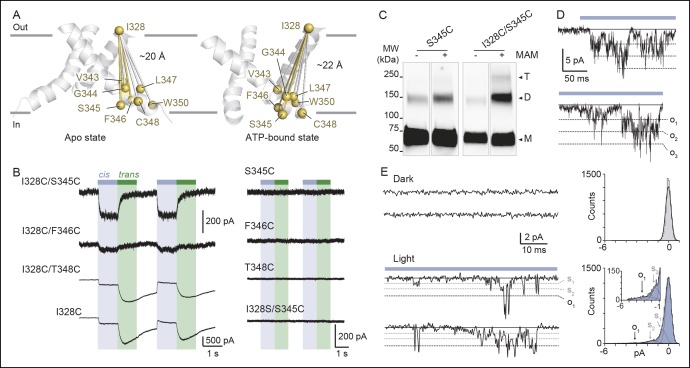
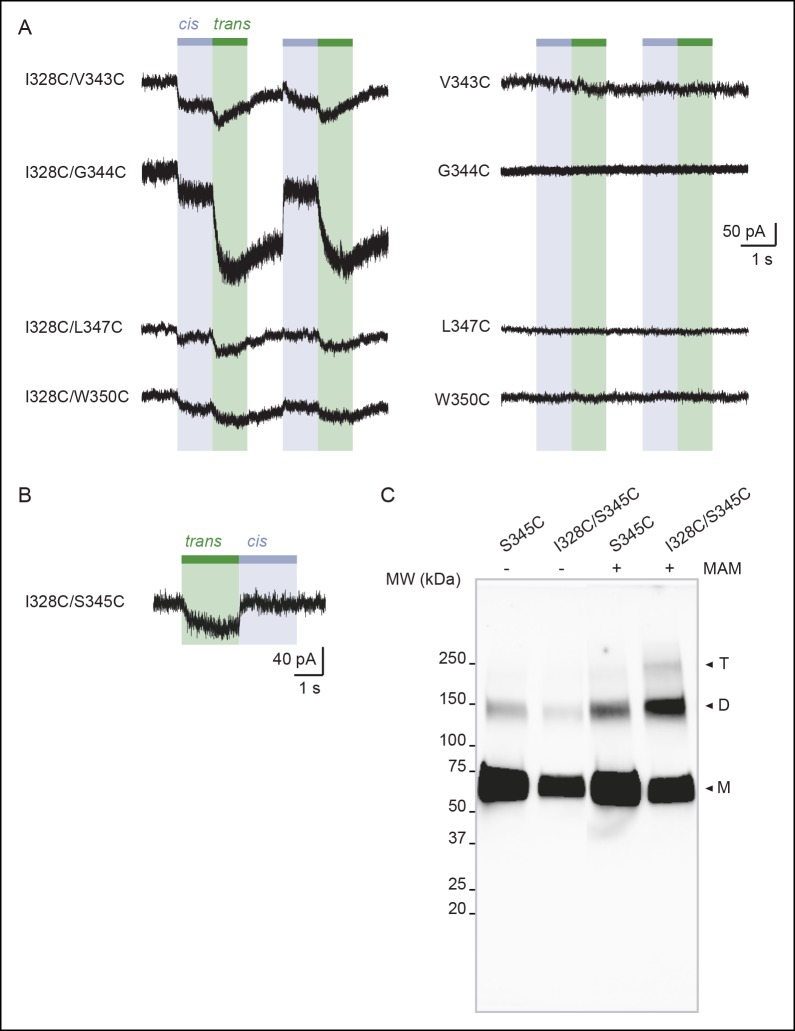
Figure 3. Shortening of the vertical distance separating adjacent TM2 ends drives channel openings.
(A) Side views of TM2 helices of a P2X2 homology model in the apo state (left) and ATP-bound state (right). The β-atoms of residues selected for cysteine substitutions are shown as yellow spheres. Indicated values are the average distances separating pairwise β-atoms from two adjacent TM2 helices (grey bridges). Highlighted bridges between residues indicate actual MAM cross-linking. For clarity, TM1 helices are omitted. (B) Whole-cell currents evoked by light at 365 nm (cis) or 525 nm (trans) in HEK cells expressing the indicated cysteine-substituted mutants treated with MAM. (C) Western blot analysis of cell-surface cross-linking of the indicated mutated subunits expressed in TSA-201 cells after treatment (+) or without treatment (-) with MAM. Monomer (M), dimer (D), and trimer (T) are indicated. Uncut gel image is shown in Figure 3—figure supplement 1C. MW, Molecular weight. (D) Single-channel currents recorded from an outside-out patch expressing the I328C/S345C mutant at -120 mV in response to 365 nm illumination. Three simultaneous openings (O) indicated by dashed black lines were detected. Black lines indicate closed channels. (E) Unitary currents (left) and corresponding all-points histograms (right) recorded before (upper) and during 365 nm illumination (lower) from the same patch expressing the I328C/S345C mutant. Sublevel openings (S1 and S2) are indicated by dotted gray lines. Inset shows expanded scale. All-points histograms were fitted to one Gaussian (upper) or to the sum of four Gaussians (lower).