Abstract
Purpose of Review
Acute and early HIV (AHI) is a pivotal time during HIV infection, yet there remain major shortfalls in diagnosis, linkage to care, and antiretroviral therapy (ART) initiation during AHI. We introduce an AHI-specific cascade, review recent evidence pertaining to the unique challenges of AHI, and discuss strategies for improving individual and public health outcomes.
Recent Findings
Presentation during AHI is common. Expanding use of 4th generation testing and pooled nucleic acid amplification testing (NAT) has led to improved AHI detection in resource-wealthy settings. Technologies capable of AHI diagnosis are rare in resource-limited settings; further development of point-of-care devices and utilization of targeted screening is needed. Rapid ART initiation during AHI limits reservoir seeding, preserves immunity, and prevents transmission. Reporting of AHI cascade outcomes is limited, but new evidence suggests that impressive rates of diagnosis, linkage to care, rapid ART initiation, and viral suppression can be achieved.
Summary
With advancements in AHI diagnostics and strong evidence for the therapeutic and prevention benefits of ART initiated during AHI, improving AHI cascade outcomes is both crucial and feasible. HIV guidelines should recommend diagnostic algorithms capable of detecting AHI and prescribe rapid, universal ART initiation during AHI.
Keywords: Acute HIV infection, HIV cascade, HIV diagnostics, linkage to care, guidelines
Introduction
Acute HIV is a very brief but critical phase of HIV infection. Historically, acute HIV infection has been defined as lasting only until the emergence of HIV-specific antibodies (1), since lack of antibodies in the presence of viral RNA was used as an operational definition of the stage of disease. However, as antibody tests have become ever more sensitive and many patients have been followed from infection onward (2), it is wiser to consider acute and early HIV infection as a package (sometimes referred to as “primary infection”), since clearly the important events that transpire after infection extend for a longer time than required for antibodies to form (3). The exact time at which acute and early infection should be considered as “established” infection has not been resolved. However, the transmission risk associated with acute and early infection lasts at least 4 months (4).
Acute HIV infection and early HIV infection (heretofore referred to as AHI) are associated with extremely high viral loads, seeding of viral reservoirs and a disproportionate contribution to onward HIV transmission. Failure to diagnose and treat persons with AHI has significant individual and public health implications. Responding to these consequences, revised US HIV management guidelines now include HIV screening algorithms to detect AHI, and recommend antiretroviral therapy (ART) regardless of CD4+ T cell counts in persons with AHI (5). The updated International AIDS Society guidelines also recommend universal ART, and the most recent European AIDS Clinical Society Guidelines advocate consideration and discussion of ART initiation with the patient with AHI (6, 7). However, prominent international guidelines, such as those from the World Health Organization (WHO), have not yet defined a diagnostic strategy nor made any specific recommendation regarding ART initiation for persons with AHI (8).
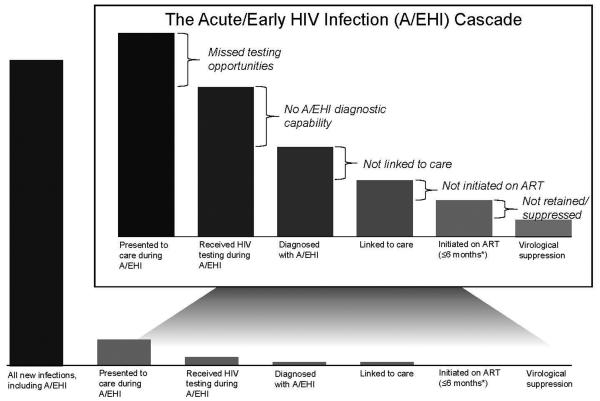
In this paper, we propose an AHI-specific cascade (Figure 1) and explore the critical concerns that could be targeted in guidelines. AHI has unique challenges. These include limited detection of AHI through HIV testing algorithms, delays in ART initiation that compromise the individual and public health benefits of ART, and limited knowledge regarding success rates of linkage to and retention in HIV care.
Figure 1. Acute HIV Infection Cascade.
There remains little data to populate the acute HIV (AHI) cascade elements. Our proposed cascade highlights not only missed opportunities for diagnosis during this critical phase of infection, but also emphasizes opportunities to improve HIV outcomes through guideline modification that recognizes the importance of AHI diagnosis and immediate linkage to care with initiation of antiretroviral therapy.
Diagnosis of acute HIV infection
Perhaps no topic related to AHI attracts more attention and frustration than identification of this stage of infection. Identifying persons with AHI is complicated by the brevity of the phase, the non-specific symptoms associated with the HIV infection, and (at first) the absence of anti-HIV antibodies. Traditional point-of-care (POC) antibody tests cannot directly address this earliest phase of infection, so the diagnosis of AHI relies on the direct detection of virus or viral antigen. (Parenthetically, discordance in particular POC rapid tests strongly suggests AHI, as discussed below).
Revised CDC guidelines outline diagnostic algorithms that detect AHI using a combination of 4th generation enzyme immunoassays (EIA) and nucleic acid testing (NAT) (9). Although WHO guidelines characterize 4th generation assays as “A1 assays” to be used where feasible for the detection of AHI, they provide no guidance for AHI screening or diagnosis (10). Expanded utilization of 4th generation assay can clearly help to diagnose persons earlier in the course of infection.
Due in part to their non-specific nature, clinical manifestations of acute infection are frequently misclassified (11, 12). In the United States, 55% of persons eventually diagnosed with AHI were missed on their first visit to the healthcare system (13). Patients in resource-limited settings also commonly seek medical care for symptoms related to acute infection, but are instead often presumptively treated for malaria (11, 14). In one survey of Kenyan adults presenting to outpatient health centers with fever, the prevalence of AHI and malaria was identical at 1.7% (95% confidence interval (CI) 0.5%−4.2%) (15). Among febrile seronegative persons presenting to outpatient clinics Uganda, 2.8 % had AHI ; in Mozambique, AHI prevalence was 3.3% (95% CI 1.3%−6.7%) (16, 17). Risk score algorithms demonstrate the utility of AHI screening among seronegative patients presenting with certain symptoms and risk behavior histories (18, 19). Among higher risk patients, such an algorithm can lead to correct and efficient diagnosis of AHI, detecting 81.0% of patients with AHI by screening only 20.1% of the targeted patient population. These AHI detection algorithms deserve more attention.
Increased targeted screening for AHI may unearth substantial missed diagnoses. In resource-limited settings, symptom-derived AHI risk scores help focus testing resources (12, 15, 18, 20). Nonetheless, AHI is rarely considered among febrile adults in sub-Saharan Africa (21). WHO guidelines recommend HIV testing in the setting of >30 days of unexplained fever with a negative malaria test, but AHI is not mentioned as a potential cause of fever of shorter duration nor is AHI screening recommended for this patient population (22). Numerous technologies are available to narrow the window of diagnosis for persons with AHI; some have been incorporated into testing algorithms in resource-wealthy settings but none are routinely applied in resource-limited settings.
Fourth generation EIAs detect p24 antigen and antibody isotypes that emerge soon after infection. These assays serve as the backbone for HIV testing algorithms in the US (9). Identifying persons earlier in the course of infection compared to previous generations, 4th generation tests substantially improve AHI detection (23-26). In a Texas emergency department, 18/78 (23%) with confirmed positive results were acutely infected and would not have been diagnosed without 4th generation assays (27). However, these assays still miss high-risk populations presenting to care in the critical gap before emergence of p24 antigen (28). Additionally, the ARCHITECT HIV Ag/Ab (Abbott Diagnostics, Chicago IL), a widely used 4th generation assay, does not discriminate between the antigen and antibody targets of the assay and thus does not distinguish between acute and established HIV. The use of a modified signal-to-cutoff ratio in 4th generation EIAs may improve detection of early HIV infection and improve discrimination between AHI and established infection (29). Ultimately though, nucleic acid amplification (NAT) testing is required to identify persons in this earlier window via detection of HIV RNA.
Use of pooled NAT (30) instead of or in conjunction with 4th generation assays identifies persons with AHI earlier than 4th generation alone (i.e. in the first two weeks of infection), and pooled specimen throughput and costs may be more suitable for AHI screening in resource-limited settings. In high- and low-prevalence settings, pooled NAT substantially increases the number of persons identified with AHI compared to standalone 4th generation testing (31, 32). Although pooling for AHI may be cost-effective (33), financial feasibility will depend on the underlying prevalence of AHI and specific pooling strategy. Ideal pooling strategies maximize batch size according to population risk profiles and HIV prevalence, with a tradeoff of increased complexity with larger pools. In a high-prevalence Thai cohort, addition of pooled NAT after 4th generation testing increased acute diagnosis by nearly 40% but also increased screening costs by 22% (31). Besides costs, laboratory-based assays introduce logistical barriers to AHI screening, requiring venipuncture and patient follow-up
Effective POC tests for AHI would allow better widespread diagnostic capacity. The Alere Determine HIV-1/2 Ag/Ab lateral flow test was designed with this goal in mind. However, extensive field-based testing demonstrated that the product lacks the ability to reliably detect p24 antigen in blood, and is also compromised by false positive results (34-36).
Three recently introduced first-generation rapid NAT devices may be more promising: The Alere Q demonstrated excellent performance for early infant diagnosis in Mozambique (37); the SAMBA semiquantitaive assay, with a 1,000 copy/ml threshold, has demonstrated adequate performance on plasma (38); and the Liat HIV Quant cartridge-based viral load assay has shown promising results for POC viral load quantification (39). The ability of these POC technologies to detect HIV RNA suggests potential for an AHI screening assay. However, none of these tests have been field tested for detection of AHI, a critical requirement.
Antiretroviral therapy for acute and early HIV infection
During AHI, the virus integrates into host cell DNA, allowing the formation of a “latent reservoir”. ART initiated in AHI is to date the most effective strategy to limit HIV reservoir seeding (40-44) and reduce the number of infected cells (2, 45-48). Timing of ART initiation is critical as reservoir size is significantly smaller and decline in the number of infected cells is much faster in those who initiated ART within the first 2 weeks (prior to HIV IgM formation) vs. later (2). Cell-associated HIV DNA levels as low as those observed in elite controllers and post-treatment controllers can be achieved through very early treatment (42, 45, 49-51). In contrast, once individuals enter the chronic HIV infection phase, despite fully suppressive ART, they maintain a large reservoir size in blood and tissue that decays little over time (52, 53), leading to a rapid recrudescence of plasma viremia when ART is interrupted (54).
Cell-associated HIV DNA levels may also predict subsequent ability to control viremia following treatment interruption (55, 56). AHI individuals who were randomized to immediate vs. deferred ART displayed significantly longer time to viral rebound and lower viral set point when ART was discontinued (49, 57-60). In addition, the actual decrease in time of exposure to ART by deferring treatment of patients with AHI is small, since most patients followed prospectively required treatment by traditional guidelines within a few years (57, 58).
CD4+ T cells depletion, immune exhaustion and immune activation occur early in HIV infection, and chances for a full recovery are reduced when ART is delayed (46, 50, 61-63). Viral escape from host immunologic response increases when HIV is left untreated leading to an alteration in the “viro-immunological” landscape that could impact natural control of the virus and efficacy of future immunotherapeutic interventions (64-66).
A related issue is the attempt to modify the course of infection with alternative, more aggressive treatment regimens. Several randomized studies comparing standard ART vs. regimens intensified with integrase and entry inhibitors showed plasma HIV RNA to decline more rapidly in the intensified arm; but all regimens were similarly effective in lowering cell-associated DNA and RNA and markers of immune activation as well as raising CD4+ T cell counts (49-51). Other non-randomized studies showed minimal to no benefit in intensifying standard ART regimens (67, 68). However, the more rapid viral load decline in blood and genital secretions with integrase inhibitor-based regimens may prove beneficial in reducing transmission risk during AHI (69).
Leveraging the acute HIV cascade for prevention
The potential to stop transmission from people with AHI is of great public health importance. Several lines of evidence suggest that HIV transmission is greatly amplified during AHI infection (4). The HIV founder virus(es) causing infection are particularly contagious (70) and the viral loads in AHI are exceptionally high (71). A series of modeling exercises suggest that patients with AHI fuel the epidemic (72) and phylogenetic analysis from Montreal (73), Switzerland (74), Amsterdam(75, 76) and San Francisco (77),all demonstrate exceptional contribution of AHI to spread of HIV in MSM. Studies from San Diego (78) and Malawi (3, 4) suggest that intervention in the first 4 months can reduce HIV spread.
A key barrier to intervention in AHI is the belief that action geared toward public health is infeasible. However, North Carolina’s Screening and Tracing Active Transmission Program has since 2002 successfully carried out statewide AHI screening and linkage to care, with 30 day linkage and ART initiation rates of 80% and 61% (79, 80). An AHI study in New York City reports similar rates between patients newly diagnosed with AHI and established HIV of linkage within 3 months (92% vs 91%) retention at 12 months (86% vs 83%, p= 0.78) and suppression (65% vs 60%) (81). Additionally, a recent study reports 111/112 participants diagnosed with AHI at a Thai clinic were initiated on ART at their first care visit, a median of 19 days from the estimated date of their exposure to HIV, with excellent subsequent retention and viral suppression outcomes (31). Other studies involving rapid linkage and ART initiation are currently in process (MP3, SEARCH, rapIT, Brigham/GHESKIO, Engage4Health, all clinicaltrials.gov).
The prevention benefits of diagnosis and care linkage during AHI extend beyond the opportunity to initiate early ART. Diagnosis during AHI coupled with risk reduction education or counseling has been associated with rapid reduction in self-reported transmission risk behaviors (82), and mathematical modeling suggests diagnosis during AHI and associated behavior change could have a significant impact on the UK MSM epidemic even in the absence of ART initiation (83).
The acute HIV cascade in practice
The concept of the HIV cascade has helped synchronize terminology and spur publication of large amounts of data on population-level HIV outcomes. However, information regarding AHI care outcomes outside of a research context remains scarce: public health surveillance data has traditionally not distinguished between AHI and established HIV diagnoses. However, when surveillance includes attempts to find infected people who are antibody negative, screening yield increases between 2-10% (30, 84-91). Yield varies according to diagnostic assays, risk profile of population (i.e. public health vs STI clinic), and underlying HIV prevalence. AHI screening yield is especially high among urban MSM in the US (84, 88, 89).
In 2008 New York City implemented citywide AHI surveillance and procedures for streamlined entry to HIV care for AHI patients. Thus far, published cascade data outcomes are limited but demonstrate 77% of the 62 city residents diagnosed with AHI were linked to care within 3 months (92). Another series of one-year cascade outcomes in a U.S. community health clinic cohort of 93 patients diagnosed with AHI reports 87% retained in care, 62% initiated on ART, and 46% virally suppressed (93). The proportions were all slightly higher for AHI patients compared to the clinics’ patients newly diagnosed with established HIV.
Addressing the limited surveillance data collection on AHI outcomes, the CDC published new case definitions in 2014 creating an HIV stage 0, defined as a sequence of concurrent or sequential discordant test results indicative of AHI (94). This development promises to lead to a dramatically improved understanding of the state of the AHI cascade in the U.S.
Conclusion
The personal and public health benefits of treatment of AHI are now well established. Indeed, as the world moves toward a universal “test and treat” strategy it would seem very unwise to purposefully leave people with AHI untreated. A large series of randomized controlled trials (95-98) have all demonstrated an advantage to earlier ART. The Strategic Timing of AntiRetroviral Treatment (START) study was stopped by the NIH DSMB because of large differences in outcomes with early treatment started at CD4 counts >500 cells/mm3. The cascade outlined in this report highlights the challenges unique to AHI: the differential diagnostic capacity according to resource setting, the highly time-sensitive nature of therapy initiation, and the opportunity to prevent transmission during a highly infectious period. There are two critical levers through which modification of guidelines could improve AHI cascade outcomes: guidelines should incorporate diagnostic algorithms that include technologies capable of AHI detection, and, once diagnosed, guidelines must emphasize expedited linkage to care and ART initiation regardless of CD4+ T cell count. Maximal treatment benefits are gained through prompt ART initiation within days of diagnosis. This window is especially critical window for persons with AHI, and this opportunity will be missed if mechanisms to prioritize and urgently start ART in acutely-infected individuals are not in place. Same-day ART has been successfully implemented in low and middle income countries (31, 99). There are substantial financial, technical, and logistical barriers involved in AHI diagnosis, linkage, and treatment; but revision of international guidelines is essential in order to begin to address these obstacles (Table 1).
Table 1.
Proposed AHI Guideline Modifications
| Diagnosis | Treatment | |
|---|---|---|
| Recommendation: |
|
|
Key points.
Diagnosis of AHI is frequently missed despite many patients presenting to care during this critical phase of infection.
Use of 4th generation EIA with pooled NAT substantially improves AHI case finding, and although these laboratory-based diagnostic tools have been incorporated into testing algorithms in the USA and Europe, the technologies are rarely available in resource-limited settings
Immediate initiation of ART during AHI is critical to limit reservoir seeding, preserve immunologic function, and reduce transmission events during this highly-infectious period.
Revisions to prominent international guidelines should define a diagnostic strategy and make specific recommendations regarding ART initiation for persons with AHI
Acknowledgements
None
Financial support and sponsorship
This work was supported by the UNC Center for AIDS Research, an NIH funded program (P30 AI50410). National Institutes of Health supported Sarah E. Rutstein (NIMH - F30 MH098731, NIGMS - T32 GM008719, and NIAID - R01 AI114320) and Christopher J. Sellers (NIAID - 5T32AI070114-08). Jintanat Ananworanich has received honorarium from Merck and ViiV Healthcare.
Footnotes
Conflicts of interest
None
References
- 1.Pilcher CD, Eron JJ, Jr., Galvin S, Gay C, Cohen MS. Acute HIV revisited: new opportunities for treatment and prevention. J Clin Invest. 2004 Apr;113(7):937–45. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One. 2012;7(3):e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilcher CD, Joaki G, Hoffman IF, Martinson FE, Mapanje C, Stewart PW, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007 Aug 20;21(13):1723–30. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011 Jul 16;378(9787):256–68. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2015. Panel on Antiretroviral Guidelines for Adults and Adolescents. [Google Scholar]
- 6.Nov, 2014. European Guidelines for treatment of HIV-infected adults in Europe: European AIDS Clinical Society. 2014.
- 7.Antiretroviral Treatment of Adult HIV Infection 2014 Recommendations of the International Antiviral Society-USA Panel: IAS-USA. 2014 doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 8.WHO . Consolidated Guidelines On HIV Prevention, Diagnosis, Treatment, And Care for Key Populations. WHO; Geneva: 2013. [PubMed] [Google Scholar]
- 9.CDC . Laboratory testing for the diagnosis of HIV infection: updated recommendations. Vol. 27. Atlanta, GA: Jun, 2014. 2014. [Google Scholar]
- 10.WHO . Service delivery approaches to HIV testing and counseling (HTC): a strategic HTC policy framework. WHO; Geneva, Switzerland: 2012. [Google Scholar]
- 11.Sanders EJ, Wahome E, Mwangome M, Thiong'o AN, Okuku HS, Price MA, et al. Most adults seek urgent healthcare when acquiring HIV-1 and are frequently treated for malaria in coastal Kenya. AIDS. 2011 Jun 1;25(9):1219–24. doi: 10.1097/QAD.0b013e3283474ed5. [DOI] [PubMed] [Google Scholar]
- 12.Powers KA, Cohen MS. Acute HIV-1 infection in sub-Saharan Africa: a common occurrence overlooked. AIDS. 2014 Jun 1;28(9):1365–7. doi: 10.1097/QAD.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 13.McKellar MS, Cope AB, Gay CL, McGee KS, Kuruc JD, Kerkau MG, et al. Acute HIV-1 infection in the Southeastern United States: a cohort study. AIDS Res Hum Retroviruses. 2013 Jan;29(1):121–8. doi: 10.1089/aid.2012.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeatman SE, Hoffman RM, Chilungo A, Lungu SR, Namadingo HC, Chimwaza AF, et al. Brief Report: Health-Seeking Behavior and Symptoms Associated With Early HIV Infection: Results From a Population-Based Cohort in Southern Malawi. J Acquir Immune Defic Syndr. 2015 May 1;69(1):126–30. doi: 10.1097/QAI.0000000000000536. ** This brief report presents critical information regarding the opportunity to improve and expand AHI screening outside of the previously-studied STI clinic setting using a population-based cohort of young Malawian women. Frequency of healthcare seeking behavior during the seroconversion period emphasizes the utility of targeted screening using valided symptom- and behavior-driven risk score algorithms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders EJ, Mugo P, Prins HA, Wahome E, Thiong'o AN, Mwashigadi G, et al. Acute HIV-1 infection is as common as malaria in young febrile adults seeking care in coastal Kenya. AIDS. 2014 Jun 1;28(9):1357–63. doi: 10.1097/QAD.0000000000000245. ** This paper highlights the frequency of AHI in the context of febrile adults presenting to care in Kenya. The prevalence of AHI as compared to malaria among patients presenting with fever highlights importance of revised screening criteria and opportunities to target AHI testing in resource-limited settings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bebell LM, Pilcher CD, Dorsey G, Havlir D, Kamya MR, Busch MP, et al. Acute HIV-1 infection is highly prevalent in Ugandan adults with suspected malaria. AIDS. 2010 Jul 31;24(12):1945–52. doi: 10.1097/QAD.0b013e32833bb732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serna-Bolea C, Munoz J, Almeida JM, Nhacolo A, Letang E, Nhampossa T, et al. High prevalence of symptomatic acute HIV infection in an outpatient ward in southern Mozambique: identification and follow-up. AIDS. 2010 Feb 20;24(4):603–8. doi: 10.1097/QAD.0b013e328335cda3. [DOI] [PubMed] [Google Scholar]
- 18.Mlisana K, Sobieszczyk M, Werner L, Feinstein A, van Loggerenberg F, Naicker N, et al. Challenges of diagnosing acute HIV-1 subtype C infection in African women: performance of a clinical algorithm and the need for point-of-care nucleic-acid based testing. PLoS One. 2013;8(4):e62928. doi: 10.1371/journal.pone.0062928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers KA, Miller WC, Pilcher CD, Mapanje C, Martinson FE, Fiscus SA, et al. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. AIDS. 2007 Oct 18;21(16):2237–42. doi: 10.1097/QAD.0b013e3282f08b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahome E, Fegan G, Okuku HS, Mugo P, Price MA, Mwashigadi G, et al. Evaluation of an empiric risk screening score to identify acute and early HIV-1 infection among MSM in Coastal Kenya. AIDS. 2013 Aug 24;27(13):2163–6. doi: 10.1097/QAD.0b013e3283629095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prins HA, Mugo P, Wahome E, Mwashigadi G, Thiong'o A, Smith A, et al. Diagnosing acute and prevalent HIV-1 infection in young African adults seeking care for fever: a systematic review and audit of current practice. Int Health. 2014 Jun;6(2):82–92. doi: 10.1093/inthealth/ihu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO . Acute Care: Integrated Management of Adolescent and Adult Illness. WHO; Geneva, Switzerland: 2009. [Google Scholar]
- 23.Kfutwah A, Lemee V, Ngono HV, De Oliveira F, Njouom R, Plantier JC. Field evaluation of the Abbott ARCHITECT HIV Ag/Ab Combo immunoassay. J Clin Virol. 2013 Dec;58(Suppl 1):e70–5. doi: 10.1016/j.jcv.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Piwowar-Manning E, Fogel JM, Richardson P, Wolf S, Clarke W, Marzinke MA, et al. Performance of the fourth-generation Bio-Rad GS HIV Combo Ag/Ab enzyme immunoassay for diagnosis of HIV infection in Southern Africa. J Clin Virol. 2015 Jan;62:75–9. doi: 10.1016/j.jcv.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel P, Bennett B, Sullivan T, Parker MM, Heffelfinger JD, Sullivan PS. Rapid HIV screening: missed opportunities for HIV diagnosis and prevention. J Clin Virol. 2012 May;54(1):42–7. doi: 10.1016/j.jcv.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haukoos JS, Lyons MS, White DA, Hsieh YH, Rothman RE. Acute HIV infection and implications of fourth-generation HIV screening in emergency departments. Ann Emerg Med. 2014 Nov;64(5):547–51. doi: 10.1016/j.annemergmed.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geren KI, Lovecchio F, Knight J, Fromm R, Moore E, Tomlinson C, et al. Identification of acute hiv infection using fourth-generation testing in an opt-out emergency department screening program. Ann Emerg Med. 2014 Nov;64(5):537–46. doi: 10.1016/j.annemergmed.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Krajden M, Cook D, Mak A, Chu K, Chahil N, Steinberg M, et al. Pooled nucleic acid testing increases the diagnostic yield of acute HIV infections in a high-risk population compared to 3rd and 4th generation HIV enzyme immunoassays. J Clin Virol. 2014 Sep;61(1):132–7. doi: 10.1016/j.jcv.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Ramos EM, Harb S, Dragavon J, Swenson P, Stekler JD, Coombs RW. Performance of an alternative HIV diagnostic algorithm using the ARCHITECT HIV Ag/Ab Combo assay and potential utility of sample-to-cutoff ratio to discriminate primary from established infection. J Clin Virol. 2013 Dec;58(Suppl 1):e38–43. doi: 10.1016/j.jcv.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilcher CD, Fiscus SA, Nguyen TQ, Foust E, Wolf L, Williams D, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005 May 5;352(18):1873–83. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 31.De Souza M, Phanuphak N, Pinyakorn S, Trichavaroj R, Pattanachaiwit S, Chomchey N, et al. Impact of nucleic acid testing relative ot antigen/antibody combination immunoassay on the detection of acute HIV infection. AIDS. 2015;29:793–800. doi: 10.1097/QAD.0000000000000616. ** This study demonstrates the added benefit of pooled NAT in the context of AHI diagnosis, as well as identifies the financial implications of pooled NAT. Almost all of the study participants successfully moved rapidly through the steps of the AHI cascade, demonstrating the feasibility of rapid linkage, ART initiation, retention and suppression in this population. [DOI] [PubMed] [Google Scholar]
- 32.Emerson B, Plough K. Detection of acute HIV-1 infections utilizing NAAT technology in Dallas, Texas. J Clin Virol. 2013 Dec;58(Suppl 1):e48–53. doi: 10.1016/j.jcv.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Hutchinson AB, Patel P, Sansom SL, Farnham PG, Sullivan TJ, Bennett B, et al. Cost-effectiveness of pooled nucleic acid amplification testing for acute HIV infection after third-generation HIV antibody screening and rapid testing in the United States: a comparison of three public health settings. PLoS Med. 2010 Sep;7(9):e1000342. doi: 10.1371/journal.pmed.1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duong YT, Mavengere Y, Patel H, Moore C, Manjengwa J, Sibandze D, et al. Poor performance of the determine HIV-1/2 Ag/Ab combo fourth-generation rapid test for detection of acute infections in a National Household Survey in Swaziland. J Clin Microbiol. 2014 Oct;52(10):3743–8. doi: 10.1128/JCM.01989-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conway DP, Holt M, McNulty A, Couldwell DL, Smith DE, Davies SC, et al. Multi-centre evaluation of the Determine HIV Combo assay when used for point of care testing in a high risk clinic-based population. PLoS One. 2014;9(4):e94062. doi: 10.1371/journal.pone.0094062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg NE, Kamanga G, Phiri S, Nsona D, Pettifor A, Rutstein SE, et al. Detection of acute HIV infection: a field evaluation of the determine(R) HIV-1/2 Ag/Ab combo test. J Infect Dis. 2012 Feb 15;205(4):528–34. doi: 10.1093/infdis/jir789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jani IV, Meggi B, Mabunda N, Vubil A, Sitoe NE, Tobaiwa O, et al. Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. J Acquir Immune Defic Syndr. 2014 Sep 1;67(1):e1–4. doi: 10.1097/QAI.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 38.Ritchie AV, Ushiro-Lumb I, Edemaga D, Joshi HA, De Ruiter A, Szumilin E, et al. SAMBA HIV semiquantitative test, a new point-of-care viral-load-monitoring assay for resource-limited settings. J Clin Microbiol. 2014 Sep;52(9):3377–83. doi: 10.1128/JCM.00593-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott L, Gous N, Carmona S, Stevens W. Laboratory evaluation of the Liat HIV Quant (IQuum) whole blood and plasma HIV-1 viral load assays for Point -of -Care testing in South Africa. J Clin Microbiol. 2015 Mar;:4. doi: 10.1128/JCM.03325-14. * This article presents promising outcomes of a new point-of-care assay for detection of HIV RNA. Although intended for use in early infant diagnosis and viral load monitoring for adult ART patients, in future iteratiorns of this device may be relevant for diagnosis of AHI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buzon MJ, Martin-Gayo E, Pereyra F, Ouyang Z, Sun H, Li JZ, et al. Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. J Virol. 2014 Sep 1;88(17):10056–65. doi: 10.1128/JVI.01046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005 May 1;191(9):1410–8. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 42.Cheret A, Bacchus-Souffan C, Avettand-Fenoel V, Melard A, Nembot G, Blanc C, et al. Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. J Antimicrob Chemother. 2015;70:2108–2120. doi: 10.1093/jac/dkv084. [DOI] [PubMed] [Google Scholar]
- 43.Archin NM, Vaidya NK, Kuruc JD, Liberty AL, Wiegand A, Kearney MF, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. PNAS. 2012;109(24):9523–8. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Archin NM, Vaidya NK, Kuruc JD, Liberty AL, Wiegand A, Kearney MF, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A. 2012 Jun 12;109(24):9523–8. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laanani M, Ghosn J, Essat A, Melard A, Seng R, Gousset M, et al. Impact of the Timing of Initiation of Antiretroviral Therapy During Primary HIV-1 Infection on the Decay of Cell-Associated HIV-DNA. Clin Infect Dis. 2015 Jun 1;60(11):1715–21. doi: 10.1093/cid/civ171. ** This study showed continued decay of cell-associated HIV DNA in a large number of early treated individuals, with fastest decay observed in those who initiated therapy within 2 weeks of infection. It highlighted the importance of immediate ART in acute HIV infection. [DOI] [PubMed] [Google Scholar]
- 46.Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013 Oct 15;208(8):1202–11. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koelsch KK, Boesecke C, McBride K, Gelgor L, Fahey P, Natarajan V, et al. Impact of treatment with raltegravir during primary or chronic HIV infection on RNA decay characteristics and the HIV viral reservoir. AIDS. 2011 Nov 13;25(17):2069–78. doi: 10.1097/QAD.0b013e32834b9658. [DOI] [PubMed] [Google Scholar]
- 48.Gianella S, von Wyl V, Fischer M, Niederoest B, Battegay M, Bernasconi E, et al. Effect of early antiretroviral therapy during primary HIV-1 infection on cell-associated HIV-1 DNA and plasma HIV-1 RNA. Antivir Ther. 2011;16(4):535–45. doi: 10.3851/IMP1776. [DOI] [PubMed] [Google Scholar]
- 49.Cheret A, Nembot G, Melard A, Lascoux C, Slama L, Miailhes P, et al. Intensive five-drug antiretroviral therapy regimen versus standard triple-drug therapy during primary HIV-1 infection (OPTIPRIM-ANRS 147): a randomised, open-label, phase 3 trial. Lancet Infect Dis. 2015 Apr;15(4):387–96. doi: 10.1016/S1473-3099(15)70021-6. ** This is the largest randomized study of standard vs. that intensified by integrase and entry inhibitors in acutely infected individuals. It observed similar outcomes in HIV RNA and cell associated HIV DNA between groups, suggesting that any suppressive regimens could be used in acute HIV infection. [DOI] [PubMed] [Google Scholar]
- 50.Markowitz M, Evering TH, Garmon D, Caskey M, La Mar M, Rodriguez K, et al. A randomized open-label study of 3-versus 5-drug combination antiretroviral therapy in newly HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2014 Jun 1;66(2):140–7. doi: 10.1097/QAI.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ananworanich J, Fauci A. HIV cure research: a formidable challenge. Virus Eradication. 2015;1:1–3. doi: 10.1016/S2055-6640(20)31152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Besson GJ, Lalama CM, Bosch RJ, Gandhi RT, Bedison MA, Aga E, et al. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis. 2014 Nov 1;59(9):1312–21. doi: 10.1093/cid/ciu585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray JM, Zaunders JJ, McBride KL, Xu Y, Bailey M, Suzuki K, et al. HIV DNA subspecies persist in both activated and resting memory CD4+ T cells during antiretroviral therapy. J Virol. 2014 Mar;88(6):3516–26. doi: 10.1128/JVI.03331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothenberger M, editor. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci USA. 2015;112:E1126–1134. doi: 10.1073/pnas.1414926112. * This unique study enrolled chronically infected, long-term virally suppressed adults who underwent ART interruption. With intensive HIV RNA monitoring and tissue biopsies, it documented a rapid recrudescence of virus from multifoci infection in gut and lymph nodes. It illustrates that late treatment is associated with signficant tissue infection that is a major barrier to achieving HIV remission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams JP, Hurst J, Stohr W, Robinson N, Brown H, Fisher M, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife. 2014;3:e03821. doi: 10.7554/eLife.03821. * In the SPARTAC study participants who initiated ART within the first 6 months of infection and subsequently interrupted treatment, pre-ART cell-associated HIV DNA was predictive of time to viral load rebound. This supports immediate ART to limit the HIV reservoir size and also suggests that total HIV DNA could potentially be used as a biomarker for HIV remission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013 Mar;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogan CM, Degruttola V, Sun X, Fiscus SA, Del Rio C, Hare CB, et al. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. J Infect Dis. 2012 Jan 1;205(1):87–96. doi: 10.1093/infdis/jir699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fidler S, Porter K, Ewings F, Frater J, Ramjee G, Cooper D, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013 Jan 17;368(3):207–17. doi: 10.1056/NEJMoa1110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grijsen ML, Steingrover R, Wit FW, Jurriaans S, Verbon A, Brinkman K, et al. No treatment versus 24 or 60 weeks of antiretroviral treatment during primary HIV infection: the randomized Primo-SHM trial. PLoS Med. 2012;9(3):e1001196. doi: 10.1371/journal.pmed.1001196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goujard C, Emilie D, Roussillon C, Godot V, Rouzioux C, Venet A, et al. Continuous versus intermittent treatment strategies during primary HIV-1 infection: the randomized ANRS INTERPRIM Trial. AIDS. 2012 Sep 24;26(15):1895–905. doi: 10.1097/QAD.0b013e32835844d9. [DOI] [PubMed] [Google Scholar]
- 61.Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;Dec10(12):e1004543. doi: 10.1371/journal.ppat.1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karris MY, Kao YT, Patel D, Dawson M, Woods SP, Vaida F, et al. Predictors of virologic response in persons who start antiretroviral therapy during recent HIV infection. AIDS. 2014 Mar 27;28(6):841–9. doi: 10.1097/QAD.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013 Jan 17;368(3):218–30. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015 Jan 15;517(7534):381–5. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberts HE, Hurst J, Robinson N, Brown H, Flanagan P, Vass L, et al. Structured observations reveal slow HIV-1 CTL escape. PLoS Genet. 2015 Feb;11(2):e1004914. doi: 10.1371/journal.pgen.1004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrari G, Korber B, Goonetilleke N, Liu MK, Turnbull EL, Salazar-Gonzalez JF, et al. Relationship between functional profile of HIV-1 specific CD8 T cells and epitope variability with the selection of escape mutants in acute HIV-1 infection. PLoS Pathog. 2011;7(2):e1001273. doi: 10.1371/journal.ppat.1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bottani GM, Oreni ML, Orofino G, Tau P, Di Nardo Stuppino S, Colella E, et al. Treatment outcome in HIV+ patients receiving 3- or 4-drug regimens during PHI. J Int AIDS Soc. 2014;17(4 Suppl 3):19778. doi: 10.7448/IAS.17.4.19778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puertas MC, Salgado M, Moron-Lopez S, Ouchi D, Munoz-Moreno JA, Molto J, et al. Effect of lithium on HIV-1 expression and proviral reservoir size in the CD4+ T cells of antiretroviral therapy suppressed patients. AIDS. 2014 Sep 10;28(14):2157–9. doi: 10.1097/QAD.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 69.Phanuphak N, Teeratakulpisarn N, van Griensven F, Chomchey N, Pinyakorn S, Fletcher JL, et al. Anogenital HIV RNA in Thai men who have sex with men in Bangkok during acute HIV infection and after randomization to standard vs. intensified antiretroviral regimens. J Int AIDS Soc. 2015;18(1):19470. doi: 10.7448/IAS.18.1.19470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlson JM, Schaefer M, Monaco DC, Batorsky R, Claiborne DT, Prince J, et al. HIV transmission. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science. 2014 Jul 11;345(6193):1254031. doi: 10.1126/science.1254031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen MS, Dye C, Fraser C, Miller WC, Powers KA, Williams BG. HIV treatment as prevention: debate and commentary--will early infection compromise treatment-as-prevention strategies? PLoS Med. 2012;9(7):e1001232. doi: 10.1371/journal.pmed.1001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volz EM, Ionides E, Romero-Severson EO, Brandt MG, Mokotoff E, Koopman JS. HIV-1 transmission during early infection in men who have sex with men: a phylodynamic analysis. PLoS Med. 2013 Dec;10(12):e1001568. doi: 10.1371/journal.pmed.1001568. discussion e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brenner BG, Roger M, Routy JP, Moisi D, Ntemgwa M, Matte C, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007 Apr 1;195(7):951–9. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 74.Kouyos RD, von Wyl V, Yerly S, Boni J, Taffe P, Shah C, et al. Molecular epidemiology reveals long-term changes in HIV type 1 subtype B transmission in Switzerland. J Infect Dis. 2010 May 15;201(10):1488–97. doi: 10.1086/651951. [DOI] [PubMed] [Google Scholar]
- 75.Bezemer D, van Sighem A, Lukashov VV, van der Hoek L, Back N, Schuurman R, et al. Transmission networks of HIV-1 among men having sex with men in the Netherlands. AIDS. 2010 Jan 16;24(2):271–82. doi: 10.1097/QAD.0b013e328333ddee. [DOI] [PubMed] [Google Scholar]
- 76.English S, Katzourakis A, Bonsall D, Flanagan P, Duda A, Fidler S, et al. Phylogenetic analysis consistent with a clinical history of sexual transmission of HIV-1 from a single donor reveals transmission of highly distinct variants. Retrovirology. 2011;8:54. doi: 10.1186/1742-4690-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hollingsworth TD, Pilcher CD, Hecht FM, Deeks SG, Fraser C. High Transmissibility During Early HIV Infection Among Men Who Have Sex With Men-San Francisco, California. J Infect Dis. 2015 Jun 1;211(11):1757–60. doi: 10.1093/infdis/jiu831. * This article compares the duration of HIV infection in a group of cases who had transmitted HIV with controls from the general HIV population in San Francisco, demonstrating an odds ratio of 15.2 for transmission during early HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Little SJ, Kosakovsky Pond SL, Anderson CM, Young JA, Wertheim JO, Mehta SR, et al. Using HIV networks to inform real time prevention interventions. PLoS One. 2014;9(6):e98443. doi: 10.1371/journal.pone.0098443. ** This important study used phylogenetic, clinical and social network data from a large primary HIV cohort to develop a risk score to identify those at high risk of transmitting HIV. This measure shows promise as a technique for identifying those who will benefit most from expedited ART initiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gay C, Dibben O, Anderson JA, Stacey A, Mayo AJ, Norris PJ, et al. Cross-sectional detection of acute HIV infection: timing of transmission, inflammation and antiretroviral therapy. PLoS One. 2011;6(5):e19617. doi: 10.1371/journal.pone.0019617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuruc JD, Hightow L, McCoy SI, Pittard DV, McGee KS, Ashby R, et al. APHA. Boston: 2006. Acute HIV-rapid intervention and entry into health care- a unique approach. [Google Scholar]
- 81.Westheimer E, Peters P, Robbins R, Braunstein SL. CROI. Seattle: Feb, 2015. A High Proportion of Persons Diagnosed With Acute HIV Achieve Viral Suppression; pp. 23–26. [Google Scholar]
- 82.Pettifor A, Corneli A, Kamanga G, McKenna K, Rosenberg NE, Yu X, et al. HPTN 062: A Pilot Randomized Controlled Trial Exploring the Effect of a Motivational-Interviewing Intervention on Sexual Behavior among Individuals with Acute HIV Infection in Lilongwe, Malawi. PLoS One. 2015;10(5):e0124452. doi: 10.1371/journal.pone.0124452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.White PJ, Fox J, Weber J, Fidler S, Ward H. How Many HIV infections may be averted by targeting primary infection in men who have sex with men? Quantification of changes in transmission-risk behavior, using an individual-based model. J Infect Dis. 2014 Dec 1;210(Suppl 2):S594–9. doi: 10.1093/infdis/jiu470. * This mathematical modeling study investigated the impact of sexual behavior change alone (without ART) on transmission in British MSM, and found that monthly HIV testing and the resulting increase in diagnosis during primary HIV infection would reduce HIV transmission by 49-52%. These findings highlight the importance of HIV diagnosis as a prevention tool distinct from the additional benefits of antiretroviral therapy for prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patel P, Klausner JD, Bacon OM, Liska S, Taylor M, Gonzalez A, et al. Detection of acute HIV infections in high-risk patients in California. J Acquir Immune Defic Syndr. 2006 May;42(1):75–9. doi: 10.1097/01.qai.0000218363.21088.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Priddy FH, Pilcher CD, Moore RH, Tambe P, Park MN, Fiscus SA, et al. Detection of acute HIV infections in an urban HIV counseling and testing population in the United States. J Acquir Immune Defic Syndr. 2007 Feb 1;44(2):196–202. doi: 10.1097/01.qai.0000254323.86897.36. [DOI] [PubMed] [Google Scholar]
- 86.Pilcher CD, Price MA, Hoffman IF, Galvin S, Martinson FE, Kazembe PN, et al. Frequent detection of acute primary HIV infection in men in Malawi. AIDS. 2004 Feb 20;18(3):517–24. doi: 10.1097/00002030-200402200-00019. [DOI] [PubMed] [Google Scholar]
- 87.Fiscus SA, Pilcher CD, Miller WC, Powers KA, Hoffman IF, Price M, et al. Rapid, real-time detection of acute HIV infection in patients in Africa. J Infect Dis. 2007 Feb 1;195(3):416–24. doi: 10.1086/510755. [DOI] [PubMed] [Google Scholar]
- 88.Stekler J, Swenson PD, Wood RW, Handsfield HH, Golden MR. Targeted screening for primary HIV infection through pooled HIV-RNA testing in men who have sex with men. AIDS. 2005 Aug 12;19(12):1323–5. doi: 10.1097/01.aids.0000180105.73264.81. [DOI] [PubMed] [Google Scholar]
- 89.Patel P, Mackellar D, Simmons P, Uniyal A, Gallagher K, Bennett B, et al. Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA, 2006-2008. Arch Intern Med. 2010 Jan 11;170(1):66–74. doi: 10.1001/archinternmed.2009.445. [DOI] [PubMed] [Google Scholar]
- 90.Stevens W, Akkers E, Myers M, Motloung T, Pilcher CD, Venter F, editors. Third IAS Conference on HIV Pathogenesis and Treatment. Rio de Janeiro, Brazil: 2005. High prevalence of undetected, acute HIV infection in a South African primary care clinic. [Google Scholar]
- 91.De Souza R, Pilcher CD, Fiscus SA. International Conference on AIDS. Toronto, Canada: 2006. Rapid and efficient acute HIV detection by 4th generation Ag/Ab ELISA. [Google Scholar]
- 92.Sabharwal CJ, Bodach S, Braunstein SL, Sepkowitz K, Shepard C. Entry into care and clinician management of acute HIV infection in New York City. AIDS Patient Care STDS. 2012 Mar;26(3):129–31. doi: 10.1089/apc.2011.0380. [DOI] [PubMed] [Google Scholar]
- 93.Axelrad JE, Mimiaga MJ, Grasso C, Mayer KH. Trends in the spectrum of engagement in HIV care and subsequent clinical outcomes among men who have sex with men (MSM) at a Boston community health center. AIDS Patient Care STDS. 2013 May;27(5):287–96. doi: 10.1089/apc.2012.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Selik RM, Mokotoff E, Branson BM, Owen SM, Whitmore S, Hall HI. Revised Surveillance Case Definition for HIV Infection — United States, 2014. MMWR. 2014 2014 Apr 11;63:1–10. ** In this report, the US CDC added for the first time a "stage 0" to their HIV case definitions, defined as early HIV infection. In doing so, they created a framework for national surveillance of acute HIV cascade outcomes. [PubMed] [Google Scholar]
- 95.Cohen MS, Smith MK, Muessig KE, Hallett TB, Powers KA, Kashuba AD. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here? Lancet. 2013 Nov 2;382(9903):1515–24. doi: 10.1016/S0140-6736(13)61998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Severe P, Juste MA, Ambroise A, Eliacin L, Marchand C, Apollon S, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010 Jul 15;363(3):257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Starting antiretroviral treatment early improves outcomes for HIV-infected individuals. NIH; Bethesda, MD: 2015. [cited 2015 May 29, 2015]; Available from: http://www.nih.gov/news/health/may2015/niaid-27.htm. [Google Scholar]
- 98.Danel C, Gabillard D, Le Carrou J, Anglaret X, Moh R, Eholie S, et al. CROI. Seattle, WA: 2015. Early ART and IPT in HIV-Infected African Adults With High CD4 Count (Temprano Trial) [Google Scholar]
- 99.Havlir D, Currier JS. CROI. Seattle, WA: Feb, 2015. Complications of HIV Infection and Antiretroviral Therapy; pp. 23–26. [PMC free article] [PubMed] [Google Scholar]