Abstract
Obesity has increased in prevalence worldwide, attributed in part to the influences of an obesity-promoting environment and genetic factors. While obesity and overweight increasingly seem to be the norm, there remain individuals who resist obesity. We present here an overview of data supporting the idea that hypothalamic neuropeptide orexin A (OXA; hypocretin 1) may be a key component of brain mechanisms underlying obesity resistance. Prior work with models of obesity and obesity resistance in rodents has shown that increased orexin and/or orexin sensitivity is correlated with elevated spontaneous physical activity (SPA), and that orexin-induced SPA contributes to obesity resistance via increased non-exercise activity thermogenesis (NEAT). However, central hypothalamic orexin signaling mechanisms that regulate SPA remain undefined. Our ongoing studies and work of others support the hypothesis that one such mechanism may be upregulation of a hypoxia-inducible factor 1 alpha (HIF-1α)-dependent pathway, suggesting that orexin may promote obesity resistance both by increasing SPA and by influencing the metabolic state of orexin-responsive hypothalamic neurons. We discuss potential mechanisms based on both animal and in vitro pharmacological studies, in the context of elucidating potential molecular targets for obesity prevention and therapy.
Keywords: Orexin (hypocretin), Hypothalamus, Obesity, Spontaneous physical activity
Spontaneous Physical Activity and Orexin
Despite genetic influences and obesigenic environments, propensity for obesity varies among humans and animals, with some individuals resisting obesity [1–6]. Obesity resistance is positively correlated with elevated spontaneous physical activity (SPA), defined as all movement not associated with formal exercise; SPA contributes to obesity resistance through an increase in non-exercise activity thermogenesis (NEAT) [Reviewed in 7]. The thermogenic effect of subtle but chronic increased movement has an important influence on energy balance, and NEAT contributes significantly to obesity resistance [8,5,9,10]. While noteworthy studies in both humans and rodents have demonstrated that differences in activities of daily living and propensity for general movement can predict obesity resistance [11,12,5,13], the underlying central neural mechanisms driving SPA remain relatively undefined.
Many brain regions, neurotransmitters, and neuropeptides are thought to contribute to SPA and obesity resistance [14]. The hypothalamic neuropeptide orexin (hypocretin), initially of interest in energy balance due to its effect on feeding and arousal, has proven to play an integrative role in energy balance and expenditure [15,16]. Throughout the past decade, work in our laboratory and elsewhere has collectively demonstrated the importance of orexin in obesity resistance [17–19,9]. However, to date the cell signaling mechanisms through which orexin effects long- and short-term change in SPA is unknown. Evidence from studies of ischemia and oxidative stress have suggested that orexin signaling can influence transcription factors involved in resistance to these stressors, and that these pathways might be critically involved in orexin effects on metabolism. The focus of this review will be to evaluate hypothalamic orexin signaling cascades that potentially underlie mechanisms through which orexin confers obesity resistance.
Orexin and Obesity Resistance
The link between susceptibility to obesity and interindividual differences in total body movement between obese and lean subjects was demonstrated in a landmark paper by J.A. Levine et al. [13]. Lean individuals were found to expend 270 to 475 more calories (kcal) per day than did obese people. Weight gain or weight loss by either group did not alter basal propensity for movement, suggesting SPA is somehow biologically determined. Support for the notion that intrinsic differences in SPA contribute to obesity resistance has come from work utilizing a polygenic rodent model of obesity, first described over a decade ago by Levin et al. [20]. Many rat strains show variability in weight gain when fed a high-energy diet [21]; some rats develop diet-induced obesity (DIO) while diet-resistant (DR) rats remain lean. Inherent variability in body weight among male Sprague-Dawley rats led Levin and colleagues to selectively breed rats with high or low rates of weight gain, ultimately resulting in two lines of animals diverging on body weight gain despite comparable caloric intake [20,22]. While these selectively-bred DIO and DR rats do not show differential SPA or weight gain when fed chow, after exposure to high-fat diet, DIO rats significantly decrease SPA and NEAT and become obese [19]. Levin’s original selectively-bred rat lines have been developed into commercially available models, including Levin DIO/DR rats (which retain a high-fat diet requirement for differential development of obesity) and obesity prone (OP) or obesity resistant (OR) rats, which develop differential obesity even on chow diets. Early work with DIO/DR rats (including work leading to the development of selectively bred OP/OR animals) suggests physiological differences in sympathetic activation and monoaminergic function exist between individual rats even prior to exposure to a high-energy diet, and that these differences in CNS function may be related to their propensity for obesity [23–26].
One of the major findings explaining the difference in weight gain in this model is that OR animals exhibit greater SPA than do more obesity-prone controls [27]. Several lines of evidence suggest orexin contributes to the SPA phenotype. Injection of orexin A (OXA) into the rostral lateral hypothalamus (rLH), paraventricular hypothalamic nucleus, nucleus accumbens, or third ventricle is known to stimulate SPA in both monogenetic and polygenetic rodent models [28–32], and both DR and OR rats show higher SPA response to orexin than do obese controls [19,9]. Indirect calorimetry shows that OXA-induced SPA results in increased oxygen consumption, CO2 production, and thermogenesis [29]. Additionally, increased OXA responsiveness in OR rats is due in part to higher levels of orexin receptor expression, particularly in the rLH [17,9]. While OXA effects on SPA can be elicited in several specific brain sites, much of our work has thus focused on the rLH.
An interesting aspect of the OP/OR model is that Levin et al. did not set out to specifically breed animals that differed in physical activity; this phenotype was an unexpected byproduct of selection for genes promoting weight gain or resistance to obesity. However, the differential orexin responsivity observed in these animals has been found in other rodent models specifically selected for physical activity. Our laboratory recently described a model of high activity (HA) and low activity (LA) rats, using the naturally occurring variability in SD rat phenotypes [33], much as was done with the early DIO/DR studies. Rats were screened for baseline SPA propensity, and animals that exhibited more than 120 min or less than 90 min total SPA over 24 h designated HA and LA, respectively. In these rats, HA animals showed higher baseline energy expenditure, and baseline SPA in all animals was significantly correlated with lean body mass and total body weight [33]. Subsequent tests showed that HA rats were more responsive to rLH orexin than were LA rats, and mRNA analysis showed HA rats had higher lateral hypothalamic prepro-orexin expression [33]. When challenged with high fat diet, HA animals showed greater resistance to fat mass gain (relative to lean mass gain) than did LA animals [33]. Where the HA/LA animals exploited naturally occurring variability, selective breeding similar to that used in development of the OP/OR model has been used to generate rats selectively bred for aerobic capacity and wheel running propensity [34]. Recent investigations of these animals have shown that, as with HA/LA and OP/OR rats, orexin may be critically involved in the phenotype [35]. Where rats with low aerobic capacity (LCR) show propensity for cardiovascular disease and development of metabolic syndrome, those bred for high aerobic capacity (HCR) are obesity resistant, remaining leaner than their LCR counterparts [35], especially on high fat diet. HCR animals are intrinsically more active than LCR animals, and show greater NEAT response to orexin injection [35]. The association of greater orexin expression or sensitivity with resistance to obesity is a common thread in the phenotypes of these animal models. That these models are polygenic is also important. Much work has been performed using monogenic rodent obesity models, but monogenic forms of human obesity are rare [36,37]. As such, the observation that orexin is important in the multiple obesity-resistant polygenic phenotypes suggests this mechanism is more relevant to human obesity phenotypes.
Together, the evidence suggests that underlying genetic differences contribute to obesity resistance, that orexin is an important component of this variability, and that the polygenic rodent models described above are suitable for testing potential orexin-mediated brain mechanisms of obesity resistance. Work with these animal models has clearly defined a direct link between orexin signaling and neuromodulation of SPA; however, the cellular signaling pathways through which orexin mediates SPA and obesity resistance still remain largely unknown [9,38]. We present here evidence from multiple in vitro investigations, including data from our ongoing work and that from other non-orexin focused rodent models, to delineate potential mechanisms through which orexin might affect obesity resistance.
Orexins and Signal Transduction
The orexins consist of two peptides, OXA and orexin B (OXB; hypocretin 2); both are produced by post-translation modification from a common precursor, prepro-orexin [39–41]. Expression of prepro-orexin within the CNS appears to be limited to a subset of cells in the lateral and perifornical hypothalamus [40,42], while OXA- and OXB-immunoreactive fibers are abundant in both hypothalamic and extra-hypothalamic regions [42–44]. Orexin peptides are endogenous ligands for two G protein-coupled receptors, orexin receptors 1 and 2 (OX1R and OX2R; respectively); orexin receptors are widely and differentially distributed throughout the brain [39,45,46]. Pharmacological data supports that OX1R has a higher selectivity for OXA, while OX2R binds with similar affinity to both orexins [39]. Although it appears that OXB can induce SPA to some degree [32], and we do not dismiss a potential role for OXB in obesity resistance, at this time the contribution of OXB in promotion of energy expenditure is unclear. This review therefore focuses on OXA; as such only potential OXA signal transduction mechanisms will be directly addressed here.
Two central questions have persisted in studying the role of the orexin system in obesity. First, what pathways are activated by OXA action at the orexin receptors, and second, what are the functional outcomes of this activation? Data from in vitro work has begun to address these issues. In neuronal cultures and recombinant cell lines, the most immediate response upon OXA binding to either receptor is an increase of intercellular Ca2+, via phospholipase C (PLC) and protein kinase C (PKC) activation [47]. As with any G-protein coupled receptor, the pathways activated by OX1R and OX2R depend on the specific G-subunits coupled to each. Early investigations using transfection models demonstrated that an OXA/OX1R response was non-pertussis toxin (PTX) sensitive, indicating Gi/o activation, while an OXA/OX2R response appeared to involve both PTX-sensitive and non-PTX sensitive G-proteins inhibiting adenylyl cyclase [48–50]. Further work shows that OX1/2R can couple to excitatory Gq/11 subunits, whereas OX2R can couple to inhibitory Gi/Go subunits thought to be responsible for K+ efflux and membrane hyperpolarization in neurons [51]. In rat adrenocortical cells, OXA has been shown to activate adenylate cyclase/protein kinase A (PKA), indicating a Gs response [51]. Studies with hypothalamic tissue from food deprived Wistar rats demonstrate that energy status can modify orexin signaling responses by stimulating distinct G-α subunit responses [52].
Recently, OXA has been demonstrated to activate mitogen-activated protein kinase (MAPK) pathways [53]. Signal profiling of recombinant human OX1R and OX2R receptors has demonstrated that orexin can activate extracellular receptor kinase 1/2 (ERK1/2) and p38, protein members of the MAPK cascade [54]. An OX1R overexpression model in Chinese hamster ovary cells has shown that orexin activation of ERK1/2 can take place via a PLC/PKC, Ras, Src and PI3K pathway [55]. Studies of human embryonic kidney (HEK-293) cells transfected with human OX2R demonstrate that OXA activation of ERK1/2 is predominantly a Gq/11, Gs and Gi process, while p38 initiation is independent of Gq/11 and Gi activation [54]. Collectively, these findings suggest that OX1R and OX2R G-α subunit activation by OXA may regulate regionally distinct and tissue specific orexin responses.
Orexin-mediated signaling and energy balance
OXA Activates MAPKs
Given the multiple physiological processes and second messenger pathways potentially activated by orexin, it is not surprising that OXA has pleiotropic effects [31,56,57]. However, little is known about the short- and long-term effects of OXA signaling on intracellular neuronal metabolic status or the physiological relevance of this signaling to SPA. Collectively, emerging evidence indicates that activation of OX1R/OX2R by OXA alters proteins involved in intracellular metabolic function [39]. The in vitro models described above suggest that OXA activation of MAPKs might represent one link between orexin and cellular mechanisms mediating long-term energy balance. MAPKs, a kinase family of signaling kinases integrating signaling transduction cascades, are traditionally known for their participation in cellular proliferation, but are continually being identified in novel CNS roles. The in vitro data discussed above shows that OXA can activate MAPKs, and several studies have shown that increased MAPK activity is correlated with increased obesity resistance. The MAPK inhibitor MAPK-phosphatase-1 (MKP-1) is an immediate-early gene that dephosphorylates MAPKs and inhibits their activity in the nucleus. Mice globally lacking MKP-1 thus have increased MAPK activity. These mice express higher levels of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) in skeletal muscle, show increases in skeletal muscle oxidative metabolism, have enhanced energy expenditure and physical movement, and are resistant to diet-induced obesity [58,59]. Pharmacological experiments exploring effects of OXA activated MAPKs, and the distinct contributions of the two receptor subtypes in these processes, are now ongoing in our lab.
Possible Role of PGC-1α
Previous and ongoing work suggests orexin MAPK pathways involve PGC-1α, a tissue-specific and inducible transcriptional coactivator for several nuclear receptors. However, whether PGC-1α is a critical component of orexin effects on neuronal metabolism remains to be explored. PGC-1α is expressed in heart, kidney, brown adipose tissue, skeletal muscle, and throughout the brain [60]. PGC-1α is a key transcriptional regulator of mitochondria and oxidative metabolic pathways [61]. PGC-1α functions as a direct link between external physiological stimuli and regulation of mitochondrial biogenesis, can simultaneously upregulate genes that protect against oxidative stress, and is a key facilitator for production of ATP [62–65]. This cofactor is also known to play key roles in energy metabolism, hepatic gluconeogenesis, and cholesterol homoeostasis. Alterations in PGC-1α are associated with pathologies such as obesity, diabetes, and chronic neurodegenerative diseases [62–65]. While the specific function of PGC-1α in brain and neuronal metabolism is still under investigation, there is increasing interest in its role in neuronal survival and systemic energy balance [66,60,62,67,68,61,64,69,65]. Data suggest that global inactivation of PGC-1α leads to neurodegeneration, partially due to the loss of protection against oxidative stress damage and impaired nutritional regulation of hypothalamic expression of genes that regulate systemic energy balance [68,64,69].
OXA Activates HIF1α
While a specific role for PGC-1α in the hypothalamus and how it may contribute to the central control of obesity is unclear at this time, data suggests that PGC-1α might be an important part of a recently described link between OXA and the transcription factor hypoxia-inducible factor 1α (HIF-1α). OXA has recently been shown to increase the expression of HIF-1α in hypothalamic tissue [70]. While increases in HIF-1α are usually associated with hypoxia, OXA induction of HIF-1α occurs in normal, non-hypoxic hypothalamic tissue [70]. OXA has been shown to be neuroprotective in cerebral cortex and in hypothalamic cell culture following oxidative stressors [71,38], potentially through activation of HIF-1α. Recent studies have shown that in conjunction with MAPK, PGC-1α is important in regulating HIF-1α expression [72,62,73,61]. As described above, OXA effects on MAPK pathways might result in increased PGC-1α. PGC-1α is known to participate in the regulation of HIF-1α in peripheral tissue [61] and presumably could do so in the hypothalamus as well.
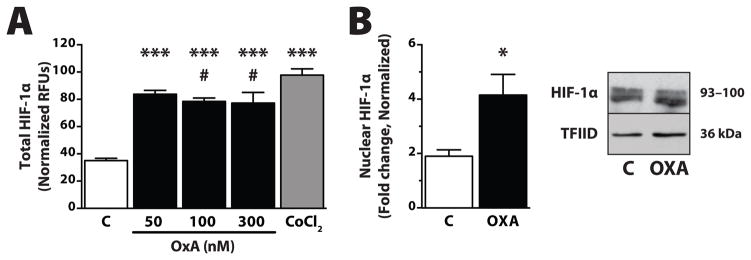
Several lines of evidence suggest OXA effects on HIF-1α could represent another link between orexin and cellular metabolic signaling pathways relevant to obesity. We and others have linked OXA to energy metabolism, showing that OXA induces HIF-1α expression in hypothalamic tissue in vitro (Figure 1), and that this results in increased ATP production via oxidative phosphorylation [74,70,38] In separate rodent studies, mice with the neuron-specific loss of the HIF-1α inhibitor asparaginyl hydroxylase factor (FIH) had reduced body weight and were protected against high-fat-diet-induced weight gain [75]. Additionally, mice lacking functional HIF-1α and HIF-2α proteins in arcuate nucleus pro-opiomelanocortin (POMC) neurons (POMC/HIFβ mice) have impaired energy expenditure, hyperphagia, and increased fat mass [76]. In the same study, viral overexpression of HIF-1α in the mediobasal hypothalamus resulted in obesity resistance during HFD feeding. The specific role of OXA in these models has not been fully evaluated, but current literature suggests that OXA effects on HIF signaling cascades could alter central mechanisms of energy expenditure in response to various metabolic stressors such as high fat diets.
Fig. 1.
OXA increases both total and nuclear HIF-1α protein within 2 h in an immortalized hypothalamic cell line. (A) Protein levels were determined by an in-cell ELISA assay (R&D Systems, Minneapolis, MN USA) following OXA treatment (Phoenix Pharmaceuticals, Burlingame, CA USA; 50, 100, or 300 nM) or the positive control CoCl2 (Sigma, St. Louis, MO USA; 150 μM). Briefly, intact cells were fixed with 4% formalin following OXA treatment, incubated with primary antibody (anti-HIF-1α or anti-cytochrome-C) followed by secondary antibody (each containing two different distinctly labeled conjugates). Cells were assayed by reading the relative fluorescent units (RFU) for each secondary antibody. Values normalized to cytochrome-C housekeeping protein; n = 3–6/group; *** p < 0.0001 vs. control; # p = 0.22 vs. CoCl2. (B) Western blot analysis for nuclear HIF-1α, following OXA (300 nM for 2 h) treatment. Nuclear fractions were prepared (Thermo-Pierce, Rockford, IL USA) and 30 μg total protein was used to determine changes in nuclear (activated) HIF-1α (Novus, Littleton, CO USA; 1:1000) compared to control (C). Nuclear TATA binding protein TFIID (Santa Cruz Biotechnology, Dallas, TX USA) used as loading control. n = 4/group; * p < 0.05 vs. control
Conclusion
The brain is exceptionally sensitive to oxidative stress caused by changes in both peripheral and intra-neuronal metabolism. The relationship between intracellular energy sensing within specific responsive neurons or brain sites is still poorly understood, but is an emerging field of study in which orexin might play a pivotal role. The hypothalamus is a complex neuroendocrine tissue, and while mechanistic studies within this region of the brain are exceptionally challenging, in vitro pharmacology models utilizing hypothalamic cell lines provide very powerful molecular tools when integrated with relevant animal models. Determining the mediators of orexin effects on cellular signaling pathways influencing energy expenditure could translate into therapies for physiological disorders in which orexin plays a role, such as those designed to increase obesity resistance by increasing responsiveness to orexin-induced SPA.
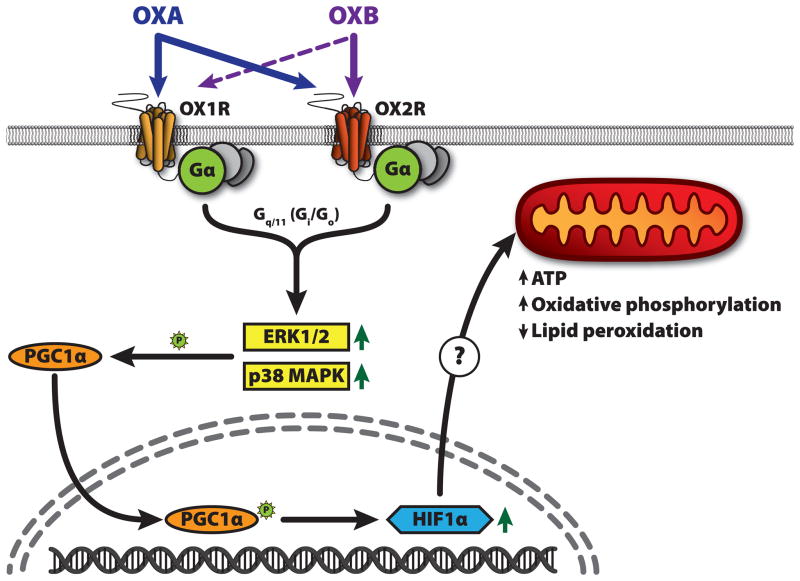
While some of the mechanisms above have not yet been confirmed in brain tissue, the likelihood they are present in neurons is high. As outlined above and summarized in Figure 2, current data from multiple independent in vitro and rodent models support the hypothesis that OXA-mediated increases in energy expenditure, and thus the obesity resistance properties of orexin, could depend in part on signaling cascades involving MAPKs, PGC-1α, and HIF-1α. Collectively, these independent lines of evidence support the idea that OXA actions on responsive neurons trigger pleiotropic effects on gene expression and second messenger pathways important in regulating intracellular neuronal metabolism, which is ultimately manifested in increased SPA and obesity resistance. Further investigation of orexin involvement in the signaling pathways outlined here will provide insight into mechanisms influencing metabolic status of OXA-responsive neurons, and elucidate how this ultimately influences energy expenditure and propensity for obesity.
Fig. 2.
Schematic representation of the hypothesized orexin signaling pathways. Orexins A and B (OXA, OXB) act on orexin receptors 1 and 2 (OX1R, OX2R). Orexin is known to increase activation (phosphorylation) of the MAP kinases ERK1/2 and p38 MAPK. This activation appears to be primarily mediated by Gq/11 signaling, but Gs/Go may be more important for OX2R. Both OXA and p38 MAPK can increase HIF-1α activation and expression; the mechanism may reply on the transcriptional coactivator PGC-1α. PGC-1α functions as a direct link between external physiological stimuli and regulation of mitochondrial biogenesis, and is a key facilitator for ATP production. PGC-1α is activated by p38 MAPK, and is a known regulator of HIF-1α expression in peripheral tissue. Increased HIF-1α results in gene expression changes leading to increased oxidative phosphorylation and decreased lipid peroxidation.
Acknowledgments
Authors received support from the US Department of Veterans Affairs Rehabilitation Research and Development, Veterans Affairs grant BX001686, and R01 DK078985.
ABBREVIATIONS
- DIO
Diet-induced obese
- DR
Diet-resistant
- ERK1/2
Extracellular receptor kinase 1 and 2
- FIH
Factor inhibiting HIF
- HA
High-activity
- HCR
High caloric restriction
- HEK
Human embryonic kidney
- HIF-1α
Hypoxia-inducible factor 1 alpha
- LA
Low-activity
- LCR
Low caloric restriction
- MAPK
Mitogen-activated protein kinase
- MKP-1
MAPK-phosphatase-1
- NEAT
Non-exercise activity thermogenesis
- OP
Obesity-prone
- OR
Obesity-resistant
- OX1R
Orexin/hypocretin 1 receptor
- OX2R
Orexin/hypocretin 2 receptor
- OXA
Orexin A (Hypocretin 1)
- OXB
Orexin B (Hypocretin 2)
- PGC-1α
Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha
- PKA
Protein kinase A
- PKC
Protein kinase C
- PLC
Phospholipase C
- POMC
Pro-opiomelanocortin
- PTX
Pertussis toxin
- rLH
Rostral lateral hypothalamic area
- SPA
Spontaneous physical activity
Contributor Information
Charles J. Billington, Email: billi005@umn.edu.
Catherine M. Kotz, Email: kotzx004@umn.edu.
Joshua P. Nixon, Email: nixon049@umn.edu.
References
- 1.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322(21):1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 2.Forbes GB, Brown MR, Welle SL, Lipinski BA. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Br J Nutr. 1986;56(1):1–9. doi: 10.1079/bjn19860080. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 4.Mustelin L, Silventoinen K, Pietilainen K, Rissanen A, Kaprio J. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. Int J Obes (Lond) 2009;33(1):29–36. doi: 10.1038/ijo.2008.258. [DOI] [PubMed] [Google Scholar]
- 5.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 6.Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of nonexercise activity. The American journal of clinical nutrition. 2000;72(6):1451–1454. doi: 10.1093/ajcn/72.6.1451. [DOI] [PubMed] [Google Scholar]
- 7.Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011;214(Pt 2):206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine JA. Non-exercise activity thermogenesis (NEAT) Best Pract Res Clin Endocrinol Metab. 2002;16(4):679–702. doi: 10.1053/beem.2002.0227. [DOI] [PubMed] [Google Scholar]
- 9.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R889–899. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- 10.Levin BE. Orexins: neuropeptides for all seasons and functions. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R885–888. doi: 10.1152/ajpregu.00344.2006. [DOI] [PubMed] [Google Scholar]
- 11.Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, Tataranni PA. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab. 2000;85(3):1087–1094. doi: 10.1210/jcem.85.3.6447. [DOI] [PubMed] [Google Scholar]
- 12.Kotz CM. Integration of feeding and spontaneous physical activity: role for orexin. Physiol Behav. 2006;88(3):294–301. doi: 10.1016/j.physbeh.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307(5709):584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 14.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R699–710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- 15.Nixon JP, Kotz CM, Novak CM, Billington CJ, Teske JA. Neuropeptides controlling energy balance: orexins and neuromedins. Handbook of experimental pharmacology. 2012;209(209):77–109. doi: 10.1007/978-3-642-24716-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C. Brain orexin promotes obesity resistance. Ann N Y Acad Sci. 2012;1264(1):72–86. doi: 10.1111/j.1749-6632.2012.06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Leighton CE, Boland K, Teske JA, Billington C, Kotz CM. Behavioral responses to orexin, orexin receptor gene expression, and spontaneous physical activity contribute to individual sensitivity to obesity. Am J Physiol Endocrinol Metab. 2012;303(7):E865–874. doi: 10.1152/ajpendo.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9(1):64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am J Physiol Endocrinol Metab. 2006;290(2):E396–403. doi: 10.1152/ajpendo.00293.2005. [DOI] [PubMed] [Google Scholar]
- 20.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273(2 Pt 2):R725–730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 21.Schemmel R, Mickelsen O, Gill JL. Dietary obesity in rats: Body weight and body fat accretion in seven strains of rats. The Journal of nutrition. 1970;100(9):1041–1048. doi: 10.1093/jn/100.9.1041. [DOI] [PubMed] [Google Scholar]
- 22.Ricci MR, Levin BE. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2003;285(3):R610–618. doi: 10.1152/ajpregu.00235.2003. [DOI] [PubMed] [Google Scholar]
- 23.Levin BE, Sullivan AC. Glucose-induced norepinephrine levels and obesity resistance. Am J Physiol. 1987;253(3 Pt 2):R475–481. doi: 10.1152/ajpregu.1987.253.3.R475. [DOI] [PubMed] [Google Scholar]
- 24.Hassanain M, Levin BE. Dysregulation of hypothalamic serotonin turnover in diet-induced obese rats. Brain Res. 2002;929(2):175–180. doi: 10.1016/s0006-8993(01)03387-x. [DOI] [PubMed] [Google Scholar]
- 25.Levin BE. Obesity-prone and -resistant rats differ in their brain [3H]paraminoclonidine binding. Brain Res. 1990;512(1):54–59. doi: 10.1016/0006-8993(90)91169-h. [DOI] [PubMed] [Google Scholar]
- 26.Levin BE. Reduced norepinephrine turnover in organs and brains of obesity-prone rats. Am J Physiol. 1995;268(2 Pt 2):R389–394. doi: 10.1152/ajpregu.1995.268.2.R389. [DOI] [PubMed] [Google Scholar]
- 27.Teske JA, Billington CJ, Kuskowski MA, Kotz CM. Spontaneous physical activity protects against fat mass gain. Int J Obes (Lond) 2012;36(4):603–613. doi: 10.1038/ijo.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050(1–2):156–162. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 29.Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286(4):E551–559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- 30.Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, et al. Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience. 2006;142(1):29–36. doi: 10.1016/j.neuroscience.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept. 2002;104(1–3):27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- 32.Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res. 1999;821(2):526–529. doi: 10.1016/s0006-8993(99)01131-2. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Leighton CE, Boland K, Billington C, Kotz CM. High and low activity rats: Elevated intrinsic physical activity drives resistance to diet induced obesity in non-bred rats. Obesity (Silver Spring) 2012 doi: 10.1002/oby.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307(5708):418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 35.Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, et al. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58(3):355–367. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farooqi IS. Monogenic human obesity syndromes. Prog Brain Res. 2006;153:119–125. doi: 10.1016/S0079-6123(06)53006-7. [DOI] [PubMed] [Google Scholar]
- 37.Farooqi IS. Monogenic human obesity. Front Horm Res. 2008;36:1–11. doi: 10.1159/0000115333. [DOI] [PubMed] [Google Scholar]
- 38.Butterick TA, Nixon JP, Billington CJ, Kotz CM. Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model. Neuroscience letters. 2012;524(1):30–34. doi: 10.1016/j.neulet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 40.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Lecea L, Sutcliffe JG, Fabre V. Hypocretins/orexins as integrators of physiological information: lessons from mutant animals. Neuropeptides. 2002;36(2–3):85–95. doi: 10.1054/npep.2002.0892. [DOI] [PubMed] [Google Scholar]
- 42.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nixon JP, Smale L. A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct. 2007;3:28. doi: 10.1186/1744-9081-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cutler DJ, Morris R, Sheridhar V, Wattam TA, Holmes S, Patel S, et al. Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides. 1999;20(12):1455–1470. doi: 10.1016/s0196-9781(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 45.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438(1–2):71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 46.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 47.Holmqvist T, Akerman KE, Kukkonen JP. High specificity of human orexin receptors for orexins over neuropeptide Y and other neuropeptides. Neuroscience letters. 2001;305(3):177–180. doi: 10.1016/s0304-3940(01)01839-0. pii. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, et al. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92(3):259–266. doi: 10.1254/jphs.92.259. [DOI] [PubMed] [Google Scholar]
- 49.Voisin T, Rouet-Benzineb P, Reuter N, Laburthe M. Orexins and their receptors: structural aspects and role in peripheral tissues. Cell Mol Life Sci. 2003;60(1):72–87. doi: 10.1007/s000180300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lund PE, Shariatmadari R, Uustare A, Detheux M, Parmentier M, Kukkonen JP, et al. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J Biol Chem. 2000;275(40):30806–30812. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- 51.Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG. Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacol Rev. 2006;58(1):46–57. doi: 10.1124/pr.58.1.4. [DOI] [PubMed] [Google Scholar]
- 52.Karteris E, Machado RJ, Chen J, Zervou S, Hillhouse EW, Randeva HS. Food deprivation differentially modulates orexin receptor expression and signaling in rat hypothalamus and adrenal cortex. Am J Physiol Endocrinol Metab. 2005;288(6):E1089–1100. doi: 10.1152/ajpendo.00351.2004. [DOI] [PubMed] [Google Scholar]
- 53.Hilairet S, Bouaboula M, Carriere D, Le Fur G, Casellas P. Hypersensitization of the Orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J Biol Chem. 2003;278(26):23731–23737. doi: 10.1074/jbc.M212369200. [DOI] [PubMed] [Google Scholar]
- 54.Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS. The signalling profile of recombinant human orexin-2 receptor. Cell Signal. 2008;20(9):1651–1661. doi: 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Ammoun S, Johansson L, Ekholm ME, Holmqvist T, Danis AS, Korhonen L, et al. OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol Endocrinol. 2006;20(1):80–99. doi: 10.1210/me.2004-0389. [DOI] [PubMed] [Google Scholar]
- 56.Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, et al. Dose-response effects of orexin-A on food intake and the behavioural satiety sequence in rats. Regul Pept. 2000;96(1–2):71–84. doi: 10.1016/s0167-0115(00)00203-2. [DOI] [PubMed] [Google Scholar]
- 57.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61(2):162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 58.Roth RJ, Le AM, Zhang L, Kahn M, Samuel VT, Shulman GI, et al. MAPK phosphatase-1 facilitates the loss of oxidative myofibers associated with obesity in mice. The Journal of clinical investigation. 2009;119(12):3817–3829. doi: 10.1172/JCI39054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu JJ, Roth RJ, Anderson EJ, Hong EG, Lee MK, Choi CS, et al. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 2006;4(1):61–73. doi: 10.1016/j.cmet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30(4):145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 61.O’Hagan KA, Cocchiglia S, Zhdanov AV, Tambuwala MM, Cummins EP, Monfared M, et al. PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci U S A. 2009;106(7):2188–2193. doi: 10.1073/pnas.0808801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C, Lin JD. PGC-1 coactivators in the control of energy metabolism. Acta Biochim Biophys Sin (Shanghai) 2011;43(4):248–257. doi: 10.1093/abbs/gmr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Onyango IG, Lu J, Rodova M, Lezi E, Crafter AB, Swerdlow RH. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta. 2010;1802(1):228–234. doi: 10.1016/j.bbadis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 64.Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, et al. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66(3):352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 66.Houten SM, Auwerx J. PGC-1alpha: turbocharging mitochondria. Cell. 2004;119(1):5–7. doi: 10.1016/j.cell.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 67.Luo Y, Zhu W, Jia J, Zhang C, Xu Y. NMDA receptor dependent PGC-1alpha up-regulation protects the cortical neuron against oxygen-glucose deprivation/reperfusion injury. Journal of molecular neuroscience : MN. 2009;39(1–2):262–268. doi: 10.1007/s12031-009-9196-5. [DOI] [PubMed] [Google Scholar]
- 68.Ma D, Li S, Lucas EK, Cowell RM, Lin JD. Neuronal inactivation of peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha) protects mice from diet-induced obesity and leads to degenerative lesions. J Biol Chem. 2010;285(50):39087–39095. doi: 10.1074/jbc.M110.151688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582(1):46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sikder D, Kodadek T. The neurohormone orexin stimulates hypoxia-inducible factor-1 activity. Genes Dev. 2007;21(22):2995–3005. doi: 10.1101/gad.1584307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan LB, Dong HL, Zhang HP, Zhao RN, Gong G, Chen XM, et al. Neuroprotective effect of orexin-A is mediated by an increase of hypoxia-inducible factor-1 activity in rat. Anesthesiology. 2011;114(2):340–354. doi: 10.1097/ALN.0b013e318206ff6f. [DOI] [PubMed] [Google Scholar]
- 72.Caretti A, Morel S, Milano G, Fantacci M, Bianciardi P, Ronchi R, et al. Heart HIF-1alpha and MAP kinases during hypoxia: are they associated in vivo? Exp Biol Med (Maywood) 2007;232(7):887–894. pii. [PubMed] [Google Scholar]
- 73.Minet E, Michel G, Mottet D, Raes M, Michiels C. Transduction pathways involved in Hypoxia-Inducible Factor-1 phosphorylation and activation. Free radical biology & medicine. 2001;31(7):847–855. doi: 10.1016/s0891-5849(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 74.Kodadek T, Cai D. Chemistry and biology of orexin signaling. Mol Biosyst. 2010;6(8):1366–1375. doi: 10.1039/c003468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang N, Fu Z, Linke S, Chicher J, Gorman JJ, Visk D, et al. The asparaginyl hydroxylase factor inhibiting HIF-1alpha is an essential regulator of metabolism. Cell Metab. 2010;11(5):364–378. doi: 10.1016/j.cmet.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang H, Zhang G, Gonzalez FJ, Park SM, Cai D. Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation. PLoS Biol. 2011;9(7):e1001112. doi: 10.1371/journal.pbio.1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]