Figure 3. Interactions through N- and C-terminal flexible regions mediate PepMV assembly.
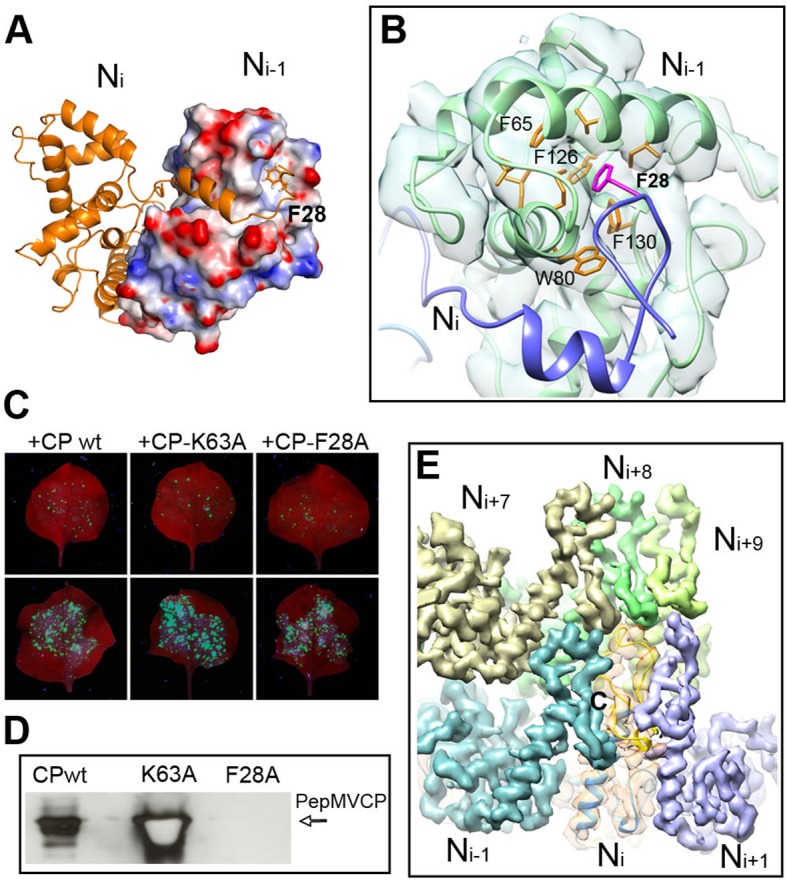
(A) Ni subunit links to a hydrophobic groove in the Ni-1 subunit via the N-terminal arm. (B) In the Ni-1 subunit a pocket of hydrophobic residues allocates F28 from the Ni adjacent subunit. (C) Trans-complementation assays show that F28A mutant allows for cell-to-cell movement. (D) The analysis by Western blot of virion preparations show no signal for fully assembled virions in the trans-complementation with F28A mutant. PepMVCP mutant K63A is a positive control. (E) Six segmented densities for PepMVCP are seen from the inner side of the virion. The subunit Ni is depicted semi-transparent and includes a ribbon representation for the atomic model for PepMV CP. PepMV CP, Pepino mosaic virus coat protein.
