Abstract
Background
Women at risk for breast cancer report elevated psychological distress, which has been adversely associated with cancer-relevant behaviors and biology.
Purpose
The present study sought to examine the effects of a 10-week cognitive behavioral stress management (CBSM) group intervention on distress among women with a family history of breast cancer.
Methods
Participants were randomly assigned to CBSM (N= 82) or a wait-list comparison group (N=76). Baseline to post-intervention effects of CBSM on depressive symptoms and perceived stress were examined using hierarchical regression.
Results
CBSM participants reported significantly lower post-treatment depressive symptoms (β=−0.17, p<0.05) and perceived stress (β= − 0.23, p<0.05) than wait-list comparison participants. Additionally, greater relaxation practice predicted lower distress.
Conclusions
Group-based CBSM intervention is feasible and can reduce psychological distress among women with a family history of breast cancer. The present findings represent an encouraging avenue for the future application of CBSM. (Clinicaltrials.gov number NCT00121160)
Keywords: Breastneoplasmsrisk, Cognitive behavioralstress management, Group psychotherapy, Distress, Relaxation practice, Female
Women at elevated risk for breast cancer (i.e., healthy women with a family history of breast cancer) report elevated levels of chronic distress (e.g., perceived stress, depressive symptomology, and elevated cancer worry) [1–4]. In general, this distress is largely due to the fact that women with a family history of breast cancer have elevated breast cancer risk perceptions [5]. High levels of perceived breast cancer risk are associated with high levels of cancer worry and general distress [4, 6, 7]. While the level of distress among high-risk women varies [8, 9] and may not reach clinical significance in all cases [10], elevated chronic distress is relevant for women at risk because elevated chronic distress has been associated with many health behaviors (e.g., screening) [11, 12] and biological outcomes (e.g., immune function, elevated cortisol levels, DNA damage) relevant to breast cancer risk [13–16].
In the general population, elevated chronic distress is associated with increased alcohol consumption [17], increased high-fat food consumption [18], decreased exercise [19], and increased body mass index [20, 21]. Elevated chronic distress is also associated with suppressed immune function [22] and dysregulated cortisol [23], both of which can support breast tumor progression [24, 25]. Among women at elevated risk for breast cancer, high levels of distress have been associated with suboptimal screening [26, 27]. While women cannot change their family history, they may be able to change the way they manage the stressors in their lives and potentially influence behavioral and biological factors associated with risk for breast cancer.
Despite the deleterious effects of psychological distress on health behaviors and biological outcomes that are relevant for women at risk for breast cancer, only a few pilot studies have sought to treat psychological distress among this population [28–31]. One multicenter pilot study reported that supportive-expressive group therapy was successful in reducing cancer worry, anxiety, and depression among 67 women identified as BRCA1 and BRCA2 mutation carriers who were either still at risk for breast cancer or were breast cancer survivors 1 year or more post-treatment [28]. We are aware of only one randomized controlled trial that addressed chronic distress among women at high risk for breast cancer [32]. In this study, women were randomized to one of three arms: a comparison group, medical information alone, or medical information in combination with written psychological self-help information in an information packet. Participants who received both the psychosocial self-help and medical information reported a significant decrease in cancer worry compared to women who received only medical information. However, the comparison group women who received no information also reported a significant decrease in cancer worry. These equivocal results suggest that more research is warranted. We are aware of no randomized controlled trials of group intervention to reduce distress among women at elevated risk for breast cancer.
Women with a family history of breast cancer should benefit from cognitive behavioral interventions that treat distress [33]. Cognitive behavioral stress management (CBSM) is a group intervention that combines cognitive behavioral therapy, coping skills training, techniques to improve social support, and training in various relaxation techniques, including mindfulness meditation [34]. CBSM has been shown to be effective in reducing general anxiety and distress as well as facilitating positive affect among breast cancer survivors [34] and in reducing the prevalence of depression among women with early-stage breast cancer (who entered the study with relatively low levels of distress; [35]). In the latter study, CBSM was found to be particularly effective for those participants who had the most confidence in their relaxation skills as a result of the intervention [36].
The purpose of the present study was to investigate the effects of CBSM on reducing distress among women at elevated risk for breast cancer due to family history. We hypothesized that participation in the intervention would be associated with decreased general and cancer-specific distress among women with a family history of breast cancer compared to a wait-list comparison group. We also hypothesized that the effects would be most pronounced for those who practiced the relaxation techniques the most.
Method
Participants
Participants were recruited from the greater Seattle area through a variety of means including letters sent via mail, flyers posted in the community, newspaper and radio advertisements, community health events, brochures distributed at medical centers, word of mouth, and employee newsletters. Participants were recruited based on self-reported elevated levels of distress. Eligible participants for the study were between the ages of 18 and 60 and reported having any family history of breast cancer, a healthy immune system, and elevated levels of distress. Because most women overestimate their actual risk [7, 37, 38], we were most interested in perceived risk, not actual risk; data were not collected on family history patterns to calculate objective risk. Participants were screened for elevated general or cancer-specific distress using the 4-item Perceived Stress Scale (PSS; [39]) and the Cancer Worry Scale [40]. Cutoff scores for both screening instruments were 1/2 standard deviation above the population mean and are described in more detail below. Exclusion criteria included prior diagnosis of cancer or autoimmune disease, current major depressive episode, history of psychotic disorder, smoking or substance dependence, consuming more than 10 drinks of alcohol a week, and previous hepatitis A diagnosis or hepatitis A vaccination. All study procedures were approved by the Hutchinson Center Institutional Review Board. Informed consent was obtained in writing from all participants before study entry. The present study was registered at clinicaltrials.gov (NCT# 01048528). Data in the present manuscript are from two related NIH-funded studies (funded by grants K07 CA107085-01 and 1 R21 CA134813-01A2). Because the studies were identical, with the exception of the R21 in vitro immune assessment, we included participants from both the K07 and R21 studies in the present manuscript. The two studies had comparable selection criteria, methodology, and participant demographics.
Design
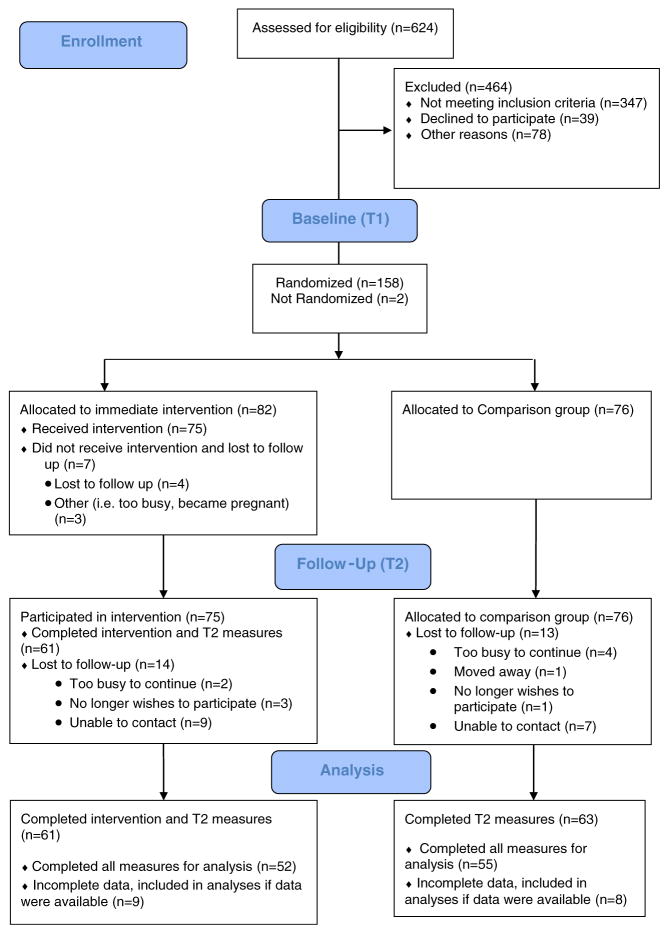
Eligible participants completed a baseline (T1) questionnaire and then were randomized to either a 10-week structured CBSM intervention or a wait-list comparison group. Outcome variables were collected at baseline (T1) and immediately post-intervention or 10-week waiting period (T2). Comparison group women did not have any contact with the researchers during the initial intervention period beyond being contacted to collect T2 outcome variables and were offered the full CBSM intervention after completing questionnaires at all time points. The present study is part of a larger study examining the effects of CBSM on antibody response to hepatitis A vaccine, which was administered after the intervention or waiting period, at T2. Participant flow through T1 and T2 of the study is illustrated in Fig. 1. Attrition did not differ significantly by condition at time 2, χ2(1, 155)=0.42, p=0.52. The present manuscript includes only data from T1 to T2.
Fig. 1.
Participant flowchart
Procedures
Women randomized to the intervention condition were asked to attend 10 structured, 2-hour CBSM sessions. Participants met weekly in closed groups of 4–10 women. The CBSM intervention was based on an existing intervention for women with early-stage breast cancer [35] but targeted for women at elevated risk for breast cancer (e.g., by including risk-relevant examples in the text). Intervention components (displayed in Table 1) were similar to the existing CBSM intervention and included education on awareness of the effects of stress, cognitive reframing, cognitive coping skills training, assertiveness training, anger management, and various relaxation techniques (including progressive muscle relaxation, guided imagery, and mindfulness meditation). In addition, participants were given a set of audio CDs with all relaxation exercises for home practice along with weekly log sheets to record home relaxation practice. On the weekly log sheet, the participants indicated the practice date, what their stress level was (on a scale from 1, “No stress” to 7, “Extremely stressed”) both before and after practice. Participants also noted whether they used the CD provided to them. Participants were not asked to record what exercise they used; however, they were encouraged to try each of the different relaxation techniques as they learned them, and then use the ones which they found most helpful.
Table 1.
Components of CBSM intervention by week
| Week | Topic | Activities and discussion |
|---|---|---|
| 1 | Introduction, overview, and rationale for stress management |
|
| 2 | Stress and awareness |
|
| 3 | Automatic thoughts and cognitive distortions |
|
| 4 | Cognitive restructuring: changing distorted thoughts to more rational ones |
|
| 5 | Introduction to coping strategies |
|
| 6 | Coping strategies |
|
| 7 | Social support |
|
| 8 | Anger management |
|
| 9 | Assertiveness training |
|
| 10 | Wrap-up |
|
All CBSM sessions were led by female licensed clinical psychologists, master-level social workers, postdoctoral fellows, or psychology interns. All sessions were audiotaped with participants’ consent and reviewed to monitor adherence to the intervention protocol using a module-specific intervention integrity checklist. All group leaders were trained in CBSM intervention protocols and met regularly with Dr. McGregor for supervision.
Assessment
Baseline Questionnaire
Participants provided demographic (e.g., race/ethnicity, marital status) and general health information (height, weight) prior to the start of the intervention. Additionally, participants answered questions regarding previous outpatient mental health treatment, current prescription medication for depression, and lifetime depression diagnoses. Finally, participants were asked to describe their self-reported health using the following item, “How would you rate your health right now?” Response options ranged from 1 (“excellent”) to 5 (“poor”) [41].
Life Events Checklist
The Life Events Checklist (LEC) is a 42-item checklist of potentially traumatic life events in the past 2 years [42]. Example items include “major personal injury or illness” and “change in residence.” Previous research has established that the LEC has exhibited adequate temporal stability and good convergence with an established measure of trauma history. Researchers have also demonstrated that scores on the LEC are associated with variables known to be correlated with measures of psychological distress and PTSD symptoms [43, 44]. The LEC was administered prior to the start of the intervention. In the present sample, the LEC demonstrated sufficient internal reliability (Cronbach’s α=0.76).
Center for Epidemiological Studies Depression Scale
Depressive symptoms were measured with the Center for Epidemiological Studies Depression Scale (CES-D), a 20-item self-report measure [45]. Participants report the frequency of symptoms in the past week on a scale from 1 (rarely or none of the time) to 4 (most or all of the time). Example items include “I had trouble keeping my mind on what I was doing” and “I felt lonely.” The CES-D is a widely used screening tool for depression with a score of 16 or greater considered clinically significant [45]. In the present sample, the CES-D demonstrated good internal reliability (Cronbach’s α=0.89).
Perceived Stress
Two versions of the Perceived Stress Scale (PSS) were used—one (the 4-item) for screening before study entry and one (the 10-item) for the baseline and follow-up questionnaire. The 4-item PSS was used to screen potential participants over the phone during a screening interview. The 4-item version of the PSS was developed for screening and has validated community sample norms [46]. Items indicate feelings and thoughts regarding lack of predictability as well as a sense of being overloaded. For screening in the present study, the cutoff score was 6 (1/2 SD above the mean), based on the mean PSS for women aged 45–54 (the mean age of the present sample) in a US normative sample (mean=4.4, SD=2.9; [39, 46]). In the present sample, the 4-item PSS demonstrated acceptable internal reliability (Cronbach’s α=0.70).
The 10-item PSS, a self-report scale that measures stress appraisal of participants’ lives in the past month [46], was used in the larger baseline and follow-up questionnaires. Items indicate feelings and thoughts regarding lack of predictability as well as a sense of being overloaded. Response options ranged from 0 (never) to 4 (very often). The mean PSS for women aged 45–54 (the mean age of the present sample) in a US normative sample was=12.6, SD=6.1 [39, 46]. In the present sample, the 10-item PSS demonstrated good internal consistency (Cronbach’s α=0.85).
Perceived Breast Cancer Risk
Three items were used to assess perceived risk for breast cancer. These items were modeled after the questions from Weinstein [47]. The first question asked of the participants was “Compared to most women, what do you think the chances are that you will get breast cancer someday?” Response options ranged from 1 (“much lower than average”) to 5 (“much higher than average”). The second question asked women “What do you think the chances are that you will have breast cancer someday?” The response options to this question were similar to the second question and ranged from 0 (“0 %”) to 9 (“100 %”). The third question asked women “On a scale of 0 to 100 (0 meaning no chance and 100 meaning definitely), what do you think your chances of getting breast cancer are?” A three-item scaled score was created for perceived risk by converting the items to Z scores, adding the Z scores, and dividing by 3. In the present sample, internal consistency (Cronbach’s α=0.72) was comparable to that demonstrated in a larger community-based sample (Cronbach’s α=0.82; [7]).
Cancer Worry Scale
The Cancer Worry Scale is a 4-item self-report scale designed to assess worry about the risk of breast cancer and the extent of interference the worry has on daily functioning [40]. Participants rate items on a scale of 1 (rarely or not at all) to 4 (a lot). The Cancer Worry Scale was used during the phone screening interview to assess cancer-specific distress for screening purposes. The mean total breast cancer worry score from a community-based sample of women in the Seattle area (mean=5.51 [SD=1.62]; [7]) was used to determine a cutoff score of 7. The Cancer Worry Scale was also administered in the baseline and follow-up questionnaires. In the present sample, internal consistency (Cronbach’s α=0.68) was comparable to that demonstrated in a larger community-based sample (Cronbach’s α=0.73; [7]).
Analytic Plan
The distributions of all variables were examined for outliers and normality. Descriptive statistics were completed, and success of randomization was tested using independent t tests for continuous variables and Pearson χ2 or Fisher’s exact test for categorical variables. We then examined theoretically based baseline covariates previously shown to be related to changes in distress including self-reported health [48], past depression diagnosis and antidepressant medication use [49, 50], ethnicity and race [51], marital status [52], prior mental health treatment [53–55], and stressful life events [43, 44]. For the purposes of covariate analysis, we combined demographic categories with small cell sizes. For ethnicity, individuals were categorized as Caucasian or Other. For marital status, married and living with a partner were combined. Individuals who were single, separated, divorced, or widowed made up a second category. Finally, with regard to unemployment status, participants were categorized as employed or unemployed. Self-reported health, lifetime depression, current depression medications, ethnicity and race, marital status, past mental health treatment, and life events were included a priori as control variables in all analyses involving changes in distress. Missing data in all regressions were handled with listwise deletion.
-
Does CBSM reduce depressive symptoms, perceived stress, or breast cancer worry?
After determining that groups did not differ at baseline (T1) on any outcome variables, except CES-D score, hierarchical regression analyses assessed whether depressive symptoms, perceived stress, and breast cancer worry in the intervention group were significantly different than in the comparison group at follow-up (T2). The T2 score was modeled as the outcome, the T1 score as the covariate (in addition to including the aforementioned covariates), and the treatment group as the final predictor in the model.
-
Is CBSM more effective among participants who practice relaxation more?
Participants recorded relaxation practice on log sheets which they turned in each week during the intervention. Total relaxation practice was calculated by summing the total number of relaxation practices outside of session. Next, we tested a hierarchical regression in which the postintervention score was modeled as the outcome, the baseline score as the covariate, as well as the aforementioned a priori covariates and main effects of assigned group (intervention vs. comparison). In the final block, the total amount of relaxation practice was entered as the predictor of interest.
-
Intent-to-treat analysis
An intent-to-treat (ITT) analysis [56] was performed. When T2 data was missing, the baseline data were carried forward as an assumption of nonresponse or return to baseline. Intent-to-treat analyses included all randomized participants with complete T1 data. ITT analysis prevents overoptimistic estimates of the efficacy of an intervention because it takes into account the fact that noncompliance and protocol deviations are likely to occur in actual clinical practice.
Results
Sample Description and Group Analyses
Demographic information is shown in Table 2. Independent sample t tests revealed no significant differences between the intervention and comparison groups for demographic (Table 2) or baseline assessments of distress variables (Table 3) except the CES-D score. Additionally, no significant differences were present between the intervention and comparison groups at baseline for self-reported health (t=−0.25, p=0.81), height (t=−0.37, p=0.71), or weight (t=1.79, p= 0.07). On average, participants attended 8.43 (SD=1.76) sessions and practiced relaxation 3.85 (SD=2.02) times per week.
Table 2.
Pretreatment demographic variables by treatment group
| Variables | Comparison (N=76) | Treatment (N=82) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | t (p) | |
| Age | 43.62 (10.65) | 42.84 (10.43) | 0.46 (0.64) |
| Education | 16.62 (2.03) | 17.18 (2.37) | −1.59 (0.11) |
| N (%) | N (%) | Fisher’s exact test (p) | |
| Ethnicity/race | 1.97 (0.85) | ||
| Latino | 1 (1.3) | 6 (7.4) | |
| White | 62 (81.6) | 69 (85.2) | |
| Black or African-American | 4 (5.3) | 2 (2.5) | |
| Asian | 5 (6.6) | 4 (4.9) | |
| Other | 5 (6.6) | 5 (6.2) | |
| Prefer not to answer | 0 (0.0) | 1 (1.2) | |
| Marital/partner status | 3.72 (0.62) | ||
| Single | 26 (34.2) | 32 (39.5) | |
| Married | 37 (48.7) | 35 (43.2) | |
| Living with a partner | 5 (6.6) | 8 (9.9) | |
| Separated | 0 (0.0) | 1 (1.2) | |
| Divorced | 7 (9.2) | 4 (4.9) | |
| Widowed | 1 (1.3) | 0 (0.0) | |
| Employment status | 3.54 (0.48) | ||
| Full-time | 51 (67.0) | 57 (71.2) | |
| Part-time | 11 (14.5) | 10 (13.8) | |
| Self-employed | 6 (7.9) | 3 (3.8) | |
| Not employed | 6 (7.9) | 9 (11.2) | |
| Retired | 2 (2.6) | 0 (0.0) | |
| Pearson’s χ2 | |||
| Previous mental health treatment | 3.06 (0.08) | ||
| Yes | 42 (55.3) | 55 (67.9) | |
| No | 33 (43.4) | 24 (29.6) |
Data presented represent the most complete data available, as some participants chose not to answer demographic questions
Table 3.
Baseline (T1) and follow-up (T2) outcome variable means for the comparison (C) and treatment (T) groups from multivariate ANOVA with a priori covariates
| T1 mean (SD)
|
T2 mean (SD)
|
|||||
|---|---|---|---|---|---|---|
| C | T | F(1, 110) | C | T | F(1, 110) | |
| N | 55 | 57 | 55 | 57 | ||
| CES-D | 13.36 (8.26) | 15.51 (9.31) | 3.00* | 13.35 (7.77) | 12.82 (10.25) | 3.38* |
| PSS | 17.36 (5.50) | 19.26 (5.87) | 1.62 | 16.67 (5.81) | 15.81 (5.99) | 2.14* |
| BCW | 5.87 (1.99) | 5.86 (1.70) | 0.78 | 5.73 (1.80) | 5.30 (1.24) | 1.07 |
| PBCR | −0.03 (0.77) | 0.00 (0.85) | 1.55 | 0.09 (1.00) | −0.10 (0.82) | 1.16 |
CES-D Center for Epidemiologic Studies Depression Scale, PSS Perceived Stress Scale, BCW breast cancer worry, PBCR perceived breast cancer risk (means are reported as Z scores)
p<0.05
Primary Results
-
Does CBSM reduce depressive symptoms, perceived stress, or breast cancer worry?
Three regression tests assessed whether depressive symptoms, perceived stress, or breast cancer worry in the intervention group were significantly lower than the comparison group following CBSM. The first regression revealed that group membership significantly predicted levels of T2 depressive symptoms (β= −0.17, p<0.05), explaining a total of 34 % of the variance of T2 depressive symptoms when controlling for baseline depressive symptoms and theoretically based covariates. Second, group membership significantly predicted T2 perceived stress (β= −0.21, p<0.05), explaining an additional 4 % of the variance above and beyond T1 perceived stress and the covariates included in the model. Group membership did not significantly predict T2 breast cancer worry (β= −0.17, p= 0.055), but the trend approached statistical significance. Finally, group membership did not significantly predict perceived risk (β= −0.11, p=0.086). These results are displayed in Table 4.
Paired sample t tests revealed that individuals within the immediate treatment group experienced significant decreases in depressive symptoms (t(60)=2.02, p=0.048), perceived stress (t(59)=4.38, p<0.001), and breast cancer worry (t(60)=2.98, p=0.004). However, there was not a significant change in perceived breast cancer risk among group participants (t(59)=1.33, p=0.189). On average, group participants decreased 2.79 points on the CES-D, 3.63 points on the PSS, and 0.64 points on the measure of breast cancer worry.
-
Is CBSM more effective among participants who practice relaxation more?
A final set of three regression tests examined the effect of relaxation practice on posttreatment symptoms within the immediate intervention group. The first regression examined T2 depressive symptoms. Greater practice was significantly related to lower T2 depressive symptoms (β= −0.34, p<0.05), explaining an additional 9 % of the variance above and beyond baseline depressive symptoms and relevant covariates.
The second regression examined postintervention perceived stress. Greater practice was significantly related to lower T2 perceived stress (β= −0.32, p<0.05), explaining an additional 8 % of the variance above the control variables. Additionally, greater practice was significantly related to lower T2 breast cancer worry (β= −0.30, p=0.041). Finally, greater relaxation practice was not associated with a change in perceived breast cancer risk (β=0.07, p=0.538). These results are presented in Table 5.
Next, for graphical purposes, a median split was created among intervention participants to graph changes in the outcome variable of interest by level of relaxation practice. The sample was divided into three groups: intervention low practice (N=28), intervention high practice (N=24), and comparison (N=59). The relationship between the practice group and the change in depressive symptoms is shown in Fig. 2 and the change in perceived stress in Fig. 3.
Paired sample t tests revealed that there were significant decreases in depressive symptoms (t(23)=4.25, p<0.001) and perceived stress (t(23)=5.22, p<0.001) among the 24 individuals in the high-practice (38 or more total hours of relaxation) group. Additionally, these individuals also exhibited a decrease in breast cancer worry that approaches statistical significance (t(23)=2.02, p=0.055). However, there were no significant changes in perceived risk within this group. On average, participants who were in the high-practice group decreased 6.96 points on the CES-D, 5.63 points on the PSS, and 0.58 points on the measure of breast cancer worry.
-
Intent-to-treat analysis
Regression analyses using an ITT approach yielded more conservative results. Group membership significantly predicted levels of T2 perceived stress (β= −0.152, p=0.033) as expected. Interestingly, group membership also significantly predicted levels of T2 breast cancer risk in the ITT analysis (β= −0.103, p=0.045) but not our primary analyses (β= −0.11, p=0.086). This is likely due to a significant outlier on T1 perceived breast cancer risk that did not complete T2 measures and was excluded in our primary analyses. Group membership did not significantly predict levels of T2 depressive symptoms (β= −0.123, p=0.090) or breast cancer worry (β= −0.119, p=0.09), but both of these associations were in the expected direction based upon our hypotheses.
Table 4.
Hierarchical linear regressions of psychological distress following participation in CBSM
| Regression equation numbera | Outcome variable | N | R2 | β | ΔR2 | F of ΔR2 | p |
|---|---|---|---|---|---|---|---|
| 1 | T2 depressive symptoms | 119 | 0.34 | −0.17 | 0.03 | 4.23 | 0.042 |
| 2 | T2 perceived stress | 118 | 0.33 | −0.21 | 0.04 | 5.95 | 0.016 |
| 3 | T2 breast cancer worry | 119 | 0.28 | −0.17 | 0.03 | 3.76 | 0.055 |
| 4 | T2 perceived breast cancer risk | 114 | 0.62 | −0.11 | 0.01 | 3.01 | 0.086 |
Delta analyses indicate the additional amount of variance explained by adding additional variables to the regression equation
All regression equations controlled for baseline levels of outcome variable of interest (1: depressive symptoms, 2: perceived stress, 3: breast cancer worry, 4: perceived breast cancer risk) in the first block of equation and a priori covariates in block 2. Group (treatment or control) was the final predictor in the model
Table 5.
Hierarchical linear regressions of relaxation practice predicting psychological distress
| Regression equationa | Outcome variable | N | R2 | β | ΔR2 | F of ΔR2 | p |
|---|---|---|---|---|---|---|---|
| 1 | T2 depressive symptoms | 53 | 0.44 | −0.34 | 0.09 | 6.30 | 0.016 |
| 2 | T2 perceived stress | 52 | 0.38 | −0.32 | 0.08 | 5.16 | 0.029 |
| 3 | T2 breast cancer worry | 53 | 0.39 | −0.30 | 0.07 | 4.47 | 0.041 |
| 4 | T2 perceived breast cancer risk | 52 | 0.63 | 0.07 | 0.00 | 0.39 | 0.538 |
Delta analyses indicate the additional amount of variance explained by adding additional variables to the regression equation
All regression equations controlled for baseline levels of outcome variable of interest (1: depressive symptoms, 2: perceived stress, 3: breast cancer worry, 4: perceived breast cancer risk) in the first block of equation and a priori covariates in block 2. Group (treatment or control) was entered in block 3, and relaxation was the final predictor in the model
Fig. 2.
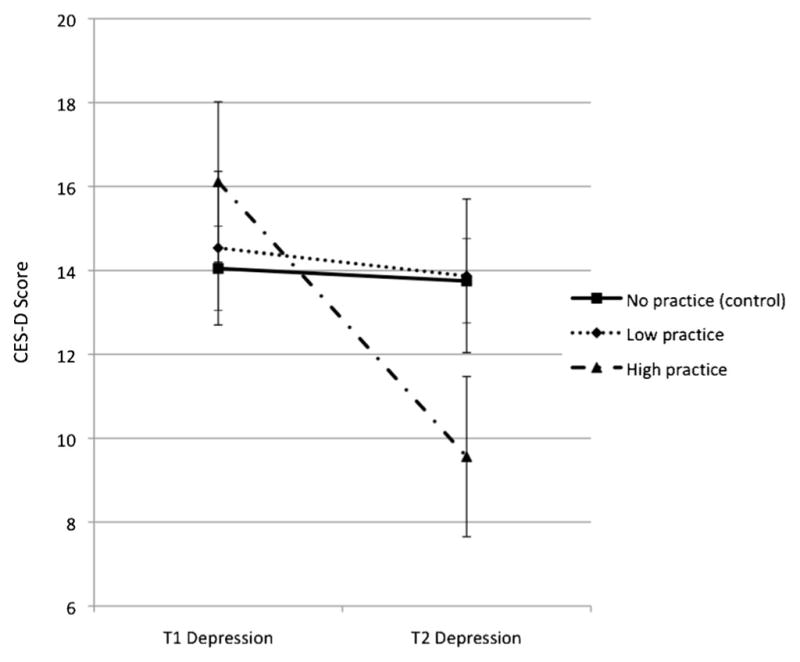
Changes in depressive symptoms from baseline (T1) to follow-up (T2) among comparison, intervention low-relaxation practice, and intervention high-relaxation practice groups. The intervention high-practice group reported significantly lower T2 depressive symptoms than the comparison or intervention low-practice group. Note: median total practice=38 h
Fig. 3.
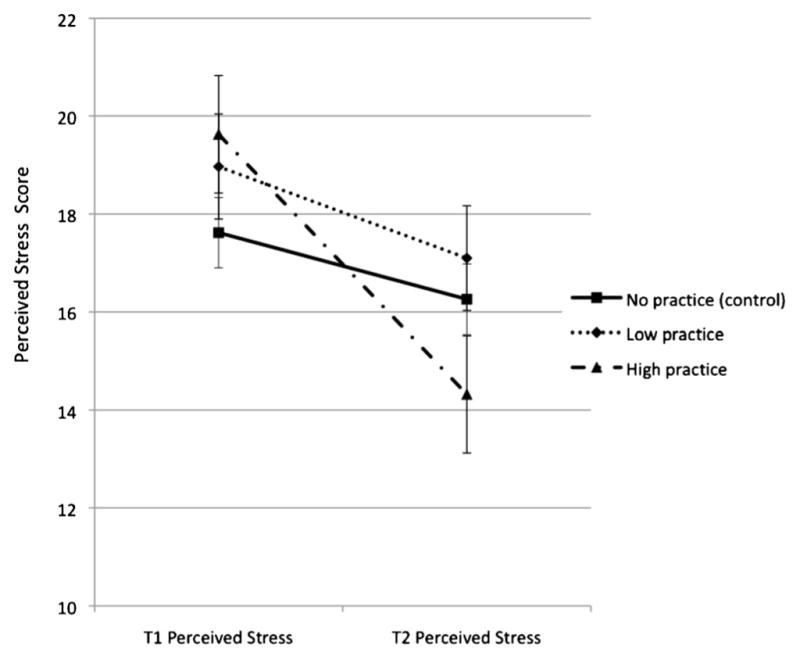
Changes in perceived stress from baseline (T1) to follow-up (T2) among comparison, intervention low-relaxation practice, and intervention high-relaxation practice groups. The intervention high-practice group reported significantly lower T2 Perceived Stress Scale scores than the comparison or intervention low-practice group. Note: median total practice=38 h
Discussion
The present randomized clinical trial is, to our knowledge, the first to report that a group-based CBSM intervention proven effective among breast cancer survivors can reduce depressive symptoms and perceived stress among women with an elevated risk of breast cancer due to family history who were reporting elevated distress at baseline. The changes in depressive symptoms and perceived stress reported between baseline and posttreatment time points were small but clinically meaningful [57–59], with Cohen’s f2 effect sizes of 0.04 (depressive symptoms) and 0.07 (perceived stress). While modest, the changes in depressive symptoms and perceived stress are not unexpected given that the women in the sample were not experiencing a major life stressor, like a breast cancer diagnosis, at baseline. Thus, the modestly elevated levels of depressive symptoms and perceived stress at baseline did not have as much room for improvement. The magnitude of the changes in these distress measures is similar to the Esplen study [28], which also utilized an intervention, supportive-expressive group therapy, which had been shown effective (also with greater magnitude due to their greater baseline distress) in reducing distress among breast cancer survivors [60].
Unlike the Esplen and other pilot interventions studies [28, 31], we did not see a significant reduction in cancer worry as a result of the intervention in the present study. However, the trend was in the expected direction and approached statistical significance. The lack of significant change in cancer worry, while unexpected, makes sense if one considers the sample in the present study. Women in the present study were recruited from the community, not a high-risk clinic. The 70 women in the Esplen study were all BRCA1 or BRCA2 mutation carriers recruited from a high-risk clinic, and the groups were mixes of women with and without a prior breast or ovarian cancer diagnosis. Thus, their risk perceptions were likely higher on average than the women in the present study.
Since perceived risk of breast cancer is associated with elevated levels of cancer worry [7], we expected a significant decrease in perceived risk and cancer worry. Previous work has demonstrated that most women overestimate their risk of breast cancer [7, 37, 38]. Overestimating breast cancer risk could be considered a cognitive distortion, one that should, like other maladaptive cognitive distortions, respond well to CBSM techniques. The fact that we did not see significant reductions in perceived risk (data not shown) or cancer worry as a result of CBSM in the present study is likely due to the fact that women were eligible for the present study if they had any family history of breast cancer (i.e., they did not need to be a mutation carrier or have a history of breast or ovarian cancer as in the Esplen study). Thus, their risk perceptions may not have been as great. The women in the present study were screened for elevated general distress or cancer worry at study entry, but often their general distress (e.g., due to work, chronically ill children, aging parents) was more salient than their cancer worry. Thus, it is not surprising that cancer worry was not influenced in the present study as greatly as it was for the women in the Esplen study [28].
Relaxation practice proved to be an important predictor of change in distress following the intervention. Intervention group women who practiced relaxation more reported greater decreases in depressive symptoms and perceived stress compared to those who practiced less or the comparison group women. Similar relaxation practice effects were reported in a CBSM study with HIV-positive men [61, 62]. Interventions with mindfulness-based stress reduction have also reported greater practice associated with greater reductions in psychological distress [63, 64]. Collectively, these papers suggest that participants’ relaxation practice outside of session may be an important component of psychological interventions, such as CBSM, to reduce psychological distress. It could be that greater relaxation practice potentiates the effects of CBSM by creating and maintaining a relaxed state. From this relaxed place, the participants may be better able to use the cognitive techniques learned in the program. However, this needs to be tested empirically in future research. Beneficial practice effects have also been seen among women at risk for breast cancer who practiced a problem-solving training pilot intervention more [29].
The findings from the present study are promising. To our knowledge, this is the first known application of CBSM with a sample of healthy women at elevated risk of breast cancer. Women at elevated risk for breast cancer represent a unique subset of individuals who are more likely to experience chronic distress. High-risk women with passive and palliative coping styles, excessive breast self-examination, and overestimation of breast cancer risk are more likely to report higher chronic distress [65]. While reductions in cancer worry did not reach statistical significance among CBSM intervention participants in the present study, it is likely that CBSM would be effective at reducing cancer worry among women recruited from a high-risk clinic who report higher levels of cancer worry. Future studies should include women from high-risk clinics who report greater distress, and thus, have the potential for more benefit.
The present study represents several unique strengths. First, while most studies of distress among women with a family history of breast cancer have recruited from high-risk clinics, we recruited our sample of participants from the community, thus increasing the generalizability of our findings. Second, we utilized a randomized pre- and post-study design to best capture the dynamics of outcomes. Third, we measured both general and cancer-specific distress, as well as relaxation practice, a moderator of intervention effectiveness.
The present study also had a number of limitations. Our sample was largely composed of White participants and we did not corroborate their actual risk for breast cancer by collecting family history patterns with genetic counseling. Thus, we cannot generalize our findings to minority and/or high-risk mutation-carrier women. Additionally, future work should also evaluate the effects of CBSM on cancer-relevant health behaviors, such as diet and exercise behavior, and biological outcomes, such as improvements in immune function, cortisol levels, and DNA repair processes. We did not have a placebo or attention comparison group. We also did not directly assess whether women in the wait-list comparison group had access to mental health services between the baseline (T1) and follow-up (T2) assessment. As an indirect measure, we found three women who reported past use of outpatient mental health services at T2 that had not reported use of these services at baseline. However, the women who reported new use of mental health services at T2 did not report significant changes in any of the outcome variables. Furthermore, data on retention of effects are not presented here. Previous studies among breast cancer survivors have reported that effects of CBSM are durable, lasting through 1 year follow-up [34]. Future work should provide data on retention of effects after the end of the intervention period. Finally, though the comparison group women were offered the full CBSM intervention after completing questionnaires at all time points, we did not continue to collect data from comparison participants while they participated in the CBSM intervention.
In summary, group-based cognitive behavioral stress management can reduce psychological distress among women with a family history of breast cancer, especially among women who practice relaxation regularly. Given that elevated and chronic distress can interfere with health behaviors and biological outcomes relevant for breast cancer risk, the present findings suggest CBSM as an encouraging avenue of therapy for women at elevated risk for breast cancer.
Acknowledgments
This study was funded by grants from the National Cancer Institute (K07 CA107085-01 (McGregor) and 1 R21 CA134813-01A2 (McGregor)).
Footnotes
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards
Authors Bonnie A. McGregor, Ph.D.; Emily D. Dolan, Ph.D.; Timothy S. Sannes, Ph.D.; Krista B. Highland, Ph.D.; Denise L. Albano, M.P.H.; Alison A. Ward, Ph.D.; Anna M. Charbonneau, Ph.D.; Mary W. Redman, Ph.D.; Karly M. Murphy, M.S.; and Rachel M. Ceballos, Ph.D., declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1.Cukier YR, et al. Factors associated with psychological distress among women of African descent at high risk for BRCA mutations. J Genet Couns. 2013;22(1):101–107. doi: 10.1007/s10897-012-9510-1. [DOI] [PubMed] [Google Scholar]
- 2.Patenaude AF, et al. Young adult daughters of BRCA1/2 positive mothers: What do they know about hereditary cancer and how much do they worry? Psychooncology. 2013;22(9):2024–2031. doi: 10.1002/pon.3257. [DOI] [PubMed] [Google Scholar]
- 3.Decruyenaere M, et al. Cognitive representations of breast cancer, emotional distress and preventive health behaviour: A theoretical perspective. Psychooncology. 2000;9(6):528–536. doi: 10.1002/1099-1611(200011/12)9:6<528::aid-pon486>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Erblich J, Bovbjerg DH, Valdimarsdottir HB. Looking forward and back: Distress among women at familial risk for breast cancer. Ann Behav Med. 2000;22(1):53–59. doi: 10.1007/BF02895167. [DOI] [PubMed] [Google Scholar]
- 5.Bennett P, et al. Long-term cohort study of women at intermediate risk of familial breast cancer: Experiences of living at risk. Psycho-Oncology. 2010;19(4):390–398. doi: 10.1002/pon.1588. [DOI] [PubMed] [Google Scholar]
- 6.Metcalfe KA, et al. The impact of having a sister diagnosed with breast cancer on cancer-related distress and breast cancer risk perception. Cancer. 2013;119(9):1722–1728. doi: 10.1002/cncr.27924. [DOI] [PubMed] [Google Scholar]
- 7.McGregor BA, et al. Optimism, perceived risk of breast cancer, and cancer worry among a community-based sample of women. Health Psychol. 2004;23(4):339–344. doi: 10.1037/0278-6133.23.4.339. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher KE, et al. A path analysis of factors associated with distress among first-degree female relatives of women with breast cancer diagnosis. Health Psychol. 2006;25(3):413–424. doi: 10.1037/0278-6133.25.3.413. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, et al. Psychological distress among healthy women with family histories of breast cancer: Effects of recent life events. Psychooncology. 2004;56:182–191. doi: 10.1002/pon.870. [DOI] [PubMed] [Google Scholar]
- 10.Coyne JC, et al. Distress and psychiatric morbidity among women from high-risk breast and ovarian cancer families. J Consult Clin Psychol. 2000;68(5):864–874. [PubMed] [Google Scholar]
- 11.Erblich J, Bovbjerg DH, Valdimarsdottir HB. Psychological distress, health beliefs, and frequency of breast self-examination. J Behav Med. 2000;23(3):277–292. doi: 10.1023/a:1005510109233. [DOI] [PubMed] [Google Scholar]
- 12.Bowen DJ, et al. The relationship between perceived risk, affect, and health behaviors. Cancer Detect Prev. 2004;28(6):409–417. doi: 10.1016/j.cdp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Bovbjerg DH, Valdimarsdottir H. Familial cancer, emotional distress, and low natural cytotoxic activity in healthy women. Ann Oncol. 1993;4(9):745–752. doi: 10.1093/oxfordjournals.annonc.a058659. [DOI] [PubMed] [Google Scholar]
- 14.Bovbjerg DH, et al. Stronger cortisol responses to laboratory stressors are independently predicted by breast-cancer specific intrusions and family history. The Annual Meeting and Scientific Sessions of the Society of Behavioral Medicine; San Francisco, CA. 2006. [Google Scholar]
- 15.Flint MS, Bovbjerg DH. DNA damage as a result of psychological stress: Implications for breast cancer. Breast Cancer Res. 2012;14(5):320. doi: 10.1186/bcr3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James GD, et al. The rate of urinary cortisol excretion at work is persistently elevated in women at familial risk for breast cancer. Am J Hum Biol. 2008;20:478–480. doi: 10.1002/ajhb.20737. [DOI] [PubMed] [Google Scholar]
- 17.Dawson DA, Grant BF, Ruan WJ. The association between stress and drinking: Modifying effects of gender and vulnerability. Alcohol Alcohol. 2005;40(5):453–460. doi: 10.1093/alcalc/agh176. [DOI] [PubMed] [Google Scholar]
- 18.Wardle J, et al. Stress, dietary restraint and food intake. J Psychosom Res. 2000;48(2):195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 19.Stetson BA, et al. Prospective evaluation of the effects of stress on exercise adherence in community-residing women. Health Psychol. 1997;16(6):515–520. doi: 10.1037//0278-6133.16.6.515. [DOI] [PubMed] [Google Scholar]
- 20.Kouvonen A, et al. Relationship between work stress and body mass index among 45,810 female and male employees. Psychosom Med. 2005;67(4):577–583. doi: 10.1097/01.psy.0000170330.08704.62. [DOI] [PubMed] [Google Scholar]
- 21.Smith AW, Baum A, Wing RR. Stress and weight gain in parents of cancer patients. Int J Obes (Lond) 2005;29(2):244–250. doi: 10.1038/sj.ijo.0802835. [DOI] [PubMed] [Google Scholar]
- 22.Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: Physiological pathways and mechanisms. Psychosom Med. 2011;73(9):724–730. doi: 10.1097/PSY.0b013e318235be76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dettenborn L, et al. Heightened cortisol responses to daily stress in working women at familial risk for breast cancer. Biol Psychol. 2005;69(2):167–179. doi: 10.1016/j.biopsycho.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Disis ML, Park KH. Immunomodulation of breast cancer via tumor antigen specific Th1. Cancer Res Treat. 2009;41(3):117–121. doi: 10.4143/crt.2009.41.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran TJ, et al. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res. 2000;60(4):867–872. [PubMed] [Google Scholar]
- 26.Andersen MR, et al. Breast cancer worry and mammography use by women with and without a family history in a population-based sample. Cancer Epidemiol Biomarkers Prev. 2003;12(4):314–320. [PubMed] [Google Scholar]
- 27.Price MA, et al. Predictors of breast cancer screening behavior in women with a strong family history of the disease. Breast Cancer Res Treat. 2010;124(2):509–519. doi: 10.1007/s10549-010-0868-1. [DOI] [PubMed] [Google Scholar]
- 28.Esplen MJ, et al. A multicenter study of supportive-expressive group therapy for women with BRCA1/BRCA2 mutations. Cancer. 2004;101(10):2327–2340. doi: 10.1002/cncr.20661. [DOI] [PubMed] [Google Scholar]
- 29.Kash KM, et al. Psychological counseling strategies for women at risk of breast cancer. J Natl Cancer Inst Monogr. 1995;17:73–79. [PubMed] [Google Scholar]
- 30.Wellisch DK, et al. Depression and anxiety symptoms in women at high risk for breast cancer: Pilot study of a group intervention. Am J Psychiatry. 1999;156(10):1644–1645. doi: 10.1176/ajp.156.10.1644. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz MD, et al. The impact of a brief problem-solving training intervention for relatives of recently diagnosed breast cancer patients. Ann Behav Med. 1998;20(1):7–12. doi: 10.1007/BF02893803. [DOI] [PubMed] [Google Scholar]
- 32.Appleton S, et al. A randomised controlled trial of a psychoeducational intervention for women at increased risk of breast cancer. Br J Cancer. 2004;90(1):41–47. doi: 10.1038/sj.bjc.6601519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bovbjerg DH, Valdimarsdottir HB. Interventions for health individuals at familial risk for cancer. In: Baum A, Andersen BL, editors. Psychosocial Interventions for Cancer. Washington, D.C: American Psychological Association; 2001. pp. 305–320. [Google Scholar]
- 34.Antoni MH, et al. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am J Psychiatry. 2006;163(10):1791–1797. doi: 10.1176/ajp.2006.163.10.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antoni MH, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol. 2001;20(1):20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- 36.Antoni MH, et al. How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol. 2006;74(6):1143–1152. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black WC, Nease RF, Jr, Tosteson AN. Perceptions of breast cancer risk and screening effectiveness in women younger than 50 years of age. J Natl Cancer Inst. 1995;87(10):720–731. doi: 10.1093/jnci/87.10.720. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd S, et al. Familial breast cancer: A controlled study of risk perception, psychological morbidity and health beliefs in women attending for genetic counselling. Br J Cancer. 1996;74(3):482–487. doi: 10.1038/bjc.1996.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapam S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park: Sage; 1988. [Google Scholar]
- 40.Lerman C, et al. Psychological side effects of breast cancer screening. Health Psychol. 1991;10(4):259–267. doi: 10.1037//0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- 41.DeSalvo KB, et al. Assessing measurement properties of two single-item general health measures. Qual Life Res. 2006;15(2):191–201. doi: 10.1007/s11136-005-0887-2. [DOI] [PubMed] [Google Scholar]
- 42.Holmes T. The Schedule of Recent Experience. 1986. The Department of Psychiatry and Behavioral Sciences, University of Washington School of Medicine; Seattle: The University of Washington Press; 1981. [Google Scholar]
- 43.Gray MJ, et al. Psychometric properties of the life events checklist. Assessment. 2004;11(4):330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- 44.Pillow DR, Zautra AJ, Sandler I. Major life events and minor stressors: Identifying mediational links in the stress process. J Pers Soc Psychol. 1996;70(2):381–394. doi: 10.1037//0022-3514.70.2.381. [DOI] [PubMed] [Google Scholar]
- 45.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 46.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 47.Weinstein N. Conceptualizing and Measuring Risk Perceptions. National Cancer Institute’s “Workshop on Conceptualizing (and Measuring) Perceived Risk”; Washington, DC. 2003. [Google Scholar]
- 48.Ruo B, et al. Depressive symptoms and health-related quality of life: The Heart and Soul Study. JAMA. 2003;290(2):215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reimherr FW, et al. Antidepressant efficacy of sertraline: A double-blind, placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression. J Clin Psychiatry. 1990;51(Suppl B):18–27. [PubMed] [Google Scholar]
- 50.Schuurmans J, et al. A randomized, controlled trial of the effectiveness of cognitive-behavioral therapy and sertraline versus a waitlist control group for anxiety disorders in older adults. Am J Geriatr Psychiatry. 2006;14(3):255–263. doi: 10.1097/01.JGP.0000196629.19634.00. [DOI] [PubMed] [Google Scholar]
- 51.Avis NE, et al. Quality of life in diverse groups of midlife women: Assessing the influence of menopause, health status and psychosocial and demographic factors. Qual Life Res. 2004;13(5):933–946. doi: 10.1023/B:QURE.0000025582.91310.9f. [DOI] [PubMed] [Google Scholar]
- 52.Molloy GJ, et al. Marital status, gender and cardiovascular mortality: Behavioural, psychological distress and metabolic explanations. Soc Sci Med. 2009;69(2):223–228. doi: 10.1016/j.socscimed.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costanzo ES, et al. Adjusting to life after treatment: Distress and quality of life following treatment for breast cancer. Br J Cancer. 2007;97(12):1625–1631. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jim HS, et al. History of major depressive disorder prospectively predicts worse quality of life in women with breast cancer. Ann Behav Med. 2012;43(3):402–408. doi: 10.1007/s12160-011-9333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Dorn RA, et al. The relationship between outpatient mental health treatment and subsequent mental health symptoms and disorders in young adults. Adm Policy Ment Health. 2010;37(6):484–496. doi: 10.1007/s10488-010-0291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee YJ, et al. Analysis of clinical trials by treatment actually received: Is it really an option? Stat Med. 1991;10(10):1595–1605. doi: 10.1002/sim.4780101011. [DOI] [PubMed] [Google Scholar]
- 57.Hays RD, Woolley JM. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmaco Economics. 2000;18(5):419–423. doi: 10.2165/00019053-200018050-00001. [DOI] [PubMed] [Google Scholar]
- 58.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 59.Beresford SA, et al. Seattle 5 a Day worksite program to increase fruit and vegetable consumption. Prev Med. 2001;32(3):230–238. doi: 10.1006/pmed.2000.0806. [DOI] [PubMed] [Google Scholar]
- 60.Classen C, et al. Supportive-expressive group therapy and distress in patients with metastatic breast cancer: A randomized clinical intervention trial. Arch Gen Psychiatry. 2001;58(5):494–501. doi: 10.1001/archpsyc.58.5.494. [DOI] [PubMed] [Google Scholar]
- 61.Cruess S, et al. Reductions in herpes simplex virus type 2 antibody titers after cognitive behavioral stress management and relationships with neuroendocrine function, relaxation skills, and social support in HIV-positive men. Psychosom Med. 2000;62(6):828–837. doi: 10.1097/00006842-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 62.Lutgendorf SK, et al. Cognitive-behavioral stress management decreases dysphoric mood and herpes simplex virus-type 2 antibody titers in symptomatic HIV-seropositive gay men. J Consult Clin Psychol. 1997;65(1):31–43. doi: 10.1037//0022-006x.65.1.31. [DOI] [PubMed] [Google Scholar]
- 63.Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31(1):23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- 64.Rosenzweig S, et al. Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68(1):29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 65.den Heijer M, et al. Long-term psychological distress in women at risk for hereditary breast cancer adhering to regular surveillance: A risk profile. Psycho-oncology. 2013;22(3):598–604. doi: 10.1002/pon.3039. [DOI] [PubMed] [Google Scholar]