Abstract
Human reproduction is a tightly controlled process of stepwise evolution with multiple, mostly yet unknown milestones and checkpoints. Healthy halpoid gametes have to be produced by the parents, which will fuse to form the diploid zygote that implants in the female uterus and grows to become first an embryo, then a fetus and finally matures into a newborn. There are several known risk factors that interfere with normal production of gametes, spermatocytes or oocytes, and often cause embryonic mortality and fetal demise at an early stage. Yet some embryos with chomosomal abnormalities can develop beyond the critical first trimester of pregnancy and, while those with supernumary chromosomes in their hyperdiploid cells will be spontaneously aborted, a small fraction of fetuses with an extra chromosome continues to grow to term and will be delivered as a liveborn baby.
While minor clinical symptoms displayed by children with trisomies are manageable for many parents, the burden of caring for a child with numerical chromosome abnormalities can be overwhelming to partners or individual families. It also poses a significant financial burden to the society and poses ethical dilemma.
In this communication, we will review the progress that has been made in the development of molecular techniques to test individual fetal cells for chromosomal imbalances. We will focus our discussion on the direct visualization of chromosome-specific DNA sequences in live or fixed specimens using fluorescence in situ hybridization (FISH) and, more specifically, talk about the groundbreaking progress that in recent years has been achieved towards an improved diagnosis with novel, chromosome-specific DNA probes.
Keywords: Pregnancy, Chromosomal imbalance, Aneuploidy, Perinatal diagnosis, Molecular cytogenetics, Fluorescence in situ hybridization (FISH), DNA probes
Mini Review
Numerical chromosome aberrations are rarely compatible with early human development and life. Most commonly, chromosomally imbalanced human zygotes or embryos carry chromosomal monosomies or trisomies leading to either failed nidation or fetal demise. Published estimates state that as many as half of the 15–20% of recognized pregnancy failures are due to numerical chromosome aberrations [1]. A few well known exceptions are embryos carrying an extra chromosome 13, 18, 21, X or Y, which lead to phenotypical abnormalities and clinically recognizable symptoms [1–6].
However, incidences of trisomy occur disproportionately among the 22 human autosomes. Studies karyotyping thousands of live birth or spontaneous abortuses, sometimes referred to as ‘product of conception (POC)’, show that trisomies involving chromosome 16 are the by far most common abnormality and are found in 31% of spontaneous abortions [1] compared to trisomy 13 or 21 found in only 4.1% and 10.5% of spontaneous abortions. Chromosome 16 trisomy occurs in 1–2% of all human conceptions and is thus most common autosomal trisomy found in first trimester miscarriages [7,8].
Studies of human preimplantation embryos at the day 3-stage could demonstrate a maternal age dependent increase in the number of embryos carrying cells with an extra chromosome 16 [9] providing further evidence that most trisomy 16 pregnancies originate as a consequence of a maternal meiosis I non-disjunction [10] and are generally not compatible with life [1,11].
However, some embryos which survive early in utero carry a rare trisomy 16 mosaic aberration, containing both euploid and trisomic cell lines with abnormal expression of imprinted genes [12,13]. Such cases include true mosaics, cases with confined placental mosaicism (CPM) and uniparental disomy (UPD) [7].
A trisomy 16 mosaicism is usually caused by a remarkable process termed ‘trisomy rescue‘, where loss of a chromosome 16 in one of the trisomic cells of the early embryo results in a euploid cell line. The final distribution of trisomy 16 cells in the placenta and the fetus depends on the embryonic stage when trisomy rescue occurs and either one of the two maternal chromosomes or the paternal chromosome can be lost [7,14]. When a trisomy 16 conceptus is rescued, the result may be maternal uniparental disomy 16 (UPD(16)mat), i.e, both homologues of chromosomes 16 are inherited from the mother [15,16] and a lifeborn child may have a distinct phenotypic effect [7,14,17]. While, a significant number of fetuses with prenatally diagnosed mosaic trisomy 16 have a good outcome with a milder phenotypical appearance [6,18], the mosaic trisomy 16 has also been associated with a severe pregnancy complication called ‘preclampsia‘ [13] emphasizing a need for rapid and accurate genetic analysis of fetal chromosomes as a further relevant tool for counselling the mother [10,11,14,19,20].
For more than two decades, our laboratories and others have been involved in the design of genetic tests analyzing the karyotypes of human sperm, oocytes, preimplantation embryos, fetal cells and tumor specimens based on fluorescence in situ hybridization (FISH) [3,21–26].
The FISH technique is based on hybridization of non-isotopically labeled nucleic acid probes and detection by fluorescence microscopy [27]. Sources of DNA probes can be any modified oligonucleotide or chunks of cloned DNA identified as chromosome- or gene-specific sequence. The goal of DNA probe optimization is to increase probe specificity and signal intensity. While small synthetic oligonucleotide probes have the advantage of rapid diffusion and thus shorter hybridization times, the small number of fluorescent moieties bound to intracellular targets often results in weak signals.
An ideal, high performance DNA probe is highly chromosome-specific and works well with a spectrum of biological specimens ranging from archival to fresh samples that may have undergone complex aging and fixation procedures [28]. Our laboratories and others have worked with a variety of cloned or PCR-amplified DNA sequences targeting tandemly-repeated pancentromeric clusters of alpha satellite DNA [21,29–32] for chromosome enumeration. Early probes were often cloned in plasmids which allow inserts in the kilobase (kb) range to be stably propagated [33,34]. However, some alphoid DNA probes face limitations of use in cases where existing heteromorphisms lead to one strong and one very weak signal, the latter of which might be easily missed [35,36].
The family of short DNA satellite repeats II/III is an attractive, large hybridization target for chromosome enumeration, even in the presence of heteromorphisms [35], but remains limited to just a handful of human chromosomes such as chromosomes 1, 9, 16 and Y. The plasmid clone pHUR98 is an example of a successfully used chromosome 16 satellite II/III DNA repeat probe [37–39].
We gained extensive experience preparing FISH probes from yeast artificial chromosome (YAC) clones or P1 clones [40–43]. However, existing physical maps for these clones do not cover the heterochromatic regions of the human genome and most probes will target single copy sequences which result in weaker signals.
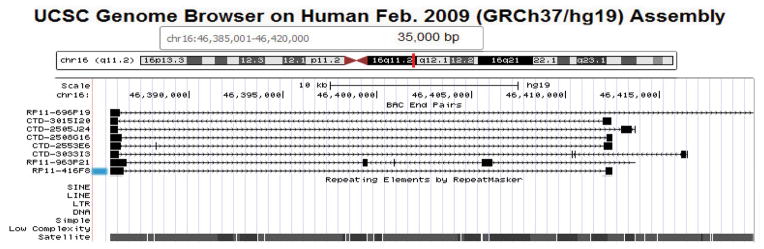
Other approaches for chromosome enumeration have been described such as comparative genomic hybridization [44], but none of them can compete with the above describe FISH and direct visualization in terms of speed, low complexity and cost. We recently investigated the use of bioinformatics tools and mining of data in existing databases. We chose to search the genome database at UC Santa Cruz [45], which shows alignments of paired end-sequenced bacterial artificial chromosome clones (BACs) [46,47] with the provisionally finished draft of the human genome sequence [48]. In our most recent approach to prepare high performance DNA probes that supersede preexisting probes with regard to probe specificity and signal-to-noise ratios, we attempt to identify BAC clones from the pericentromeric regions of different chromosomes that are free of non-chromosome-specific short or long interspersed repeat sequences (SINE’s, LINE’s) and contain ‘pure’ satellite DNA (Figure 1) [49–51].
Figure 1.
The Graphic User Interface (GUI) of the UC Santa Cruz online Genome Browser indicating the position of BAC clone RP11-416F8 (indicated by the blue mark on the left) along the draft sequence of human chromosome 16.
As the example in Figure 1 illustrates, the alignment of paired end-sequences from BAC clone RP11-416F8 suggests an insert of about 26.678 kb of human DNA mapping to chromosome 16, band q11.2 [51]. The insert is predicted to be comprised entirely of satellite DNA. We isolated the BAC DNA and labeled it with biotin using a Bioprime kit (Life Technologies, La Jolla, CA). The probe, when hybridized to metaphase spreads prepared from the fibroblast cell line WI-38 and stained with avidin-FITC following our published protocol [24], showed two very bright, specific signals per cell (arrows in Figure 2B) which were easy to count in the microscope by eye. Other probes, which have been prepared using a similar bioinformatics approach for chromosomes 10, X and Y can easily be combined with this chromosome 16-specific probe [50–52].
Figure 2.
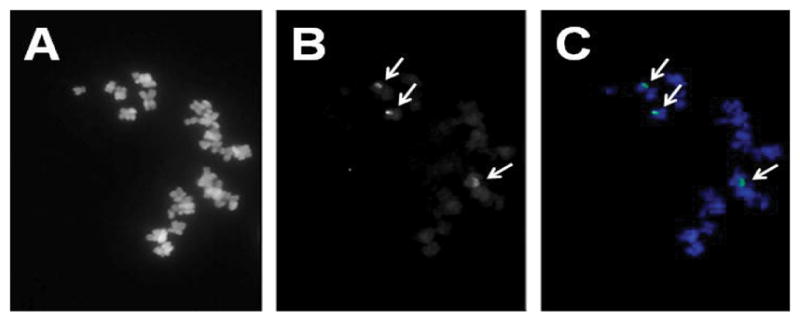
FISH result using a biotinylated probe prepared from BAC clone RP11-416F8. A) DAPI image showing the DNA/metaphase chromosomes; B) Three chromosome-specific green fluorescence signals (arrows) after staining with avidin-FITC; C) Overlay of the DAPI and FITC images.
The fact that the WI-38 human diploid cell line was derived by Leonard Hayflick from normal female embryonic (3 months gestation) lung tissue in 1962 does not necessarily imply that the particular batch of WI-38 cells used in our studies was diploid [53]. As Sigma Aldrich, a major supplier of WI-38 cells for research and vaccine production describes the cells on their web site as 'Fibroblast-like, 2n=46, diploid except at high passage number', karyotype alterations have to be anticipated at high number of passages [54]. The observation of three chromosome 16-specific signals in WI-38 cells matches the primary goal of our technical developments, i.e., an assay able to detect trisomy 16 in fetal tissues.
In summary, despite the fact that most of the human heterochromatin remains unchartered territory, simple data mining approaches can identify potential DNA probes for chromosome enumeration that are easy and inexpensive to prepare by the less experienced laboratory and result in FISH signals of unprecedented specificity and intensity.
Acknowledgments
We gratefully acknowledge the help provided by students, staff and participating guests in the Weier labs at LBNL and UCSF. We wish to thank Dr. T. Groesser, LBNL, for providing the WI-38 fibroblast cells.
Funding
This work was supported in parts by a grant from the Director, Office of Energy Research, Office of Health and Environmental Research, U.S. Department of Energy, under contract DE-AC02-05CH11231 and NIH grant CA168345 (to HUW).
Abbreviations
- BAC
Bacterial Artificial Chromosome
- CPM
Confined Placental Mosaicism
- DAPI
4,6-Diamino-2-Phenylindole
- FISH
Fluorescence In Situ Hybridization
- FITC
Fluorescein Isothiocyanate
- UPD
Uniparental Disomy
Footnotes
Disclaimer
This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor The Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or The Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof, or The Regents of the University of California.
References
- 1.Pflueger SMV. Cytogenetics of Spontaneous Abortion. In: Gerson SL, Keagle MB, editors. The Principles of Clinical Cytogenetics. Humana Press; Totowa NJ, USA: 2005. [Google Scholar]
- 2.Hassold TJ, Jacobs PA. Trisomy in man. Ann Rev Genet. 1984;18:69–97. doi: 10.1146/annurev.ge.18.120184.000441. [DOI] [PubMed] [Google Scholar]
- 3.Pinkel D, Landegent J, Collins C, Fuscoe J, Segraves R, et al. Fluorescence in situ hybridization with human chromosome-specific libraries: detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci USA. 1988;85:9138–9142. doi: 10.1073/pnas.85.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen B, Bryndorf T, Philip J, Lundsteen C, Hansen W. Rapid prenatal diagnosis of trisomy 18 and triploidy in interphase nuclei of uncultured amniocytes by non-radioactive in situ hybridization. Prenatal Diagnosis. 1992;12:241–250. doi: 10.1002/pd.1970120403. [DOI] [PubMed] [Google Scholar]
- 5.Johnson MP, Childs MD, Robichaux AGr, Isada NB, Pryde PG, et al. Viable pregnancies after diagnosis of trisomy 16 by CVS: lethal aneuploidy compartmentalized to the trophoblast. Fetal Diagn Ther. 1993;8:102–108. doi: 10.1159/000263756. [DOI] [PubMed] [Google Scholar]
- 6.Rieubland C, Francis D, Houben L, Corrie S, Bankier A, et al. Two cases of trisomy 16 mosaicism ascertained postnatally. Am J Med Genet A. 2009;149A:1523–1528. doi: 10.1002/ajmg.a.32925. [DOI] [PubMed] [Google Scholar]
- 7.Benn P. Trisomy 16 and trisomy 16 Mosaicism: a review. Am J Med Genet. 1998;79:121–133. [PubMed] [Google Scholar]
- 8.Lathi RB, Westphal LM, Milki AA. Aneuploidy in the miscarriages of infertile women and the potential benefit of preimplanation genetic diagnosis. Fertil Steril. 2008;89:353–357. doi: 10.1016/j.fertnstert.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 9.Benadiva CA, Kligman I, Munne S. Aneuploidy 16 in human embryos increases significantly with maternal age. Fertil Steril. 1996;66:248–255. [PubMed] [Google Scholar]
- 10.Robinson WP, Barrett IJ, Bernard L, Telenius A, Bernasconi F, et al. Meiotic origin of trisomy in confined placental mosaicism is correlated with presence of fetal uniparental disomy, high levels of trisomy in trophoblast, and increased risk of fetal intrauterine growth restriction. Am J Hum Genet. 1997;60:917–927. [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson MP, Childs MD, Robichaux AGr, Isada NB, Pryde PG, et al. Viable pregnancies after diagnosis of trisomy 16 by CVS: lethal aneuploidy compartmentalized to the trophoblast. Fetal Diagn Ther. 1993;8:102–108. doi: 10.1159/000263756. [DOI] [PubMed] [Google Scholar]
- 12.Kalousek DK1. Pathogenesis of chromosomal mosaicism and its effect on early human development. Am J Med Genet. 2000;91:39–45. doi: 10.1002/(sici)1096-8628(20000306)91:1<39::aid-ajmg7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Blair JD, Langlois S, McFadden DE, Robinson WP. Overlapping DNA methylation profile between placentas with trisomy 16 and early-onset preeclampsia. Placenta. 2014;35:216–222. doi: 10.1016/j.placenta.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Gardner RJM, Sutherland GR, Shaffer LG. Oxford Monographs in Medical Genetics. Oxford: Oxford University Press, UK; 1989. Chromosome Abnormalities and Genetic Counselling. [Google Scholar]
- 15.Yong PJ, Barrett IJ, Kalousek DK, Robinson WP. Clinical aspects, prenatal diagnosis, and pathogenesis of trisomy 16 mosaicism. J Med Genet. 2003;40:175–182. doi: 10.1136/jmg.40.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel E. A new genetic concept: uniparental disomy and its potential effect, isodisomy. Am J Med Genet. 1980;6:137–143. doi: 10.1002/ajmg.1320060207. [DOI] [PubMed] [Google Scholar]
- 17.Yong PJ, Marion SA, Barrett IJ, Kalousek DK, Robinson WP. Evidence for imprinting on chromosome 16: the effect of uniparental disomy on the outcome of mosaic trisomy 16 pregnancies. Am J Med Genet. 2002;112:123–132. doi: 10.1002/ajmg.10702. [DOI] [PubMed] [Google Scholar]
- 18.Langlois S, Yong PJ, Yong SL, Barrett I, Kalousek DK, et al. Postnatal follow-up of prenatally diagnosed trisomy 16 mosaicism. Prenat Diagn. 2006;26:548–558. doi: 10.1002/pd.1457. [DOI] [PubMed] [Google Scholar]
- 19.Wolstenholme J. An audit of trisomy 16 in man. Prenat Diagn. 1995;15:109–121. doi: 10.1002/pd.1970150202. [DOI] [PubMed] [Google Scholar]
- 20.Gada Saxena S, Desai K, Shewale L, Ranjan P. Pre-implantation genetic screening using fluorescence in situ hybridization in couples of Indian ethnicity: Is there a scope? J Hum Reprod Sci. 2014;7:25–29. doi: 10.4103/0974-1208.130812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weier HU, Kleine HD, Gray JW. Labeling of the centromeric region on human chromosome 8 by in situ hybridization. Hum Genet. 1991;87:489–494. doi: 10.1007/BF00197174. [DOI] [PubMed] [Google Scholar]
- 22.Wyrobek AJ, Robbins WA, Mehraein Y, Pinkel D, Weier HU. Detection of sex chromosomal aneuploidies X-X, Y-Y, and X-Y in human sperm using two-chromosome fluorescence in situ hybridization. Am J Med Genet. 1994;53:1–7. doi: 10.1002/ajmg.1320530102. [DOI] [PubMed] [Google Scholar]
- 23.Munne S, Grifo J, Cohen J, Weier HU. Chromosome abnormalities in human arrested preimplantation embryos: a multiple-probe FISH study. Am J Hum Genet. 1994;55:150–159. [PMC free article] [PubMed] [Google Scholar]
- 24.Weier JF, Hartshorne C, Nguyen HN, Baumgartner A, Polyzos AA, et al. Analysis of human invasive cytotrophoblasts using multicolor fluorescence in situ hybridization. Methods. 2013;64:160–168. doi: 10.1016/j.ymeth.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Weier JF, Weier HU, Jung CJ, Gormley M, Zhou Y, et al. Human cytotrophoblasts acquire aneuploidies as they differentiate to an invasive phenotype. Dev Biol. 2005;279:420–432. doi: 10.1016/j.ydbio.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Fotra R, Gupta S, Koul S. Analysis of the chromosomal aneuploidy by interphase fluorescence in situ hybridization (FISH) in squamous cell carcinoma of the cervix in Jammu region of J and K state. J Cancer Res Ther. 2014;10:317–323. doi: 10.4103/0973-1482.136612. [DOI] [PubMed] [Google Scholar]
- 27.Manuelidis L. Chromosomal localization of complex and simple repeated human DNAs. Chromosoma. 1978;66:23–32. doi: 10.1007/BF00285813. [DOI] [PubMed] [Google Scholar]
- 28.Tkachuk DC, Pinkel D, Kuo WL, Weier HU, Gray JW. Clinical applications of fluorescence in situ hybridization. Genetic Analysis, Techniques and Applications. 1991;8:67–74. doi: 10.1016/1050-3862(91)90051-r. [DOI] [PubMed] [Google Scholar]
- 29.Willard HF, Smith KD, Sutherland J. Isolation and characterization of a major tandem repeat family from the human X chromosome. Nucleic Acids Research. 1983;11:2017–2033. doi: 10.1093/nar/11.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe J, Darling SM, Erickson RP, Craig IW, Buckle VJ, et al. Isolation and characterization of an alphoid centromeric repeat family from the human Y chromosome. Journal of Molecular Biology. 1985;182:477–485. doi: 10.1016/0022-2836(85)90234-7. [DOI] [PubMed] [Google Scholar]
- 31.Waye JS, Durfy SJ, Pinkel D, Kenwrick S, Patterson M, et al. Chromosome-specific alpha satellite DNA from human chromosome 1: hierarchical structure and genomic organization of a polymorphic domain spanning several hundred kilobase pairs of centromeric DNA. Genomics. 1987;1:43–51. doi: 10.1016/0888-7543(87)90103-0. [DOI] [PubMed] [Google Scholar]
- 32.Munne S, Sultan KM, Weier HU, Grifo JA, Cohen J, et al. Assessment of numeric abnormalities of X, Y, 18, and 16 chromosomes in pre-implantation human embryos before transfer. Am J Obstet Gynecol. 1995;172:1191–1199. doi: 10.1016/0002-9378(95)91479-x. discussion 1199–1201. [DOI] [PubMed] [Google Scholar]
- 33.Weier HU, Segraves R, Pinkel D, Gray JW. Synthesis of Y chromosome-specific labeled DNA probes by in vitro DNA amplification. J Histochem Cytochem. 1990;38:421–426. doi: 10.1177/38.3.2406338. [DOI] [PubMed] [Google Scholar]
- 34.Weier H-U, Rosette CD, Matsuta M, Zitzelsberger H, Matsuta M, et al. Generation of highly specific DNA hybridization probes for chromosome enumeration in human interphase cell nuclei: isolation and enzymatic synthesis of alpha satellite DNA probes for chromosome 10 by primer directed DNA amplification. Methods in Mol Cell Biol. 1994;4:231–248. [Google Scholar]
- 35.Weier HU, Gray JW. A degenerate alpha satellite probe, detecting a centromeric deletion on chromosome 21 in an apparently normal human male, shows limitations of the use of satellite DNA probes for interphase ploidy analysis. Analyt Cell Path. 1992;4:81–86. [PubMed] [Google Scholar]
- 36.Cacheux V, Tachdjian G, Druart L, Oury JF, Serero S, et al. Evaluation of X, Y, 18, and 13/21 alpha satellite DNA probes for interphase cytogenetic analysis of uncultured amniocytes by fluorescence in situ hybridization. Prenatal Diagnosis. 1994;14:79–86. doi: 10.1002/pd.1970140202. [DOI] [PubMed] [Google Scholar]
- 37.Moyzis RK, Albright KL, Bartholdi MF, Cram LS, Deaven LL, et al. Human chromosome-specific repetitive DNA sequences: novel markers for genetic analysis. Chromosoma. 1987;95:375–386. doi: 10.1007/BF00333988. [DOI] [PubMed] [Google Scholar]
- 38.Fung J, Weier HU, Goldberg JD, Pedersen RA. Multilocus genetic analysis of single interphase cells by spectral imaging. Hum Genet. 2000;107:615–622. doi: 10.1007/s004390000416. [DOI] [PubMed] [Google Scholar]
- 39.Fung J, Weier HU, Pedersen RA. Detection of structural and numerical chromosome abnormalities in interphase cells using spectral imaging. Journal of Histochemistry and Cytochemistry. 2001;49:797–798. doi: 10.1177/002215540104900616. [DOI] [PubMed] [Google Scholar]
- 40.Sternberg NL. Cloning high molecular weight DNA fragments by the bacteriophage P1 system. Trends Genet. 1992;8:11–16. doi: 10.1016/0168-9525(92)90018-y. [DOI] [PubMed] [Google Scholar]
- 41.Sternberg N1, Shepherd NS. Construction of bacteriophage P1 libraries with large inserts. Curr Protoc Hum Genet. 2001;Chapter 5(Unit 5) doi: 10.1002/0471142905.hg0503s09. [DOI] [PubMed] [Google Scholar]
- 42.Weier HU, Rhein AP, Shadravan F, Collins C, Polikoff D. Rapid physical mapping of the human trk protooncogene (NTRK1) to human chromosome 1q21-q22 by P1 clone selection, fluorescence in situ hybridization (FISH), and computer-assisted microscopy. Genomics. 1995;26:390–393. doi: 10.1016/0888-7543(95)80226-c. [DOI] [PubMed] [Google Scholar]
- 43.O'Brien B, Jossart GH, Ito Y, Greulich-Bode KM, Weier JF, et al. ‘Chromosomal Rainbows’ detect oncogenic rearrangements of signaling molecules in thyroid tumors. The Open Cell Signaling Journal. 2010;2:13–21. doi: 10.2172/1011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wells D, Sherlock JK, Handyside AH, Delhanty JD. Detailed chromosomal and molecular genetic analysis of single cells by whole genome amplification and comparative genomic hybridisation. Nucl Acids Res. 1999;27:1214–1218. doi: 10.1093/nar/27.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.genome.ucsc.edu
- 46.Ioannou PA, Amemiya CT, Garnes J, Kroisel PM, Shizuya H, et al. A new bacteriophage P1-derived vector for the propagation of large human DNA fragments. Nat Genet. 1994;6:84–89. doi: 10.1038/ng0194-84. [DOI] [PubMed] [Google Scholar]
- 47.Osoegawa K, Mammoser AG, Wu C, Frengen E, Zeng C, et al. A bacterial artificial chromosome library for sequencing the complete human genome. Genome Res. 2001;11:483–496. doi: 10.1101/gr.169601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baumgartner A, Weier JF, Weier HU. Chromosome-specific DNA repeat probes. J Histochem Cytochem. 2006;54:1363–1370. doi: 10.1369/jhc.6A6974.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng H, Weier HUG, Kwan J, Wang M, O’Brien B. Data Mining Empowers the Generation of a Novel Class of Chromosome-specific DNA Probes. J Data Mining Genom Proteom. 2011;2:108. [Google Scholar]
- 50.O'Brien B, Zeng H, Polyzos AA, Lemke KH, Weier JF, et al. Bioinformatics tools allow targeted selection of chromosome enumeration probes and aneuploidy detection. J Histochem Cytochem. 2013;61:134–147. doi: 10.1369/0022155412470955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu JH, Zeng H, Lemke KH, Polyzos AA, Weier JF, et al. Chromosome-specific DNA repeats: rapid identification in silico and validation using fluorescence in situ hybridization. Int J Mol Sci. 2012;14:57–71. doi: 10.3390/ijms14010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng H, Weier JF, Wang M, Kassabian HJ, Polyzos AA, et al. Bioinformatic tools identify chromosome-specific DNA probes and facilitate risk assessment by detecting aneusomies in extra-embryonic tissues. Curr Genomics. 2012;13:438–445. doi: 10.2174/138920212802510510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wadman M. Cell Division. Nature. 2013;498:422–427. doi: 10.1038/498422a. [DOI] [PubMed] [Google Scholar]
- 54.http://www.sigmaaldrich.com/catalog/product/sigma/90020107?lang=en®ion=US