107:205–215. doi: 10.1152/jn.00452.2011. http://dx.doi.org/10.1152/jn.00452.2011. [DOI] [PubMed] [Google Scholar]
- Zeng W, Mak DOD, Li Q, Shin DM, Foskett JK, Muallem S. A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gbetagamma. Curr Biol. 2003;13:872–876. doi: 10.1016/s0960-9822(03)00330-0. [DOI] [PubMed] [Google Scholar]
- Zhang C, Rønnekleiv OK, Kelly MJ. Kisspeptin inhibits a slow afterhyperpolarization current via protein kinase C and reduces spike frequency adaptation in GnRH neurons. Am J Physiol Endocrinol Metab. 2013;304:E1237–E1244. doi: 10.1152/ajpendo.00058.2013. http://dx.doi.org/10.1152/ajpendo.00058.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shapiro MS. Activity-dependent transcriptional regulation of M-type (Kv7) K(+) channels by AKAP79/150-mediated NFAT actions. Neuron. 2012;76:1133–1146. doi: 10.1016/j.neuron.2012.10.019. http://dx.doi.org/10.1016/j.neuron.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Lau PM, Bi GQ. Gain in sensitivity and loss in temporal contrast of STDP by dopaminergic modulation at hippocampal synapses. Proc Natl Acad Sci USA. 2009;106:13028–13033. doi: 10.1073/pnas.0900546106. http://dx.doi.org/10.1073/pnas.0900546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ouyang M, Ganellin CR, Thomas SA. The slow afterhyperpolarization: a target of β1-adrenergic signaling in hippocampus-dependent memory retrieval. J Neurosci. 2013;33:5006–5016. doi: 10.1523/JNEUROSCI.3834-12.2013. http://dx.doi.org/10.1523/JNEUROSCI.3834-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. http://dx.doi.org/10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]