Abstract
BACKGROUND
Hemolytic disease of the fetus and newborn, classically caused by maternal–fetal incompatibility of the Rh blood group D antigen, can be prevented by RhIG prophylaxis. While prophylactic practices for pregnant women with serologic weak D phenotypes vary widely, RHD genotyping could provide clear guidance for management. This analysis evaluated the financial implications of using RHD genotyping to guide RhIG prophylaxis among pregnant females.
STUDY DESIGN AND METHODS
A Markov-based model was constructed to evaluate the costs of RHD genotyping for pregnant females with serologic weak D phenotypes to inform RhIG prophylaxis. Using a comparison strategy of managing these women conservatively as D−, direct medical costs were assessed over 10- and 20-year periods for a simulated population of US women. One-way and probabilistic sensitivity analyses were used to assess the robustness of conclusions.
RESULTS
Using base-case variables, RHD genotyping for pregnant women with serologic weak D phenotypes is expected to marginally reduce overall costs. RHD genotyping these patients, rather than conservatively managing them as D−, would be cost-saving when the cost of genotyping is below $256. Genotyping would decrease net costs among non-Hispanic Caucasian females (−$0.17/pregnancy), but would increase costs among non-Hispanic African Americans (+$0.51/pregnancy), non-Hispanic American Indian/Alaskans (+$0.10/pregnancy), and Hispanics (+$0.37/pregnancy). Incorporating RHD genotyping would not significantly impact costs among Asians and Hawaiians/Pacific Islanders.
CONCLUSIONS
Using RHD genotyping to guide RhIG prophylaxis among pregnant women with serologic weak D phenotypes may be clinically beneficial without increasing overall costs.
In the United States, rates of hemolytic disease of the fetus and newborn (HDFN) have declined dramatically since the introduction of Rh immunoprophylaxis to prevent maternal–fetal alloimmunization.1,2 HDFN is caused by the transplacental passage of maternal IgG alloantibodies directed against fetal blood group antigens3 and classically involves incompatibility of the Rh blood group D (RhD) antigen.4 Fetomaternal hemorrhage in a D− pregnant female with a D+ fetus can induce production of anti-D, leading to potentially severe HDFN and possible fetal death during current or future pregnancies.3
The American College of Obstetrics and Gynecology recommends antenatal and postpartum use of RhIG for D− pregnant females with D+ fetuses.4 D− females requiring transfusion are also only provided with D− red blood cells (RBCs). RhD alloimmunization during pregnancy has declined dramatically and now occurs in approximately six of every 1000 live births.2
While guidelines for administration of RhIG to D− women are clear, prophylactic practices for pregnant women who have serologic weak D phenotypes vary. A serologic weak D phenotype is defined as absent or weak (<2+) reactivity of RBCs with an anti-D reagent in initial testing, but moderate to strong agglutination with addition of antihuman globulin (weak D test), if performed. In the United States, a small minority of individuals have an altered RHD gene, which encodes expression of D antigen that may type as weaker than expected, depending on the method and reagent used for detection.5-9 While a 2006 American College of Obstetrics and Gynecology recommendation states that women with weak D phenotypes should be considered D+ and not be given RhIG,4 AABB (formerly known as American Association of Blood Banks) Standards state that “the test for weak D is unnecessary when testing the patient,” thereby encouraging the conservative management of these women as D−.10 A 2012 survey from the College of American Pathologists demonstrated a lack of consistent practices for the management of pregnant women with serologic weak D phenotypes.11 Developing standard practices for the management of these individuals has implications for patient health as well as financial consequences.
Further complicating the design of an appropriate management strategy for individuals with serologic weak D phenotypes is the extensive genetic variability in RHD alleles associated with this phenotype, since only certain alleles are associated with any risk of D alloimmunization. A recently convened AABB and College of American Pathologists work group concluded that individuals with serologic weak D phenotypes associated with alleles that encode weak D Types 1, 2, or 3 could be safely managed as D+ and has proposed incorporating RHD genotyping in the management of pregnant women presenting with D typing discrepancies.12
In this analysis, we evaluated the financial implications of using RHD genotyping to guide the management of pregnant females identified as having serologic weak D phenotypes. We modeled a dynamic US population undergoing pregnancies, births, and deaths, tracking pregnancy-related costs and events. The model also incorporated race and ethnicity-dependent probabilities of alternative RHD genotypes to realistically estimate financial impact across the entire US population.
MATERIALS AND METHODS
A Markov-based decision tree model was constructed (TreeAge Pro Suite 2014, TreeAge Software, Williamstown, MA) to compare the expected financial impact of two alternative strategies for the management of pregnant women identified as having serologic weak D phenotypes (and thus potentially at risk of RhD alloimmunization). Under both strategies, costs for routine serologic testing to classify pregnant women as D+ or D− were incorporated, according to current AABB standards (no separate weak D test required). Under a conservative strategy, all pregnant females with a serologic weak D phenotype were treated as D− and thus considered to be at risk of RhD alloimmunization. Under an alternative strategy, RHD genotyping was conducted on samples from patients with a serologic weak D phenotype. Those found by genotyping to have alleles encoding a weak D Type 1, 2, or 3 phenotype were managed as D+. Thus, although RHD genotyping would add to initial testing expenses, fewer individuals would require RhIG prophylaxis, decreasing costs of managing RhD alloimmunization risk.
Markov models have been used extensively to simulate recurring processes13 and are thus well suited to modeling a population undergoing repeated pregnancies, births, and deaths over time. This model simulated a representative population of females in the United States, incorporating an “initial population” supplemented annually by “incident cohorts” of females born in that year. Simulated individuals were assigned an RhD type at the start of the simulation (initial population) or upon their introduction to the model (annual cohorts) based on published race/ethnicity-specific rates in the US population (Table 1).
TABLE 1. Input variables: base-case values and ranges used in sensitivity analyses.
| Variable | Base-case value | Range (sensitivity analysis)* | Source |
|---|---|---|---|
| Background demographic characteristics† | |||
| Initial population size | 160,477,237 | (120,357,928-200,596,546) | 14 |
| Annual female birth cohort | 1,925,056 | (1,443,792-2,406,320) | 14 |
| Age distribution (initial population) | 14 | ||
| Racial distribution/Hispanic origin (age-dependent) | 14 | ||
| Mortality rates (age, race, Hispanic origin-dependent) | 15 | ||
| Pregnancy characteristics | |||
| Birth rates (age, race, Hispanic origin-dependent) | 16 | ||
| Proportion of out-of-hospital births (%) | |||
| Caucasian (non-Hispanic) | 2.05 | (2-2.1) | 17 |
| African American (non-Hispanic) | 0.49 | (0.44-0.54) | 17 |
| Asian | 0.54 | (0.49-0.59) | 17 |
| Hawaiian/Pacific Islander | 0.54 | (0.49-0.59) | 17 |
| Hispanic | 0.46 | (0.41-0.51) | 17 |
| American Indian/Alaskan (non-Hispanic) | 0.81 | (0.76-0.86) | 17 |
| Proportion of births with inadequate prenatal care (%) | |||
| Caucasian (non-Hispanic) | 2.4 | (2.2-2.6) | 18 |
| African American (non-Hispanic) | 5.9 | (5.7-7.1) | 18 |
| Asian | 2.1 | (1.9-2.3) | 18 |
| Hawaiian/Pacific Islander | 7.0 | (6.8-7.2) | 18 |
| Hispanic | 4.0 | (3.8-4.2) | 18 |
| American Indian/Alaskan (non-Hispanic) | 7.0 | (6.8-7.2) | 18 |
| D− (inclusive of weak D, %) | |||
| Caucasian (non-Hispanic) | 17.3 | (17.0-17.6) | 19 |
| African American (non-Hispanic) | 7.1 | (6.9-7.3) | 19 |
| Asian | 1.7 | (1.5-1.9) | 19 |
| Hawaiian/Pacific Islander | 1.7 | (1.5-1.9) | 9,19 |
| Hispanic | 7.3 | (7.1-7.5) | 19 |
| American Indian/Alaskan (non-Hispanic) | 9.7 | (9.5-9.9) | 19 |
| Weak D (%) | |||
| Caucasian (non-Hispanic) | 0.4 | (0.2-1) | 5,6 |
| African American (non-Hispanic) | 0.57 | (0.37-0.77) | 7 |
| Asian | 0.01 | (0-0.03) | 20-22 |
| Hawaiian/Pacific Islander | 0.01 | (0-0.03) | 9,20-22 |
| Hispanic | 0.8 | (0.6-1) | 8 |
| American Indian/Alaskan (non-Hispanic) | 0.8 | (0.6-1) | 8 |
| Weak D Type 1, 2, or 3 (among serologic weak D, %) | |||
| Caucasian (non-Hispanic) | 93.5 | (90-97) | 5 |
| African American (non-Hispanic) | 0 | (0-1) | 23 |
| Asian | 0 | (0-1) | 20-22 |
| Hawaiian/Pacific Islander | 0 | (0-1) | 9,20-22 |
| Hispanic | 37.5 | (33-41) | 8 |
| American Indian/Alaskan (non-Hispanic) | 37.5 | (33-41) | 8 |
| Proportion of D− mothers giving birth to D+ babies (%) | |||
| Caucasian (non-Hispanic) | 59.0 | (54-64) | Calculated from 24 |
| African American (non-Hispanic) | 70.9 | (65-75) | Calculated from 24 |
| Asian | 94.5 | (90-99) | Calculated from 24 |
| Hawaiian/Pacific Islander | 94.5 | (90-99) | Calculated from 24 |
| Hispanic | 72.7 | (67-77) | Calculated from 24 |
| American Indian/Alaskan (non-Hispanic) | 72.7 | (67-77) | Calculated from 24 |
| Proportion of pregnancies with vaginal bleeding (%) | 25 | (20-30) | 25 |
| Testing/product costs ($) | |||
| Initial testing | |||
| ABO group | 12.12 | (9.09-15.15) | 26 |
| RhD type | 12.12 | (9.09-15.15) | 26 |
| Antibody screen | 12.12 | (9.09-15.15) | 26 |
| Antibody identification | 31.57 | (23.68-39.46) | 26 |
| Additional RhD testing | |||
| RHD genotyping test | 250 | (100-500) | Assumed |
| Cord blood RhD typing | 30.33 | (22.75-37.91) | 25 |
| Blood products | |||
| RhIG (300 μg dose) | 162 | (121.50-202.50) | 25 |
| RhIG administration | 9.60 | (7.20-12.00) | 25 |
Probabilistic sensitivity analysis (Table 4) incorporates variation in pregnancy characteristics and testing or product costs.
Each year, a portion of females became pregnant, among which a smaller portion were at risk for sensitization to fetal RhD antigens. Pregnancies, antenatal (e.g., ABO, RhD type, antibody screen) and postpartum blood testing (e.g., cord blood RhD testing), and potential risk of RhD alloimmunization were tracked for females over 10- and 20-year periods, along with any associated costs. Costs were assessed separately under the two alternative strategies.
Model structure
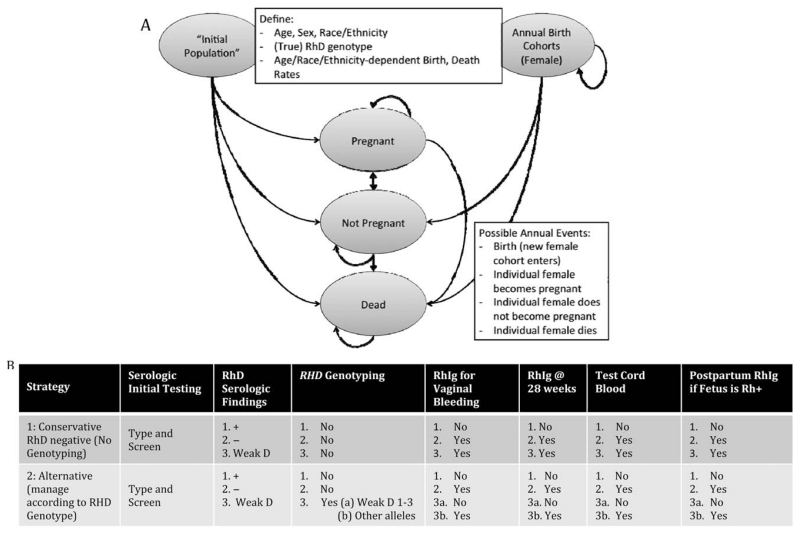
Under both strategies for managing possible RhD alloimmunization, simulated females traversed the same model, diagrammed in Fig. 1A. Over a series of 1-year cycles, each annual birth cohort joined a population of females present at the start of the simulation. Individuals were initially characterized by an age, sex (female), race/ethnicity, and RhD type (D+; D−; serologic weak D phenotype whose RHD genes encoded weak D Type 1, 2, or 3; or serologic weak D phenotype whose RHD genes did not encode weak D Types 1, 2, or 3), based on published data of the prevalence of each category in the population. Age and race/ethnicity-specific pregnancy and death rates were also defined, as described in Table 1. The model defined the following race/ethnicity categories, according to US census bureau classifications: Caucasian (non-Hispanic), African American (non-Hispanic), Asian, Hawaiian/Pacific Islander, American Indian/Alaskan (non-Hispanic), and Hispanic. Because data were not available for the distribution of RhD types of Hawaiians/Pacific Islanders and for American Indians/Alaskans, we assumed that RhD types among these individuals followed the same distributions as Asians and Hispanics, respectively. While there is genetic evidence of similarities between these racial/ethnic groups,9 we conducted sensitivity analyses to evaluate the impact of variation in these variables.
Fig. 1. (A) Diagram of Markov model. An initial population of females is supplemented annually by birth cohorts. Simulated individuals are characterized by age, sex, race/ethnicity, and RHD genotype (positive, negative; weak D Type 1, 2, or 3; or other than weak D Type 1, 2, or 3). Birth and death rates are defined. Each cycle, females could become pregnant, not become pregnant, or die, with transition probabilities based on US Census data. Individual paths through the model were tracked, as costs were accumulated. (B) Components of management by strategy. All pregnant females would receive a type and screen and be classified as serologic D+, D−, or weak D. Under a conservative strategy, no follow-up RHD genotyping would be performed; both serologic D− and serologic weak D patients would be managed as D−. Under an alternative strategy, genotyping would be performed on serologic weak D females; only those females not genotyping as weak D Type 1, 2, or 3 would be managed as D−.
Each year, females could follow one of three paths, each defined by particular Markov state transitions: the individual could: 1) become pregnant, 2) not become pregnant, or 3) die. Pregnancy rates were assumed to be 0 for females below age 10 or above age 49. For each individual, pregnancies and births were tracked.
During a pregnancy, females underwent initial blood testing and had the possibility of receiving RhIG at 28 weeks, in the event of vaginal bleeding, and after fetal cord blood typing, as described (Fig. 1B). The likelihood of undergoing any of these events during pregnancy depended on an individual’s background characteristics. Costs associated with each procedure were accumulated over the course of the simulation, both at the individual level and at the cohort level.
Pregnancy-related events
As part of standard prenatal care, pregnant females were assumed to undergo initial blood testing (ABO group and RhD type and an antibody screen). While we incorporated prior pregnancies, we assumed that females did not have any existing alloantibodies requiring antibody identification. Because prior alloimmunization rates would be the same under both scenarios and would be managed the same way, this assumption did not affect differences in financial outcomes between the two strategies.
Under the alternative strategy, RHD genotyping would be conducted as a follow-up to ABO and RhD typing for females whose RBCs reacted weaker than expected with anti-D (<2+, i.e., serologic weak D). We assumed that all samples could be classified as either weak D Type 1, 2, 3 or not weak D Type 1, 2, 3 by RHD genotyping, as described by the RhD workgroup.12 In addition, we assumed that RHD genotyping would need to be performed only once for a female typing as a serologic weak D and that this would occur at her first pregnancy. Prophylactic treatment during future pregnancies was assumed to rely on results from this initial genotyping. This assumption is reasonable if the female seeks care at the same facility for a subsequent pregnancy or if information is sufficiently documented in her medical history and shared. Under the conservative strategy, females with serologic weak D phenotypes were managed as D−.
Pregnant females managed as D− were assumed to be prophylactically treated with RhIG (300 μg) at 28 weeks of pregnancy. In addition, all pregnant females who were managed as D− and experienced vaginal bleeding (25% of pregnancies) received a second dose of RhIG at the time of bleeding to prevent RhD sensitization.25
We also accounted for some pregnant females not seeking prenatal care and some pregnancies not occurring in the hospital. These variables were race/ethnicity dependent. Females not seeking prenatal care but still delivering in the hospital were assumed to undergo initial blood testing (ABO group and RhD type and antibody screen) at a later time during their pregnancy. However, we assumed that pregnant females who were being managed as D− might miss their dose of prophylactic RhIG at 28 weeks. Women who did not deliver within a hospital facility traversed the model, but were assumed not to accumulate any costs.
At the time of delivery, cord blood from newborns delivered by females who had been managed as D− would be RhD typed. In the event that the fetus was D+, the mother would receive another dose of RhIG. Under the conservative strategy, this subset of mothers included D− individuals and those with serologic weak D. However, under the alternative RHD genotyping strategy, this subset included only D− mothers and those mothers who were not found to be weak D Type 1, 2, or 3 by genotyping. Mothers with serologic weak D who had been genotyped as weak D Type 1, 2, or 3 were managed as D+ and thus did not receive RhIG prophylaxis and did not have their newborn’s cord blood collected or tested.
For each race/ethnicity category, we calculated the expected probability of a D− mother giving birth to an D+ child by using published allele frequencies24 to estimate the portion of males who were homozygous (RHD/RHD) and hemizygous (RHD/−). We then calculated the portion of matings that would lead to a D+ child. For simplicity, we assumed that all matings were within the same race/ethnicity category. Expected probabilities for each race/ethnicity category are provided in Table 1.
Input variables
Individual patients were tracked as they traversed the model, experiencing pregnancy-related events and accumulating associated expenses, which were discounted to the beginning of the simulation and expressed in 2013 USD. Costs were discounted at a rate of 3% per year. The analysis focused on the perspective of a hospital, with each component of the pregnancy—initial blood testing, RHD genotyping, cord blood testing, and RhIG prophylaxis—being associated with a cost. Only direct medical expenses were included, and these were estimated by 2013 Medicare reimbursement rates26,27 wherever possible. We assumed that the RHD genotype method was 100% sensitive and 100% specific, and thus simulated individuals did not become RhD sensitized. Because the comparison strategy involved a conservative approach for pregnant females with a serologic weak D phenotype, we assumed that RhD sensitization would also not occur under this strategy. Input variables are provided in Table 1.
Statistical analysis
Under the base-case scenario, strategies were analyzed over 10- and 20-year periods to reflect estimated financial outcomes over policy-relevant midrange and a long-range periods. A total of 100,000 individual trials were used for each simulation run. Costs were expressed per pregnant female, per live birth, and for a comprehensive patient population, as well as for individual race/ethnicity categories.
One-way sensitivity analysis was used to evaluate the impact of variation in the cost of RHD genotyping and the cost of RhIG on overall financial outcomes. Probabilistic sensitivity analysis, using 10,000 samples of 10,000 trials each, evaluated the impact of uncertainty in input variables. Costs were varied by 25% in either direction using an adjustment factor sampled from a triangular distribution (mode, 1; minimum, 0.75; maximum, 1.25). Efficacy estimates and incidence rates were drawn from beta distributions, using the 95% confidence interval (CI) reported by the original data source wherever possible.
RESULTS
Under the base-case scenario, a strategy incorporating RHD genotyping for pregnant females with a serologic weak D phenotype is marginally cost-saving, compared to a conservative strategy of management as D− (Table 2). Over a 10-year period under the conservative strategy, the per-pregnancy cost of testing and RhIG administration averaged across the entire simulated population is $34.75, while a strategy incorporating genotyping would cost an average of $0.02 less. While the costs associated with pre- and postpartum laboratory testing would increase by $0.42 with the addition of RHD genotyping, this increase would be outweighed by a savings of $0.45 from decreased RhIG use. Across the entire US population, the RHD genotyping strategy is associated with a net cost savings of $645,034 over 10 years. Over a 20-year period, RHD genotyping for pregnant women with a serologic weak D phenotype was associated with an average cost savings of $0.04 per pregnancy. For the US population over this time period, expected savings amount to $2.01 million.
TABLE 2. Base-case results: costs associated with alternative management strategies—per pregnancy and for the entire population.
| Period | Strategy 1: conservative (no genotyping) |
Strategy 2: RHD genotyping for weak D |
Change in costs associated with RHD genotyping* |
|---|---|---|---|
| 10 years | |||
| Mean individual pregnancy | |||
| Total costs ($) | 34.75 | 34.73 | −0.02 |
| Testing costs ($) | 18.04 | 18.46 | 0.42 |
| RhIG costs ($) | 16.71 | 16.26 | −0.45 |
| Entire population | |||
| Total costs (million $) | 923.91 | 923.26 | −0.65 |
| Testing costs (million $) | 479.61 | 490.85 | 11.25 |
| RhIG costs (million $) | 444.30 | 432.41 | −11.89 |
| 20 years | |||
| Mean individual pregnancy | |||
| Total costs ($) | 20.22 | 20.18 | −0.04 |
| Testing costs ($) | 10.51 | 10.80 | 0.29 |
| RhIG costs ($) | 9.71 | 9.38 | −0.33 |
| Entire population | |||
| Total costs (million $) | 994.12 | 992.11 | −2.01 |
| Testing costs (million $) | 516.95 | 531.13 | 14.18 |
| RhIG costs (million $) | 477.17 | 460.97 | −16.19 |
Change in costs defined as “Strategy 2: RHD genotyping for serologic weak D” – “Strategy 1: conservative (no genotyping).” Negative changes are indicative of cost savings associated with genotyping.
Cost savings associated with RHD genotyping varied substantially by race/ethnic group over a 10-year period (Table 3). While among non-Hispanic Caucasian females, RHD genotyping is expected to decrease average net costs per pregnancy by $0.17, RHD genotyping will increase average net costs among non-Hispanic African Americans by $0.51. RHD genotyping is expected to increase net costs for non-Hispanic African Americans, American Indians/Alaskans, and Hispanics. Incorporating RHD genotyping is expected to result in no significant cost increases among Asians and Hawaiians/Pacific Islanders.
TABLE 3. Base-case results: costs associated with alternative management strategies over a 10-year period—per pregnancy by racial/ethnic group.
| Racial/ethnic group | Strategy 1: conservative (no genotyping) |
Strategy 2: RHD genotyping for weak D |
Change in costs associated with RHD genotyping* |
|---|---|---|---|
| Caucasian (non-Hispanic) female | |||
| Total costs ($) | 41.19 | 41.02 | −0.17 |
| Testing costs ($) | 18.47 | 18.79 | 0.32 |
| RhIG costs ($) | 22.72 | 22.23 | −0.49 |
| African American (non-Hispanic) female | |||
| Total costs ($) | 26.26 | 26.77 | 0.51 |
| Testing costs ($) | 16.99 | 17.50 | 0.51 |
| RhIG costs ($) | 9.26 | 9.27 | 0.00 |
| Asian female | |||
| Total costs ($) | 19.13 | 19.14 | 0.01 |
| Testing costs ($) | 16.31 | 16.33 | 0.02 |
| RhIG costs ($) | 2.82 | 2.81 | 0.00 |
| Hawaiian/Pacific Islander female | |||
| Total costs ($) | 18.85 | 18.85 | 0.01 |
| Testing costs ($) | 16.23 | 16.23 | 0.01 |
| RhIG costs ($) | 2.62 | 2.62 | 0.00 |
| American Indian/Alaskan (non-Hispanic) female | |||
| Total costs ($) | 29.67 | 29.77 | 0.10 |
| Testing costs ($) | 16.33 | 17.04 | 0.71 |
| RhIG costs ($) | 13.34 | 12.73 | −0.61 |
| Hispanic female | |||
| Total costs ($) | 28.51 | 28.88 | 0.37 |
| Testing costs ($) | 17.77 | 18.48 | 0.71 |
| RhIG costs ($) | 10.74 | 10.40 | −0.34 |
Change in costs defined as “Strategy 2: RHD genotyping for serologic weak D” – “Strategy 1: conservative (no genotyping).” Negative changes are indicative of cost savings associated with genotyping.
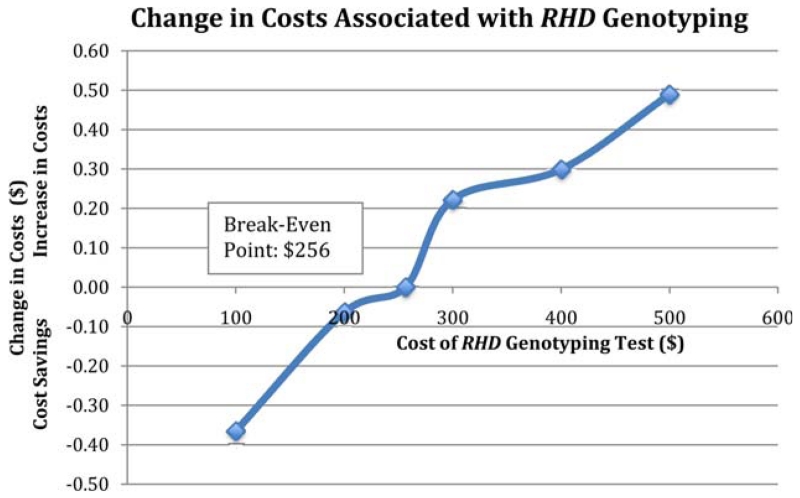
One-way sensitivity analysis (Fig. 2) demonstrates that over a 10-year period, incorporating RHD genotyping for pregnant females with a serologic weak D phenotype would be cost-saving as long as the genotyping assay cost was less than $256. Probabilistic sensitivity analysis (Table 4), which incorporated variability in pregnancy characteristics and costs, demonstrated that RHD genotyping is not likely to substantially affect overall financial outcomes. This analysis showed that for an entire population over 10 years, RHD genotyping for management of pregnancies in which the woman is known to have a serologic weak D phenotype is expected to add $1.09 million (95% CI, −3.67 to 5.85) to overall costs.
Fig. 2. One-way sensitivity analysis varying the cost of RHD genotyping. At a cost of less than $256, an RHD genotyping assay is expected to be cost-saving compared to no genotyping.
TABLE 4. Probabilistic sensitivity analysis: costs associated with alternative management strategies—per pregnancy and for the entire population*.
| Period | Strategy 1: conservative as D− (no genotyping) |
Strategy 2: RHD genotyping for weak D |
Change in costs associated with RHD genotyping† |
|---|---|---|---|
| 10 years | |||
| Mean individual pregnancy | |||
| Total costs ($) | 34.10 (22.76 to 48.06) | 34.14 (22.81 to 48.23) | 0.04 (0.01 to 0.07) |
| Testing costs ($) | 17.76 (13.93 to 21.99) | 18.16 (14.14 to 22.70) | 0.41 (0.40 to 0.42) |
| RhIG costs ($) | 16.34 (7.36 to 27.87) | 15.97 (7.13 to 27.44) | −0.37 (−0.39 to −0.34) |
| Entire population | |||
| Total costs (million $) | 899.54 (600.53 to 1268.05) | 900.63 (601.89 to 1272.40) | 1.09 (−3.67 to 5.85) |
| Testing costs (million $) | 468.48 (367.52 to 580.19) | 479.21 (372.93 to 598.83) | 10.73 (9.18 to 12.29) |
| RhIG costs (million $) | 431.07 (194.07 to 735.35) | 421.42 (723.82 to 723.82) | −9.65 (−13.50 to −5.79) |
| 20 years | |||
| Mean individual pregnancy | |||
| Total costs ($) | 20.05 (13.56 to 28.39) | 20.07 (13.57 to 28.38) | 0.02 (−0.01 to 0.05) |
| Testing costs ($) | 10.47 (8.23 to 13.00) | 10.70 (8.37 to 13.32) | 0.23 (0.22 to 0.24) |
| RhIG costs ($) | 9.58 (4.34 to 16.46) | 9.37 (4.21 to 16.19) | −0.21 (−0.23 to −0.19) |
| Entire population | |||
| Total costs (million $) | 985.13 (666.17 to 1394.92) | 986.09 (666.97 to 1394.67) | 0.97 (−4.21 to 6.14) |
| Testing costs (million $) | 514.31 (404.49 to 639.06) | 525.61 (411.16 to 654.71) | 11.31 (9.60 to 13.01) |
| RhIG costs (million $) | 470.82 (213.15 to 808.69) | 460.48 (795.77 to 795.77) | −10.34 (−14.54 to −6.14) |
Data are reported as point estimates (95% CI).
Change in costs defined as “Strategy 2: RHD genotyping for serologic weak D” – “Strategy 1: conservative (no genotyping).” Negative changes are indicative of cost savings associated with genotyping.
DISCUSSION
Management of pregnant women at risk of RhD alloimmunization with RhIG has been shown to dramatically reduce rates of HDFN.2 However, as demonstrated by a recent survey of hospital laboratories, many transfusion services are currently managing pregnant females known to have a serologic weak D phenotype as D−, although many of these women may not be at risk of RhD alloimmunization and may not benefit from RhIG prophylaxis.11 This practice, which seeks to prevent alloimmunization of women with partial D phenotypes, results in unnecessary and unwarranted use of RhIG.
This analysis compared the financial implications of two alternative strategies for the prevention of RhD alloimmunization among pregnant females known to have a serologic weak D phenotype. The first strategy reflected a commonly used conservative approach with all D− females and females with serologic weak D phenotypes (<2+ reactivity or only reactive by weak D testing) being managed as at risk of RhD alloimmunization. The second strategy incorporated RHD genotyping with preventative management provided only to D− females and females with a serologic weak D phenotype who did not have alleles encoding weak D Type 1, 2, or 3.
Although incorporating RHD genotyping in the testing procedures for pregnant females with a serologic weak D phenotype adds an additional expense, reduced spending on RhIG balances out net costs when assessed across the population. Were this strategy to be implemented across the hospital-delivering US population, RHD genotyping would likely not add significant costs and may lead to modest savings over longer time periods. RHD genotyping would add a one-time testing cost for each pregnant female with a serologic weak D phenotype, but prevent unnecessary costs associated with RhIG during every subsequent pregnancy.
The model also demonstrated that because of the differential distributions of RHD alleles by race and ethnicity, the financial impact of RHD genotyping would vary across these groups of individuals. Among non-Hispanic Caucasians, who are relatively common in the US pregnant female population and have high rates of serologic weak D phenotypes with alleles encoding weak D Type 1, 2, or 3 (and, thus, a high rate of pregnant females not at risk of RhD alloimmunization), RHD genotyping is expected to reduce unnecessary RhIG use, saving a mean of $0.17 per pregnancy. However, non-Hispanic African Americans and Hispanics with a serologic weak D have a lower likelihood of carrying weak D Type 1, 2, or 3 alleles,7 being more likely to carry partial D or weak D 4.0 alleles. Thus, for these populations, RHD genotyping for women with serologic weak D phenotypes may increase initial testing costs compared to conservative management, without providing additional long-term financial benefit from reduced RhIG use.
Our analysis suggests that while implementing RHD genotyping is an essentially cost-neutral strategy, any cost savings from genotyping are expected to increase slightly over time. RHD genotyping would need to be performed only once per lifetime, even if women have multiple pregnancies. Thus, costs per pregnancy would decrease over time due to reduced spending on testing and RhIG. Costs over the entire population associated with RHD genotyping are also expected to be somewhat lower over a 20-year period than a 10-year period. Estimates over the entire population incorporate additional costs (and potential savings) from individuals entering the pool of pregnant women and getting tested for the first time, but also account for individuals leaving the pool of pregnant women who are no longer experiencing savings from reduced RhIG use. However, since the majority of the pregnant population under the 10-year analysis remained in the pregnant population under the 20-year analysis, cost estimates across the two periods were similar. These trends were also observed in the probabilistic sensitivity analyses and across race/ethnic groups.
Our analysis likely resulted in conservative estimates of possible cost savings associated with genotyping and did not account for additional health benefits of avoiding RhIG when it is not necessary. The true costs of RHD genotyping per ever-pregnant female may be lower than those incorporated in the model, and these costs are likely to decrease over time, as genotyping becomes more fully integrated and available within the hospital system. In addition, we have not included possible additional benefits, such as eliminated risk of transfusion-transmitted infections through RhIG and possible reduced use of D− RBCs in the event of necessary transfusions.
In the base-case scenario, the model assumed that initial testing among pregnant women would identify those individuals presenting with a serologic weak D phenotype. No additional testing with the indirect antiglobulin test (IAT) was included, as IAT testing of the RBCs when determining the RhD type of pregnant women is not required according to AABB standards.10 However, depending on the testing performed, some pregnant women with serologic weak D phenotypes are likely to be missed, and may instead be classified and treated as D−. Our probabilistic sensitivity analyses incorporated substantial variation in the number of pregnant women detected as having a serologic weak D phenotype, and this variation did not substantially affect financial outcomes.
This model evaluates RHD genotyping to guide management of RhIG prophylaxis for women with serologic weak D phenotypes and does not address those pregnant women with serologic weak D that are undetected and type as D− by routine testing. RHD genotyping of all D− women, as well as those with serologic weak D phenotypes, would be required to comprehensively identify all women for whom RhIG prophylaxis would not be clinically beneficial. The strategy evaluated in this model also does not address those individuals with partial D phenotypes with RBCs that type strongly D+ and are at risk for anti-D and would be better served by management as D−. Identifying D+ women with a partial D phenotype would require additional RHD genotyping of all D+ women.
Although RHD genotyping is available, market pricing and reimbursement rates for the test have not been determined. This analysis has demonstrated that such an assay may have clinical value for the management of RhD alloimmunization among pregnant females without adding a significant financial burden.
ACKNOWLEDGMENT
We appreciate the review and thoughtful comments of R. Sue Shirey on this study and the manuscript.
ABBREVIATION
- HDFN
hemolytic disease of the fetus and newborn
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.Crowther CA, Middleton P, McBain RD. Anti-D administration in pregnancy for preventing Rhesus alloimmunisation. Cochrane Database Syst Rev. 2013;2:CD000020. doi: 10.1002/14651858.CD000020.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Moise KJ., Jr. Management of rhesus alloimmunization in pregnancy. Obstet Gynecol. 2008;112:164–76. doi: 10.1097/AOG.0b013e31817d453c. [DOI] [PubMed] [Google Scholar]
- 3.Bowman J. Thirty-five years of Rh prophylaxis. Transfusion. 2003;43:1661–6. doi: 10.1111/j.0041-1132.2003.00632.x. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006;108:457–64. doi: 10.1097/00006250-200608000-00044. [DOI] [PubMed] [Google Scholar]
- 5.Wagner FF, Gassner C, Müller TH, et al. Molecular basis of weak D phenotypes. Blood. 1999;93:385–93. [PubMed] [Google Scholar]
- 6.Jenkins CM, Johnson ST, Bellissimo DB, et al. Incidence of weak D in blood donors typed as D positive by the Olympus PK 7200. Immunohematology. 2005;21:152–4. [PubMed] [Google Scholar]
- 7.Chou ST, Jackson T, Vege S, et al. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–71. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 8.Cruz BR, Chiba AK, Moritz E, et al. RHD alleles in Brazilian blood donors with weak D or D-negative phenotypes. Transfus Med. 2012;22:84–9. doi: 10.1111/j.1365-3148.2011.01129.x. [DOI] [PubMed] [Google Scholar]
- 9.Friedlaender JS, Friedlaender FR, Reed FA, et al. The genetic structure of Pacific Islanders. PLoS Genet. 2008;4:e19. doi: 10.1371/journal.pgen.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roseff SD. Standards for blood banks and transfusion services. 29th AABB; Bethesda (MD): 2014. [Google Scholar]
- 11.Sandler SG, Roseff SD, Domen RE, et al. Policies and procedures related to testing for weak D phenotypes and administration of Rh immune globulin: results and recommendations related to supplemental questions in the comprehensive transfusion medicine survey of the College of American Pathologists. Arch Pathol Lab Med. 2014;138:620–5. doi: 10.5858/arpa.2013-0141-CP. [DOI] [PubMed] [Google Scholar]
- 12.Sandler SG, Flegel WA, Westhoff CM, et al. It’s time to phase in RHD genotyping for patients with a serologic weak D phenotype. Transfusion. 2015;55:680–9. doi: 10.1111/trf.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397–409. doi: 10.2165/00019053-199813040-00003. [DOI] [PubMed] [Google Scholar]
- 14.US Census Bureau . Table PEPASR6H: annual estimates of the resident population by sex, age, race, and Hispanic origin for the United States and States: April 1, 2010 to July 1, 2013. US Census Bureau, Population Division; Washington (DC): Jun, 2014. [cited 2014 Jul 17]. Available from: http://fact-finder2.census.gov. [Google Scholar]
- 15.Centers for Disease Control and Prevention . Underlying cause of death 1999-2013 on CDC WONDER online database. Centers for Disease Control and Prevention, National Center for Health Statistics; Atlanta (GA): Released 2014 [cited 2014 Jul 17]. Available from: http://wonder.cdc.gov/ucd-icd10.html. [Google Scholar]
- 16.Hamilton BE, Martin JA, Osterman MJ, et al. Births: preliminary data for 2013. 2. Vol. 63. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System; Hyattsville (MD): May 29, 2014. [cited 2014 Jul 17]. Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr63/nvsr63_02.pdf. [PubMed] [Google Scholar]
- 17.MacDorman MF, Mathews TJ, Declercq E. Trends in out-of-hospital births in the United States, 1990-2012. National Center for Health Statistics; Hyattsville (MD): Mar, 2014. NCHS Data Brief, Number 144. 2014 [cited 2014 Aug 7]. Available from: http://www.cdc.gov/nchs/data/databriefs/db144.pdf%3E. [Google Scholar]
- 18.US Department of Health and Human Services. Health Resources and Services Administration. Maternal and Child Health Bureau . Child health USA 2013: prenatal care utilization. US. Department of Health and Human Services; Rockville (MD): 2013. [cited 2014 Aug 15]. Available from: http://mchb.hrsa.gov/chusa13/health-services-utilization/p/prenatal-care-utilization.html%3E. [Google Scholar]
- 19.Garratty G, Glynn SA, McEntire R. ABO and Rh(D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion. 2004;44:703–6. doi: 10.1111/j.1537-2995.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- 20.Yan L, Wu J, Zhu F, et al. Molecular basis of D variants in Chinese persons. Transfusion. 2007;47:471–7. doi: 10.1111/j.1537-2995.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu JJ, Hong XZ, Xu XG, et al. Molecular basis of partial D phenotypes in Chinese. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006;14:587–91. [PubMed] [Google Scholar]
- 22.Ye SH, Wu DZ, Wang MN, et al. A comprehensive investigation of RHD polymorphisms in the Chinese Han population in Xi’an. Blood Transfus. 2014;12:396–404. doi: 10.2450/2013.0121-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid ME, Halter Hipsky C, Hue-Roye K, et al. Genomic analyses of RH alleles to improve transfusion therapy in patients with sickle cell disease. Blood Cells Mol Dis. 2014;52:195–202. doi: 10.1016/j.bcmd.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid ME, Lomas-Francis C. Blood group antigens and antibodies: a guide to clinical relevance and technical tips. 2nd SBB Books; New York: 2007. [Google Scholar]
- 25.Hawk AF, Chang EY, Shields SM, et al. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122:579–85. doi: 10.1097/AOG.0b013e31829f8814. [DOI] [PubMed] [Google Scholar]
- 26.American Association of Blood Banks . Medicare 2014 hospital outpatient PPS final rule - summary of major provisions affecting transfusion medicine and cellular therapies. AABB; Bethesda (MD): 2014. [cited 2014 Aug 16]. Available from: http://www.aabb.org/advocacy/reimbursementinitiatives/Pages/14hoppsrulefinal.aspx. [Google Scholar]
- 27.Centers for Medicare & Medicaid Services . Hospital outpatient prospective payment system: addendum A update. Centers for Medicare & Medicaid Services; 2012. [cited 2014 Aug 15]. Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Addendum-A-and-Addendum-B-Updates-Items/2012-July-Addendum-A.html?DLPage=3&DLSort=2&DLSortDir=descending. [Google Scholar]