Abstract
Several anti-PD1/PD-L1 monoclonal antibodies (MAb) are currently providing evidence of clinical benefit in subsets of cancer patients. The mode of action of these MAbs is to inhibit PD1 on immune cells interacting with PD-L1 on tumor cells. These MAbs are either designed or engineered to eliminate antibody-dependent cell-mediated cytotoxicity (ADCC), which, however, has been implicated as an important mechanism in several highly effective MAb-mediated cancer therapies. A fully human anti-PD-L1 MAb would potentially be able to block PD-L1/PD1 interactions and also mediate the ADCC lysis of tumor cells. MSB0010718C (designated avelumab) is a fully human IgG1 anti-PD-L1 MAb. The studies reported here demonstrate (a) the ability of avelumab to lyse a range of human tumor cells in the presence of PBMC or NK effectors; (b) IFNγ can enhance tumor cell PD-L1 expression and in some cases enhance ADCC tumor cell lysis; (c) purified NK cells are potent effectors for avelumab; (d) similar levels of avelumab-mediated ADCC lysis of tumor cells are seen using purified NK as effectors from either healthy donors or cancer patients; (e) very low levels of avelumab-mediated lysis are seen using whole PBMCs as targets; this finding complements results seen in analyses of PBMC subsets of patients receiving avelumab; and (f) the addition of IL12 to NK cells greatly enhances avelumab-mediated ADCC. These studies thus provide an additional mode of action for an anti-PD-L1 MAb and support the rationale for further studies to enhance avelumab-mediated ADCC activity.
Keywords: checkpoint inhibitor, anti-PD-L1, antibody-dependent cellular cytotoxicity
Introduction
The recent FDA approvals of immune checkpoint inhibitor monoclonal antibodies (MAb) constitute a major advance in the immunotherapy of cancer. Ipilimumab (anti-CTLA-4) has shown significant clinical benefit in metastatic melanoma (1), and several MAbs directed against programmed cell death protein-1 ligand (PD-L1) and PD1 have demonstrated clinical benefit in patients with melanoma, Hodgkin's lymphoma, lung and bladder carcinomas, and other tumor types (2-14). The mode of action of these anti-PD-1/PD-L1 MAbs is to inhibit the interaction of PD1 on immune cells with PD-L1 on tumor cells, thus reducing or eliminating immunosuppressive signals, and leading to enhanced immune cell activation. These fully human or humanized MAbs are either of the IgG4 isotype, which does not mediate antibody-dependent cell-mediated cytotoxicity (ADCC), or of the IgG1 isotype and specifically engineered to eliminate ADCC activity. This has been done to obviate any potential toxicity that may result from ADCC-mediated lysis of subsets of immune cells that express PD-L1. ADCC, however, has been shown to play a major role in immune-mediated antitumor responses in many preclinical studies, and has been implicated as an important mechanism of action for several highly effective and most widely employed MAb-mediated therapies of cancer (15-24).
Examples of MAbs with ADCC properties include trastuzumab (Herceptin), which targets the Her2/neu molecule on metastatic breast cancer cells; rituximab (Rituxan), which targets CD20 on lymphoma cells; and cetuximab (Erbitux), which targets EGF-R on KRAS wild-type colorectal cancer (CRC) cells and squamous cell cancer of the head and neck cells. It should be pointed out that while all of these MAbs have demonstrated clear clinical benefit, and have been approved by the FDA for their respective indications, all three target molecules are expressed on non-tumor cell populations.
A fully human anti-PD-L1 MAb would potentially be able to block the interaction of PD1 on immune cells with PD-L1 on tumor cells, while also potentially mediating the ADCC lysis of tumor cells. MSB0010718C (designated avelumab) is a fully human IgG1 anti-PD-L1 MAb with potential ADCC properties. A dose-escalation Phase I study of avelumab in 117 patients (including expansion cohorts) has recently been completed at the National Institutes of Health's Clinical Center (NCT01772004) (25). Subjects received doses ranging from 1 mg/kg to 20 mg/kg. While results are preliminary, clinical benefit in terms of RECIST responses and prolonged disease stabilization were observed in several tumor types (manuscript in preparation). Moreover, the toxicity profile appeared similar to that of other anti-PD1/PD-L1 MAbs. Analyses of 127 immune cell subsets in peripheral blood mononuclear cells (PBMC) at days 15, 43, 84, and 128 post-initiation of therapy with avelumab showed little, if any, changes from pre-treatment levels (manuscript in preparation); these analyses were conducted in subsets of patients receiving all dose levels of the MAb.
The studies reported here were conducted to determine whether anti-PD-L1 avelumab has the ability to lyse human tumor cells via the ADCC mechanism and to determine which factors may have an influence on this activity.
Materials and Methods
Cell lines and culture
Human tumor cell lines (MDA-MB-231, A431, ASPC-1, PANC-1, H441, H226, SW900, H460, H520, H157, CaLU1, COLO205, HT29, SW403, SW116, HBT 1, HBT 4, HBT 5) were purchased from American Type Culture Collection (Manassas, VA). All cell cultures were free of mycoplasma and maintained in RPMI 1640 medium (Corning Cellgro, Manassas, VA) supplemented with 10% fetal calf serum (Gemini Bio-Products, West Sacramento, CA), 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine (Corning Cellgro). PBMCs from healthy volunteer donors were obtained from the National Institutes of Health Clinical Center Blood Bank (NCT00001846). PBMCs from cancer patients were obtained from non-small cell lung cancer (NSCLC) patients enrolled in a previously described clinical trial prior to the initiation of therapy (26). The National Cancer Institute Institutional Review Board approved the trial procedures, and informed consent was obtained in accordance with the Declaration of Helsinki.
Antibodies and flow cytometric analysis
Antibodies used were as follows: anti-PD-L1 avelumab and matching IgG1 isotype control were obtained from EMD Serono (Boston, MA) as part of a Cooperative Research and Development Agreement with the Laboratory of Tumor Immunology and Biology, National Cancer Institute. Other MAbs were obtained as follows: anti-PD-L1-APC (clone 29E.2A3) (BioLegend, San Diego, CA), anti-CD16-FITC (clone CB16), anti-CD56-APC (clone CMSSB), anti-CD3-APC-eF780 (clone SK7), anti-TIM3-eF450 (clone F38-2E2), and anti-CD16 neutralizing Ab (clone B73.1) (eBioscience, San Diego, CA). For flow analysis, 1×106 cells were stained in 100 μL staining buffer (PBS, 1% BSA) for 30 minutes at 4° C. All flow cytometry Abs were used at ½ the recommended volume for a 100 μL test. Prior to staining, cells were incubated with 5 μL per test of Human TruStain FcX (BioLegend) for 15 minutes at 4° C. Live / dead discrimination was accomplished via the inclusion of Live Dead Fixable Stain Aqua (Invitrogen, Grand Island, NY). Events were acquired on an LSRII flow cytometer (BD Bioscience, San Jose, CA) and all downstream analysis was performed in FlowJo 9.5.2 (Treestar Inc., Ashland, OR). The voltage settings on the LSRII may vary between experiments. In order to have a reproducible MFI value for PD-L1 expression on all tumor cell lines, we therefore normalized the MFI values for all experiments by making a ratio between the MFI for stained cells divided by the MFI for control stained cells (27). In addition, each human tumor cell line was scored on a quartile scale (ranging from 1 to 4) for both % PD-L1 positive cells and PD-L1 MFI. The combined score of % PD-L1 positive cells and MFI (ranging from 2 to 8) was termed the PD-L1 score for the tumor cell line.
In vitro ADCC assay
PBMC effectors were thawed the evening prior to the assay and allowed to rest overnight in RPMI 1640 medium containing 10% human AB serum (Omega Scientific, Tarzana, CA) and 200U/mL IL2 (Peprotech, Burlington, Canada). NK effectors were isolated using the Human NK Cell Isolation (negative selection) Kit 130-092-657 (Miltenyi Biotech, San Diego, CA) following the manufacturer's protocol, resulting in >90% purity, and allowed to rest overnight in RPMI 1640 medium containing 10% human AB serum. Human tumor cell lines were used as targets using PBMCs or purified NK cells as effectors, with avelumab or control antibody. A 4-h 111In-release assay was used. Target cells were labeled with 20 μCi 111In-oxyquinoline (GE Healthcare, Silver Spring, MD) at 37°C for 20 minutes, and used as targets at 3000 cells/well in 96-well round-bottom culture plates (28). We used effector cell:target cell (E:T) ratios of 100, 50, 25, and 12.5:1. Assays were performed for 4 hours in RPMI medium (Mediatech, Manassas, VA) supplemented with fetal bovine serum (Gemini Bio-Products, W Sacramento, CA), glutamine and antibiotics (Mediatech). Spontaneous release was determined by incubating target cells with medium alone, and complete lysis by incubation with 0.05% Triton X-100. Specific ADCC lysis was determined using the following equation:
Initial studies were carried out using 40 μg/ml of avelumab. Titration experiments revealed that similar effects could be obtained at 2 μg/ml and with E:T ratios of 25:1. These conditions were employed in subsequent experiments. The avelumab concentration or E:T ratios were also varied if PBMCs or purified NK cells were used as effectors. In experiments indicating IL12 stimulation of NK cells, isolated NK cells were cultured overnight in RPMI 1640 medium containing 10% human AB serum and 10 ng/mL recombinant human IL12 (R&D, Minneapolis, MN). In experiments indicating IFNγ treatment of tumor targets, tumor cell lines were treated with 20 ng/mL recombinant human IFNγ (R&D) for 24 hours prior to their use in the assay. When CD16 neutralization is indicated, the CD16 neutralizing Ab was added at the same time as avelumab.
CTL assay
The MUC-1-specific A24-restricted T-cell line and details for its use in CTL assays has been described previously (29).
FcγRIIIa (CD16) genotyping
DNA was extracted from peripheral blood using the QIAamp DNA Blood Mini kit (Qiagen, CA), and stored at −80°C until use. The polymorphism of CD16 that is a valine (V) versus phenylalanine (F) substitution at amino acid position 158 was determined by performing allele-specific droplet digital polymerase chain reaction (ddPCR) using the TaqMan array for CD16 (rs396991) (Life Technologies, Grand Island, NY) (30). A master reaction mix was prepared, and 1 μl of genotyping DNA was added. The PCR reaction was performed on a BioRad T100 thermal cycler (BioRad, Hercules, CA) for 40 cycles at 95°C for 10 min, 94°C for 30 s, and 60°C for 1 min. The plate was read on a BioRad QX200 droplet reader. Data were analyzed with BioRad QuantaSoft 1.5.
Statistical analyses
Statistical analyses were performed in GraphPad Prism 5. All p values were calculated using a paired Student's t test.
Results
Tumor cell surface expression of PD-L1 determines sensitivity to ADCC mediated by the anti-PD-L1 MAb avelumab
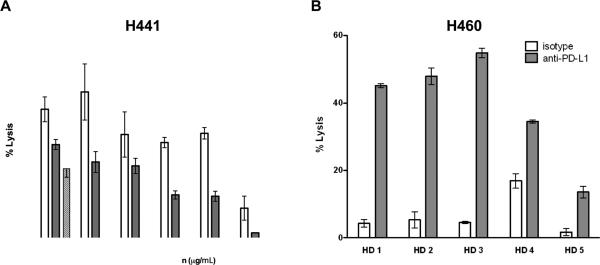
As an antibody of the IgG1 isotype, avelumab was evaluated for the ability to induce ADCC lysis of human tumor cell targets expressing PD-L1. ADCC was evaluated in relationship to the level of PD-L1 surface expression of tumor cells using as effectors PBMCs from several healthy donors and cancer patients. Flow cytometric analysis of a panel of 18 human tumor cell lines encompassing five different tumor types revealed that human carcinoma cell lines express a broad range of PD-L1 % positive cells and PD-L1 cell surface densities (Tables 1 and 2). As seen in Tables 1 and 2, a broad range of PD-L1 mean fluorescence intensities (MFI) was identified among the cell lines that were determined to be 100% positive for PD-L1 expression. This broad range in PD-L1 MFIs suggested the possibility that those cell lines with the highest cell surface expression would be the most sensitive to ADCC mediated by avelumab. To test this hypothesis, we performed in vitro ADCC assays utilizing whole PBMCs as effectors as described in Materials and Methods. The PD-L1-high cell line H441 was sensitive to ADCC mediated by avelumab (Fig. 1A), over a range of antibody concentrations and effector:target ratios, while the PD-L1-low cell line ASPC1 was resistant to lysis (see Table 1). We performed ADCC assays on the PD-L1-high H460 cell line with PBMCs derived from five healthy donors. We found considerable variability among the PBMCs of donors in their capacity to induce ADCC, with or without avelumab, indicating that characteristics intrinsic to effector cells can influence lytic potential independently of target cell characteristics (Fig. 1B). In an additional experiment, PBMC effectors from the same donor were either rested overnight or treated overnight with 200 U/ml of rhIL2. As can be seen in Supplementary Table S1, there was no difference in the avelumab-mediated ADCC lysis between the two treatments.
Table 1.
Human tumor cell lines express variable amounts of PD-L1 and are differentially susceptible to ADCC mediated by anti-PD-L1 MSB0010718C (avelumab) in combination with human PBMC effectors
| Cell line | Tissue type | % positive cells | Normalized MFI | PD-L1 score | ADCC (% lysis) |
|---|---|---|---|---|---|
| MDA-MB-231 | breast | 100 | 72 | 7 | 43.7 |
| A431 | epidermoid | 85 | 28 | 6 | 18.6 |
| ASPC-1 | pancreatic | 18 | 2 | 2 | 0 |
| PANC-1 | pancreatic | 41 | 3 | 3 | 5.7 |
| H441 | lung | 100 | 40 | 6 | 46.4 |
| H226 | lung | 90 | 21 | 5 | 26.9 |
| H157 | lung | 94 | 9 | 5 | 44.4 |
| CaLU1 | lung | 100 | 109 | 8 | 38.1 |
Eight human tumor cell lines were subjected to flow cytometric analysis using anti-PD-L1 MAb (Biolegend, San Diego, CA) to determine the expression of PD-L1. Data are presented as % PD-L1-positive cells and normalized PD-L1 MFI (ratio of PD-L1 MFI to control MFI). Each tumor cell line was also scored on a quartile scale (ranging from 1 to 4) for both % PD-L1-positive cells and PD-L1 MFI. The combined score of % PD-L1-positive cells and MFI (ranging from 2 to 8) was termed the PD-L1 score for the tumor cell line. Each cell line was subjected to an in vitro ADCC assay as described in Materials and Methods with PBMCs as effectors. Data shown are derived from 40 μg/mL avelumab with an effector:target ratio of 100:1. Lysis shown is the mean of triplicate wells. No lysis was observed in the presence of avelumab and absence of PBMCs.
Table 2.
Treatment of human tumor cell lines with IFNγ increases PD-L1 surface expression in all cell lines tested, but increases ADCC sensitivity using anti-PD-L1 avelumab plus PBMCs in only some of the cell lines
| Cell line | Tissue type | % positive cells | Normalized MFI | PD-L1 score | ADCC (% lysis) |
|---|---|---|---|---|---|
| SW900 | lung | 100 | 38 | 6 | 37.1 |
| SW900 + IFNγ | lung | 100 | 71 | 7 | 28 |
| H460 | lung | 93 | 11 | 5 | 26.7 |
| H460 + IFNγ | lung | 100 | 20 | 5 | 24.3 |
| H520 | lung | 63 | 6 | 4 | 3.1 |
| H520 + IFγ | lung | 97 | 15 | 5 | 6.5 |
| COLO205 | colon | 3 | 3 | 2 | 4.7 |
| COLO205 + IFNγ | colon | 82 | 12 | 5 | 18.4 |
| HT29 | colon | 14 | 3 | 2 | 1.6 |
| HT29 + IFNγ | colon | 92 | 18 | 5 | 19.6 |
| SW403 | colon | 2 | 2 | 2 | 1.3 |
| SW403 + IFNγ | colon | 57 | 6 | 4 | 2.7 |
| SW1116 | colon | 24 | 2 | 2 | 4.8 |
| SW1116 + IFNγ | colon | 92 | 13 | 5 | 6.7 |
| HTB1 | bladder | 99 | 12 | 5 | 7.7 |
| HTB1 + IFNγ | bladder | 99 | 31 | 6 | 20.6 |
| HTB4 | bladder | 100 | 17 | 5 | 27.9 |
| HTB4 + IFNγ | bladder | 100 | 53 | 7 | 24.4 |
| HTB5 | bladder | 97 | 9 | 5 | 7.8 |
| HTB5 + IFNγ | bladder | 100 | 15 | 5 | 13.3 |
Ten human tumor cell lines were untreated or treated with 20 ng/mL IFNγ for 24 hours prior to flow cytometry and being used in in vitro ADCC assays as described in Materials and Methods. Data are presented as % PD-L1-positive cells and normalized PD-L1 MFI (ratio of PD-L1 MFI to control MFI). Each tumor cell line was also scored on a quartile scale (ranging from 1 to 4) for both % PD-L1-positive cells and PD-L1 MFI. The combined score of % PD-L1-positive cells and MFI (ranging from 2 to 8) was termed the PD-L1 score for the tumor cell line. All data were analyzed using PBMCs as effectors, an avelumab concentration of 40 μg/mL, and an effector:target ratio of 100:1. Lysis shown is the mean of triplicate wells. No lysis was observed in the presence of avelumab and absence of PBMCs.
Figure 1.
Anti-PD-L1 Ab concentration, effector to target ratio, and donor PBMC effector activity influence ADCC mediated by avelumab. A, Titration of avelumab concentrations and different PBMC:tumor target ratios. In vitro ADCC assay using the PD-L1-high H441 human lung cancer cell line as a target. Data shown are the mean±SD of triplicate wells. B, In vitro ADCC assay using the PD-L1-high H460 human lung cancer cell line as a target and PBMCs from five different healthy donors (HD) as effectors. Data shown are the mean±SD of triplicate wells using 2 μg/mL avelumab or 2 μg/mL of isotype control human IgG1 MAb (see Methods) and an effector:target ratio of 25:1.
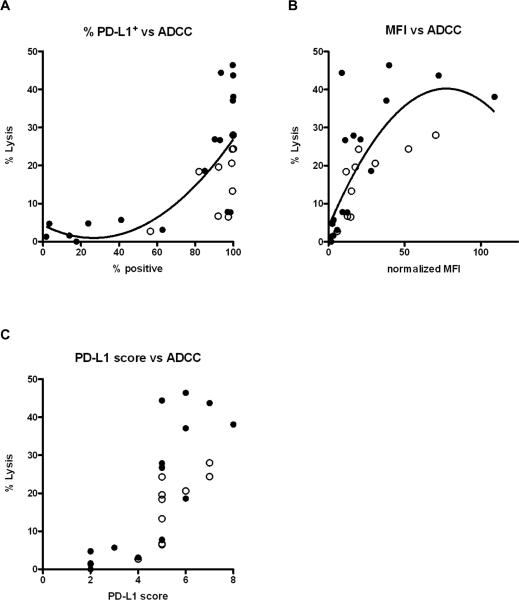
We then performed ADCC assays on the panel of 18 tumor cell lines listed in Tables 1 and 2. While the % PD-L1-positive tumor cells was somewhat predictive of avelumab-mediated ADCC sensitivity, cells lines that were 100% positive for PD-L1 displayed a broad range of ADCC sensitivities (Fig. 2A and Tables 1 and 2). PD-L1 MFI, on the other hand, was a stronger predictor of avelumab-mediated ADCC sensitivity, with high MFI being the greater predictor of ADCC sensitivity (Fig. 2B and Tables 1 and 2). A PD-L1 score was developed to integrate both of these factors (% PD-L1 positive cells and MFI) into a single parameter as described in Materials and Methods. PD-L1 scores of 6 or greater were predictive of ADCC sensitivity, while scores of 4 or less were predictive of little if any lysis (Spearman's rank correlation showed P < 0.0001 and r = 0.82) (Fig. 2C). Taken together, these data demonstrate that avelumab induces ADCC lysis in a broad range of carcinoma types, and that PD-L1 surface expression as both % positive cells and MFI is a predictor of sensitivity to ADCC. This finding has potential implications in scoring biopsies by immunohistochemistry as a potential marker for anti-PD-L1 efficacy in patients.
Figure 2.
PD-L1 MFI is a stronger predictor of sensitivity to ADCC mediated by avelumab as compared to percentage of PD-L1 positive tumor cells. Each dot represents lysis of a different human tumor cell line. A total of 18 human tumor cell lines were evaluated, and 10 of these were assayed both with and without pre-treatment with IFNγ (see Tables 1 and 2). Thus there are a total of 28 cell line targets in each figure. The experiments were carried out using PBMCs from different healthy donors, and all tumor cell lines were evaluated multiple times. Tumor targets treated with IFNγ are denoted by a white circle (O). A, Correlation between % PD-L1-positive tumor cells and % ADCC lysis (p<0.0001, r=0.799). B, Correlation between PD-L1 tumor cell MFI and % ADCC lysis (p<0.0001, r=0.811). C, Correlation between the PD-L1 score and % lysis (p<0.0001, r=0.826). The PD-L1 score was derived by scoring each cell line for % positive cells and normalized MFI on a quartile scale ranging from 1–4. The combined score of % PD-L1-positive cells and MFI, ranging from 2–8, was considered the PD-L1 score for the cell line. All data were analyzed using PBMCs from normal donors as effectors, an avelumab concentration of 40 μg/mL, and an effector:target ratio of 100:1. All correlations show the p value and Spearman's rank correlation coefficient. The best-fit lines were determined using a centered second order polynominal (quadratic) model in GraphPad Prism.
IFNγ treatment of tumor-cell targets increases PD-L1 surface expression, but does not uniformly increase ADCC lysis mediated by avelumab
It has been reported that tumor PD-L1 expression may be driven by IFNγ produced by tumor-infiltrating lymphocytes (31, 32). Ten human tumor cell lines were treated with IFNγ and in vitro ADCC assays were carried out with avelumab and PBMCs as effectors. IFNγ treatment increased PD-L1 MFI in all cell lines tested – as much as 6-fold in some cases (Table 2, p=0.002). As seen in Table 2, however, lysis mediated by avelumab using PBMC effectors was increased in only some tumor cell lines (COLO205, HT29, and HTB1) following IFNγ treatment, but not in several others. These data indicate that an increase in PD-L1 expression upon tumor cell exposure to IFNγ does not always increase tumor-cell sensitivity to the ADCC mechanism. It has also been reported that the transcriptional program induced by IFNγ upregulates MHC Class I expression (33). Since NK cells (the main effector cells in ADCC) sense Class I as a negative costimulatory signal, this represents one possible explanation for IFNγ–mediated protection from NK-cell lysis in some tumor cell lines.
Isolated NK cells are significant effectors of ADCC mediated by avelumab
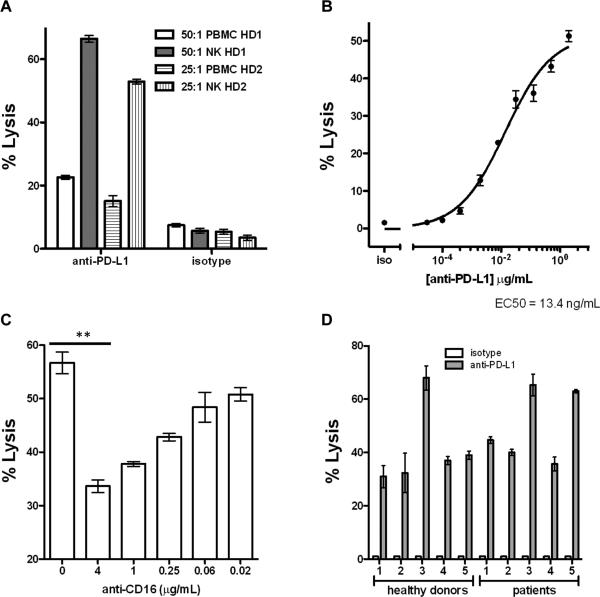
The previous experiments utilized PBMCs as effectors in ADCC assays. Many cells in PBMCs do not possess true effector activity (e.g., B cells, CD4+ cells), thus lowering the actual effector:target ratio in a given assay. We therefore utilized magnetic selection kits to isolate NK cells from healthy donor PBMCs. When we compared isolated NK cells with PBMCs from the same donors in in vitro ADCC assays, we found that purified NK cells produced significantly greater lytic activity with avelumab compared to whole PBMCs (Fig. 3A). To elucidate an avelumab antibody concentration within the physiologic range in patients for use in the NK cell-mediated ADCC assay, we performed antibody titrations on the human tumor cell lines H441, H460, and HBT4 with concentrations ranging from 20 μg/mL to 0.03 ng/mL. The average EC50 for the three tested lines was 6 ng/mL of avelumab, ranging from 1 ng/mL to 13.4 ng/mL (Fig. 3B, as an example, and Supplementary Fig. S1).
Figure 3.
NK cells are efficient effectors of ADCC mediated by avelumab. A, NK cells were purified from PBMCs from two healthy donors (HD) using negative magnetic selection. In vitro ADCC assays were performed using the PD-L1-high H460 human lung cancer cell line as a target, and either whole PBMCs or purified NK cells from the same donor as effectors. Data shown are the mean±SD of triplicate wells using 2 μg/mL avelumab concentration with the designated effector:target ratios. B, Dose-response curve for the H441 human lung cancer cell line using purified NK cells as effectors at an effector:target ratio of 25:1. EC50 = 13.4 ng/mL. Data shown are the mean±SD of triplicate wells. C, In vitro ADCC activity against H441 is reduced by the inclusion of increasing amounts of a CD16 neutralizing Ab. Data shown are the mean±SD of triplicate wells using avelumab at a concentration of 8 ng/mL. D, Purified NK cells from cancer patients mediate ADCC induced by avelumab as effectively as those isolated from healthy donors. Isolated NK cells from five healthy donors and five lung cancer patients were used in in vitro ADCC assays against the H441 human lung cancer cell line. Data shown are the mean±SD of triplicate wells using 20 ng/mL avelumab concentration at an effector:target ratio of 20:1.
In order to evaluate whether avelumab could have any negative effects on immune cells, whole PBMCs from five normal donors were used as targets in ADCC assays employing autologous purified NK cells as effectors with avelumab or isotype control MAb. Effector:target ratios of 25:1, 12.5:1 and 6.25:1 were used for all five donors. No lysis of whole PBMCs above 2.8% was seen for any of the five donors (data not shown).
NK cell-mediated ADCC lysis occurs when CD16 (FcγRIII) on NK effectors interact with the Fc portion of antibodies opsonizing (or bound to) target cells. Effective activation of NK cells to mediate lysis requires significant positive stimulation resulting from the binding and clustering of multiple CD16 receptors (23). To confirm whether CD16 engagement was a critical component of ADCC lysis mediated by avelumab, we isolated NK effectors and used them in an in vitro ADCC assay targeting the PD-L1-high lung cancer cell line H441 in the presence or absence of anti-CD16 antibody. The addition of the CD16 neutralizing antibody significantly inhibited ADCC lysis, demonstrating that CD16 ligation is a major mechanism of action for ADCC lysis mediated by avelumab (Fig. 3C).
NK cells isolated from cancer patients can maintain avelumab-mediated ADCC activity
Cancer patients, particularly those with advanced metastatic disease, can display significant defects in immune system functionality. As fully functional NK cells are a necessary component of an effective ADCC response, poor NK lytic capacity in cancer patients could limit the effectiveness of this therapeutic mechanism of action. We therefore isolated NK cells from five healthy donors and from five NSCLC patients prior to therapy who were enrolled in a Phase I clinical trial at NCI (NCT00923741) (26) and used the isolated NK cells in an in vitro ADCC assay. As seen in Fig. 3D, there was no detectable difference in avelumab-mediated ADCC lysis between NK cells from the five healthy donors and from the five NSCLC patients. These data indicate that patients with advanced NSCLC can potentially maintain avelumab-mediated NK cell ADCC. It should be noted that close to 100% of the H441 human lung carcinoma cell line used in this study expresses HLA-Class I, which most likely explains the low endogenous level of antibody-independent NK lysis.
Differentiating CTL tumor cell lysis from avelumab-mediated ADCC lysis
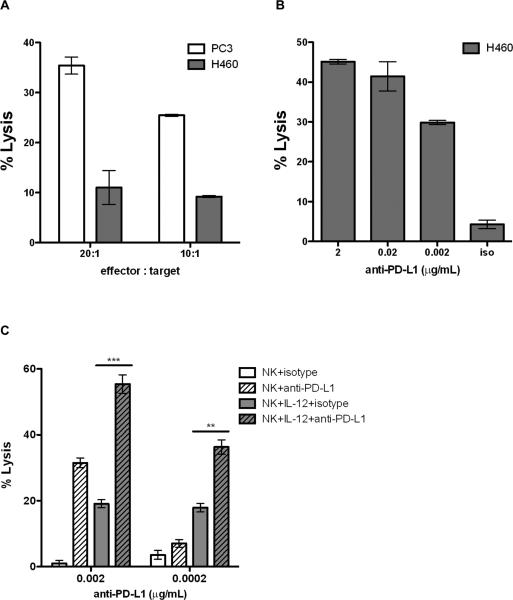
The capacity of tumors to escape CTL lysis via low or no expression of MHC class I suggests that ADCC may be an important mechanism to clinically target these tumors. To test this hypothesis, we investigated ADCC and CTL in the H460 lung cancer cell line, which is a MUC-1+ HLA-A24+ tumor cell line. The H460 lung cancer cell line has previously been shown to express high levels of the transcription factor brachyury, and low levels of MHC class I, and to be resistant to CTL- and drug-mediated lysis (34, 35). As seen in Fig. 4A, despite its MUC-1 positivity, H460 is relatively insensitive to MUC-1–specific A24-restricted CTL lysis. PC3, a MUC-1+ HLA-A24+ prostate cancer cell line, was used as a positive control for CTL and is lysed by the MUC-1–specific T-cell line (Fig. 4A). In spite of resistance to CTL-mediated lysis, H460 is a PD-L1-positive cell line, and it is effectively lysed by purified NK cells in combination with avelumab (Fig. 4B). These data demonstrate that ADCC lysis may be a mechanism to target tumor cells that are resistant to CTL lysis.
Figure 4.
Tumor cells relatively insensitive to CTL-mediated lysis are efficiently lysed via ADCC mediated by avelumab. A, The HLA-A24+, MUC-1+ human tumor cell lines PC3 (prostate) and H460 (lung) were used as targets in in vitro CTL assays with a MUC-1–specific HLA-A24 CD8+ T-cell line as described in Materials and Methods. Effector:target ratios used were 20:1 and 10:1. The mean % CTL lysis ± SD of triplicate assays are shown. While the PC3 line is sensitive to the CTL lysis, the H460 line is not. B, Concurrently, the H460 cell line was used as a target in an in vitro ADCC assay with purified NK cells as effectors. Data shown are for an effector:target ratio of 20:1 at three different concentrations of avelumab. Isotype control Ab was used at 2 μg/mL. Data shown are the mean±SD of triplicate wells. C, Treatment of purified NK effectors with IL12 significantly increases ADCC activity mediated by avelumab. Purified NK cells were treated overnight with 10 ng/mL recombinant human IL12. ADCC activity against the PD-L1+ H460 human lung cancer cell line was determined using control and IL12-treated NK cells as effectors at a 20:1 ratio at the indicated avelumab concentrations. Data shown are the mean±SD of triplicate wells. Results were analyzed using a parametric unpaired t test for the relevant comparisons (**, p<0.01; ***, p<0.001).
IL12 stimulation of NK effectors increases ADCC lysis
IL12 is a pleomorphic cytokine that has previously been demonstrated to increase the activity of multiple immune cell subsets (36, 37). We have recently published on the in vivo efficacy of a tumor-seeking IL12–based therapeutic known as NHS-IL12 (38). This ability to deliver IL12 directly to the tumor, combined with the known relationship between IL12 and NK activity, suggested that combining avelumab with an IL12–based therapy might effectively potentiate ADCC lysis. To test this hypothesis, we isolated NK cells from a healthy donor and treated them overnight with 10 ng/mL recombinant human IL12. Flow cytometric analysis of IL12–stimulated and control NK cells demonstrated that IL12 stimulation increased the CD16+CD56+TIM-3+ NK population from 12% to 52%. This increase in the fully functional NK cell population led to significant increases in avelumab-mediated ADCC lysis of the H460 cell line as compared to unstimulated NK cells (Fig. 4C). This difference was particularly apparent at low avelumab concentrations (0.2 ng/mL) that did not induce lysis in the presence of unstimulated NK cells. In addition, an ADCC assay of the human colorectal carcinoma cell line HT29 showed upregulation of lysis from 4.6% to 18.9% after the NK cells were treated with IL12, but the same magnitude of increased lysis was seen using NK cells treated both with avelumab and with the isotype control (Supplementary Table S2). The human pancreatic carcinoma cell line ASPC-1, which expresses extremely low levels of PD-L1 (18% positive but a normalized MFI of 2), was used as a negative control for ADCC (see Table 1), and displayed no lysis with untreated NK cells and very low lysis (≈10%) with IL12-treated NK cells in combination with either avelumab or isotype control (Supplementary Table S2). Thus, our conclusion is that the increased lysis in these cases is due to increased activity of the NK cells, but not to increased avelumab-mediated ADCC activity. These data provide proof of concept that an IL12–based therapeutic such as NHS-IL12 potentially could be combined with avelumab to enhance the ADCC activity of avelumab.
Healthy donors with the FcγRIIIa-158 V/V genotype display higher avelumab-mediated ADCC lysis of tumor cells than donors with the F/F genotype
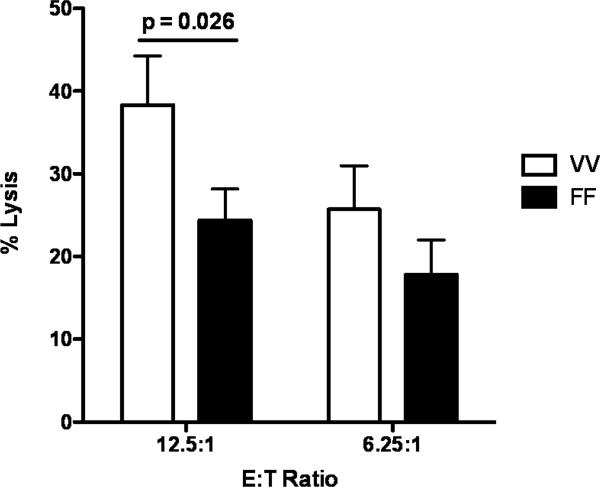
One factor that can contribute to the variability of the levels of cytotoxicity and ADCC mediated by NK cells from different individuals is CD16 polymorphism. The FcγRIIIa-158 V/V genotype has been shown to correlate with the objective response rate and progression-free survival in trastuzumab-based therapy (39). We therefore wanted to investigate if this would be the case for avelumab-mediated ADCC as well. Twenty healthy donors were genotyped for FcγRIIIa-158 polymorphism. Of these, only three displayed the V/V genotype. An ADCC assay was performed with purified NK cells from these three donors and three additional donors with the F/F genotype, and showed higher lysis with NK cells from donors with the V/V genotype (p=0.026) (Fig. 5). Since we were only able to identify three donors with the V/V genotype, these results are preliminary as discussed below.
Figure 5.
Healthy donors with the FcγRIIIa-158 V/V genotype display higher lysis of tumor cells than donors with the F/F genotype. Twenty healthy donors were genotyped for FcγRIIIa-158 polymorphism. An ADCC assay using the H441 human lung cancer cell line as a target was performed with purified NK cells from 3 donors with the V/V genotype and 3 donors with the F/F genotype. Avelumab was used at 0.5 ng/mL, and results are shown at E:T ratios of 12.5:1 and 6.25:1. Data shown are the mean±SD for each group. Results were analyzed using a parametric unpaired t test, p=0.026.
Discussion
ADCC is well established as a major mode of tumor cell killing with NK cells (36, 40) being the major effector cell population. This has been demonstrated in numerous human in vitro systems using purified NK cells as effectors, and in animal models in which the depletion of NK cells leads to the loss of antitumor activity. While such depletion studies cannot be carried out in patients, numerous studies have implicated ADCC as a major mechanism of the antitumor activity of trastuzumab, rituximab, and cetuximab (15-24). It has been shown that the Fcγ receptor on human NK cells is an integral part of the ADCC mechanism. Prior studies (41) have demonstrated that specific polymorphisms in the FcγRIIa and IIIa of NK cells correlate with enhanced clinical outcomes employing trastuzumab, rituximab, as well as cetuximab in patients with Her2+ breast cancer, lymphoma, and KRAS wild-type CRC, respectively. It is currently believed that tumor cell killing of all three of these MAbs is mediated by receptor-ligand signaling interactions, as well as by immune-mediated ADCC. To our knowledge, the other anti-PD-L1 MAbs currently being employed clinically were specifically designed to eliminate any ADCC activity as a precaution against unwanted toxicities. The extensive Phase I study of over 100 patients just completed employing avelumab revealed no toxicity over that observed with other MAbs targeting the PD1/PD-L1 axis (25). Moreover, little, if any, reduction of immune cell populations was observed.
The studies reported here demonstrate (a) the ability of avelumab to lyse a range of human tumor cells, including lung, breast, and bladder carcinomas in the presence of PBMC or NK effectors; (b) IFNγ can enhance both the percent of tumor cells expressing PD-L1 and the PD-L1 MFI, and in some cases, but not all, can enhance MAb-mediated ADCC tumor cell lysis; (c) purified NK cells were more potent mediators of avelumab tumor cell lysis vs PBMCs; (d) similar levels of avelumab-mediated ADCC lysis of tumor cells was seen using effectors (either PBMCs or purified NK cells) from either healthy donors or cancer patients; (e) very low levels of avelumab-mediated lysis were seen using PBMCs as targets; this finding complements results seen in analyses of PBMC subsets of patients receiving avelumab; and (f) the addition of IL12 to NK cells greatly enhanced avelumab-mediated ADCC.
The main objective of the studies reported here was to evaluate the ability of avelumab to mediate ADCC lysis of human tumor targets. The ADCC activity can vary using different donor effector cells, as well as different tumor targets. We have also shown that, in some cases, one can enhance the levels of PD-L1 on tumor cells by the addition of IFNγ, which in turn enhanced ADCC activity mediated by avelumab. Moreover, we sought to demonstrate that exogenous factors such as the addition of IL12 to NK cells can also modify avelumab-mediated ADCC activity. Furthermore, as shown in Fig. 4, the level of MHC class I on tumor cells can also modify avelumab-mediated ADCC activity.
Many factors can contribute to the variability of the levels of cytotoxicity and ADCC mediated by NK cells from different individuals. These factors include CD16 polymorphism. The FcγRIIIa-158 V/V genotype has been shown to be significantly correlated with the objective response rate and progression-free survival in trastuzumab-based therapy (39). As seen in Fig. 5, healthy donors with the FcγRIIIa-158 V/V genotype displayed higher avelumab-induced lysis of tumor cells than donors with the F/F genotype. These results are preliminary, since we were only able to identify three donors with the V/V genotype. More definitive studies will require a larger and more homogeneous cohort of patients to identify a link, if any, between CD16 polymorphism and patient benefit.
Other factors that affect NK ADCC and cytotoxicity include inhibitory NK receptors (KIR). The KIRs include the killer Ig-like receptors (KIR2DL1, KIR2DL2, KIR2DL3, and KIR3DL1), each specific for a different group of HLA class I alleles; the leukocyte Ig-like receptor (LIR-1) that shows a broad HLA class I specificity, and the lectin-like heterodimer CD94/NKG2A that recognizes the HLA class 1b, HLA-E molecules (42). Expression of PD1, as well as the maturation of NK cells, also contributes to the variability of the background killing. This report is focused on the correlation of the expression of PD-L1 on tumor cells and the ability of avelumab to mediate ADCC against these tumor cells. Other factors affecting NK cells are under active investigation. The studies reported here were carried out with avelumab at different concentrations and using effector cells from different healthy donors, but similar ADCC effects were obtained from different donors in separate experiments using an avelumab concentration of 2 μg/ml and E:T ratio of 25:1, as shown in Figs. 1B, 3A and 4B.
While the studies reported here indicate little or no lysis by avelumab when whole PBMCs were used as targets, this does not preclude the possibility that subpopulations of immune cell subsets that express PD-L1 would be subject to some degree of lysis. PD-L1 expression on immune cells, however, has been associated with both immune activation and as a marker of an immunosuppressive cell (43). Moreover, as shown in Tables 1 and 2, the level of PD-L1 expression as percent cells positive or MFI may not always correlate with anti-PD-L1–mediated lysis. The results from larger randomized clinical studies in specific patient cohorts will define any concerns related to toxicity vs. clinical benefits.
The potential exists, however, to enhance the avelumab-mediated ADCC activity and thus clinical benefit of avelumab. Numerous studies have shown that a range of cytokines can enhance NK cell function. Systemic IL2 has long been known to activate NK cells (44). In our in vitro system, pre-treating the PBMC effector cells overnight with IL2 did not increase the avelumab-induced ADCC activity (Supplementary Table S1). It has been shown that IL21 can enhance the in vitro ADCC activity of both trastuzumab and cetuximab (45). Studies are now emerging that IL15 can greatly enhance NK cells in both experimental systems and clinical studies (46, 47). IL12 has been shown in both in vitro and in vivo experimental studies to enhance NK activity and enhance the ADCC activity of cetuximab and rituximab (37, 48-50). A recent preclinical study (38) of a tumor-targeting MAb – IL12 immunocytokine (NHS-IL12) – showed enhanced NK and CD8 T-cell responses leading to a reduction of tumor masses. This IL12 immunocytokine is currently being evaluated in a Phase I trial (NCT01417546). Recent preclinical studies have shown (manuscript in preparation) that the combination of avelumab and the NHS-IL12 immunocytokine led to greater antitumor activity than the use of either alone.
In addition to cytokines, other immunotherapeutics, such as cancer vaccines, can enhance the pro-inflammatory environment at the tumor site. Studies have shown that vaccines can enhance the level of CD8+ T cells at the tumor site, and the additional use of an anti-PD1/PD-L1 MAb could inhibit negative signaling on T cells, leading to enhanced T-cell activity (51, 52). It is also possible that the use of vaccine in combination with an anti-PD-L1 MAb capable of mediating ADCC would potentially lead to enhanced NK-mediated killing at the tumor site. Vaccine-mediated T-cell lysis of tumor cells is mediated by the presence of peptide-MHC complexes on the surface of tumor cells; it is well established that one mechanism of immune escape is that subpopulations of tumor cells have extremely low or are devoid of MHC expression (53). NK lysis of tumor cells is accomplished, however, when tumor cells are deficient in MHC class I (33, 54). These observations further support the potential use of approaches using vaccines or other immunotherapeutics in combination with an anti-PD-L1 MAb capable of mediating ADCC.
Supplementary Material
Acknowledgments
The authors thank Diane J. Poole for her technical assistance. The authors also thank Debra Weingarten for her editorial assistance in the preparation of this manuscript.
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health
Footnotes
Author Contributions
Conception and design: JS, KYT
Development of methodology: BB, CJ, MF, KYT
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): BB, CJ, MF, CRH, JLG, KYT
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): BB, CJ, MF, CRH, JLG, KYT, JS
Writing, review, and/or revision of the manuscript: BB, CJ, CRH, JLG, KYT, JS
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): JS, CJ
Study supervision: JS
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQ, Hwu W-JJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Eng J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–8. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manusow JS, Khoja L, Pesin N, Joshua AM, Mandelcorn ED. Retinal vasculitis and ocular vitreous metastasis following complete response to PD-1 inhibition in a patient with metastatic cutaneous melanoma. J Immunother Cancer. 2014;2:41. doi: 10.1186/s40425-014-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min L, Hodi FS. Anti-PD1 following ipilimumab for mucosal melanoma: durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res. 2014;2:15–8. doi: 10.1158/2326-6066.CIR-13-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–9. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 10.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PDL1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 11.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Eng J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. New Eng J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 2010;70:4481–9. doi: 10.1158/0008-5472.CAN-09-3704. [DOI] [PubMed] [Google Scholar]
- 16.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–75. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123:678–86. doi: 10.1182/blood-2013-08-519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Garff-Tavernier M, Decocq J, de Romeuf C, Parizot C, Dutertre CA, Chapiro E, et al. Analysis of CD16+CD56dim NK cells from CLL patients: evidence supporting a therapeutic strategy with optimized anti-CD20 monoclonal antibodies. Leukemia. 2010;25:101–9. doi: 10.1038/leu.2010.240. [DOI] [PubMed] [Google Scholar]
- 19.Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, et al. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med. 2012;11:307. doi: 10.1186/1479-5876-11-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shuptrine CW, Surana R, Weiner LM. Monoclonal antibodies for the treatment of cancer. Semin Cancer Biol. 2012;22:3–13. doi: 10.1016/j.semcancer.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–72. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veeramani S, Wang S-YY, Dahle C, Blackwell S, Jacobus L, Knutson T, et al. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118:3347–9. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vivier E, Ugolini S, Blaise D, Chabannon C. Targeting natural killer cells and natural killer T cells in cancer. Nature Rev Immunol. 2012;12:239–52. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward E, Mittereder N, Kuta E, Sims GP, Bowen MA, Dall'Acqua W, et al. A glycoengineered anti-CD19 antibody with potent antibody-dependent cellular cytotoxicity activity in vitro and lymphoma growth inhibition in vivo. Br J Haematol. 2011;155:426–37. doi: 10.1111/j.1365-2141.2011.08857.x. [DOI] [PubMed] [Google Scholar]
- 25.Heery CR, O'Sullivan Coyne GH, Madan RA, Schlom J, von Heydebreck A, Cuillerot JM, et al. Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies.. J Clin Oncol; 2014 ASCO Annual Meeting; Chicago, IL. 2014. 2014. p. 5s. abstr 3064); 2014. [Google Scholar]
- 26.Madan RA, Tsang K-Y, Bilusic M, Vergati M, Poole DJ, Jochems C, et al. Effect of talactoferrin alfa on the immune system in adults with non-small cell lung cancer. Oncologist. 2013;18:821–2. doi: 10.1634/theoncologist.2013-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan LY, Yam EKF, Choo ABH. Normalized median fluorescence: an alternative flow cytometry analysis method for tracking human embryonic stem cell states during differentiation. Tissue Eng Part C Methods. 2013;19:156–65. doi: 10.1089/ten.TEC.2012.0150. [DOI] [PubMed] [Google Scholar]
- 28.Qi CF, Nieroda C, De Filippi R, Greiner JW, Correale P, Schlom J, et al. Macrophage colony-stimulating factor enhancement of antibody-dependent cellular cytotoxicity against human colon carcinoma cells. Immunol Lett. 1995;47:15–24. doi: 10.1016/0165-2478(95)00054-9. [DOI] [PubMed] [Google Scholar]
- 29.Jochems C, Tucker JA, Vergati M, Boyerinas B, Gulley JL, Schlom J, et al. Identification and characterization of agonist epitopes of the MUC1-C oncoprotein. Cancer Immunol Immunother. 2014;63:161–74. doi: 10.1007/s00262-013-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–97. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–82. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 32.Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PloS One. 2013;9:e88557. doi: 10.1371/journal.pone.0088557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devaiah BN, Singer DS. CIITA and Its dual roles in MHC gene transcription. Front Immunol. Dec 20. 2012;4:476. doi: 10.3389/fimmu.2013.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roselli M, Fernando RI, Guadagni F, Spila A, Alessandroni J, Palmirotta R, et al. Brachyury, a driver of the epithelial-mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res. 2012;18:3868–79. doi: 10.1158/1078-0432.CCR-11-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton DH, Huang B, Fernando RI, Tsang K-Y, Palena C. WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition. Cancer Res. 2014;74:2510–9. doi: 10.1158/0008-5472.CAN-13-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf SF, Sieburth D, Sypek J. Interleukin 12: a key modulator of immune function. Stem Cells. 1994;12:154–68. doi: 10.1002/stem.5530120203. [DOI] [PubMed] [Google Scholar]
- 38.Fallon J, Tighe R, Kradjian G, Guzman W, Bernhardt A, Neuteboom B, et al. The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget. 2014;5:1869–84. doi: 10.18632/oncotarget.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 40.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol. 2012;6:1. doi: 10.1186/1756-8722-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 43.Heeren AM, Koster BD, Samuels S, Ferns DM, Chondronasiou D, Kenter GG, et al. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical cancer. Cancer Immunol Res. 2015;3:48–58. doi: 10.1158/2326-6066.CIR-14-0149. [DOI] [PubMed] [Google Scholar]
- 44.Koehn TA, Trimble LL, Alderson KL, Erbe AK, McDowell KA, Grzywacz B, et al. Increasing the clinical efficacy of NK and antibody-mediated cancer immunotherapy: potential predictors of successful clinical outcome based on observations in high-risk neuroblastoma. Front Pharmacol. 2012;3:91. doi: 10.3389/fphar.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe M, Kono K, Kawaguchi Y, Mizukami Y, Mimura K, Maruyama T, et al. Interleukin-21 can efficiently restore impaired antibody-dependent cell-mediated cytotoxicity in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2010;102:520–9. doi: 10.1038/sj.bjc.6605502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waldmann TA. Interleukin-15 in the treatment of cancer. Expert Rev Clin Immunol. 2014;10:1689–701. doi: 10.1586/1744666X.2014.973856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waldmann TA. The shared and contrasting roles of IL2 and IL15 on the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res. 2015;3:219–27. doi: 10.1158/2326-6066.CIR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ansell SM, Geyer SM, Maurer MJ, Kurtin PJ, Micallef INM, Stella P, et al. Randomized phase II study of interleukin-12 in combination with rituximab in previously treated non-Hodgkin's lymphoma patients. Clin Cancer Res. 2006;12:6056–63. doi: 10.1158/1078-0432.CCR-06-1245. [DOI] [PubMed] [Google Scholar]
- 50.Luedke E, Jaime-Ramirez AC, Bhave N, Roda J, Choudhary MM, Kumar B, et al. Cetuximab therapy in head and neck cancer: immune modulation with interleukin-12 and other natural killer cell-activating cytokines. Surgery. 2012;152:431–40. doi: 10.1016/j.surg.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh LF, et al. Safety, correlative markers and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. 2015;21:712–20. doi: 10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, et al. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 2014;74:2974–85. doi: 10.1158/0008-5472.CAN-13-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrone S, Whiteside TL. Tumor microenvironment and immune escape. Surg Oncol Clin N Am. 2007;16:755–74, viii. doi: 10.1016/j.soc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Mentlik James A, Cohen AD, Campbell KS. Combination immune therapies to enhance anti-tumor responses by NK cells (Article 481). Front Immunol. 2013;4:1–12. doi: 10.3389/fimmu.2013.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.