Abstract
Background
The aim of the study is to investigate differences in clinical presentation, disease stage and survival of operable pancreatic cancer patients with new onset DM compared to long standing diabetes mellitus (DM) and non diabetics.
Methods
A prospectively maintained pancreatic cancer surgery database of a tertiary care teaching hospital from January 2006 to August 2012 was reviewed. Only patients with a histological diagnosis of pancreatic carcinoma (PC) were included in final analysis. DM was defined as HbA1c >6.5% or any patient on anti-diabetic treatment regardless of HbA1c value. New onset DM was defined when diagnosed within two preceding years of surgery. Patients were stratified into two groups: DM and non DM. Among the DM patients, patients with new onset DM were further stratified and studied separately. Staging of PC was performed according to the 6th edition of AJCC. Survival of patients with PC was determined by reviewing medical records. Patients and their families were contacted if there was no existing follow-up.
Results
Eighty-six patients (n=55, 63.9% male) with a mean age of 62 years (range, 29-85 years) underwent pancreatic cancer surgery during the study period. Of the 86 patients, 30 (34%) had DM of which eight patients (9% overall) had new onset DM. DM patients tended to be older compared to non DM patients (67.8 vs. 58.5 years, P=0.0005). The majority of non DM patients were symptomatic (98.2%), and there was a tendency for DM group patients to be asymptomatic at presentation (13.3% vs. 1.8%, P=0.05). Abdominal pain was less common in DM patients compared to non DM patients (30% vs. 53.6%, P=0.04). The median duration of new onset DM prior to diagnosis of PC was 2 months (range, 1-23 months). There was a tendency for DM patients to present at an early stage (stage I and stage II) (P=0.08). There was no difference in survival (P=0.17) for new onset DM compared to long standing DM and non DM patients.
Conclusions
DM patients tend to be older and are less likely to present with abdominal pain. Asymptomatic presentation and early stage disease tends to occur in DM patients. A larger sample size is required to determine if survival of new onset DM patients differs from long standing and non DM patients.
Keywords: Diabetes mellitus (DM), pancreatic cancer
Introduction
Diabetes mellitus (DM) has been widely associated with pancreatic cancer—both as a cause as well as effect. Eight percent to 64% of individuals with pancreatic cancer are diabetic (1-4). Studies have reported an incidence of pancreatic cancer in new onset DM to be 5-14% (5,6). The association was particularly strong within 2 years of DM diagnosis in one large United States cohort study, accounting for 58% (86/149) of identified pancreatic cancer (7). Pancreatic cancer has a poor prognosis because it is usually diagnosed at an advanced stage and is unresectable (8). It remains of particular interest to study the occurrence of new onset DM in operable pancreatic cancer as this group of patients are at the maximum benefit of early diagnosis and improving outcomes. Lee et al. has concluded that elderly patients, patients with lower body mass index (BMI), patients with a history of weight loss and without a family history of DM are at the highest risk of developing pancreas cancer among all the DM patients (9). It is possible that application of pancreatic cancer screening programmes in all patients with new onset DM could positively affect outcomes. Occurrence of long standing DM or new onset DM in metastatic pancreatic cancer does not affect treatment or outcomes; while in locally advanced pancreatic cancer its role remains unproven in absence of evidence based data on neo-adjuvant protocols. This study is done to identify if there are any difference in clinical presentation, stage of disease and long term outcomes of operable pancreatic cancer patients with new onset DM compared to long standing DM and non diabetic patients.
Methods
A prospectively maintained pancreatic cancer surgery database from January 2006 to August 2012 was reviewed. DM was defined as HbA1c >6.5% (10) or any patient on anti-diabetic treatment regardless of HbA1c value. New onset DM was defined when diagnosed within two preceding years of surgery (5-7). Only patients with a final histology of PC were included in our study. The following information was recorded: age at surgery, gender, smoking status, family history of pancreatic cancer, presence of preoperative symptoms, presence of new onset DM, stage of disease, date of last follow-up and date of death. Patients were stratified into two groups: DM and non DM. Among the DM patients, patients with new onset DM were studied separately. Survival of patients with PC was determined by review of medical records and phone calls to patients’ family members if needed. Staging of PC was performed according to the 6th edition of AJCC (11).
Statistical analysis
Patient characteristics were summarized using proportions, mean ± standard deviation (SD), or median interquartile range (IQR), (when appropriate). Kruskal-Wallis test was conducted to compare the difference of continuous variables among the groups. For categorical variables, chi-square or Fisher’s exact test was used to examine the difference in proportions among the groups. The log rank test was used to test the survival difference among the non DM, long standing DM and new onset DM patients. All statistical analyses were carried out with Stata 10.0 at 5% level of significance.
Results
Eighty-six patients (n=55, 63.9% male) with a mean age of 62 years (range, 29-85 years) underwent a pancreatic resection during the study period. Thirteen patients (15.1%) had a history of smoking and one patient (1.2%) had a family history of pancreatic cancer. Table 1 shows the demographic and clinical characteristics of PC patients with and without DM. Of the 86 patients, 30 (34%) had DM. Pancreas cancer in patients with DM tend to occur at an older age compared to patients without DM (67.8 vs. 58.5 years, P=0.0005). Proportion of asymptomatic patients was higher in the DM group compared to non DM group (13.3% vs. 1.8%, P=0.05). Abdominal pain was less common in DM patients compared to non DM patients (30% vs. 53.6%, P=0.04). Amongst the 30 patients with DM, eight patients (9%) had new onset DM and one patient was clinically asymptomatic for pancreas cancer. Of the seven patients who were symptomatic; weight loss and jaundice (four patients each), loss of appetite (three patients), abdominal pain (two patients) and back pain, abdominal mass and cholangitis (one patient each) were presenting symptoms. In these eight patients, the median duration of DM prior to pancreatic cancer diagnosis was 2 months (range, 1-23 months). In the 22 patients with long standing DM, the median duration of DM prior to pancreatic cancer diagnosis was 4 years (range, 2-10 years).
Table 1. Demography and clinical presentation of pancreas cancer patients with and without DM.
| Patient characteristics | DM (n=30) | Non DM (n=56) | P value |
|---|---|---|---|
| Age in years [range] | 67.8 [37-85] | 58.5 [29-85] | 0.0005 |
| Gender (male/female) | 19/11 | 36/20 | 1.00 |
| Presenting symptoms (%) | |||
| Jaundice | 15 (50.0) | 30 (53.6) | 0.82 |
| Abdominal pain | 9 (30.0) | 30 (53.6) | 0.04 |
| Weight loss | 15 (50.0) | 26 (46.4) | 0.82 |
| Loss of appetite | 9 (30.0) | 20 (35.7) | 0.64 |
| Abdominal lump | 4 (13.3) | 3 (5.4) | 0.23 |
| Cholangitis | 1 (3.3) | 1 (1.8) | 1.00 |
| Asymptomatic | 4 (13.3) | 1 (1.8) | 0.05 |
| Smoker (%) | 3 (10.0) | 10 (17.9) | 0.53 |
| Family history of pancreatic cancer (%) | Nil | 1 (1.8) | 1.00 |
Histopathological staging of the patients according to DM status revealed majority of new onset and long standing DM patients had stage I or II disease (Figure 1). There was a tendency for early stage disease (stage I or stage II) to be more common in patients with DM compared to non DM (83.3% vs. 64.3%, P=0.08). There was no difference in 5 years survival of new onset DM patients compared to patients with non DM and long standing DM (P=0.17) (Figure 2). The only patient who was asymptomatic with new onset DM survived 14 months.
Figure 1.
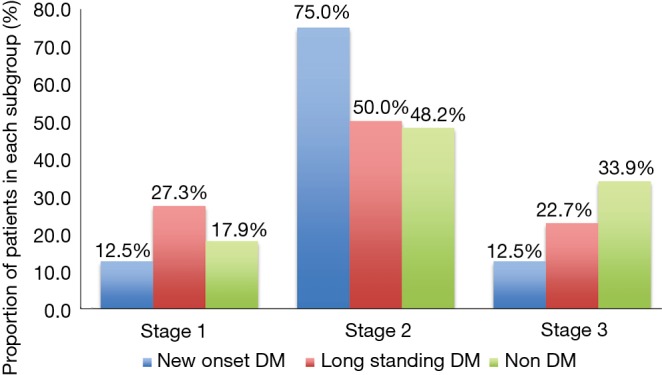
Histopathological stage of pancreas cancer patients according to diabetes mellitus (DM) status.
Figure 2.
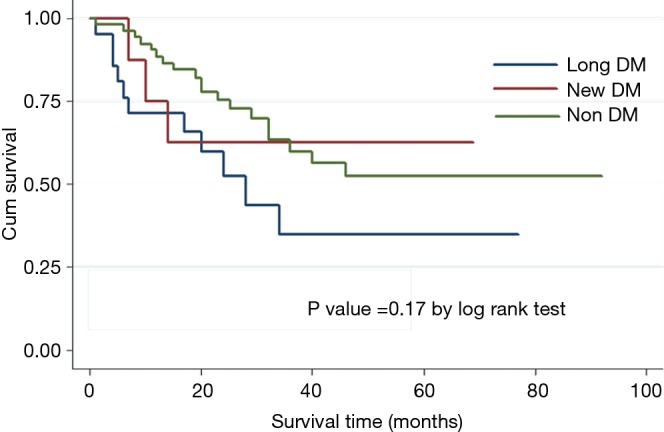
Survival of pancreatic cancer patients according to diabetes mellitus (DM) status.
Discussion
Our study has shown that pancreas cancer patients with DM tend to be older and abdominal pain is less likely to be the presenting symptom. Patients with DM are also more likely to be diagnosed before symptoms manifest and there is a tendency for early stage (stage I and II) diagnosis; however there is no difference in 5 years survival of new onset DM patients compared to patients with non DM and long standing DM.
Pancreatic cancer is the 5th and 6th most common causes of cancer mortality in male and female in Singapore respectively (12). Despite advances in technology and improvement in surgical outcomes, prognosis remains poor. The only chance of cure is surgical resection and 5-year survival following a curative surgery for pancreatic cancer approaches 25% (13). Studies have shown that DM patients are at a 2.7 to 3.2 fold increased risk of developing pancreatic cancer (14-16). In fact, new onset DM is identified to have a strong association with developing pancreatic cancer (7,17). The DM associated with pancreatic cancer is often not associated with known risk factors of diabetes like family history and obesity. The relationship between DM and pancreatic cancer is complex and intercalated, comprising an entire spectrum of metabolic, immunologic, and hormonal alterations intimately related to tumour growth and progression. The exact pathogenesis for development of DM in pancreatic cancer patients is unclear. A meta-analysis demonstrated elevated serum c-peptide to insulin ratio was associated with pancreatic cancer; implicating peripheral insulin resistance in the mechanism of DM associated pancreatic cancer (18). The response to insulin resistance causes a hyperinsulin state and insulin is known to be a potent growth factor and mediator of many tumour progression pathways (19-21). Anti-diabetic drugs such as insulin have been attributed as a contributing factor for increased risk of pancreatic cancer (16). A recent study has concluded that pancreatic cancer causes paraneoplastic β-cell dysfunction by shedding adrenomedullin(+)/CA19-9(+) exosomes into circulation that inhibit insulin secretion, likely through adrenomedullin-induced ER stress and failure of the unfolded protein response (22).
The prevalence of DM and new onset DM in pancreatic cancer is 34% and 9% respectively. Patients with DM are less likely to present with abdominal pain. While majority of the patients with pancreas cancer are symptomatic at presentation, asymptomatic patients are more likely to be diabetic. In our study it remains unclear if presence of diabetic autonomic neuropathy contributed to the lower incidence of abdominal pain or concomitant use of over the counter analgesic medications consumed for unrelated medical problems e.g., joint pain etc which is also more prevalent in old population. It is likely that old patients without abdominal pain are less likely to seek early medical attention and hence more likely to present at an advanced disease stage. However in our experience, early stage (stage I and II) was still more common in patients with DM. This illustrates the fact that DM patients are more likely to present at an early stage. Various authors have examined the relationship between DM status and pancreatic cancer survival and have yielded conflicting results. Busaidy et al. found that diabetes was a significant independent risk factor for increased overall mortality [OR, 1.55 (1.15-2.07)] and disease-specific mortality [OR, 1.37 (1.00-1.89)] (23). Chu et al. similarly reported that DM increased mortality [HR, 1.55 (1.02-2.35)] (24). On the other hand, Dandona et al. found no difference in overall survival among surgically resected patients with DM [HR, 0.87 (0.66-1.14)] (25). Similarly, both McWilliams et al. and Ganti et al. reported no observed association between history of DM and survival (26,27). It is possible that DM patients are more likely to experience perioperative complications with increase in perioperative mortality and hence any benefit of early stage presentation is hence nullified with eventual no difference in long term survival. We found that DM patients with PC tend to present with early stage disease but 5 years survival does not differ in new onset DM patients compared to both long standing DM and non DM patients. Our study is limited by small sample size and includes only patients from the operative database and hence unable to determine the cause effect relationship of DM and pancreatic cancer. Surveillance for pancreatic cancer in individuals with new onset DM cannot be recommended based on our study due to the uncertainty of pancreatic cancer incidence with respect to the incidence of new adult-onset DM in large populations and a lack of data showing improvement in survival from this surveillance strategy. A multicenter study with large sample size and inclusion of all the patients with PC which controls for immediate perioperative mortality could help identify if new onset DM patients enjoy any long term survival benefit.
Conclusions
In conclusion, DM patients tend to be older and are less likely to present with abdominal pain. Asymptomatic presentation and early stage disease tends to occur in DM patients. A larger population based multi-institutional cohort study is needed to explore if survival of new onset DM patients differ from long standing and non DM patients and whether new onset DM patients should be routinely screened for pancreas cancer for early diagnosis to improve outcomes.
Acknowledgements
We would like to acknowledge Tan Tock Seng Hospital Clinical Research Unit statistician Miss Mira Shen for assisting with analysis of data for this manuscript.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Noy A, Bilezikian JP. Clinical review 63: Diabetes and pancreatic cancer: clues to the early diagnosis of pancreatic malignancy. J Clin Endocrinol Metab 1994;79:1223-31. [DOI] [PubMed] [Google Scholar]
- 2.Kim TD, Oh HJ, Kim KH, et al. Clinical characteristics of pancreatic cancer according to the presence of diabetes mellitus. Korean J Gastroenterol 2004;43:35-40. [PubMed] [Google Scholar]
- 3.Cetin M, Colak R, Bayram F, et al. High prevalence of diabetes in patients with pancreatic cancer in central Anatolia, Turkey. Diabetes Res Clin Pract 2002;58:97-100. [DOI] [PubMed] [Google Scholar]
- 4.Permert J, Ihse I, Jorfeldt L, et al. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg 1993;159:101-7. [PubMed] [Google Scholar]
- 5.Ogawa Y, Tanaka M, Inoue K, et al. A prospective pancreatographic study of the prevalence of pancreatic carcinoma in patients with diabetes mellitus. Cancer 2002;94:2344-9. [DOI] [PubMed] [Google Scholar]
- 6.Damiano J, Bordier L, Le Berre JP, et al. Should pancreas imaging be recommanded in patients over 50 years when diabetes is discovered because of acute symptoms? Diabetes Metab 2004;30:203-7. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Vittinghoff E, Bertenthal D, et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol 2006;4:1366-72; quiz 1301. [DOI] [PubMed] [Google Scholar]
- 8.Gudjonsson B. Cancer of the pancreas. 50 years of surgery. Cancer 1987;60:2284-303. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Kim SA, Park HY, et al. New-onset diabetes patients need pancreatic cancer screening? J Clin Gastroenterol 2012;46:e58-61. [DOI] [PubMed] [Google Scholar]
- 10.WHO Guidelines Approved by the Guidelines Review Committee. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, et al, editors. AJCC Cancer Staging Manual. 6th edition. Berlin/Heidelberg/New York/London/Paris/Tokyo/Hong Kong: Springer-Verlag, 2002. [Google Scholar]
- 12.National Registry of Diseases Office (NRDO). Singapore Cancer Registry Interim Annual Registry Report Trends in Cancer Incidence in Singapore 2005-2009. Singapore Cancer Registry, 2011. [Google Scholar]
- 13.David M, Lepage C, Jouve JL, et al. Management and prognosis of pancreatic cancer over a 30-year period. Br J Cancer 2009;101:215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan MM, Bondy ML, Wolff RA, et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol 2007;102:2696-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamal MM, Yoon EJ, Vega KJ, et al. Diabetes mellitus as a risk factor for gastrointestinal cancer among American veterans. World J Gastroenterol 2009;15:5274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D, Yeung SC, Hassan MM, et al. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009;137:482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao KF, Lai SW, Li CI, et al. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol Hepatol 2012;27:709-13. [DOI] [PubMed] [Google Scholar]
- 18.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem 2008;114:63-70. [DOI] [PubMed] [Google Scholar]
- 19.Powell DR, Suwanichkul A, Cubbage ML, et al. Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J Biol Chem 1991;266:18868-76. [PubMed] [Google Scholar]
- 20.Stoeltzing O, Liu W, Reinmuth N, et al. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol 2003;163:1001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohmura E, Okada M, Onoda N, et al. Insulin-like growth factor I and transforming growth factor alpha as autocrine growth factors in human pancreatic cancer cell growth. Cancer Res 1990;50:103-7. [PubMed] [Google Scholar]
- 22.Javeed N, Sagar G, Dutta SK, et al. Pancreatic Cancer-Derived Exosomes Cause Paraneoplastic β-cell Dysfunction. Clin Cancer Res 2015;21:1722-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busaidy N, Yazbeck C, Shah P, et al. Survival of resectable pancreatic cancer patients with diabetes. J Clin Oncol 2006;24:abstr 4098.
- 24.Chu CK, Mazo AE, Goodman M, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol 2010;17:502-13. [DOI] [PubMed] [Google Scholar]
- 25.Dandona M, Linehan D, Hawkins W, et al. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas 2011;40:931-7. [DOI] [PubMed] [Google Scholar]
- 26.McWilliams RR, Matsumoto ME, Burch PA, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer 2010;116:5054-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganti AK, Potti A, Koch M, et al. Predictive value of clinical features at initial presentation in pancreatic adenocarcinoma: a series of 308 cases. Med Oncol 2002;19:233-7. [DOI] [PubMed] [Google Scholar]


