Abstract
Epidemiological investigations regarding temperature influence on human health have focused on mortality rather than morbidity. In addition, most information comes from developed countries despite the increasing evidence that climate change will have devastating impacts on disadvantaged populations living in developing countries. In the present study, we assessed the impact of daily temperature range on upper and lower respiratory infections in Cordoba, Argentina, and explored the effect modification of socio-economic factors and influence of airborne particles We found that temperature range is a strong risk factor for admissions due to both upper and lower respiratory infections, particularly in elderly individuals, and that these effects are more pronounced in sub-populations with low education level or in poor living conditions. These results indicate that socio-economic factors are strong modifiers of the association between temperature variability and respiratory morbidity, thus they should be considered in risk assessments.
Keywords: daily temperature range, respiratory infections, socio-economic conditions, education, morbidity
Introduction
Many studies have investigated the effects of extreme temperatures and mortality (McMichael et al., 2008; Ostro et al., 2009; Zanobetti et al., 2013); however, a limited number of investigations have evaluated the effect of extreme temperatures on morbidity. Moreover, the findings of studies on the association of cardiovascular or respiratory morbidity with temperature have been conflicting. For example, in some studies high temperatures increased hospital admissions for cardiovascular and respiratory disorders (Lin et al., 2009). Whereas, in another study a strong effect was observed for respiratory diseases, but no effect was found for cardiovascular diseases (Michelozzi et al., 2009). In contrast, other studies reported no association for cardiovascular diseases and only a weak association with respiratory diseases (Kovats et al., 2004, Linares and Diaz, 2008).
Several temperature exposure metrics have been studied as risk factors for respiratory diseases including daily mean, maximum, minimum, equivalent or apparent temperature. Recent studies suggest that the frequency of extreme heat waves will increase in a warmer climate, resulting in greater variability in surface temperatures (Anderson, 2011; Ganguly et al., 2009; Meehl and Tebaldi, 2004), therefore suggesting that temperature variability is an appropriate indicator of weather conditions and climate change, providing even more information than mean temperature alone. This is because trends in mean surface temperature can be due to changes in either maximum or minimum temperature, or relative changes in both (Braganza el at., 2004). However, few studies have examined the potential health effects associated to temperature variability. In many Asian countries, large changes in daily mean temperature were associated with mortality and with emergency admissions (Liang et al., 2009; Lim et al., 2012, Wang et al., 2013). In a prospective cohort study of older men, elevated mean temperature and increased temperature variability were associated with longer QTc, a cardiovascular marker of ventricular repolarization (Mehta et al., 2014). Therefore, in the present paper we examined the effects of daily temperature range (DTR), defined as the difference between maximum and minimum temperature within 1 day. It is highly likely that temperature does not act alone in determining health, but rather in concert with other weather parameters, anthropogenic air pollutants, and aerosols of biological origin. Indeed, there is growing recognition that bio-aerosols may contribute to health effects related to ambient PM exposures partly through their own direct toxic effects and/or in combination with other PM that carries biologically-derived materials which may elicit unfavorable effects (Rosa et al., 2008).
In recent years, epidemiological studies have focused on developing countries due to the increasing evidence that climate change will affect more deeply the health of the most disadvantaged populations (Bell et al., 2008). Urban agglomerations from medium and low-income countries represent particularly risky environments due to socio-economic conditions (in addition to weather variables and pollution) and contribute to population vulnerability. Among other factors, nutritional status, water quality and availability, air conditioning use, access to health care systems, cost of living, and education have been reported as modifiers of the temperature-related morbidity (Jaffe et al., 2003, Ruijsbroek et al., 2011). Moreover, low-income countries have the highest rate of urban growth, which further underscores the need to understand better, how these populations respond to extreme weather conditions. Although living conditions in developing countries are recognized as strong modifiers of individual vulnerability to the occurrence of extreme events, the influence of these socio-economic factors on the temperature-related morbidity has been scarcely studied. There have only been a few studies performed in Argentina regarding morbidity, and they only considered the influence of weather variables on general hospital emergency consults (Rusticucci et al., 2002), asthma (Hoffman et al., 1983) or cardiovascular hospitalizations (Piccolo et al., 1988), disregarding the influence of air pollution exposures.
In a previous study, we found that low education levels increased the risk of respiratory infections in children from Cordoba, Argentina (Amarillo and Carreras, 2012). In the present study, we assessed the impact of DTR on population morbidity in Cordoba, a medium-sized city in Argentina, while controlling for several variables. We further explored the effect modification of socio-economic vulnerability indicators on DTR-related respiratory diseases. We decided to assess respiratory infections as a health outcome, since it has already been established that in Cordoba respiratory morbidity is responsible for 21–25 % of total hospital admissions each year (Coarasa et al., 2010).
Methods
Study area
The City of Cordoba is located in the center of Argentina (31°24′S, 64°11′W) at an altitude of approximately 400 m above sea level. It has a population of 1.4 million and an irregular topography. Its general structure is funnel-shaped, with an increasing positive slope from the center towards the surrounding areas. This somewhat concave formation reduces the air circulation and causes frequent thermal inversions during the autumn and winter seasons. The climate is sub-humid, with an average annual rainfall of 790 mm, concentrated mainly in summer. The mean annual temperature is 17.4°C and the prevailing winds come from the NE, S, and SE.
The main air pollution source in Cordoba City is traffic (Stein and Toselli, 1996). As expected, primary-pollutant concentrations, such as CO, NOx and in part PM10, are strongly correlated with traffic density (Olcese and Toselli, 2002). Cordoba City also has important stationary sources, including metallurgic and mechanical industries that are located in surrounding areas.
Study population and health outcomes
Health data were obtained from the City of Cordoba public and medical care system, comprising 95 health care centers distributed throughout the urban and suburban areas of the City. The information included the health center location as well as the patient age, gender, and principal diagnosis of admission. Because personal identifiers were not available for individual patients, readmissions could not be identified and removed; as a result, all admissions were included and were considered independent for the purpose of our analysis. Informed consent was not necessary according to current Argentinian ethical guidelines.
Although respiratory diseases are a major cause of morbidity worldwide, the rate of lower respiratory infections is higher in developing countries (Rosa et al., 2008). Our study population included all patients diagnosed with upper or lower respiratory infections from January 1, 2005 to December 31, 2011. Respiratory infections were classified according to the International Classification of Diseases, 9th Revision (ICD-9 code) and diagnosed as otitis (H65 and H66), pharyngitis (J02), sinusitis (J01) and laryngitis (J04), which were grouped as upper respiratory tract infections (URI) and bronchitis (J20 and J21) and pneumonia (J18), which were categorized as lower respiratory tract infections (LRI).
We used the most updated socio-economic data available for Cordoba City, recorded in the socio-demographic Census of 2008 (DGEC, 2008). During this census, home residents were asked to complete a survey to assess their socio-economic status, which was then grouped and saved at neighborhood level. Therefore for the present study, we extracted the socio-economical characteristics regarding adult education and family overall living conditions assigned to each neighborhood, and used them to characterize the health centers according to their geographical location. Ruijsbroek et al. (2011) proposed that the highest education level obtained by the adults at home is a good indicator of the family socio-economic status. This indicator was divided into four categories: percentages of adults with no formal education, percentages of adults who completed elementary school, secondary education, or with a university degree. To study family-level living conditions, we assessed the following characteristics: “overcrowded” defined as percentage of residences with more than three people living in the same room; general “living conditions” as percentage of hovels or cabins, and; “housing conditions” as percentage of residences without toilet.
Meteorological data
Weather data were obtained from the meteorological station of the National Meteorological Service located at the Cordoba Airport, 9.5 km north from the city center (−31.31° S, −64.21° W, altitude 484 masl). This meteorological station is considered as a source of the most reliable data. For the present study, we calculated daily temperature range (DTR) and considered the influence of relative humidity (RH), atmospheric pressure (P) wind speed (W), and rain (R).
In other Latin American cities, the most robust association between air pollutants and morbidity have been observed for ambient particulate matter (PM) (Martins et al., 2004). Considering that PM is one of the main pollutants in Cordoba City, which is influenced by temperature; we included PM in the model in order to separate the effects of both ambient temperature and PM. We collected PM10 mass data for the period August 2011 through January 2013 in the City of Cordoba on glass fiber filters using a medium volume sampler with a flow rate of 0.2 m3 min−1. The concentration of particles was determined by differences in the filter weights before and after the 24 h exposures divided by the filtered air volume. Since there was no information on PM10 concentration for the period 2005–2010, we used the data collected during the period August 2011 through January 2013 to develop a PM10 prediction model. Subsequently, this model was employed to predict PM10 levels for the entire study period. Toward this end, we used visibility, relative humidity, and wind speed as main predictors while controlling for day of the week, season and long-term trend. We cross validated the PM10 prediction model by randomly selecting 10% of the observed data and compared them to those predicted by the model using the remaining 90% of the data. The cross validation correlation coefficient was R=0.65.
Statistical analysis
We applied a time series analysis to estimate the association between daily DTR and daily counts of upper and lower respiratory diseases. The data were analyzed using Generalized Additive Models (GAM) with a quasi-Poisson distribution link function. To control for seasonality we fitted a cubic penalized spline of date with equally spaced knots, with four degrees of freedom per year. We used a dummy variable for weekday, to capture day of the week effects, i.e., hospital admissions showed a minimum on Sundays because during weekends only a few health care centers remain open. Overall, we controlled for holidays, day of the week, seasonality and long-term trends in the time-series analysis.
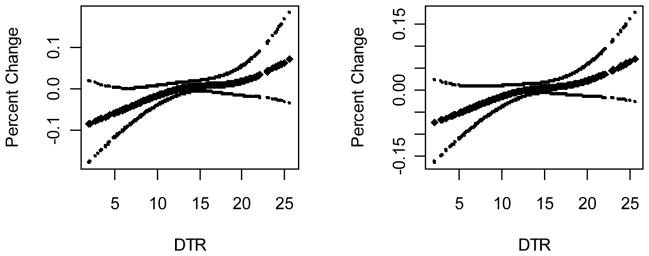
First, we explored graphically the relationship between daily temperature range (DTR) and respiratory morbidity, allowing for a non-linear relationship using a penalized spline (Fig 1). Since we observed that the relationship between DTR and the outcomes was close to linear, we included this parameter in the model as a linear term, which facilitates the reporting of the effects.
Figure 1.
Plots of the smoothing function of daily temperature range with 95% CIs for upper respiratory diseases (left panel) and lower respiratory diseases (right panel).
As primary analysis, we run the model only with DTR as main predictor on respiratory diseases. Other meteorological variables were sequentially entered in the model and were included if they were significant predictors or confounders of the relationship between DTR and respiratory infections. Thus, we controlled for same day relative humidity because high humidity together with high temperature adds to discomfort and heat stress. We also controlled for the linear same-day PM10 concentration since it contributes significantly to respiratory infections admissions (Amarillo and Carreras, 2012) and is one of the main urban pollutants in Cordoba City. After testing with a penalized spline whether the relationship with the outcome was non-linear using a penalized spline, both parameters, relative humidity and PM10, were included into the model as linear terms. The analyses were performed using statistical software R 2.12.1.
The temperature effects on respiratory morbidity have been observed over a lag period of up to 10 days (Halonen et al., 2010). Thus, the number of admissions on a given day depends on the same day effect of that day’s temperature, but also on the effect of the lagged temperature, that is the previous day’s temperature, two previous days, etc. We first checked the lagged effect (from lag 0 to lag 10) of DTR using an unconstrained distributed lag model by including all the 10 lags of DTR in the same model; based on this result we then selected the lag with the strongest effect to perform the sensitivity analysis. We also considered the possibility that moving averages over a period longer than one day may be better predictors of the association between DTR and respiratory morbidity. Therefore, we computed the moving averages as averages of the exposure lags. For example, the 2-day moving average (lag 0–1) was computed as the mean of the same and previous days; the 3-day moving average (lag 0–2) included lag 0, 1, and 2, and so on, up to the 10-day moving average.
For secondary analysis, we ran the same base model (same day DTR as main predictor adjusted for same day RH and PM; all variables included as linear terms) stratified by season, age group and socio-economic conditions. Seasonal patterns have often been observed in respiratory diseases, mainly for upper respiratory infections (Bell et al., 2008; Basu, 2009; Makinen et al., 2009; Du Prel et al., 2009). Therefore, we performed a stratified analysis considering a warm period from October to April and a cold period, from May to September. Similarly, we stratified the data by age groups, since it has been shown that age modifies the relationship between temperature and health. We considered four age categories: young children (<6 years old); school aged children (≥6 to ≤18 years old); adults (≥19 to ≤59 years old), and; elderly (≥60 years old). Finally, because socio-economic status has a strong influence on the individual susceptibility to weather events (Stafoggia et al., 2008; Zanobetti et al., 2009; Zanobetti et al., 2013) we examined whether cases with different socio economic status had different respiratory morbidity rates. Effect estimates are reported as percent change in LRI or URI for a 10°C increase in DTR.
Results
As presented above, the associations between DTR and occurrence of respiratory tract infections were studied among the population attending health care centers in Cordoba during the period January 2005 through December 2011. Daily means of meteorological variables and PM10 exposures during the study period are shown in Table 1. In Cordoba City, seasons are markedly different: while winters are dry, cold and windy, particularly in July and August, summers are very hot, humid and temperatures higher than 30 °C are common from November to February.
Table 1.
Seasonal meteorological conditions during the sampling period.
| Warm | Cold | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | |
| Mean T (°C) | 21.2 | 3.7 | 7.6 | 31.6 | 12.7 | 4.3 | −0.1 | 28.1 |
| Maximum T (°C) | 27.9 | 4.6 | 12.1 | 42.4 | 20.1 | 5.5 | 1.2 | 38.0 |
| Minimum T (°C) | 15.3 | 3.9 | 1.0 | 24.5 | 6.2 | 4.4 | −5.5 | 18.8 |
| DTR (°C) | 12.4 | 3.9 | 2.3 | 24.6 | 13.8 | 4.9 | 1.8 | 25.6 |
| Relative Humidity (%) | 63.1 | 15.2 | 19.4 | 96.8 | 55.9 | 16.6 | 15.6 | 96.6 |
| Atmospheric Pressure (hP) | 958.2 | 4.3 | 944.1 | 976.8 | 961.9 | 5.9 | 946.0 | 982.6 |
| Wind (km/h) | 13.5 | 4.8 | 4.5 | 32.5 | 16.3 | 5.2 | 2.8 | 34.9 |
| Rain (mm) | 3.1 | 10.1 | 0.0 | 149.0 | 0.4 | 2.1 | 0.0 | 25.0 |
| PM10 (μg/m3) | 34.4 | 5.1 | 2.3 | 47.2 | 36.0 | 4.9 | 14.1 | 48.4 |
During the study period, 867,617 patients that attended the health care centers due to URI or LRI were included in the analysis. A detailed description of the number of cases corresponding to each disease, their distribution by period and age as well as the socioeconomic characteristics of the population is presented in Table 2. URI showed no difference in the number of admission between the warm and cold periods, while more LRI cases were observed during the cold period. The frequency of admissions due to URI was high in young and school-aged children, while LRI were more frequent only in young children. Regarding the level of education attained by adults, most of them finished elementary school and only a small proportion had no education at all. Finally, regarding living conditions, most families experienced overcrowding and only a very small percentage had serious sanitary problems.
Table 2.
Descriptive statistics of the individual characteristics of the study subjects.
| URI | LRI | ||||
|---|---|---|---|---|---|
|
| |||||
| n subjects | % | n subjects | % | ||
| Diseases | |||||
| Otitis | 64,253 | 7.4 | Pneumonia | 18,567 | 2.1 |
| Pharynx | 358,155 | 41.4 | Bronchitis | 321,307 | 37.2 |
| Larynx | 56,425 | 6.5 | |||
| Sinusitis | 45,513 | 5.3 | |||
|
| |||||
| Period | |||||
| Warm | 258,707 | 29.9 | 126,963 | 14.7 | |
| Cold | 265,639 | 30.7 | 212,911 | 24.6 | |
|
| |||||
| Age | |||||
| Children | 190,242 | 22. | 217,610 | 25.2 | |
| Youth | 180,755 | 20.9 | 62,916 | 7.3 | |
| Adult | 140,611 | 16.3 | 47,562 | 5.5 | |
| Elderly | 12,738 | 1.5 | 11,786 | 1.4 | |
|
| |||||
| Educationa | |||||
| None | 3,999 | 1.9 | |||
| Elementary | 86,122 | 40.5 | |||
| Secondary | 75,656 | 35.6 | |||
| University | 46,920 | 22.1 | |||
|
| |||||
| Socio-economicb | |||||
| Overcrowded | 101,982 | 7.8 | |||
| Living conditions | 19,578 | 1.5 | |||
| Housing conditions | 11,545 | 0.9 | |||
adults (> 18 years old) at home that completed each level
proportion of homes with unfavorable conditions
Table 3 shows the increased risk in upper and lower respiratory infections for a 10°C increase in DTR with and without adjustments for other weather variables and PM10. A 10°C-increase in DTR was related to a 6.2% (95% CI: 2.8, 9.6) increase in risk for URI admissions and a 4.4% (95% CI: 0.9, 8.0) increase in risk for LRI admissions. Relative humidity had a protective effect on both types of respiratory infections and when combined with DTR in the model, the risk of infections decreased to half. Exposure to PM10 was also a significant predictor so it was included in the regression model as an indicator of air pollution levels.
Table 3.
Percent increase (and 95% CI) in admissions due to upper (URI) and lower (LRI) respiratory infections associated with a 10 °C increase in DTR, a 10% increase in RH or a 10 μg/m3 increase in PM10
| URI | LRI | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| % change | 95% CI | % change | 95% CI | |||
| DTR a | 6.2 | 2.8 | 9.6 | 4.4 | 0.9 | 8.0 |
| RH a | −2.6 | −3.6 | −1.7 | −1.6 | −2.7 | −0.6 |
| PM10 a | 6.0 | 1.9 | 11.5 | 5.8 | 1.3 | 11.9 |
| DTR adjusted for RH b | 2.9 | 0.8 | 6.8 | 2.9 | 1.0 | 6.9 |
| DTR adjusted for RH and PM10 c | 2.9 | 0.8 | 6.7 | 2.9 | 1.0 | 6.9 |
All parameters were included as linear terms, with lag 0.
single predictor models
with adjustments for RH and PM10
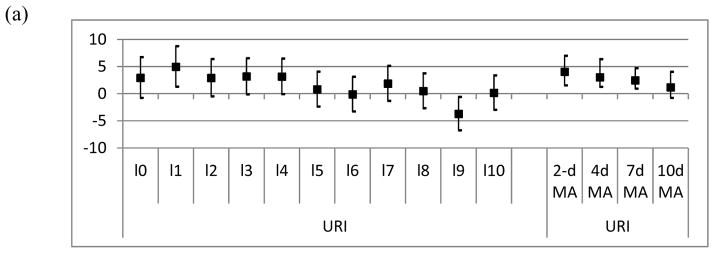
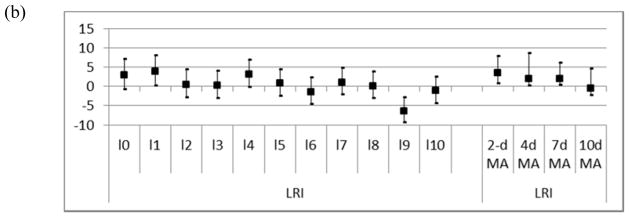
To account for DTR lagged effects on respiratory infections we explored the relationship up to 10 days prior the event using an unconstrained distributed lag model. In addition, we examined whether DTR has a cumulative effect using DTR moving averages as main predictors. Figures 2a and b show the percent increase in admissions for upper and lower respiratory diseases for a 10°C increase in DTR for the 10 lag days included in the unconstrained distributed lag model and for the moving averages of 2, 4, 7, and 10 days. When looking at the 10 day lags, URIs and LRIs showed the strongest association on the day before the event. For averages of exposure, the strongest associations were observed for the average of the same and previous day of exposure (URIs 4.1%, CI: 1.6, 7.0 and LRIs 3.5%, CI: 0.6, 7.8) and declined with averages including longer time periods.
Figure 2.
Percent increase in risk of morbidity due to upper respiratory infections (a) and lower respiratory infections (b), for individual day lags (lag0–lag10) from an unconstrained distributed lag model and for moving averages (2dMA–10dMA). The squares represent the central estimate and the vertical lines the 95 % CI. (Model adjusted for same day RH and PM)
Previous studies demonstrated that the number of admissions due to URIs and LRIs varied significantly with seasons (Amarillo and Carreras, 2012). Therefore, we examined the effect of same day DTR separately on warm and cold periods (Figure 3). The risk for both upper and lower respiratory infections was higher during warm season, with a 5.2% (CI: 1.0, 11.7) and 7.3% (CI: 0.5, 14.4) increase in risk for admissions, respectively.
Figure 3.
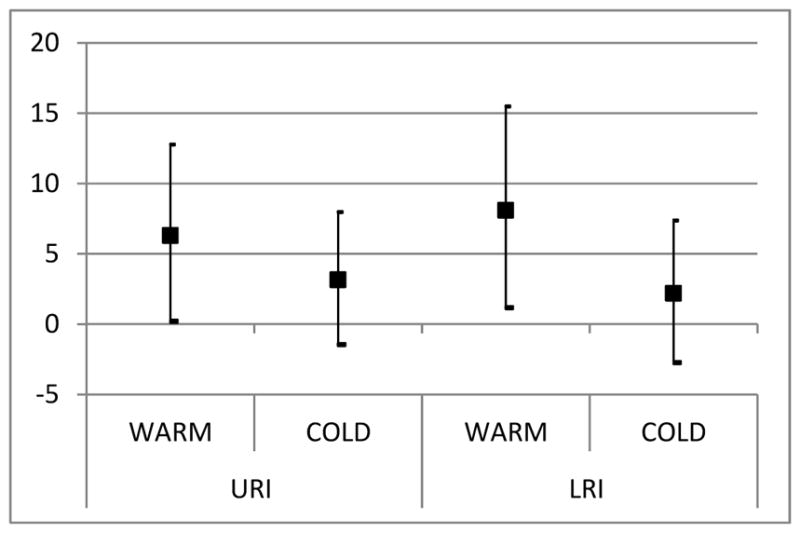
Percent increase in admissions for upper and lower respiratory infections for a 10°C increase in DTR by seasons. (Model adjusted for same day RH and PM, for all age groups)
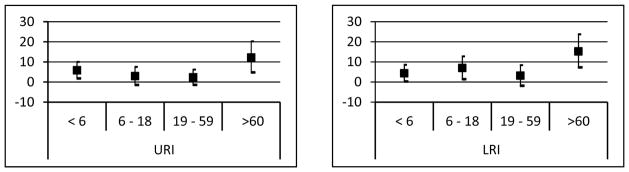
We further examined the association between same day DTR and respiratory diseases by age group (Figure 4). We found the highest increase in elderly subjects aged 60 and over with an 11.4% (95% CI: 4.1, 19.3) increase in URI and a 14.2% (95% CI: 6.3, 22.6) increase in LRI for a 10°C increase in temperature range. Children under 6 years old were significantly affected by DTR, with a 5.2% (95% CI: 1.2, 9.3) increase in URIs admissions, while school aged children were significantly affected by DTR with a 5.9% (CI: 0.4, 11.6) increase in LRIs.
Figure 4.
Percent increase in admissions for upper respiratory infections (left) and lower respiratory infections (right) for a 10°C increase in same day DTR by age groups. (Model adjusted for same day RH and PM)
Table 4 compares morbidity risk for upper and lower respiratory infections for a 10°C increase in DTR by education attainment and socio-economic conditions of the population for all ages. For education level, the strongest effect on upper and lower respiratory infections was observed in the health centers whit higher proportion of people with no formal education; the associations decrease with the increase in the education level of population, but a positive association was still present in those centers with high proportion of people with university degrees. On the other hand, each of the socio-economic vulnerability indicators was associated with a risk increase over a range of 2 to 7%.
Table 4.
Percent increase in risk of upper (URI) and lower (LRI) respiratory infections (95% CI) for every 10 °C increase in same day DTR, by education and socio-economic vulnerability indicators of the population. (Models adjusted for same day RH and PM10, for all age groups)
| URI | LRI | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| % change | 95% CI | % change | 95% CI | |||
| Education | ||||||
| None | 4.1 | 3.8 | 4.7 | 4.4 | 4.2 | 4.7 |
| Elementary | 3.4 | 3.0 | 3.9 | 3.4 | 3.2 | 4.0 |
| Secondary | 2.5 | 2.4 | 2.8 | 3.3 | 3.0 | 3.7 |
| University | 2.0 | 1.8 | 2.2 | 2.0 | 1.7 | 2.2 |
| Socio-economica | ||||||
| Overcrowded | 1.9 | 1.8 | 2.0 | 2.8 | 2.7 | 2.9 |
| Living conditions | 2.9 | 2.6 | 3.3 | 0.3 | −0.1 | 0.7 |
| Housing conditions | 8.1 | 7.5 | 8.7 | 7.5 | 7.2 | 8.0 |
comparing health centers where most homes have more than three people living in the same room, or are similar to hovels or does not have toilet.
Discussion
Although the impact of temperature on human health have been widely explored, there is few evidence on the effects of DTR which is considered an important index of climate change. To our knowledge, this is the first study in a developing country that investigates the effect of short-term variations in temperature, a factor that can easily trigger respiratory infections particularly from the upper respiratory tract. Our results provide evidence that DTR is an independent risk factor for respiratory infections, after controlling for relative humidity and PM10. It has been suggested that sudden temperature changes can cause inflammatory nasal responses (Graudenz et al., 2006) and induce the onset of a respiratory event (Ge et al., 2013), which may be one of the reasons that DTR is associated with respiratory admissions.
We observed that PM10 was also a significant predictor of both URI and LRI. Studies conducted in developed countries, demonstrated that ambient particulate matter induces epithelial injury and inflammatory cytokine production (Frampton et al., 1999). However, since these effects depend largely on the particle composition, the results may not be valid in developing countries where the composition of airborne particles is very different due to high pollutant loadings and much more wind-blown coarse dust (Harrison et al., 1997). In addition, the level of confounding by PM may differ by city due to domestic and local adaptation factors (Ye et al., 2012). In a previous work, we observed a consistent association of particles with respiratory diseases in children (Amarillo and Carreras, 2012); in this study, we reinforce this and we found that airborne particle exposure is significant risk factor of the DTR effects on respiratory infection, suggesting the need to control for this variable when assessing respiratory diseases exposure risk. On the other hand, respiratory infections were negatively associated with high relative humidity levels. Similar results were already observed in Buenos Aires, where humidity was negatively related to both cardiovascular and respiratory mortality (Rusticucci et al., 2002).
We conducted an analysis to study the distributed lag pattern of DTR up to 10 days after the event and found the strongest association on the following day after the event, with URIs being more affected than LRI. Previous studies also found that the sudden change in temperature favored the onset of new cases of upper respiratory infections (Danielides et al., 2002). The different effects of temperature variability on URI and LRI could be due to the different etiological pattern and often biphasic nature of LRI development. In a previous study on respiratory mortality in Buenos Aires, stronger associations were observed also on the first three days following the event and the effect estimates diminished as days pass, which is consistent with the underlying biological mechanism leading to high temperature related deaths (Abrutzki et al., 2012).
Regarding the population susceptibility to DTR, we found that the risk for hospital admissions due to upper and lower respiratory infections was respectively two and three times higher for the elderly than for the rest of the population, which is consistent with their low thermoregulation along with a decrease of appropriate immune responses to environmental factors due to aging (Lim et al., 2012). Previous studies also reported the strongest temperature impact in the older age categories (Abrutzki et al., 2012; Bell et al., 2008; Michelozzi et al., 2009; Zanobetti et al., 2012). These results are significant from a public health point of view since the proportion of elderly population is increasing worldwide. Therefore, considering the expected climatic changes it is of paramount importance to understand the heath impact of temperature fluctuations, especially on this susceptible population.
From the public health point of view, it is important to identify periods with high risk for respiratory infections, considering not only temperature limits but also other factors that contribute to morbidity. Our results showed a stronger association with DTR during the warm period than the cold period, meaning that in Cordoba City there is a higher risk for URIs and particularly LRIs during spring and summer months than during fall and winter. Strong associations between respiratory mortality and high temperature are already well established (Medina Ramon and Schwartz, 2007; Basu, 2009). However, the associations with respiratory morbidity were less investigated and most studies have been performed in northern hemisphere countries with cool climates. In general, an overall increase in morbidity has been consistently associated with heat waves (Ye et al., 2012). In agreement with our findings, in a study conducted in Australia, a country with warm and dry climate similar to Argentina, authors reported higher respiratory admissions during the warm season (Hansen et al., 2012). Nevertheless, more data are needed to fully understand the impact of temperature variations on respiratory health in people living in warm climates, controlling for cofactors such as pollen and other allergens as well as their synergistic effects with temperature and PM.
We expected that socio-economic status would modify the temperature-morbidity relationship. Higher respiratory morbidity was observed among the less-educated individuals, indicating that those living in families whose adults have fewer years of attained education have less wealth and poor life quality. It has been mentioned that persons with more years of attained education generally have greater wealth (O’Neill et al., 2013). Our results are consistent with this general pattern and are also similar to the trend observed in a study made in Sao Paulo, Brazil (Bell et al., 2008); although these authors mentioned that the pattern of vulnerability by education may differ by cities or regions due to co-variation of other socioeconomic factors. The lack of sanitary services was the strongest modifier of the respiratory infections-DTR relationship, which lends support to the biologic plausibility that a higher exposure to bacteria and virus due to scarce personal hygiene can contribute to respiratory morbidity. These results confirm that people living in families with low socio-economic backgrounds experience poor general health. Socioeconomic inequalities in mortality and morbidity have already been found in Europe and Brazil (Martins, et al. 2004; Zanobetti and Schwartz, 2000). However, further studies using a combination of individual and neighborhood-level indicators are required. Moreover, considering that the nature of susceptibility by socioeconomic position is far more complex than what can be capture by a single marker, and could depend more on preexisting medical conditions than on social factors.
Our study has several limitations. First, it is retrospective, thus we cannot be sure of the accuracy of diagnosis or respiratory diseases, which may have influenced the degree of association between weather variables and diseases. The second limitation is that the present study focused only on public health care centers admissions, thus representing only the low- to middle-income portion of the population. However, while we suspect that this population is probably the most susceptible to extreme temperature conditions, it would be important to investigate whether they are actually more vulnerable. Another limitation may be that meteorological data were obtained from fixed monitoring sites and were assumed uniform across regions. Similarly, we did not account for spatial variability among residential locations or in individual daily activity patterns, including exposure to indoor temperatures. Furthermore, since we did not have actual PM10 measurements for the entire study period, we used a prediction model to estimate the PM10 exposures for the entire study. It is worth noting that our predictions were lower than the levels measured recently (101±14 μg/m3) by López et al. (2011). Therefore, considering that we already found evidence of adverse health effects when using estimated PM10 concentrations, we can expect that the actual PM10 data could yield much stronger effects. Finally, we did not control for allergens and viral infections that are responsible for respiratory infections. However, since it has been demonstrated that in urban areas one of the several causes of the rise in morbidity associated with allergic respiratory diseases is the increased presence of particles (D’Amato et al., 2010), we decided to focus on triggers of respiratory infections that can actually be controlled. Therefore, the results of the present study could help policy makers to legislate and regulate one of the main factors contributing to respiratory infections.
Our study provides new information on vulnerability factors influencing the temperature-related morbidity in populations from southern hemisphere countries. Indeed, it suggests that the effects of temperature variations fall disproportionately on those persons at relative social disadvantage. Our findings reinforce the need to find effective ways of promoting a healthier lifestyle among people from lower socioeconomic backgrounds in order to decrease health disparities.
Acknowledgments
This work received partial support from Consejo Nacional de Investigaciones Científicas y Tecnológicas (Grant #11220090100999), Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba (Grant #30720130100458CB) and USEPA grant RD-834798. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. The authors thank also the support from Harvard University Clean Air Research Center.
References
- Abrutzky R, Dawidowski L, Matus P, Lankao PR. Health effects of climate and air pollution in Buenos Aires: A first time series analysis. J Environ Prot Ecol. 2012;3:262–271. [Google Scholar]
- Amarillo AC, Carreras H. The effect of airborne particles and weather conditions on pediatric respiratory infections in Cordoba, Argentine. Environ Poll. 2012;170:217–221. doi: 10.1016/j.envpol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Anderson BT. Intensification of seasonal extremes given a 2°C global warming target. Clim Change. 2001;112:325–337. [Google Scholar]
- Basu R. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ Health. 2009;16:8–40. doi: 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, O’Neill MS, Ranjit N, Borja-Aburto VH, Cifuentes LA, Gouveia NC. Vulnerability to heat-related mortality in Latin America: a case-crossover study in Sao Paulo, Brazil, Santiago, Chile and Mexico City, Mexico. Int J Epidemiol. 2008;37:796–804. doi: 10.1093/ije/dyn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Samet JM. An exposure assessment study of ambient heat exposure in an elderly population in Baltimore, Maryland. Environ Health Perspect. 2002;100:1219–1224. doi: 10.1289/ehp.021101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga ALF, Zanobetti A, Schwartz J. The time course of weather related deaths. Epidemiology. 2002;12:662–667. doi: 10.1097/00001648-200111000-00014. [DOI] [PubMed] [Google Scholar]
- Braganza K, Karoly DJ, Arblaster JM. Diurnal temperature range as an index of global climate change during the twentieth century. Geophys Res Lett. 2004;31:13–17. [Google Scholar]
- Burkart K, Canario P, Breitner S, Schneider A, Scherber K, Andrade H, Alcoforado MJ, Endlicher W. Interactive short-term effects of equyivalent temperature and air pollution on human mortality in Berlin and Lisbon. Environ Pollut. 2013;183:54–63. doi: 10.1016/j.envpol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Coarasa A, Giugno H, Cutri A, Loto Y, Torres F, Giubergia V, Ossorio MF, Durán P, González Pena H, Ferrero F. Validation of a clinical prediction tool to evaluate severity in children with wheezing Arch. Argent Pediatr. 2010;108:1668–3501. doi: 10.1590/S0325-00752010000200005. [DOI] [PubMed] [Google Scholar]
- Danielides V, Nousia CS, Patrikakos G, Bartzokas A, Lolis CJ, Milionis HJ, Skevas A. Effect of meteorological parameters on acute laryngitis in adults. Acta Oto-Laryngol. 2002;122:655–660. doi: 10.1080/000164802320396358. [DOI] [PubMed] [Google Scholar]
- DGEC. Dirección General De Estadísticas y Censos. Censo Provincial de Población 2008. 2008 Retrieved 21 January 2014, from http://estadistica.cba.gov.ar/Población/Censo2008/tabid/462/language/es-AR/Default.aspx.
- Du Prel JB, Puppe W, Gröndahl B, Knuf M, Weigl JA, Schaaff F, Schmitt HJ. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis. 2009;49:861–8. doi: 10.1086/605435. [DOI] [PubMed] [Google Scholar]
- Frampton MW, Ghio AJ, Samet JM, Carson JL, Carter JD, Devlin RB. Effects of aqueous extracts of PM (10) filters from the Utah valley on human airway epithelial cells. Am J Physiol. 1999;277:960–967. doi: 10.1152/ajplung.1999.277.5.L960. [DOI] [PubMed] [Google Scholar]
- Ganguly AR, Steinhaeuser K, Erickson DJ, Branstetterc M, Parisha ES, Singha N, Drakec JB, Buja L. Higher trends but larger uncertainty and geographic variability in 21st century temperature and heat waves. Proc Natl Acad Sci U S A. 2009;106:15555–15559. doi: 10.1073/pnas.0904495106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WZ, Xu F, Zhao ZH, Zhao JZ, Kan HD. Association between diurnal temperature range and respiratory tract infections. Biomed Environ Sci. 2013;26:222–225. doi: 10.3967/0895-3988.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Graudenz GS, Landgraf RG, Jancar S. The role of allergic rhinitis in nasal responses to sudden temperature changes. J Allergy Clin Inmunol. 2006;118:1126–1132. doi: 10.1016/j.jaci.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Halonen JI, Zanobetti A, Sparrow D, Vokonas P, Schwartz J. Relationship between outdoor temperature and blood pressure. Occupat Environ Med. 2010;68:296–301. doi: 10.1136/oem.2010.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Bi P, Nitschke M, Pisaniello D, Ryan P, Sullivan T, Barnett AG. Particulate air pollution and cardiorespiratory hospital admissions in a temperate Australian city: A case-crossover analysis. Sci Total Environ. 2012;1:48–52. doi: 10.1016/j.scitotenv.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Harrison RM, Deacon AR, Jones MR. Sources and process affecting concentrations of PM10 and PM2,5 particulate matter in Birmingham (UK) Atmos Environ. 1997;31:4103–4117. [Google Scholar]
- Hoffmann J, Arnolt R, Daguerre M, Calcagno L. Correlaciones Entre los Ataques de Asma en Pediatría y las Condiciones Meteorológicas en Rosario. Meteorológica. 1983;14:87–89. [PubMed] [Google Scholar]
- Jaffe DH, Singer ME, Rimm AA. Air pollution and emergency department visits for asthma among Ohio Medicaid recipients, 1991–1996. Env Res. 2003;91:21–28. doi: 10.1016/s0013-9351(02)00004-x. [DOI] [PubMed] [Google Scholar]
- Kovats SR, Hajat S, Wilkinson P. Contrasting patterns of mortality and hospital admissions during hot weather and heat waves in Greater London, UK. J Occup Environ Med. 2004;61:893–898. doi: 10.1136/oem.2003.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal M, Harasawa H. Future climate change scenarios for Asia as inferred from selected coupled atmosphere-ocean global climate models. J Meteorol Soc Jpn. 2001;79:219–227. [Google Scholar]
- Leitte AM, Petrescu C, Franck U, Richter M, Suciu O, Ionovici R, Herbarth O, Schlink U. Respiratory health, effects of ambient air pollution and its modification by air humidity in Drobeta-Turnu Severin, Romania. Sci Total Environ. 2009;15:4004–4011. doi: 10.1016/j.scitotenv.2009.02.042. [DOI] [PubMed] [Google Scholar]
- Liang WM, Liu WP, Kuo HW. Diurnal temperature range and emergency room admissions for chronic obstructive pulmonary disease in Taiwan. Int J Biometeorol. 2009;53:17–23. doi: 10.1007/s00484-008-0187-y. [DOI] [PubMed] [Google Scholar]
- Lim YH, Hong YC, Kim H. Effects of diurnal temperature range on cardiovascular and respiratory hospital admissions in Korea. Sci Total Environ. 2012;417:55–60. doi: 10.1016/j.scitotenv.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Lin HC, Lin CC, Chen CS, Lin HC. Seasonality of pneumonia admissions and its association with climate: an eight-year nationwide population-based study. Chronobiol Int. 2009;26:1647–59. doi: 10.3109/07420520903520673. [DOI] [PubMed] [Google Scholar]
- Linares C, Diaz J. Impact of high temperatures on hospital admissions: comparative analysis with previous studies about mortality (Madrid) Eur J Public Health. 2008;18:317–322. doi: 10.1093/eurpub/ckm108. [DOI] [PubMed] [Google Scholar]
- López ML, Ceppi S, Palancar GG, Olcese LE, Tirao G, Toselli B. Elemental concentration and source identification of PM10 and PM2.5 by SR-XRF in Córdoba city, Argentina. Atmos Environ. 2011;45:5450–5457. [Google Scholar]
- Mäkinen TM, Juvonen R, Jokelainen J, Harju TH, Peitso A, Bloigu A, Silvennoinen-Kassinen S, Leinonen M, Hassi J. Cold temperature and low humidity are associated with increased occurrence of respiratory tract infections. Respir Med. 2009;103:456–62. doi: 10.1016/j.rmed.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Makowski K, Wild M, Ohmura A. Diurnal temperature range over Europe between 1950 and 2005. Atmos Chem Phys. 2008;8:6483–6498. [Google Scholar]
- Martins MC, Fatigati FL, Véspoli TC, Martins LC, Pereira LA, Martins MA, Saldiva PH, Braga AL. Influence of socioeconomic conditions on air pollution adverse health effects in elderly people: an analysis of six regions in São Paulo, Brazil. J Epidemiol Commun H. 2004;58:41–46. doi: 10.1136/jech.58.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Wilkinson P, Kovats RS, Pattenden S, Hajat S, Armstrong B, Vajanapoom N, Niciu EM, Mahomed H, Kingkeow C. International study of temperature, heat and urban mortality: the ‘ISOTHURM’ project. Int J Epidemiol. 2008;37:1121–1131. doi: 10.1093/ije/dyn086. [DOI] [PubMed] [Google Scholar]
- Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 2004;305:994–997. doi: 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- Mehta AJ, Kloog I, Zanobetti A, Coull BA, Sparrow D, Vokonas P, Schwartz J. Associations between Changes in City and Address Specific Temperature and QT Interval - The VA Normative Aging Study. PLoS ONE. 2014;9:e106258. doi: 10.1371/journal.pone.0106258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelozzi P, Accetta G, De Sario M, D’Ippoliti D, Marino C, Baccini M, Biggeri A, Ross Anderson H, Katsouyanni K, Ballester F, Bisanti L, Cadum E, Forsberg B, Forastiere F, Goodman P, Hojs A, Kirchmayer U, Medina S, Paldy A, Schindler C, Sunyer J, Perucci C. High Temperature and Hospitalizations for Cardiovascular and Respiratory Causes in 12 European Cities. Am J Resp Critical Care Med. 2009;179:383–389. doi: 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- Medina-Ramon M, Schwartz J. Temperature, temperature extremes, and mortality: a study of acclimatation and effect modification in 50 US cities. Occup Environ Med. 2007;64:827–833. doi: 10.1136/oem.2007.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcese LE, Toselli BM. Some Aspects of Air Pollution in Córdoba, Argentina. Atmos Env. 2002;36:299–306. [Google Scholar]
- O’Neill MS, Zanobetti A, Schwartz J. Modifiers of the temperature and mortality association in seven US cities. Am J Epidemiol. 2003;157:1074–82. doi: 10.1093/aje/kwg096. [DOI] [PubMed] [Google Scholar]
- Ostro BD, Roth LA, Green RS, Basu R. Estimating the mortality effect of the July 2006 California heat wave. Environ Res. 2009;109:614–619. doi: 10.1016/j.envres.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Patz JA, Engelberg D, Last J. The effects of changing weather on public health. Ann Rev Pub Health. 2000;21:271–303. doi: 10.1146/annurev.publhealth.21.1.271. [DOI] [PubMed] [Google Scholar]
- Piccolo MC, Perillo GM, Ramon CG, Di Dio V. Outbreaks of asthma attacks and meteorologic parameters in Bahia Blanca, Argentina. Ann Allergy. 1988;60:107–110. [PubMed] [Google Scholar]
- Rosa AM, Ignotti E, de Souza Hacon S, Albuquerque de Castro H. Analysis of hospitalizations for respiratory diseases in Tangará da Serra, Brazil. J Bras Pneumol. 2008;34:575–582. doi: 10.1590/s1806-37132008000800006. [DOI] [PubMed] [Google Scholar]
- Ruijsbroek A, Wijga AH, Kerkhof M, Koppelman GH, Smit HA, Droomers M. The development of socio-economic health differences in childhood: results of the Dutch longitudinal PIAMA birth cohort. BMC Public Health. 2011;12:11–225. doi: 10.1186/1471-2458-11-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusticucci M, Barrucand M. Observed trends and changes in Temperature Extremes over Argentina. J Climate. 2004;17:4099–4107. [Google Scholar]
- Rusticucci M, Betolli L, Harris MA. Association between Weather Conditions and the Number of Patients at the Emergency Room in an Argentine Hospital,” Int. J Biometeorol. 2002;46:42–51. doi: 10.1007/s00484-001-0113-z. [DOI] [PubMed] [Google Scholar]
- Stafoggia M, Forastiere F, Agostini D, Caranci N, de’Donato F, Demaria M, Michelozzi P, Miglio R, Rognoni M, Russo A, Perucci CA. Factors affecting in-hospital heat-related mortality: a multi-city casecrossover analysis. J Epidemiol Community Health. 2008;62:209–215. doi: 10.1136/jech.2007.060715. [DOI] [PubMed] [Google Scholar]
- Stein AF, Toselli B. Street level air pollution in Cordoba city, Argentina. Atmos Environ. 1996;30:3491–3495. [Google Scholar]
- Wang MZ, Zheng S, He SL, Li B, Teng HJ, Wang SG, Yin L, Shang KZ, Li TS. The association between diurnal temperature range and emergency room admissions for cardiovascular, respiratory, digestive and genitourinary disease among the elderly: a time series study. Sci Total Environ. 2013;1:456–457. doi: 10.1016/j.scitotenv.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S. Ambient temperature and morbidity: a review of epidemiological evidence. Environ Health Perspect. 2012;120:19–28. doi: 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Franklin M, Koutrakis P, Schwartz J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health. 2009;21:8–58. doi: 10.1186/1476-069X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, O’Neill M, Gronlund C, Schwartz J. Summer temperature variability and long-term survival among elderly people with chronic disease. Proc Natl Acad Sci U S A. 2012;17:6608–6613. doi: 10.1073/pnas.1113070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, O’Neill MS, Gronlund CJ, Schwartz J. Susceptibility to mortality in weather extremes: effect modification by personal and small area characteristics in a multi-city case-only analysis. Epidemiology. 2013;24:809–19. doi: 10.1097/01.ede.0000434432.06765.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Race, gender and social status as modifiers of the effects of PM10 on mortality. J Occup Environ Med. 2000;42:469–474. doi: 10.1097/00043764-200005000-00002. [DOI] [PubMed] [Google Scholar]