Abstract
Obstructive sleep apnoea (OSA) is one of the most common causes of sleep-disordered breathing (SDB) in children. It is associated with significant morbidity, potentially impacting on long-term neurocognitive and behavioural development, as well as cardiovascular outcomes and metabolic homeostasis. The low grade systemic inflammation and increased oxidative stress seen in this condition are believed to underpin the development of these OSA-related morbidities. The significant variance in degree of end organ morbidity in patients with the same severity of OSA highlights the importance of the interplay of genetic and environmental factors in determining the overall OSA phenotype. This review seeks to summarize the current understanding of the aetiology and mechanisms underlying OSA, its risk factors, diagnosis and treatment.
Keywords: Paediatric obstructive sleep apnoea (paediatric OSA), sleep-disordered breathing in childhood (SDB in childhood)
Introduction
Obstructive sleep apnoea (OSA) is a common paediatric health problem and children at risk need to be identified, investigated and treated in a timely manner because the resultant activation of inflammatory cascades can impose wide ranging effects, impacting on neurocognitive, cardiovascular and metabolic systems. The adverse consequences of paediatric OSA may not simply be confined to the child’s immediate well-being and development, but may continue to be detrimental to the patient’s long-term health in adulthood. The emphasis of this article is to summarize the latest research and developments in paediatric OSA and to provide a practical approach to the recognition, diagnosis and treatment of this condition.
Definition of obstructive sleep-disordered breathing (SDB) and clinical entities
The European Respiratory Society (ERS) taskforce on the diagnosis and management of obstructive SDB in childhood has defined obstructive SDB as “a syndrome of upper airway dysfunction during sleep, characterized by snoring and/or increased respiratory effort secondary to increased upper airway resistance and pharyngeal collapsibility” (1). This can potentially result in hypoxia, hypercarbia, increase in respiratory effort, pronounced intrathoracic pressure changes and sleep fragmentation.
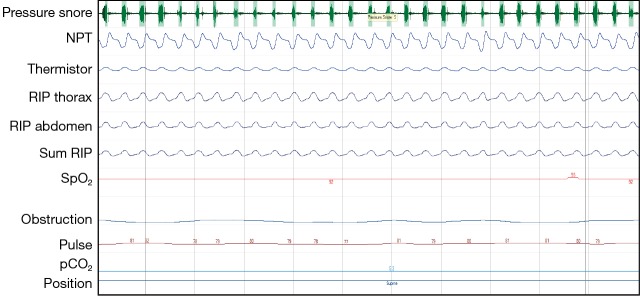
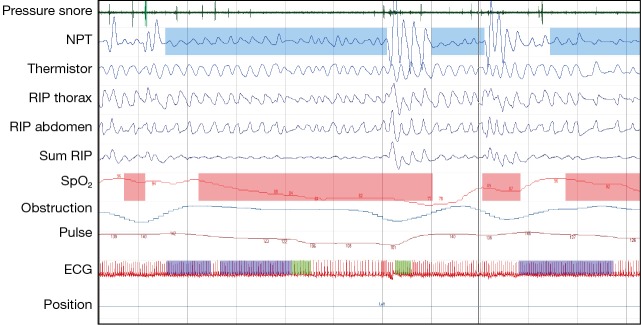
The spectrum of paediatric obstructive SDB in increasing order of severity encompasses (Figures 1,2,3):
Figure 1.
Cardiorespiratory polygraphy: snoring with flow limitation seen on the nasal pressure airflow transducer suggestive of increased upper airway resistance. Normal work of breathing (RIP thorax and RIP abdomen), normal breathing pattern (RIP thorax and RIP abdomen are in phase), stable saturations (SpO2).
Figure 2.
Cardiorespiratory polygraphy: obstructive hypopnoeas. Snoring followed by reduced flow in the presence of continued breathing effort. Notice paradoxical breathing (RIP thorax and RIP abdomen), desaturation (SpO2), breakthrough breath with breakthrough snoring and recovery of SpO2 at the end of hypopnoea.
Figure 3.
Cardiorespiratory polygraphy: run of obstructive apnoeas. Snoring and increase work of breathing (pressure flow, RIP thorax and RIP abdomen) followed by complete stop of airflow despite breathing effort. Notice paradoxical breathing (RIP thorax and RIP abdomen out of phase) and desaturations (SpO2), breakthrough breath with breakthrough snoring and recovery of SpO2 at the end of each apnoea.
Primary snoring, the mildest and most prevalent manifestation, which is defined as habitual snoring for more than 3 nights per week without apnoeas, hypopnoeas, frequent arousals or gas exchange abnormalities. Its estimated population prevalence is 7.45% (95% confidence interval: 5.75−9.61%) (2-4);
Upper airway resistance syndrome (UARS) comprises snoring, increased work of breathing and frequent arousals, without recognizable obstructive events or gas exchange abnormalities;
Obstructive hypoventilation is characterized by snoring plus elevated end-expiratory carbon dioxide partial pressure in the absence of recognizable obstructive events;
OSA syndrome manifests with recurrent events of partial or complete upper airway obstruction (hypopnoeas, obstructive or mixed apnoeas) with disruption of normal oxygenation, ventilation and sleep pattern. The prevalence of OSA has been reported to be between 1% and 5% (4,5).
Aetiology and mechanisms of OSA
Although the aetiologies of paediatric OSA are multiple, they can be broadly classified into conditions which result in intrinsic upper airway narrowing and those that result in increased upper airway collapsibility. Adenotonsillar hypertrophy is currently the most common example of the former. Magnetic resonance imaging (MRI) studies have shown that the size of the adenoids and tonsils in children with OSA is significantly increased compared to healthy controls (6). The causes of this lymphoid tissue hypertrophy are not completely understood.
Other anatomical features resulting in upper airway narrowing such as micrognathia, macroglossia, and midface hypoplasia, are often found in children with craniofacial syndromes (e.g., Treacher Collins syndrome, Crouzon syndrome, Apert syndrome, Pierre Robin sequence), achondroplasia, trisomy 21, Beckwith Wiedemann syndrome, and mucopolysaccharidoses.
To understand upper airway collapsibility, researchers have modeled the upper airway as a Starling resistor, essentially a rigid tube with a collapsible segment, the collapsible segment being the pharynx bordered by rigid upstream (nasal passages) and downstream (trachea) segments. Under conditions of flow limitation, the maximum inspiratory airflow is determined by the pressure changes upstream to the collapsible segment (nasal passages) rather than downstream changes (pressures generated in the trachea by the diaphragm). Collapse occurs when the pressure outside the collapsible segment is greater than that within the segment, and is referred to as the critical closing pressure (Pcrit). Disturbances in Pcrit are believed to play a key role in OSA pathogenesis (7). Children with OSA have been found to have significantly more collapsible upper airways with elevated (i.e., less negative) Pcrit during sleep than children without OSA (8). Increased upper airway collapsibility can be caused either by conditions leading to a decrease in muscle tone in the upper airway, such as cerebral palsy, neuromuscular disorders, or inflammatory conditions affecting the upper airways, such as allergic rhinitis and asthma.
Whilst previously, the typical paediatric OSA patient was one with adenotonsillar hypertrophy and failure to thrive, with the current obesity epidemic, there are an increasing number of children diagnosed with OSA who are obese. These patients often suffer from more prominent daytime sleepiness symptoms, similar to adult OSA patients. A recent cross-sectional, prospective multicenter study, the NANOS study, assessed the contribution of obesity and adenotonsillar hypertrophy to paediatric OSA and found that 46.6% of obese children in the community had an obstructive apnoea hypopnoea index (OAHI) >1/hr total sleep time (TST) (9). The degree of tonsillar and adenoidal hypertrophy emerged as the most important risk factors for paediatric OSA in this nonreferral cohort of obese children. Whilst the degree of obesity appeared to contribute to the risk of OSA in the single factorial analysis, it did not retain its statistical significance as a major determinant of OSA in the multivariate analysis model.
The concurrent presence of adenotonsillar hypertrophy and obesity appears to facilitate the emergence of OSA. Obesity can contribute to OSA in mechanical ways: fatty infiltrates within the upper airway structure and neck contribute to upper airway narrowing as well as pharyngeal collapsibility. Accumulation of abdominal visceral fat impinging on the chest cavity limits diaphragmatic descent, particularly when supine, and adipose tissue in the chest wall can impair lung compliance, leading to hypoventilation, atelectasis and ventilation perfusion mismatch. The consequent excessive daytime sleepiness from OSA is likely to impact on physical activity, which in turn favours more weight gain (10). Furthermore, OSA and obesity might also be linked via an imbalance between leptin and ghrelin, two hormones crucial in regulating satiety and hunger. While leptin is secreted by adipocytes and promotes satiety, ghrelin is secreted in the gut and creates a feeling of hunger. OSA has been shown to be associated with leptin resistance and increased ghrelin levels, which could favour obesity (11).
Consequences of OSA
OSA is associated with significant morbidity: impairment of neurocognitive development, school performance, and behaviour are the most commonly reported consequences of paediatric OSA, but it has also been linked with cardiovascular morbidity, metabolic consequences, and nocturnal enuresis. Children with OSA have also been shown to have increased healthcare utilization compared with their peers, with more hospital visits and more medication prescriptions, mainly for respiratory infections (12). Healthcare utilization and annual health care costs decreased following adenotonsillectomy (13).
OSA and inflammation
There is emerging evidence that OSA is a disease with chronic low grade systemic inflammation and increased oxidative stress which likely lead to the end organ morbidities described above. At the local level, nasal nitric oxide (NO), a marker of airway inflammation, is elevated in children with OSA and primary snoring compared with healthy controls (14). Levels of H2O2 in morning exhaled breath condensate of children with OSA have been shown to be elevated, suggesting increased oxidative stress (15). Systemically, the pro-inflammatory cytokines, TNF-α, IL-6, and IL-8 have been found to be elevated in the serum of OSA patients. Levels of regulatory cytokines such as IL-10 are decreased and correlate with the severity of OSA (16). The peripheral Th17/Treg ratio is skewed towards Th17 predominance, further suggesting a systemic pro-inflammatory milieu in OSA. The Th17/Treg ratio correlates with severity of OSA, and adenotonsillectomy reverses this Th17/Treg imbalance and reduces serum inflammatory cytokine levels (17). The mechanisms underlying these imbalances are not fully understood, but some interesting findings have been made recently. For example, a single nucleotide polymorphism in the TNF alpha coding region—TNF-α (308A) SNP—appears to be significantly associated with OSA (18). There is also evidence of epigenetic alterations in OSA, for instance the promoter region of the FOXP3 gene, which is crucially involved in the development of regulatory T cells, shows severity dependent increased methylation in paediatric OSA (19). OSA and obesity seem to mutually amplify the systemic inflammatory pathways, for instance monocyte chemoattractant protein 1 and plasminogen activator-inhibitor 1 levels have been found to be significantly raised in obese OSA children compared with body mass index (BMI)-matched obese children without OSA (20). It is postulated that this induction of inflammatory cascades along with increased oxidative stress gives rise to the morbidities associated with OSA.
Neurocognitive and behavioural comorbidities
It is important to consider OSA as a differential diagnosis when investigating children with behavioural problems or attention deficit hyperactivity disorder (ADHD) since there can be considerable overlap of symptoms. Even mild OSA and habitual snoring have been associated with hyper activity, difficulties concentrating, attention problems and impulsivity. The Tucson Children’s Assessment of Sleep Apnoea (TuCASA) study revealed that young people with untreated OSA had attention problems and hyperactivity, aggressive behaviours, lower social competencies, poor communication and/or diminished adaptive skills (21). This study also showed a negative correlation between apnoea-hypopnoea index (AHI) and immediate recall, full-scale intelligence quotient (IQ), performance IQ and mathematics achievements, while nocturnal hypoxaemia adversely affected nonverbal skills (22). In a prospective study in first-grade school children, OSA has been shown to be disproportionally high in children whose school performance was in the lowest 10% of their class. Strikingly, while children who were treated for OSA showed significant academic improvement, children who did not receive treatment did not improve (23). However, the Childhood Adenotonsillectomy Trial (CHAT), a randomized controlled trial that compared the effects of adenotonsillectomy versus watchful waiting in children with mild OSA, did not find any significant improvements in cognitive function after treatment, although there were improvements in behaviour, symptoms and quality of life. This may be because, due to ethical considerations, only children with mild OSA with no significant oxygen desaturations were included in the study and the follow-up period was only 7 months (24). A recent meta-analysis including 16 studies confirmed clear links between OSA and poor academic performance for core academic domains related to language, arts, maths and science in school-aged children (25). There is however, considerable phenotypic variability whereby some children with severe OSA do not appear to experience neurocognitive or behavioural morbidity, whereas some children with relatively mild OSA do. Part of this may be explained by the differential compensatory mechanisms adopted and individual variability in neuroplasticity which are influenced by genetic and environmental factors. Recent functional MRI-imaging data have demonstrated changes in cognitive and empathetic processing in children with OSA, with greater neural recruitment of regions of the brain involved in cognitive control, conflict monitoring, and attentional allocation observed in children with OSA compared with children without OSA, even when no differences were discernable on standardized neuropsychological testing by experienced child psychologists (26).
Cardiovascular comorbidities
The most severe cardiovascular consequence of OSA is pulmonary hypertension, and the resultant cor pulmonale if the OSA is left untreated. Fortunately, this is not commonly seen in children, who tend to have more subtle evidence of cardiovascular dysfunction, such as dysregulation of blood pressure (BP), cardiac remodeling and endothelial dysfunction. Amin et al. observed reduced nocturnal dipping, greater mean BP variability during wakefulness and sleep, and higher night/day systolic BP ratios, which are known risk factors for cardiovascular events in adults (27,28). Children with OSA have also been shown to have diminished cardiac output and oxygen consumption at peak exercise capacity (29). Cardiac remodeling may be due to greater overnight changes in brain natriuretic peptide (BNP) observed in children with moderate to severe OSA compared with mild OSA and controls (30). Assessment of post-occlusive hyperaemic responses in children with OSA has revealed evidence of endothelial dysfunction. Interestingly, in a study by Gozal et al., while endothelial dysfunction improved after treatment of the OSA with adenotonsillectomy in the majority of children, in those with a strong family history of cardiovascular disease it remained abnormal, once again suggesting the influence of genetic and environmental factors on phenotypic expression (31). Furthermore, a recent study suggests that even children with primary snoring already display evidence of endothelial dysfunction and have significantly higher serum isoprostanes and soluble NOX2-dp levels compared with healthy controls (32).
In the CHAT study, overnight heart rate emerged as the most sensitive parameter for determining the severity of paediatric OSA. Adenotonsillectomy did not have significant effects on cardiometabolic measures compared with watchful waiting. Again, it should be noted that children in this study only had mild OSA without significant oxygen desaturations (33).
Metabolic comorbidities
Data on metabolic consequences of paediatric OSA are less robust than in adults, and interpretation is complicated by pubertal status and the presence of obesity. Elevations in low density lipoprotein (LDL) cholesterol along with reduced levels in high density lipoprotein (HDL) cholesterol were observed in both obese and non-obese children with OSA, with significant improvements after OSA treatment (34,35). Associations between OSA and the metabolic syndrome have been shown in postpubertal adolescents (36). In young children, OSA has been shown to be associated with reduced insulin sensitivity in obese children, with improvements in homeostatic model assessment (HOMA) when OSA was treated (37). Elevated liver enzymes have also been reported which improved after treatment of OSA in the majority of these patients (38). Two other groups have recently demonstrated that the presence and severity of OSA was associated with the presence of non-alcoholic fatty liver disease, liver fibrosis independent of BMI, abdominal adiposity, metabolic syndrome and insulin resistance, while the percentage of time with oxygen saturation below 90% correlated with increased intrahepatic leukocytes, activated Kupffer cells, and circulating markers of hepatocyte apoptosis and fibrogenesis (39,40).
Excessive daytime sleepiness
With the exception of obese children who more closely resemble the adult OSA phenotype, excessive daytime sleepiness is not a frequently reported symptom in children with OSA. In children, sleep fragmentation often manifests as hyperactivity, difficulties concentrating, and irritability instead. However, they do have severity-dependent shortening of their sleep latencies (41), and there seems to be an association between the magnitude of sleep latency reduction and TNF-alpha gene polymorphisms (42).
Nocturnal enuresis
A higher prevalence of nocturnal enuresis has been reported in children with OSA (43). This may be due to the inhibitory effects of OSA on arousal responses to changes in bladder pressure, or effects of elevated BNP levels which affect the renin-angiotensin pathway, vasopressin, and excretion of sodium and water (44).
When to suspect obstructive SDB?—symptoms of OSA in children
OSA should be suspected when nocturnal symptoms of snoring, gasping, increased work of breathing or paradoxical breathing, restless sleep, witnessed apnoeas or mouth breathing are reported. Some children with OSA will also sleep with their neck in a hyperextended position to maintain their airway. Daytime symptoms of OSA are very non-specific, but together with nighttime symptoms may help alert clinicians to clinically significant obstructive SDB: hyperactivity, difficulty concentrating/learning difficulties, behavioural difficulties, excessive daytime sleepiness, and moodiness. A history of prematurity is associated with an increased risk of OSA, and there is some evidence that a family history of OSA may also be a risk factor (1).
On physical examination, findings of tonsillar hypertrophy, obesity, midface deficiency, macroglossia or mandibular hypoplasia may strengthen the suspicion of OSA (1). Other groups at risk for obstructive SBD are children with uncontrolled epilepsy, neuromuscular disorders, Prader-Willi syndrome, and complex medical conditions, such as achondroplasia, Chiari malformation, Ehlers-Danlos syndrome, mucopolysaccharidoses, and trisomy 21. In most complex conditions there is a mix of obstructive and central SBD and often a component of alveolar hypoventilation.
While adenotonsillar hypertrophy identified on MRI has been shown to be associated with OSA, there is little evidence from the literature for an association of tonsillar size evaluated subjectively during physical examination and OSA severity determined by polysomnography (PSG) (1,45). The ERS taskforce proposed that in clinical practice, techniques for objective evaluation of abnormalities predisposing for obstructive SDB, such as lateral neck X-ray, flexible nasopharyngoscopy, cephalometry, MRI or computed tomography (CT) of the upper airway, should be reserved for more complex cases (1).
Diagnosis of OSA in children
The gold standard test for the diagnosis of obstructive SDB and assessment of its severity is an overnight, attended, in-laboratory PSG study. PSGs allow for objective diagnosis and assessment of disturbances in respiratory parameters and sleep patterns, allowing for classification of patients into differential disease severities, thus enabling clinicians to tailor clinical management accordingly. The AHI is the most commonly used PSG parameter for the quantification of SDB severity. It comprises the number of mixed, obstructive and central apnoeas and hypopnoeas per hour of total sleep time. It is often helpful to report the central apnoea index and obstructive apnoea index separately. The ICSD-3 PSG criteria for diagnosis are either (I) one or more obstructive events (obstructive or mixed apnoea or obstructive hypopnoea) per hour of sleep or (II) obstructive hypoventilation, as manifest by PaCO2 >50 mmHg for >25% of sleep time, together with snoring, paradoxical thoracoabdominal movement, or flattening of the nasal airway pressure waveform implying flow limitation (46). Most sleep centers consider an obstructive AHI ≤1/hrTST to be normal, 1< AHI ≤5 to be mild OSA, 5< AHI ≤10 to be moderate OSA, and an AHI >10/hrTST as severe OSA.
Full PSGs are labour and resource intensive, requiring in hospital monitoring of the patient by skilled staff overnight, and subsequent scoring and analysis. They may also not be widely available in all countries. When PSGs are not available, possible alternatives include:
Nocturnal oximetry studies: oximetry studies have a high specificity but low sensitivity in the diagnosis of paediatric OSA (47). Three or more clusters of desaturation events ≥4% and at least three desaturations to <90% are considered abnormal (McGill criteria). However, the rate of false negative or inconclusive results is high. Nocturnal oximetry studies could help in the prioritization of treatment in resource poor countries where PSGs are not available;
Respiratory polygraphy (RP) studies: respiratory polygraphies are essentially PSGs without the EEG, EMG, and EOG sensors. They are performed commonly in Europe and are quicker to set up and score than PSGs. Some centers have reported good agreement with in-lab PSGs (48), but there is the possibility that the AHI may be underestimated due to missed hypopnoeas resulting in arousals but not desaturation (49);
Ambulatory RP or PSG: there has been a recent drive towards performing ambulatory PSG or RP studies, not only because they are seen as a less expensive alternative to in-lab PSGs, but also because the results may be more representative of the child’s typical night’s sleep at home. Data on direct comparison with in-lab PSG are still limited but a recent study by Alonso-Álvarez et al. compared home RP with in-lab PSG in 50 children clinically suspected to have OSA and showed that all the home RP studies were successful and the area under the curve was consistently >90% when various PSG cut-off values OAHI ≥1, 3 and 5/hrTST were used (50). This would suggest the validity of home RP for the diagnosis of OSA in children with a high pre-test probability of having OSA. However, more research is required to further optimize the sensitivity and specificity of home RP testing for the diagnosis of children with mild OSA;
Paediatric sleep questionnaire: this parent-filled questionnaire assesses symptoms of SDB, such as snoring, excessive daytime sleepiness, attention problems, and hyperactive behaviour in children aged 2−18 years. Its sensitivity and specificity for diagnosing OSA in otherwise healthy children are 78% and 72%, respectively, but it may be useful in predicting OSA-related neurobehavioural morbidity and its improvement after adenotonsillectomy (51);
Sleep clinical record: this is a diagnostic tool composed of physical examination, subjective symptoms and clinical history of behavioural and cognitive problems. These items are used to determine the sleep clinical score (SCS). A SCS of ≥6.5 is considered positive for OSA, with a sensitivity of 96.05% and specificity of 67.00%. This may potentially be a useful tool to screen patients for suspected OSA (52).
Whilst these investigations may be less sensitive and specific than PSGs, they can still be valuable in assessing SDB as long as clinicians are aware of their limitations.
The ERS taskforce, in line with the American Academy of Otolaryngology-Head and Neck Surgery, recommends that children with co-existing obesity, Down syndrome, craniofacial abnormalities, neuromuscular disorders, sickle cell disease, mucopolysaccharidoses or children in whom the need for treatment is unclear should have priority in accessing PSG or RP prior to adenotonsillectomy. These children should also have a PSG or RP post adenotonsillectomy due to their increased risk of persistent OSA. PSGs/RPs should also be performed in patients with persistent symptoms of OSA despite surgery and those who had moderate-to-severe OSA pre-surgery also to assess for residual OSA. PSG is also indicated before and after rapid maxillary expansion, application of orthodontic appliances, continuous positive airway pressure (CPAP) and bi-level positive pressure ventilation (BiPAP) (1).
Treatment of OSA
Most physicians would consider treatment in children with an AHI ≥5/hrTST. In children with 1< AHI ≤5, treatment may be beneficial especially in the presence of comorbidities. Individual risk factors predisposing for OSA, the presence of OSA-related co-morbidities, and the kind and severity of symptoms should determine the priority for treatment and the therapeutic strategy (53). The ERS taskforce advises a stepwise treatment approach, until complete resolution of the OSA. This may include a combination of different treatment modalities depending on severity and cause for the upper airway obstruction. The taskforce acknowledges that data on the appropriate sequence of interventions is scarce, but they propose the following steps:
Weight loss if the child is overweight or obese: there is data supporting the efficacy of weight loss as a treatment intervention in obese adolescents (54,55). However, there are currently no studies on obese younger children;
Nasal corticosteroids and/or oral montelukast: children with OSA have increased expression of leukotriene C4 synthase as well as leukotriene receptors 1 and 2 in tonsillar lymphocytes compared with controls (56). Addition of leukotriene receptor antagonists to tonsillar cells from children with OSA in vitro resulted in dose-dependent reductions in cell proliferation and secretion of TNF-α, IL-6, and IL-12. A 6 to 12 weeks course of nasal steroids and/or montelukast may reduce adenoidal size and has shown favourable results in children with mild to moderate OSA (57,58);
Adenotonsillectomy: there is good evidence that adenotonsillectomy is efficacious in children with OSA and adenotonsillar hypertrophy (59,60), and the American Academy of Pediatrics (AAP) recommends adenotonsillectomy as the first line treatment for children with adenotonsillar hypertrophy (5). Subtotal tonsillectomies, also known as tonsillotomies, have been gaining popularity recently as they have lower postoperative complication rates. However there are no well-designed studies directly comparing the two surgical approaches and their OSA treatment outcomes. Subtotal resection also carries an increased risk of tonsillar regrowth (61). Whilst adenotonsillectomy is an effective treatment, recent studies have demonstrated that although the majority of children show marked improvement in their PSG parameters post-surgery, a significant number do not achieve complete normalization (62). In otherwise healthy, non-obese children, the success rate of adenotonsillectomy is approximately 75%. Risk factors for residual OSA include obesity, severe OSA pre-surgery with an AHI of >20/hrTST, children aged >7 years, high Mallampati score, African-American ethnicity, children with asthma, craniofacial abnormalities (e.g., Pierre Robin syndrome), chromosomal abnormalities (e.g., trisomy 21), and neuromuscular disease. Families should be counselled that OSA may recur after initial postoperative improvement;
Rapid maxillary expansion or orthodontic appliances: a number of small studies have shown that rapid maxillary expansion can be efficacious in the treatment of OSA in carefully selected patients (63,64). A previous Cochrane review concluded that oral appliances are beneficial as an auxiliary treatment in children with OSA and non-syndromic craniofacial abnormalities. However, this review was only based on one study (65). A more recent meta-analysis including six studies concluded that orthodontic treatments may be effective in managing snoring and OSA. The authors noted however, that the efficacy of orthodontic treatments with regards to improving consequences of OSA e.g., neurocognitive and cardiovascular functions have not yet been systematically addressed (66);
CPAP or non-invasive positive pressure ventilation (NIPPV) for nocturnal hypoventilation: in children who have residual OSA after adenotonsillectomy, OSA related to obesity, craniofacial abnormalities, neuromuscular disorders, those who do not have significant adenotonsillar hypertrophy, or those who choose not to undergo surgery, positive airway pressure therapy is recommended. The goal is to maintain patency of the upper airway throughout the respiratory cycle, improve functional residual lung capacity and decrease work of breathing. Starting CPAP in children can be challenging, a multidisciplinary team approach works best and parental involvement and education is crucial (67). In our center, once the child has been fitted with the correct size mask, the family is given the mask to go home with so the child can play with it and practice wearing it. Once the child is happy to wear the mask for brief periods of time when awake, an inpatient admission is arranged, nocturnal CPAP is started and pressures titrated. The admission sometimes needs to be for several nights, depending on how the child tolerates CPAP. Play therapists working together with specialist nurses/respiratory therapists, and in the more challenging cases, clinical psychologists, can make the process fun and reduce anxiety. For most children with OSA, CPAP will be effective. However, if the nocturnal CO2 is significantly raised, which is more often seen in children with other co-existing conditions such as neuromuscular disease, craniofacial syndromes or obesity hypoventilation, BiPAP may be needed. In BiPAP the machine delivers a higher inspiratory pressure when the child breathes in, and a lower pressure when the child breaths out. BiPAP may also be better tolerated in children who do not tolerate CPAP due to high positive end-expiratory pressure requirements. Complications of CPAP and BiPAP include nasal congestion, rhinorrhoea, epistaxis, facial skin erythema related to the mask, discomfort from air leak, abdominal distension, and midface retrusion. It is important to monitor adherence to CPAP or BiPAP and to manage complications to optimize patient adherence. Regular long-term follow-up is necessary as pressure requirements will change and the interface will need to be upsized and adjusted with the growth and development of the child;
Tracheostomy, craniofacial surgery: craniofacial surgery has been shown to be successful in children with syndromic craniofacial abnormalities. Success rates of 95.6% have been reported in patients with micrognathia (68), but success rates tend to be lower in the presence of other abnormalities. Tracheostomy has the highest efficacy in the treatment of obstructive SDB when compared to other surgical interventions but is associated with worse quality of life and psychosocial development (69,70). Early onset complications include pneumomediastinum, pneumothorax, wound infection and bleeding, whilst late-onset complications include granulation tissue formation, tracheocutaneous fistulae, laryngo-tracheal stenosis, delayed language skills acquisition and increased rates of respiratory infections. In clinical practice, craniofacial surgery and tracheostomy are mostly reserved for the most severe cases when all other treatment options have failed.
Prognosis of OSA and recognition and management of persistent SDB
The goal of OSA treatment is complete resolution of the SDB. This may require combining treatments in a stepwise manner as proposed by the ERS taskforce. Treatment outcomes should be monitored after each intervention and include assessment of symptoms and the presence of any cardiovascular and central nervous co-morbidities and growth, as well as objective measures to detect residual OSA, such as PSG, or polygraphy, oximetry and capnography when PSG is not available. Generally, PSG is recommended 6 weeks after adenotonsillectomy, after 12 weeks of montelukast/nasal steroid treatment, 12 months after rapid maxillary expansion and after 6 months with orthodontic appliances. Children on CPAP or BiPAP should be re-evaluated at least every 12 months after initial titration (1). In children with persistent SDB after intervention it may also be worth looking for additional upper airway abnormalities, such as laryngomalacia or adenoid regrowth.
It is worth mentioning that weight gain can sometimes be seen post treatment of OSA. Katz et al. found that the BMI score increased more in children who underwent adenotonsillectomy compared with those in the watchful waiting group (71). Children who have a rapid increase in BMI postoperatively are at increased risk of having recurrent OSA (72). These findings highlight the importance of weight monitoring, nutritional counselling, and encouragement of physical activity and follow-up assessment for residual OSA.
Obstructive SDB can resolve spontaneously, particularly in children with mild OSA and adenotonsillar hypertrophy. In the CHAT study, 42% of the children in the watchful-waiting group showed normalization of their AHI score after 5 months, no longer fulfilling the PSG criteria for OSA (73). Improvements may be due to growth of the airway or regression of lymphoid tissue, routine medical care, or regression to the mean (24). Risk factors for the persistence of untreated OSA include obesity and increasing body mass index percentile, male gender, more severe OSA with an obstructive AHI >5 episodes/hour, African-American ethnicity (53) and complex underlying conditions, such as chromosomal aberrations, neuromuscular diseases or craniofacial malformations.
As alluded to earlier, it is increasingly recognized that there is considerable phenotypic variation in terms of end organ morbidity for the same degree of OSA severity. Much recent research has therefore concentrated on the identification of biomarkers for phenotype characterization; estimation of the risk of OSA related consequences and prediction of the success of treatment interventions. “Omics” technologies and new bioinformatics approaches will hopefully help in determining a patient’s risks for comorbidities and their likelihood of responding to treatment interventions. An example of the former was a study by Khalyfa et al. which showed preliminary evidence that exosomal miRNAs may be a potential biomarker of cardiovascular risk in children with OSA (74). As an example of the latter, Kheirandish-Gozal et al. discovered that a combination of serum biomarkers including MCP-1, PAI-1, MMP-9, IL-19, IL-6, adropin and osteocrin provided excellent sensitivity and moderate specificity in predicting residual OSA in obese children after adenotonsillectomy (75).
Conclusions
In summary, considerable progress has been made in the field of paediatric sleep medicine in the past few years. However, there still remain gaps in our knowledge particularly concerning the mechanistic pathways in the pathogenesis of paediatric OSA and factors influencing the phenotypic variability of the disease. A better understanding of these will help in the development of novel therapies. Further research is also needed to identify biomarkers for patients at risk for consequences of OSA as well as predictors for treatment success to enable the development of individual therapeutic approaches for the implementation of personalized medicine.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kaditis AG, Alonso Alvarez ML, Boudewyns A, et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J 2016;47:69-94. [DOI] [PubMed] [Google Scholar]
- 2.Bonuck KA, Chervin RD, Cole TJ, et al. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. Sleep 2011;34:875-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockmann PE, Urschitz MS, Schlaud M, et al. Primary snoring in school children: prevalence and neurocognitive impairments. Sleep Breath 2012;16:23-9. [DOI] [PubMed] [Google Scholar]
- 4.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:242-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130:e714-55. [DOI] [PubMed] [Google Scholar]
- 6.Slaats MA, Van Hoorenbeeck K, Van Eyck A, et al. Upper airway imaging in pediatric obstructive sleep apnea syndrome. Sleep Med Rev 2015;21:59-71. [DOI] [PubMed] [Google Scholar]
- 7.Pham LV, Schwartz AR. The pathogenesis of obstructive sleep apnea. J Thorac Dis 2015;7:1358-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus CL, McColley SA, Carroll JL, et al. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol (1985) 1994;77:918-24. [DOI] [PubMed] [Google Scholar]
- 9.Alonso-Álvarez ML, Cordero-Guevara JA, Terán-Santos J, et al. Obstructive sleep apnea in obese community-dwelling children: the NANOS study. Sleep 2014;37:943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tauman R, Gozal D. Obesity and obstructive sleep apnea in children. Paediatr Respir Rev 2006;7:247-59. [DOI] [PubMed] [Google Scholar]
- 11.Spruyt K, Sans Capdevila O, Serpero LD, et al. Dietary and physical activity patterns in children with obstructive sleep apnea. J Pediatr 2010;156:724-30, 730.e1-730.e3. [DOI] [PubMed]
- 12.Tarasiuk A, Greenberg-Dotan S, Simon-Tuval T, et al. Elevated morbidity and health care use in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2007;175:55-61. [DOI] [PubMed] [Google Scholar]
- 13.Tarasiuk A, Simon T, Tal A, et al. Adenotonsillectomy in children with obstructive sleep apnea syndrome reduces health care utilization. Pediatrics 2004;113:351-6. [DOI] [PubMed] [Google Scholar]
- 14.Gut G, Tauman R, Greenfeld M, et al. Nasal nitric oxide in sleep-disordered breathing in children. Sleep Breath 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Malakasioti G, Alexopoulos E, Befani C, et al. Oxidative stress and inflammatory markers in the exhaled breath condensate of children with OSA. Sleep Breath 2012;16:703-8. [DOI] [PubMed] [Google Scholar]
- 16.Leon-Cabrera S, Arana-Lechuga Y, Esqueda-León E, et al. Reduced systemic levels of IL-10 are associated with the severity of obstructive sleep apnea and insulin resistance in morbidly obese humans. Mediators Inflamm 2015;2015:493409. [DOI] [PMC free article] [PubMed]
- 17.Ye J, Liu H, Li P, et al. CD4(+)T-lymphocyte subsets in nonobese children with obstructive sleep apnea syndrome. Pediatr Res 2015;78:165-73. [DOI] [PubMed] [Google Scholar]
- 18.Bielicki P, MacLeod AK, Douglas NJ, et al. Cytokine gene polymorphisms in obstructive sleep apnoea/hypopnoea syndrome. Sleep Med 2015;16:792-5. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Bhattacharjee R, Khalyfa A, et al. DNA methylation in inflammatory genes among children with obstructive sleep apnea. Am J Respir Crit Care Med 2012;185:330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gileles-Hillel A, Alonso-Álvarez ML, Kheirandish-Gozal L, et al. Inflammatory markers and obstructive sleep apnea in obese children: the NANOS study. Mediators Inflamm 2014;2014:605280. [DOI] [PMC free article] [PubMed]
- 21.Perfect MM, Archbold K, Goodwin JL, et al. Risk of behavioral and adaptive functioning difficulties in youth with previous and current sleep disordered breathing. Sleep 2013;36:517-525B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kheirandish-Gozal L, De Jong MR, Spruyt K, et al. Obstructive sleep apnoea is associated with impaired pictorial memory task acquisition and retention in children. Eur Respir J 2010;36:164-9. [DOI] [PubMed] [Google Scholar]
- 23.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics 1998;102:616-20. [DOI] [PubMed] [Google Scholar]
- 24.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013;368:2366-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galland B, Spruyt K, Dawes P, et al. Sleep Disordered Breathing and Academic Performance: A Meta-analysis. Pediatrics 2015;136:e934-46. [DOI] [PubMed] [Google Scholar]
- 26.Kheirandish-Gozal L, Yoder K, Kulkarni R, et al. Preliminary functional MRI neural correlates of executive functioning and empathy in children with obstructive sleep apnea. Sleep 2014;37:587-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin R, Somers VK, McConnell K, et al. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension 2008;51:84-91. [DOI] [PubMed] [Google Scholar]
- 28.Amin RS, Carroll JL, Jeffries JL, et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med 2004;169:950-6. [DOI] [PubMed] [Google Scholar]
- 29.Evans CA, Selvadurai H, Baur LA, et al. Effects of obstructive sleep apnea and obesity on exercise function in children. Sleep 2014;37:1103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldbart AD, Levitas A, Greenberg-Dotan S, et al. B-type natriuretic peptide and cardiovascular function in young children with obstructive sleep apnea. Chest 2010;138:528-35. [DOI] [PubMed] [Google Scholar]
- 31.Gozal D, Kheirandish-Gozal L, Serpero LD, et al. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation 2007;116:2307-14. [DOI] [PubMed] [Google Scholar]
- 32.Loffredo L, Zicari AM, Occasi F, et al. Endothelial dysfunction and oxidative stress in children with sleep disordered breathing: role of NADPH oxidase. Atherosclerosis 2015;240:222-7. [DOI] [PubMed] [Google Scholar]
- 33.Quante M, Wang R, Weng J, et al. The Effect of Adenotonsillectomy for Childhood Sleep Apnea on Cardiometabolic Measures. Sleep 2015;38:1395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 2008;177:369-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zong J, Liu Y, Huang Y, et al. Serum lipids alterations in adenoid hypertrophy or adenotonsillar hypertrophy children with sleep disordered breathing. Int J Pediatr Otorhinolaryngol 2013;77:717-20. [DOI] [PubMed] [Google Scholar]
- 36.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med 2007;176:401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med 2008;177:1142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kheirandish-Gozal L, Sans Capdevila O, Kheirandish E, et al. Elevated serum aminotransferase levels in children at risk for obstructive sleep apnea. Chest 2008;133:92-9. [DOI] [PubMed] [Google Scholar]
- 39.Nobili V, Cutrera R, Liccardo D, et al. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med 2014;189:66-76. [DOI] [PubMed] [Google Scholar]
- 40.Sundaram SS, Sokol RJ, Capocelli KE, et al. Obstructive sleep apnea and hypoxemia are associated with advanced liver histology in pediatric nonalcoholic fatty liver disease. J Pediatr 2014;164:699-706.e1. [DOI] [PMC free article] [PubMed]
- 41.Gozal D, Serpero LD, Kheirandish-Gozal L, et al. Sleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathing. Sleep 2010;33:319-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalyfa A, Serpero LD, Kheirandish-Gozal L, et al. TNF-α gene polymorphisms and excessive daytime sleepiness in pediatric obstructive sleep apnea. J Pediatr 2011;158:77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeyakumar A, Rahman SI, Armbrecht ES, et al. The association between sleep-disordered breathing and enuresis in children. Laryngoscope 2012;122:1873-7. [DOI] [PubMed] [Google Scholar]
- 44.Sans Capdevila O, Crabtree VM, Kheirandish-Gozal L, et al. Increased morning brain natriuretic peptide levels in children with nocturnal enuresis and sleep-disordered breathing: a community-based study. Pediatrics 2008;121:e1208-14. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell RB, Garetz S, Moore RH, et al. The use of clinical parameters to predict obstructive sleep apnea syndrome severity in children: the Childhood Adenotonsillectomy (CHAT) study randomized clinical trial. JAMA Otolaryngol Head Neck Surg 2015;141:130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Academy of Sleep Medicine. ICSD-3, International Classification of Sleep Disorders: Diagnostic and coding manual. 3rd ed. 2014. [Google Scholar]
- 47.Nixon GM, Brouillette RT. Diagnostic techniques for obstructive sleep apnoea: is polysomnography necessary? Paediatr Respir Rev 2002;3:18-24. [DOI] [PubMed] [Google Scholar]
- 48.Alonso Alvarez ML, Terán Santos J, Cordero Guevara JA, et al. Reliability of respiratory polygraphy for the diagnosis of sleep apnea-hypopnea syndrome in children. Arch Bronconeumol 2008;44:318-23. [PubMed] [Google Scholar]
- 49.Tan HL, Gozal D, Ramirez HM, et al. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Sleep 2014;37:255-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso-Álvarez ML, Terán-Santos J, Ordax Carbajo E, et al. Reliability of home respiratory polygraphy for the diagnosis of sleep apnea in children. Chest 2015;147:1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg 2007;133:216-22. [DOI] [PubMed] [Google Scholar]
- 52.Villa MP, Paolino MC, Castaldo R, et al. Sleep clinical record: an aid to rapid and accurate diagnosis of paediatric sleep disordered breathing. Eur Respir J 2013;41:1355-61. [DOI] [PubMed] [Google Scholar]
- 53.Kaditis A, Kheirandish-Gozal L, Gozal D. Algorithm for the diagnosis and treatment of pediatric OSA: a proposal of two pediatric sleep centers. Sleep Med 2012;13:217-27. [DOI] [PubMed] [Google Scholar]
- 54.Siegfried W, Siegfried A, Rabenbauer M, et al. Snoring and Sleep Apnea in Obese Adolescents: Effect of Long-term Weight Loss-Rehabilitation. Sleep Breath 1999;3:83-8. [DOI] [PubMed] [Google Scholar]
- 55.Verhulst SL, Franckx H, Van Gaal L, et al. The effect of weight loss on sleep-disordered breathing in obese teenagers. Obesity (Silver Spring) 2009;17:1178-83. [DOI] [PubMed] [Google Scholar]
- 56.Tsaoussoglou M, Hatzinikolaou S, Baltatzis GE, et al. Expression of leukotriene biosynthetic enzymes in tonsillar tissue of children with obstructive sleep apnea: a prospective nonrandomized study. JAMA Otolaryngol Head Neck Surg 2014;140:944-50. [DOI] [PubMed] [Google Scholar]
- 57.Goldbart AD, Goldman JL, Veling MC, et al. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med 2005;172:364-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics 2008;122:e149-55. [DOI] [PubMed] [Google Scholar]
- 59.Brietzke SE, Gallagher D. The effectiveness of tonsillectomy and adenoidectomy in the treatment of pediatric obstructive sleep apnea/hypopnea syndrome: a meta-analysis. Otolaryngol Head Neck Surg 2006;134:979-84. [DOI] [PubMed] [Google Scholar]
- 60.Friedman M, Wilson M, Lin HC, et al. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg 2009;140:800-8. [DOI] [PubMed] [Google Scholar]
- 61.Zagólski O. Why do palatine tonsils grow back after partial tonsillectomy in children? Eur Arch Otorhinolaryngol 2010;267:1613-7. [DOI] [PubMed] [Google Scholar]
- 62.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med 2010;182:676-83. [DOI] [PubMed] [Google Scholar]
- 63.Katyal V, Pamula Y, Daynes CN, et al. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing and changes in quality of life with rapid maxillary expansion. Am J Orthod Dentofacial Orthop 2013;144:860-71. [DOI] [PubMed] [Google Scholar]
- 64.Villa MP, Rizzoli A, Rabasco J, et al. Rapid maxillary expansion outcomes in treatment of obstructive sleep apnea in children. Sleep Med 2015;16:709-16. [DOI] [PubMed] [Google Scholar]
- 65.Carvalho FR, Lentini-Oliveira D, Machado MA, et al. Oral appliances and functional orthopaedic appliances for obstructive sleep apnoea in children. Cochrane Database Syst Rev 2007;(2):CD005520. [DOI] [PubMed] [Google Scholar]
- 66.Huynh NT, Desplats E, Almeida FR. Orthodontics treatments for managing obstructive sleep apnea syndrome in children: A systematic review and meta-analysis. Sleep Med Rev 2016;25:84-94. [DOI] [PubMed] [Google Scholar]
- 67.Simonds AK, editor. ERS Practical Handbook of Noninvasive Ventilation. European Respiratory Society, 2015. [Google Scholar]
- 68.Tahiri Y, Viezel-Mathieu A, Aldekhayel S, et al. The effectiveness of mandibular distraction in improving airway obstruction in the pediatric population. Plast Reconstr Surg 2014;133:352e-359e. [DOI] [PubMed] [Google Scholar]
- 69.Kremer B, Botos-Kremer AI, Eckel HE, et al. Indications, complications, and surgical techniques for pediatric tracheostomies--an update. J Pediatr Surg 2002;37:1556-62. [DOI] [PubMed] [Google Scholar]
- 70.Cohen SR, Suzman K, Simms C, et al. Sleep apnea surgery versus tracheostomy in children: an exploratory study of the comparative effects on quality of life. Plast Reconstr Surg 1998;102:1855-64. [DOI] [PubMed] [Google Scholar]
- 71.Katz ES, Moore RH, Rosen CL, et al. Growth after adenotonsillectomy for obstructive sleep apnea: an RCT. Pediatrics 2014;134:282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amin R, Anthony L, Somers V, et al. Growth velocity predicts recurrence of sleep-disordered breathing 1 year after adenotonsillectomy. Am J Respir Crit Care Med 2008;177:654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chervin RD, Ellenberg SS, Hou X, et al. Prognosis for Spontaneous Resolution of OSA in Children. Chest 2015;148:1204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khalyfa A, Gozal D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J Transl Med 2014;12:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kheirandish-Gozal L, Gileles-Hillel A, Alonso-Álvarez ML, et al. Effects of adenotonsillectomy on plasma inflammatory biomarkers in obese children with obstructive sleep apnea: A community-based study. Int J Obes (Lond) 2015;39:1094-100. [DOI] [PMC free article] [PubMed] [Google Scholar]